Abstract
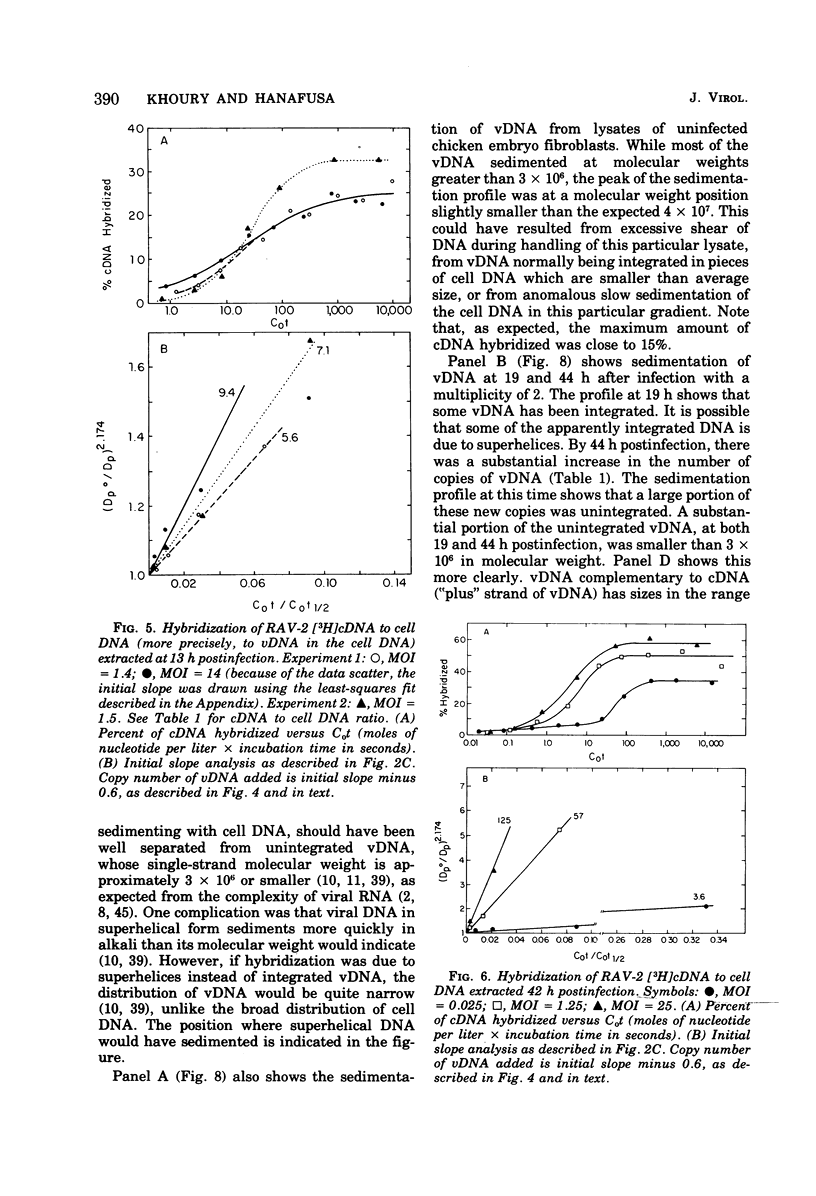
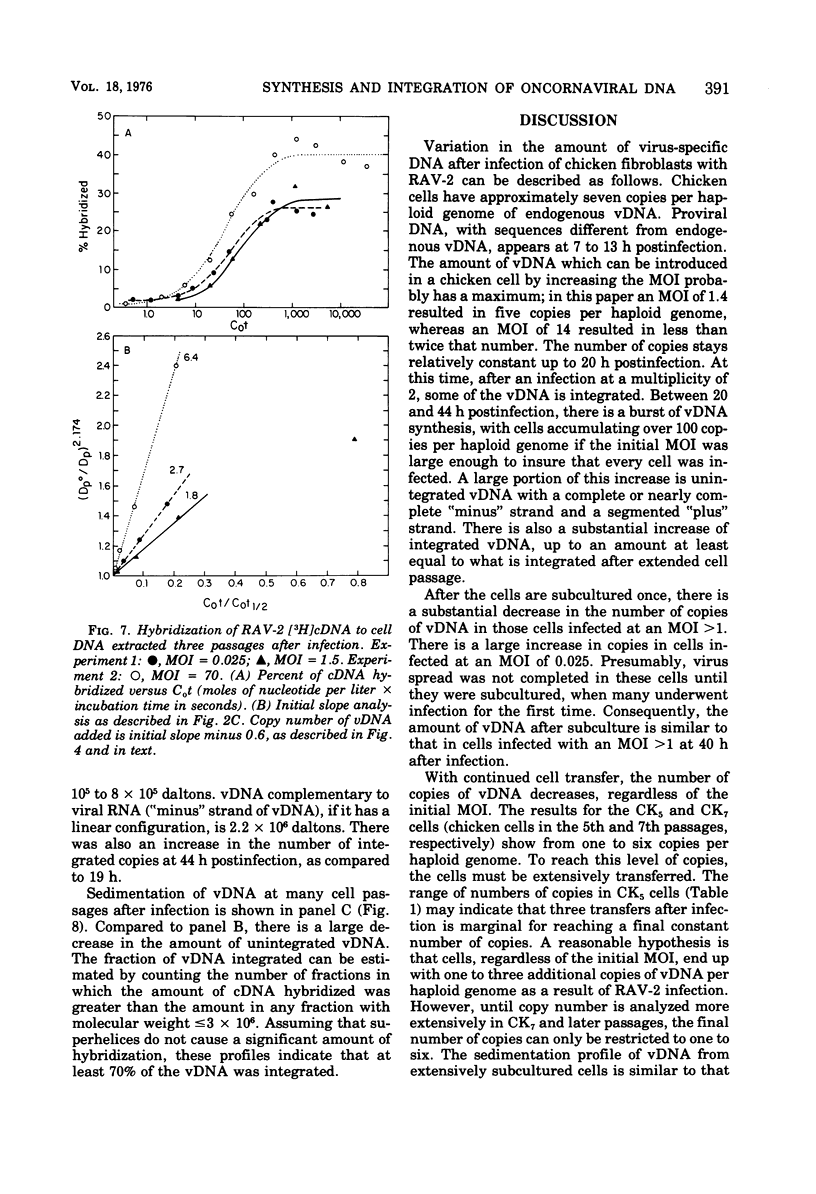
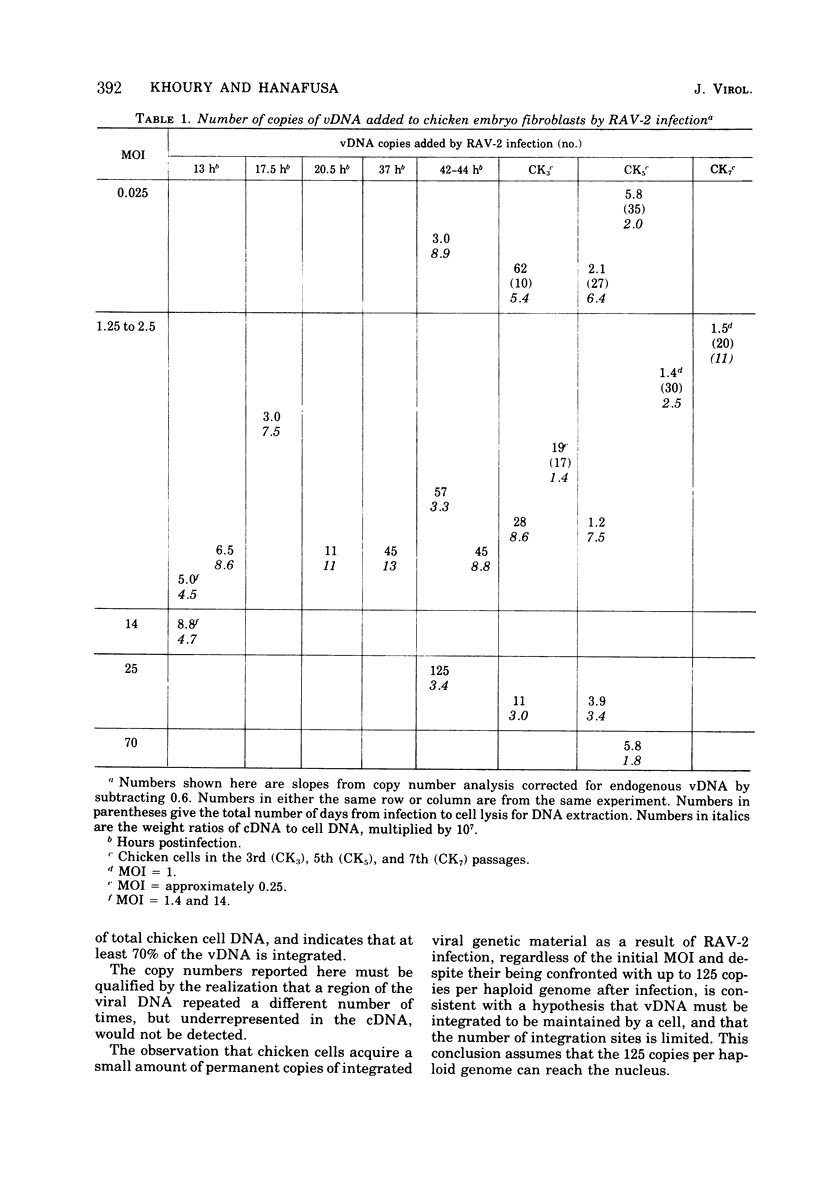
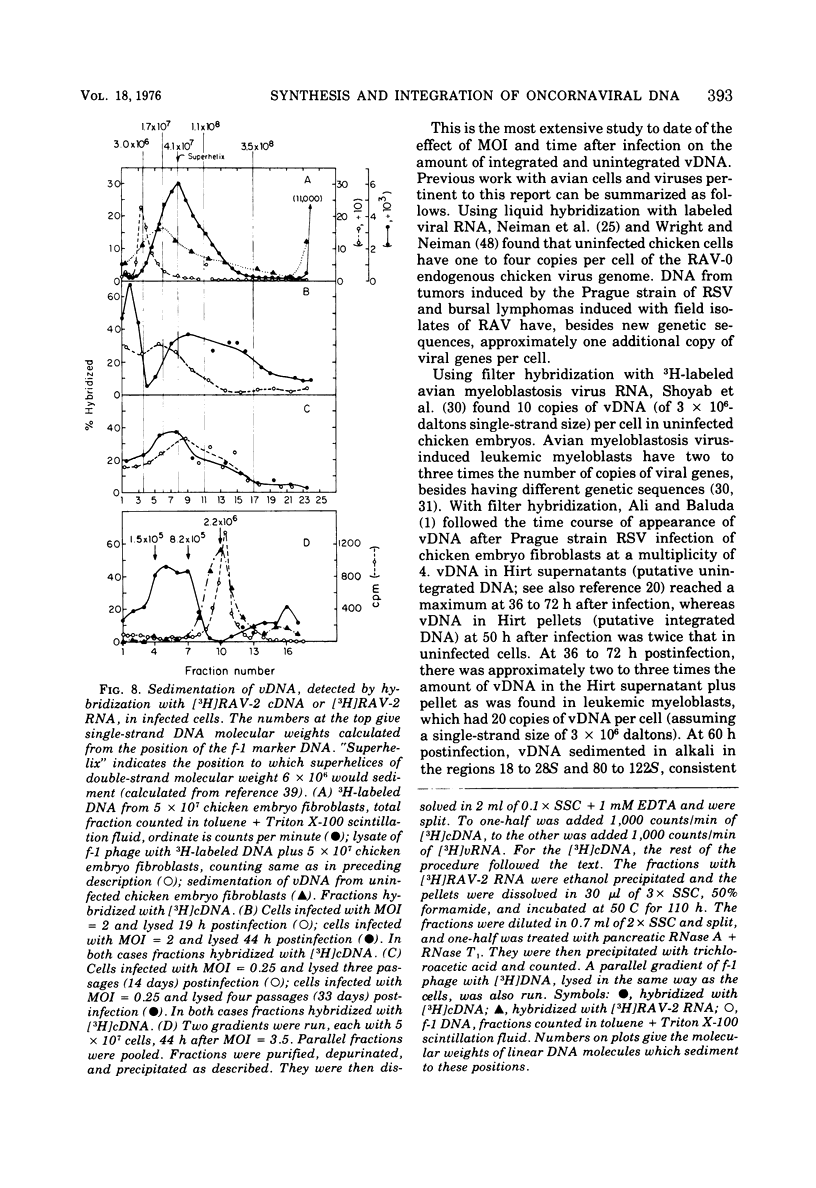
To see if integration of the provirus resulting from RNA tumor virus infection is limited to specific sites in the cell DNA, the variation in the number of copies of virus-specific DNA produced and integrated in chicken embryo fibroblasts after RAV-2 infection with different multiplicities has been determined at short times, long times, and several transfers after infection. The number of copies of viral DNA in cells was determined by initial hybridization kinetics of single-stranded viral complementary DNA with a moderate excess of cell DNA. The approach took into account the different sizes of cell DNA and complementary DNA in the hybridization mixture. It was found that uninfected chicken embryo fibroblasts have approximately seven copies, part haploid genome of DNA sequences homologous to part of the Rous-association virus 2 (RAV-2) genome. Infection with RAV-2 adds additional copies, and different sequences, of RAV -2- specific DNA. By 13 h postinfection, there are 3 to 10 additional copies per haploid genome. This number can not be increased by increasing the multiplicity of infection, and stays relatively constant up to 20 h postinfection, when some of the additional viral DNA is integrated. Between 20 and 40 h postinfection, the cells accumulated up to 100 copies per haploid genome of viral DNA. Most of these are unintegrated. This number decreases with cell transfer, until cells are left with one to three copies of additional viral DNA sequences per haploid genome, of which most are integrated. The finding that viral infection causes the permanent addition of one to three copies of integrated viral DNA, despite the cells being confronted with up to 100 copies per haploid genome after infection, is consistent with a hypothesis that chicken cells contain a limited number of specific integration sites for the oncornavirus genome.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M., Baluda M. A. Synthesis of avian oncornavirus DNA in infected chicken cells. J Virol. 1974 May;13(5):1005–1013. doi: 10.1128/jvi.13.5.1005-1013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Shoyab M., Markham P. D., Evans R. M., Droham W. N. Base sequence complexity of 35S avian myeloblastosis virus RNA determined by molecular hybridization kinetics. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):869–874. doi: 10.1101/sqb.1974.039.01.101. [DOI] [PubMed] [Google Scholar]
- Bishop J. O. Molecular hybridization of ribonucleic acid with a large excess of deoxyribonucleic acid. Biochem J. 1972 Jan;126(1):171–185. doi: 10.1042/bj1260171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D., Temin H. M. Light inactivation of focus formation by chicken embryo fibroblasts infected with avian sarcoma virus in the presence of 5-bromodeoxyuridine. Nature. 1970 Nov 14;228(5272):622–624. doi: 10.1038/228622a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Duesberg P., Vogt P. K., Beemon K., Lai M. Avian RNA tumor viruses: mechanism of recombination and complexity of the genome. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):847–857. doi: 10.1101/sqb.1974.039.01.099. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., McKerlie M. L., Salzman N. P. Characterization of Simian virus 40 DNA component II during viral DNA replication. J Mol Biol. 1973 Feb 25;74(2):95–111. doi: 10.1016/0022-2836(73)90101-0. [DOI] [PubMed] [Google Scholar]
- Gianni A. M., Smotkin D., Weinberg R. A. Murine leukemia virus: detection of unintegrated double-stranded DNA forms of the provirus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):447–451. doi: 10.1073/pnas.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni A. M., Weinberg R. A. Partially single-stranded form of free Moloney viral DNA. Nature. 1975 Jun 19;255(5510):646–648. doi: 10.1038/255646a0. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Mahy B. W., Bishop J. M., Varmus H. E. Ethidium bromide inhibits appearance of closed circular viral DNA and integration of virus-specific DNA in duck cells infected by avian sarcoma virus. Nature. 1975 Feb 13;253(5492):507–511. doi: 10.1038/253507a0. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS. 3. DETERMINING INFLUENCE OF A NEW HELPER VIRUS ON THE HOST RANGE AND SUSCEPTIBILITY TO INTERFERENCE OF RSV. Virology. 1965 Feb;25:248–255. doi: 10.1016/0042-6822(65)90203-5. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T., Kawai S. Genetic control of expression of endogenous virus genes in chicken cells. Virology. 1974 Apr;58(2):439–448. doi: 10.1016/0042-6822(74)90078-6. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Recombination between endogenous and exogenous RNA tumor virus genes as analyzed by nucleic acid hybridization. J Virol. 1975 Jun;15(6):1367–1377. doi: 10.1128/jvi.15.6.1367-1377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Plaque assay for some strains of avian leukosis virus. Virology. 1972 Apr;48(1):126–135. doi: 10.1016/0042-6822(72)90120-1. [DOI] [PubMed] [Google Scholar]
- Khoury A. T., Deering R. A. Sedimentation of DNA of Dictyostelium discoideum lysed on alkaline sucrose gradients: role of single-strand breaks in gamma ray lethality of sensitive and resistant strains. J Mol Biol. 1973 Sep 15;79(2):267–284. doi: 10.1016/0022-2836(73)90005-3. [DOI] [PubMed] [Google Scholar]
- Markham P. D., Baluda M. A. Integrated state of oncornavirus DNA in normal chicken cells and in cells transformed by avian myeloblastosis virus. J Virol. 1973 Oct;12(4):721–732. doi: 10.1128/jvi.12.4.721-732.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., McCarthy B. J. The interaction of oligodeoxynucleotides with denatured DNA. Biochim Biophys Acta. 1967 Nov 21;149(1):180–189. doi: 10.1016/0005-2787(67)90700-9. [DOI] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- Neiman P. E., Purchase H. G., Okazaki W. Chicken leukosis virus genome sequences in DNA from normal chick cells and virus-induced bursal lymphomas. Cell. 1975 Apr;4(4):311–319. doi: 10.1016/0092-8674(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Wright S. E., Purchase H. G. Studies of the interrelationship of chicken leukosis virus and host cell genomes by RNA-DNA hybridzation. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):875–883. doi: 10.1101/sqb.1974.039.01.102. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. N., Robinson H. L., Robinson W. S., Hanafusa T., Hanafusa H. DNA in uninfected and virus-infected cells complementary to avian tumor virus RNA. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2336–2340. doi: 10.1073/pnas.68.10.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A., Evans R. Acquisition of new DNA sequences after infection of chicken cells with avian myeloblastosis virus. J Virol. 1974 Feb;13(2):331–339. doi: 10.1128/jvi.13.2.331-339.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A. Separation of DNA sequences complementary to the RNA of avian myeloblastosis virus from chicken DNA by alkaline cesium chloride density sedimentation. J Virol. 1973 Sep;12(3):534–537. doi: 10.1128/jvi.12.3.534-537.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Evans R. M., Baluda M. A. Presence in leukemic cells of avian myeloblastosis virus-specific DNA sequences absent in normal chicken cells. J Virol. 1974 Jul;14(1):47–49. doi: 10.1128/jvi.14.1.47-49.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus N. A., Bonner T. I. Temperature dependence of RNA-DNA hybridization kinetics. Biochim Biophys Acta. 1972 Aug 16;277(1):87–95. doi: 10.1016/0005-2787(72)90355-3. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Goodman N. C., Cho J. R., Ruprecht R. M., Redfield R. R., Spiegelman S. The presence of unique DNA sequences after viral induction of leukemia in mice. (RNA tumor virus-nucleic acid hybridization-insertion of viral DNA). Proc Natl Acad Sci U S A. 1974 May;71(5):1705–1709. doi: 10.1073/pnas.71.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M. Mixed infection with two types of Rous sarcoma virus. Virology. 1961 Feb;13:158–163. doi: 10.1016/0042-6822(61)90049-6. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Faras A. J., Varmus H. E., Levinson W. E., Bishop J. M. Ribonucleic acid directed deoxyribonucleic acid synthesis by the purified deoxyribonucleic acid polymerase of Rous sarcoma virus. Characterization of the enzymatic product. Biochemistry. 1972 Jun 6;11(12):2343–2351. doi: 10.1021/bi00762a021. [DOI] [PubMed] [Google Scholar]
- Tereba A., Skoog L., Vogt P. K. RNA tumor virus specific sequences in nuclear DNA of several avian species. Virology. 1975 Jun;65(2):524–534. doi: 10.1016/0042-6822(75)90057-4. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Deng C. T., Bishop J. M. Synthesis, structure and function of avian sarcoma virus-specific DNA in permissive and nonpermissive cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):987–996. doi: 10.1101/sqb.1974.039.01.113. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Fan W. J., Heasley S., Bishop J. M. Synthesis of viral DNA in the cytoplasm of duck embryo fibroblasts and in enucleated cells after infection by avian sarcoma virus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3874–3878. doi: 10.1073/pnas.71.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Bishop J. M. Use of DNA-DNA annealing to detect new virus-specific DNA sequences in chicken embryo fibroblasts after infection by avian sarcoma virus. J Virol. 1974 Oct;14(4):895–903. doi: 10.1128/jvi.14.4.895-903.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Levinson W. E., Bishop J. M. Extent of transcription by the RNA-dependent DNA polymerase of Rous sarcoma virus. Nat New Biol. 1971 Sep 1;233(35):19–21. doi: 10.1038/newbio233019a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Baltimore D. Covalent linkage between ribonucleic Acid primer and deoxyribonucleic Acid product of the avian myeloblastosis virus deoxyribonucleic Acid polymerase. J Virol. 1972 Oct;10(4):622–627. doi: 10.1128/jvi.10.4.622-627.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C., Parsons J. T., Coffin J. W., Rymo L., Billeter M. A., Hofstetter H. Studies on the structure and synthesis of Rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1043–1056. doi: 10.1101/sqb.1974.039.01.120. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G. Excluded volume effects on the rate of renaturation of DNA. Biopolymers. 1971;10(4):601–613. doi: 10.1002/bip.360100402. [DOI] [PubMed] [Google Scholar]
- Wright S. E., Neiman P. E. Base-sequence relationships between avian ribonucleic acid endogenous and sarcoma viruses assayed by competitive ribonucleic acid-deoxyribonucleic acid hybridization. Biochemistry. 1974 Mar 26;13(7):1549–1554. doi: 10.1021/bi00704a035. [DOI] [PubMed] [Google Scholar]



