Abstract
Background.
Impaired functional status attenuates the relationship of systolic blood pressure (SBP) with mortality in older adults but has not been studied in middle-aged populations.
Method.
Among 10,264 stroke-free Atherosclerosis Risk in Communities participants (mean age 62.8 [5.7] years; 6,349 [62%] younger [<65 years]; 5,148 [50%] men; 2,664 [26%] Black), function was defined as good function (GF) for those self-reporting no difficulty performing functional tasks and basic or instrumental tasks of daily living; all others were defined as impaired function (IF). SBP categories were normal (<120 mmHg), prehypertension (120–139 mmHg), and hypertension (≥140 mmHg). Mortality risk associated with SBP was estimated using adjusted Cox proportional hazard models with a triple interaction between age, functional status, and SBP.
Results.
Mean follow-up was 12.9 years with 2,863 (28%) deaths. Among younger participants, 3,017 (48%) had IF; 2,279 of 3,915 (58%) older participants had IF. Prehypertension (hazard ratio [HR] = 1.48 [1.03, 2.15] p = .04) and hypertension (HR = 1.97 [1.29, 3.03] p = .002) were associated with mortality in younger GF and older (≥65 years) GF participants (prehypertension HR = 1.21 [1.06, 1.37] p = .005; hypertension HR = 1.47 [1.36, 1.59] p < .001). Among IF participants, prehypertension was not associated with mortality in younger participants (HR = 0.99 [0.85, 1.15] p = .93) and was protective in older participants (HR = 0.87 [0.85, 0.90] p < .001). Hypertension was associated with mortality in younger IF participants (HR = 1.54 [1.30, 1.82] p < .001) but not in older IF participants (HR = 0.99 [0.87, 1.14] p = .93).
Conclusions.
Compared with younger and well-functioning persons, the additional contribution of blood pressure to mortality is much lower with older age and impaired function, particularly if both are present. Functional status and age could potentially inform optimal blood pressure targets.
Key Words: Blood pressure, Mortality, Middle aged, Functional status.
The association between blood pressure (BP) and mortality in older adults remains controversial, with several studies reporting U-shaped relationships representing higher mortality risk among those with the lowest and highest BP, particularly among the oldest old (1–4). The Eighth Joint National Commission (JNC) hypertension (HTN) guidelines (5) sparked controversy (6,7) with recommendations to raise the target systolic BP (SBP) for initiating and control of HTN to 150 mmHg in persons 60 years or older without diabetes or kidney disease. The debate over BP control is not new (8,9), despite randomized controlled trials demonstrating benefits of lowering BP in hypertensive persons older than 60 years (10–12) and in hypertensive persons 80 years and older in the Hypertension in the Very Elderly Trial (HYVET) (13). Opposing arguments cite attenuated benefit or even increased mortality among older participants in treatment arms of clinical trials of HTN (10–12,14,15), associations of higher BP with better outcomes among older adults in cohort studies (16,17), and lack of generalizability to persons frequently encountered in clinical practice including those with multiple comorbidities, physical or cognitive disabilities, frailty, and those in nursing homes or assisted living facilities (8,18,19).
Age, however, may not be the best predictor of clinical risk. Measures of functional status are important vital signs of health in older adults (20), are associated with mortality and other adverse outcomes, and modify life expectancy (21,22) in older adults. Recent reports demonstrate that self-reported (fast, medium, and slow) (23) and objectively measured walking speed (24) modifies the relationship of BP with mortality in older adults, with no association observed between higher BP and mortality among slow walkers but a significantly higher risk of mortality among faster walkers with higher BP. These findings suggest that functional measures may be informative for understanding risks and benefits associated with HTN and HTN treatment in older persons. If functional measures provide information on mortality risk apart from age alone, functional status may also modify the relationship of BP to mortality in younger as well as older persons. The effect of functional status on the relationship of BP to mortality has not been examined in middle-aged populations.
The aim of this study was to examine the effects of age and functional status on the relationship of SBP to all-cause mortality in a middle-aged and older biracial population.
Method
Population
The Atherosclerosis Risk in Communities (ARIC) is a prospective study of a community-dwelling cohort of men and women at four sites in the United States (Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD) designed to investigate the natural history of atherosclerosis as previously reported (25). At baseline (1987–1989), 15,792 participants were sampled; participants were predominantly White in MD and MN. All participants in Jackson, MS were African American; African Americans were oversampled in Forsyth County to facilitate race-specific analyses. This analysis included 11,656 participants from the fourth exam (1997–1999), which was considered the baseline for this study, when self-reported functional status was ascertained. Those with prevalent stroke at the time of the functional assessment (n = 709) were excluded as patients with stroke may differ from those without stroke regarding risk associated with BP; participants who developed stroke after the index examination were not excluded. Another 683 were missing functional status data, leaving 10,264 for the current analysis.
Functional Status and Disability Definitions
Functional status was assessed using standardized questionnaires to ascertain the level of difficulty (None, Some, A lot, and Unable) performing functional measures (26,27) and instrumental and basic activities of daily living adapted from validated questionnaires (26–29). Participants were asked how much difficulty they have performing each of the following: walking room to room; transferring; dressing; feeding oneself; standing from a chair; walking ¼ mile; walking up 10 steps; stooping, crouching, kneeling; lifting or carrying 10 pounds; doing housework; preparing meals; and managing money. Good function (GF) was assigned to participants reporting “No difficulty” on all questions and impaired function (IF) otherwise.
Mortality
ARIC mortality surveillance has been previously described and was completed through December 31, 2011 (25). All participants or their proxies are contacted annually by phone. Deaths were identified through records obtained from hospitals in the ARIC surveillance catchment areas, death certificates, and interviews of next of kin for potential out-of-hospital fatal events. Death certificates from state vital statistics offices were obtained on an ongoing basis. A questionnaire was also sent to participants’ physicians to confirm out-of-hospital deaths.
BP Measurements
The average of the second and third measurements of resting BP, assessed by standardized protocols with a random-zero sphygmomanometer, was used for this analysis. SBP was analyzed as both a continuous and a categorical variable according to the American Heart Association recommendations for normal (<120mm Hg), pre-HTN (120–139 mmHg), and HTN (≥140mm Hg) (30).
Covariates
Self-reported demographics, education, alcohol use, and smoking status were ascertained using standardized interviews. Medications were recorded at each study visit. Body mass index (kg/m2) was calculated from weight and height with participants wearing lightweight clothes. Heart disease (31), stroke (32), and heart failure (33,34) were ascertained as previously described using self-report, medical records, and standardized questionnaires. Laboratory assays, including lipids, were obtained using standardized protocols; glucose was measured with the hexokinase method. Diabetes was defined as a fasting glucose level ≥126mg/dl, a random glucose level ≥200mg/dl, or current use of hypoglycemic medications. A diagnosis of HTN (for descriptive purposes) was defined as use of antihypertensive medications, SBP ≥140 or diastolic BP ≥90.
Statistical Analysis
Descriptive statistics were examined using Student’s t tests and Pearson’s chi-squared tests for continuous and categorical variables, respectively. Cox proportional hazard models with three-way interactions between functional status, SBP, and age (p < .001 for triple interaction with continuous SBP) were used along with all lower-level two-way interactions; the results were more conservative compared with those from models using two-way interactions alone. The assumption of proportionality was examined visually with log-log-survival plots and supported using Schoenfeld residual tests. Sensitivity analyses were performed using Weibull distribution accelerated failure time models which generated the same conclusions. Kaplan–Meier plots were constructed to show cumulative incidence curves. Hazard ratios (HRs) for continuous SBP were modeled using flexible parametric restricted cubic-spline survival models (35). Models were adjusted for race, sex, body mass index, smoking, diabetes, heart disease, heart failure, statin use, antihypertensive medications, and race-clustering by site was accounted for using Huber White sandwich estimators. Similar results were found with simple race- and sex-adjusted models. Sex- and race-stratified analyses were examined, and moderating effects were not supported (p value for all interactions > .15). All analyses were performed with Stata v13.1 (StataCorp, College Station, TX).
Results
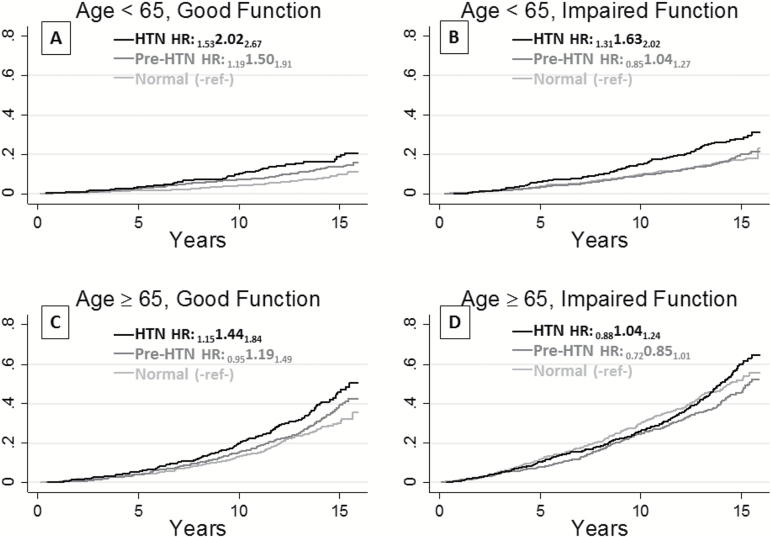
Table 1 shows characteristics of younger (<65 years) and older (≥65 years) participants stratified by Visit 4 functional status. The mean follow-up was 12.9 years. In both younger and older participants, IF was associated with older age, African American race, generally poorer health measures, and death. IF was associated with higher SBP, more so in younger than older participants, whereas IF was associated with lower diastolic BP in older participants. Figure 1 shows unadjusted, cumulative mortality incidence for the normotensive, pre-HTN, and HTN groups by age–functional status; crude HRs were similar to adjusted HRs. Mortality risk was higher in the older and IF groups.
Table 1.
Population Characteristics at the Baseline Functional Assessment (ARIC Visit 4) by Age and Functional Status
| Variable | Age < 65 Years | Age ≥ 65 Years | ||||
|---|---|---|---|---|---|---|
| Good Function (N = 3,332) | Impaired function (N = 3,017) | p Value | Good Function (N = 1,636) | Impaired Function (N = 2,279) | p Value | |
| Age, mean (SD) | 58.7 (3.2) | 59.0 (3) | <.001 | 68.5 (3) | 68.9 (3) | <.001 |
| Black, n (%) | 588 (18) | 864 (29) | <.001 | 192 (12) | 436 (19) | <.001 |
| Education, n (%) | ||||||
| <12 Years | 333 (10) | 547 (18) | <.001 | 319 (20) | 559 (25) | <.001 |
| 12 Years | 1065 (32) | 1127 (37) | 529 (32) | 742 (33) | ||
| Some college | 278 (8) | 277 (9) | 139 (9) | 216 (9) | ||
| ≥College degree | 1650 (50) | 1064 (35) | 646 (40) | 759 (33) | ||
| Male, n (%) | 1657 (50) | 970 (32) | <.001 | 924 (56) | 895 (39) | <.001 |
| Systolic BP, mmHg mean (SD) | 122.7 (17) | 126.0 (18) | <.001 | 130.4 (19) | 131.4 (19) | .128 |
| Diastolic BP, mmHg mean (SD) | 72.1 (9.7) | 72.1 (10) | .934 | 69.6 (10) | 68.6 (10) | .002 |
| Total cholesterol, mmol/L mean (SD) | 5.18 (0.9) | 5.24 (1.0) | .012 | 5.19 (0.9) | 5.20 (1.0) | .657 |
| HDL, mmol/L mean (SD) | 1.31 (0.4) | 1.30 (0.4) | .222 | 1.28 (0.4) | 1.28 (0.4) | .930 |
| LDL, mmol/L mean (SD) | 3.17 (0.9) | 3.19 (0.9) | .251 | 3.20 (0.8) | 3.16 (0.9) | .172 |
| Triglycerides, (mmol/L) mean (SD) | 1.55 (0.9) | 1.67 (1.0) | <.001 | 1.59 (1.0) | 1.68 (0.9) | .004 |
| BMI, kg/m2 mean (SD) | 27.5 (4.6) | 30.7 (6.4) | <.001 | 26.9 (4.1) | 29.2 (5.7) | <.001 |
| Current smoker n (%) | 477 (14) | 549 (18) | <.001 | 160 (10) | 269 (12) | .046 |
| Current alcohol use, n (%) | 2031 (61) | 1373 (46) | <.001 | 843 (52) | 945 (42) | <.001 |
| Hypertension, n (%) | 1,126 (34) | 1,444 (48) | <.001 | 761 (47) | 1,331 (59) | <.001 |
| Diabetes, n (%) | 329 (10) | 572 (19) | <.001 | 228 (14) | 436 (19) | <.001 |
| Heart disease, n (%) | 154 (5) | 208 (7) | <.001 | 154 (10) | 268 (12) | .015 |
| Heart failure, n (%) | 22 (1) | 77 (3) | <.001 | 18 (1) | 88 (4) | <.001 |
| Statins, n (%) | 308 (9) | 290 (10) | .600 | 214 (13) | 323 (14) | .317 |
| Hypertension meds, n (%) | 822 (25) | 1,160 (39) | <.001 | 522 (32) | 1,053 (47) | <.001 |
| Deaths, n (%) | 365 (11) | 519 (17) | <.001 | 477 (29) | 866 (38) | <.001 |
Note: ARIC = Atherosclerosis Risk in Communities; BMI = body mass index; BP = blood pressure; HDL = high density lipoprotein; LDL = low density lipoprotein.
Figure 1.
Kaplan–Meier (unadjusted) cumulative mortality incidence by systolic blood pressure group and hazard ratios for prehypertension (Pre-HTN: 120–140 mmHg) and hypertension (HTN: ≥ 140 mmHg) compared with normal blood pressure (<120 mmHg) for the same age group and functional status.
Participants With Good Functional Status
Table 2 shows the adjusted risk of death associated with BP categories comparing across the same age and functional groups, using normal SBP (<120mm Hg) as the reference. Among participants with GF, pre-HTN was associated with a 48% increase in mortality risk in younger persons, HR = 1.48 (95% confidence interval: 1.03, 2.15) p = .036, and a 21% increase in mortality risk in older persons, HR = 1.21 (1.06, 1.37) p = .005. GF younger participants with HTN (SBP ≥ 140) showed a doubling of mortality risk compared with normal SBP in younger persons, HR = 1.97 (1.29, 3.03) p = .002, and a 50% increase in mortality risk in older persons, HR = 1.47 (1.36, 1.59) p < .001, showing some attenuation of, but still statistically and clinically meaningful, increased mortality risk among the older participants. Despite attenuation of risk associated with higher BP in the older groups, differences in absolute risks associated with higher SBP were comparable for those with GF. For example, the absolute risk among the younger GF group with HTN versus normal SBP was 18% versus 9% (absolute risk difference 9%); for the older group with GF, risk among those with HTN versus normal SBP was 37% and 27%, respectively, an absolute risk difference of 10%.
Table 2.
Adjusted Hazard Ratios Estimating Mortality Risk Associated With Pre-HTN and HTN Compared With Normal Blood Pressure in the Same Age and Functional Status Groups
| Functional Group | HTN Group | Younger Participants | Older Participants | ||
|---|---|---|---|---|---|
| Deaths | Hazard Ratio | Deaths | Hazard Ratio | ||
| Good function | Normal | 139/1,569 = 9% | Reference | 137/517 = 27% | Reference |
| Pre-HTN | 165/1,314 = 13% | 1.48, p = .04 (1.03, 2.15) | 217/710 = 31% | 1.21, p = .005 (1.06, 1.37) | |
| HTN | 95/543 = 18% | 1.97, p = .002 (1.29, 3.03) | 190/517 = 37% | 1.47, p < .001 (1.36, 1.59) | |
| Impaired function | Normal | 199/1246 = 16% | Reference | 271/658 = 41% | Reference |
| Pre-HTN | 206/1,249 = 16% | 0.99, p = .930 (0.85, 1.15) | 402/1,066 = 38% | 0.87, p < .001 (0.85, 0.90) | |
| HTN | 178/679 = 26% | 1.54, p < .001 (1.30, 1.82) | 358/809 = 44% | 0.99, p = .930 (0.87, 1.14) | |
Notes: Models adjusted for race, sex, body mass index, smoking, diabetes, heart disease, heart failure, statin use, antihypertensive medications, and clustering by site.
HTN = Hypertension.
Participants With Impaired Functional Status
Among those with IF, pre-HTN was not associated with mortality in younger participants, HR = 0.99 (0.85, 1.15) p = .93, and was protective in older participants, HR = 0.87 (0.85, 0.90) p < .001. HTN remained associated with mortality in younger IF participants, HR = 1.54 (1.30, 1.82) p < .001, but was not associated with mortality in older IF participants, HR = 0.99 (0.87, 1.14) p = .93. Notably, the confidence intervals for older IF participants did not overlap with confidence intervals for older GF participants, supporting differences in associations of HTN with mortality between the older IF and the older GF groups. Differences in absolute risks associated with HTN for younger IF participants were similar to differences observed in the GF groups but not among older participants with IF. The absolute risk among the younger IF group with HTN versus normal SBP was 26% and 16%, respectively (absolute risk difference 10%). Conversely, for the older group with IF, risk for those with HTN versus normal SBP was 44% and 41%, respectively, an absolute risk difference of 3%. Thus, the absolute risk was higher in older persons and in those with IF. However, the absolute mortality increase attributed to HTN was similar and around 10% in younger participants, regardless of functional status, and in older participants with GF, whereas the absolute difference was much lower (3%) and statistically nonsignificant in older participants with IF. Consistent results were found in sensitivity analyses including the 709 participants with prevalent strokes.
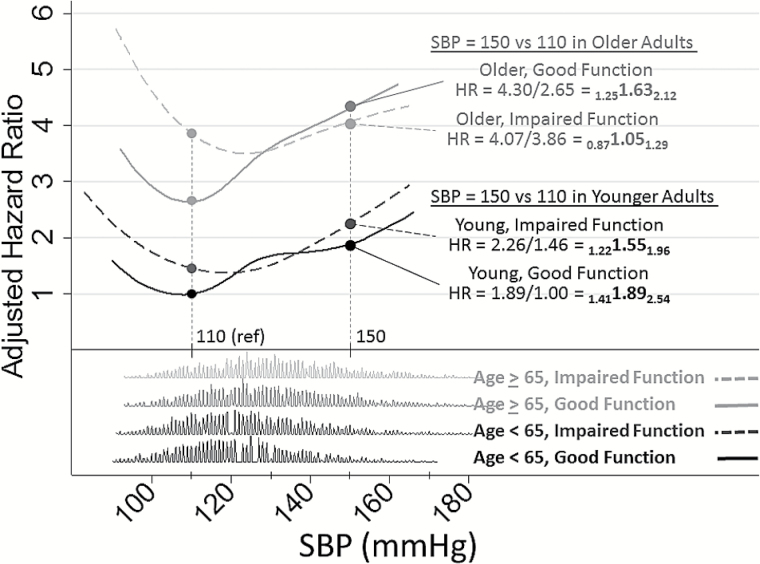
Figure 2 illustrates the estimated nonlinear relationships of continuous SBP with mortality by age and functional status. Median SBP values for each BP group were selected for displaying risk comparisons (SBP = 110 mmHg for those with SBP < 120 mmHg and SBP = 150 for those with SBP > 140 mmHg, vertical reference lines on plot). The x-axis shows continuous SBP along with distributions of SBP for each age–function group. The HRs for each of these groups are shown on the y-axis comparing any given SBP in each age–function group with the reference of a younger GF participant with SBP = 110 (HR = 1). For example, compared with a younger GF participant with SBP = 110 mmHg, an older GF participant with the same SBP had 2.6 times the mortality risk, but 4.3 times the risk for an older GF participant with SBP = 150 mmHg, indicating that the increase in risk for an older GF participant due to SBP was HR = 4.30/2.65 = 1.63 (95% confidence interval: 1.25, 2.12). Older IF adults had higher mortality risks at lower SBP and experienced a statistically nonsignificant 5% risk increase for the same SBP increase; HR = 1.05 (95% confidence interval: 0.87, 1.29). Comparing older GF versus IF adults, the increase in SBP showed a 35% lower effect on mortality risk for IF older adults; ratio of hazard ratio = 0.65 (0.60, 0.69) p < .001. Similar but attenuated reductions in SBP effects were seen comparing younger GF versus younger IF adults; ratio of hazard ratio = 0.82 (0.77, 0.86) p < .001. Of note, in older IF participants, we did not find support for increased mortality risk associated with HTN compared with normal SBP even when using a higher threshold of SBP = 160 mmHg, HR = 1.04 (0.76, 1.43), although the sample size was smaller (N = 741).
Figure 2.
Nonlinear relationships of continuous systolic blood pressure (SBP) with mortality by age and functional status. The x-axis shows continuous SBP along with distributions of SBP for each age–function group. The y-axis gives adjusted hazard ratios (HRs) for all-cause mortality across SBP by age group (<65 years vs ≥65 years) and function group (GF = good function, IF = impaired function) with reference group (HR = 1) set to those with age <65, GF and SBP = 110 mmHg. Shown are HRs with lower and upper confidence limits (LCLHRUCL) comparing SBP = 150 versus SBP = 110 for each of the four age/function groups. For example, compared with a younger GF participant with SBP = 110 mmHg, an older GF participant with the same SBP had 2.6 times the mortality risk, but 4.3 times the risk at SBP = 150 mmHg, indicating that the increase in risk for an older GF participant due to SBP was HR = 4.30/2.65 = 1.63 (95% confidence interval: 1.25, 2.12).
Discussion
In this study of middle-aged and young-old adults, both age and impaired self-reported functional status attenuated the association of SBP to mortality. In older persons and in those with IF, for whom the absolute mortality risk is high, the additional contribution of BP to mortality was much lower than in younger participants and in those with GF, although absolute risk differences were similar between older and younger persons with good functional status. The attenuation of risk associated with higher BP was especially evident both for those at older age and for those with IF. Clinicians could be misguided if using single BP targets for large segments of the population. The findings in this study suggest that both age and functional status can inform clinicians of risk associated with BP and suggest that developing a personalized approach to BP management that incorporates measurable factors, of which age and functional status appear to be important, may be a rational strategy.
In older persons and in those with impaired functional status, the reduced contribution of BP to mortality risk could be due in part to a higher risk of death from other conditions, for example, cancer and hip fractures. HTN also causes endovascular dysfunction that has been linked with cerebral hypoperfusion (36); long-standing hypoperfusion of the brain or other end organs could contribute to cell death, organ dysfunction, and mortality, potentially explaining associations of lower BP with adverse outcomes. Recent studies also suggest that lower SBP is associated with higher mortality (37) and more cognitive decline (38) in selected older populations. These findings emphasize the need to elucidate mechanisms for the heterogeneity of responses to different BP levels, especially in older persons, and to develop a more personalized approach to BP management in older populations.
A large proportion (nearly half) of study participants were described as having IF using our broad definition. If a large segment of a population with impaired physical function indeed gains little to no benefit from BP control to guideline standards, this could have an immense population health impact. We caution, however, that we only considered mortality outcomes, and uncontrolled HTN contributes to other devastating outcomes including stroke. Further studies should examine the ways in which more precise assessments of functional status might better define appropriate BP levels for subgroups in whom risks and benefits related to BP differ in mid and late life.
The current study builds upon prior studies that reported increased mortality associated with higher SBP among high-functioning adults aged 60 years or older but not among lower functioning older adults (23,24) by extending findings to middle-aged persons. Based on the collective findings, we suggest that risk associated with higher BP should not be aggregated by age thresholds but that age, functional status, and related life expectancies should be considered. Failure to account for modifying characteristics underestimates risks for some, overestimates for others, and may explain disconcordance across studies of relationships between BP or BP treatment and mortality.
Impaired functional status is related to mortality and other adverse outcomes (21,22) and may identify persons in whom risks of interventions outweigh benefits. Conversely, well-functioning older persons may benefit from procedures or treatments, including more aggressive BP control, similar to younger persons. For example, a high-functioning 80-year-old man whose life expectancy is 10 years might benefit from maintaining a SBP near 130 mmHg rather than 150 mmHg, but an 80-year-old man with IF whose life expectancy is 3 years might not live to see the benefit and may even have more adverse effects or potential harm. A growing literature cites the complexity of treating the older population and the need to consider multiple chronic conditions, time to benefit, increasing susceptibility to adverse drug effects and risk of disease, quality of life, and patient preferences (39). These considerations are often presented in the context of avoiding unnecessary interventions and burdens on older, frail persons who may not benefit and could be harmed (39). The protective direction of the association between pre-HTN and mortality, compared with the current definition of normal BP, in our study supports this view. The converse argument is equally important; high-functioning older adults with few clinical problems may achieve benefit from treatments similar to younger persons. Studies are needed to determine how functional assessments could inform clinicians of the most appropriate BP targets and how functional abilities might shift treatment targets across the life span.
Some limitations warrant discussion. Objective measures of functional status were not available and may be more sensitive than self-reported measures, particularly at higher levels of functional abilities. However, self-reported measures are widely available, acceptable in clinical settings, and are validated measures of function (29,40). The age range of ARIC participants (52–75 years at the baseline functional assessment) limits the ability to examine relationships in the oldest old, that is, in those aged 80 and older, the subpopulation in which HTN treatment has been most controversial. However, the current findings among middle-aged persons with IF add to the existing literature and should motivate further investigation in other populations. Potentially, among the oldest old with IF, higher BP targets may be beneficial although this remains to be defined. Additionally, the results could be useful in developing study designs and hypotheses to elucidate optimal treatment targets.
In summary, these findings emphasize the need to consider functional markers of age in addition to chronologic age in estimating risk of BP in both middle-aged and older adults and underscore the relevance of simple, clinically feasible measures of function.
Funding
This work was supported by the National Heart, Lung, and Blood Institute (HHSN268201100005C, HSN268201100006C, HHSN268 201100007C, HHSN268201100008C, HHSN268201100009C, H HSN268201100010C, HHSN268201100011C, HHSN268201100 012C).
Conflict of Interest
There are no conflicts for any of the authors.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
References
- 1. Langer RD, Criqui MH, Barrett-Connor EL, Klauber MR, Ganiats TG. Blood pressure change and survival after age 75. Hypertension. 1993;22:551–559. [DOI] [PubMed] [Google Scholar]
- 2. Boshuizen HC, Izaks GJ, van Buuren S, Ligthart GJ. Blood pressure and mortality in elderly people aged 85 and older: community based study. BMJ. 1998;316:1780–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mattila K, Haavisto M, Rajala S, Heikinheimo R. Blood pressure and five year survival in the very old. Br Med J (Clin Res Ed). 1988;296:887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hakala SM, Tilvis RS, Strandberg TE. Blood pressure and mortality in an older population. A 5-year follow-up of the Helsinki Ageing Study. Eur Heart J. 1997;18:1019–1023. [DOI] [PubMed] [Google Scholar]
- 5. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. doi:10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 6. Peterson ED, Gaziano JM, Greenland P. Recommendations for treating hypertension: what are the right goals and purposes? JAMA. 2014;311:474–476. doi:10.1016/j.ypmed.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 7. Ortiz E, Oparil S, James PA. Guidelines for managing high blood pressure–reply. JAMA. 2014;312:295–296. doi:10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 8. Goodwin JS. Embracing complexity: a consideration of hypertension in the very old. J Gerontol A Biol Sci Med Sci. 2003;58:653–658. [DOI] [PubMed] [Google Scholar]
- 9. Aronow WS. Commentary on “embracing complexity: a consideration of hypertension in the very old”. J Gerontol A Biol Sci Med Sci. 2003;58:659–660. [DOI] [PubMed] [Google Scholar]
- 10. Amery A, Birkenhager W, Brixko R, et al. Efficacy of antihypertensive drug treatment according to age, sex, blood pressure, and previous cardiovascular disease in patients over the age of 60. Lancet. 1986;2:589–592. [DOI] [PubMed] [Google Scholar]
- 11. Dahlöf B, Lindholm LH, Hansson L, Scherstén B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet. 1991;338:1281–1285. [DOI] [PubMed] [Google Scholar]
- 12. Staessen JA, Fagard R, Thijs L, et al. Subgroup and per-protocol analysis of the randomized European Trial on Isolated Systolic Hypertension in the Elderly. Arch Intern Med. 1998;158:1681–1691. [DOI] [PubMed] [Google Scholar]
- 13. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. doi:10.1056/NEJMoa0801369 [DOI] [PubMed] [Google Scholar]
- 14. Gueyffier F, Bulpitt C, Boissel JP, et al. Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. INDANA Group. Lancet. 1999;353:793–796. [DOI] [PubMed] [Google Scholar]
- 15. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi:10.1136/bmj.b1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peralta CA, Katz R, Newman AB, Psaty BM, Odden MC. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: the role of functional limitation in the Cardiovascular Health Study. Hypertension. 2014;64:472–480. doi:10.1161/HYPERTENSIONAHA.114.03831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabayan B, van Vliet P, de Ruijter W, Gussekloo J, de Craen AJ, Westendorp RG. High blood pressure, physical and cognitive function, and risk of stroke in the oldest old: the Leiden 85-plus Study. Stroke. 2013;44:15–20. doi:10.1161/STROKEAHA.112.663062 [DOI] [PubMed] [Google Scholar]
- 18. Douma S, Petidis K, Zamboulis C. Treatment of hypertension in the elderly. N Engl J Med. 2008;359:971–972. doi:10.1056/NEJMc081224 [DOI] [PubMed] [Google Scholar]
- 19. Messerli FH, Sulicka J, Gryglewska B. Treatment of hypertension in the elderly. N Engl J Med. 2008;359:972–973. [PubMed] [Google Scholar]
- 20. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32:46–49. [PubMed] [Google Scholar]
- 21. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keeler E, Guralnik JM, Tian H, Wallace RB, Reuben DB. The impact of functional status on life expectancy in older persons. J Gerontol A Biol Sci Med Sci. 2010;65:727–733. doi:10.1093/gerona/glq029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Odden MC, Covinsky KE, Neuhaus JM, Mayeda ER, Peralta CA, Haan MN. The association of blood pressure and mortality differs by self-reported walking speed in older Latinos. J Gerontol A Biol Sci Med Sci. 2012;67:977–983. doi:10.1093/gerona/glr245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Odden MC, Peralta CA, Haan MN, Covinsky KE. Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch Intern Med. 2012;172:1162–1168. doi:10.1001/archinternmed.2012.2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 26. Nagi S. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q. 1976;54:439–467. [PubMed] [Google Scholar]
- 27. Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556–559. [DOI] [PubMed] [Google Scholar]
- 28. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 29. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 30. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi:10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 31. Toole JF, Chambless LE, Heiss G, Tyroler HA, Paton CC. Prevalence of stroke and transient ischemic attacks in the Atherosclerosis Risk in Communities (ARIC) study. Ann Epidemiol. 1993;3:500–503. [DOI] [PubMed] [Google Scholar]
- 32. Chambless LE, Shahar E, Sharrett AR, et al. Association of transient ischemic attack/stroke symptoms assessed by standardized questionnaire and algorithm with cerebrovascular risk factors and carotid artery wall thickness. The ARIC Study, 1987–1989. Am J Epidemiol. 1996;144:857–866. [DOI] [PubMed] [Google Scholar]
- 33. Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. [DOI] [PubMed] [Google Scholar]
- 34. Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical activity and cardiovascular disease in African Americans in Atherosclerosis Risk in Communities. Med Sci Sports Exerc. 2013;45:901–907. doi:10.1249/MSS.0b013e31827d87ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Royston P, Lambert PC. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model. College Station, TX: Stata Press; 2011. [Google Scholar]
- 36. Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi:10.1097/HJH.0b013e328347cc17 [DOI] [PubMed] [Google Scholar]
- 37. Benetos A, Labat C, Rossignol P, et al. Treatment with multiple blood pressure medications, achieved blood pressure, and mortality in older nursing home residents: The PARTAGE Study. JAMA Intern Med. 2015;175:989–995. doi:10.1001/jamainternmed.2014.8012 [DOI] [PubMed] [Google Scholar]
- 38. Mossello E, Pieraccioli M, Nesti N, et al. Effects of low blood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Intern Med. 2015;175:578–585. doi:10.1001/jamainternmed.2014.8164 [DOI] [PubMed] [Google Scholar]
- 39. Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–724. doi:10.1001/jama.294.6.716 [DOI] [PubMed] [Google Scholar]
- 40. Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556–559. [DOI] [PubMed] [Google Scholar]