Abstract
The major histocompatibility complex I (MHCI) pathway, which canonically functions in innate immune viral antigen presentation and detection, is functionally pleiotropic in the central nervous system (CNS). Alternative roles include developmental synapse pruning, regulation of synaptic plasticity, and inhibition of neuronal insulin signaling; all processes altered during brain aging. Upregulation of MHCI components with aging has been reported; however, no systematic examination of MHCI cellular localization, expression, and regulation across CNS regions, life span, and sexes has been reported. In the mouse, MHCI is expressed by neurons and microglia, and MHCI components and receptors (H2-K1, H2-D1, β2M, Lilrb3, Klra2, CD247) display markedly different expression profiles across the hippocampus, cortex, cerebellum, brainstem, and retina. MHCI components, receptors, associated inflammatory transcripts (IL1α, IL1β, IL6, TNFα), and TAP (transporter associated with antigen processing) components are induced with aging and to a greater degree in female than male mice across CNS regions. H2-K1 and H2-D1 expression is associated with differential CG and non-CG promoter methylation across CNS regions, ages, and between sexes, and concomitant increased expression of proinflammatory genes. Meta-analysis of human brain aging data also demonstrates age-related increases in MHCI. Induction of MHCI signaling could contribute to altered synapse regulation and impaired synaptic plasticity with aging.
Key Words: Sex differences, MHCI, DNA methylation, Gene expression, Aging, brain
Aging is a primary risk factor for a number of central nervous system (CNS) diseases and impairments including cognitive decline, Alzheimer’s disease, and Parkinson’s disease (1). Healthy aging is not associated with overt neurodegeneration but instead is characterized by synapse loss, altered synaptic morphology, and diminished synaptic plasticity (2). The molecular mechanisms underlying these age-associated alterations with normal brain aging are not fully understood, and identification of age-related pathways could provide potential therapeutic targets to prevent deleterious aspects of brain aging and delay or treat age-associated cognitive impairment. Additionally, understanding of the fundamental shifts in cellular function with brain aging may identify mechanisms by which the brain becomes more susceptible to disease and less resilient to insult.
The major histocompatibility complex class I (MHCI) processing and presentation pathway has been extensively characterized in the immune system, and functions to detect viral antigens and destroy infected cells. It was previously believed that the CNS was immune privileged (3,4) and did not express MHCI pathway components under normal conditions. However, MHCI is normally expressed in the CNS (5) with important and functionally pleiotropic roles including activity-dependent structural remodeling of processes, synaptic refinement, synaptic plasticity (6–9), neurodevelopment and neuronal repair (10,11), neuritogenesis and neurite outgrowth (12,13), regulating neuronal insulin receptor signaling (14), stroke-induced brain damage (15), as well as the development of schizophrenia (16) and autism (17).
Previously, we have demonstrated a significant increase in MHCI processing and presentation pathway transcript expression in hippocampal synaptic preparations of aged male rats, including classical MHCI components, the invariant cofactor β2M, TAP (transporter associated with antigen processing) components, and the MHCI receptors Lilrb3 (PirB) and Klra2 (18). Transcriptome analyses also suggest similar inductions of MHCI, the antigen-processing and presentation pathway, and inflammation in the brains of aged rats and in several different mouse strains (19,20). As increased neuroinflammation with age is a hallmark of brain aging (1), the induction of MHCI signaling could represent a relatively unexplored aspect of age-related neuroinflammation that may underlie neurophysiological changes often associated with advanced age. In support of this hypothesis, recent data have shown that circulating β2M, the invariant cofactor of MHCI, is a progeronic factor that causes cognitive dysfunction and reduced neurogenesis in aged animals (21,22). The exact mechanism by which circulating β2M contributes to cognitive decline is unknown, but more broadly MHCI is involved in the negative regulation of synapse density (6), induction of long-term depression, and inhibition of long-term potentiation (7,23–25). Reduced synaptic density and impaired plasticity are observed with brain aging (2). Previously, synaptic plasticity has been shown to decrease with advanced age in C57BL/6 mice (22). Additionally, decreases in dendritic spine density and length in specific populations of dendrites within the hippocampus are evident in aged C57BL/6 mice (26). Given the known negative association between neuronal MHCI expression, synaptic plasticity, and synapse density, this suggests that aberrant MHCI expression could contribute to these functional deficits associated with brain aging, and as such, warrants further study.
With the discovery of these pleiotropic functions of MHCI in the CNS, a number of important questions remain to be addressed, including the regulation and cellular localization of MHCI in the CNS, its receptors, and downstream functional effects. This study sought to determine the cellular localization of MHCI, the expression patterns of MHCI pathway components across five regions of the CNS, the effects of age and sex on MHCI pathway expression, and to characterize potential epigenetic and inflammatory regulators of MHCI expression in the CNS.
MHCI is ubiquitously expressed across the brainstem, cerebellum, cortex, hippocampus, and retina in both male and female mice across the life span and is expressed by neurons across all regions examined with expression also evident in microglia. Quantitative expression patterns of MHCI pathway components vary significantly across brain regions, and this regional control of classical MHCI expression may be regulated through differential DNA promoter methylation. Inductions in MHCI expression with advanced age are evident in both male and female mice, with females demonstrating greater inductions of inflammatory transcript expression when compared with age-matched males. This differential induction may be regulated through both epigenetic mechanisms (CG and non-CG methylation in promoter regions) and increased proinflammatory signals. These data provide an important new avenue of research examining changes in local neuroinflammation that may affect cognitive processes with advanced age.
Experimental Procedures
Detailed methods are available in the Supplementary Material
Animals
All animal experiments were executed according to protocols approved by the Penn State University Institutional Animal Care and Use Committee. Male and female C57BL/6 mice aged 3, 12, and 24 months were purchased from the National Institute on Aging colony at Charles River Laboratories (Wilmington, MA). All animals were housed in the Pennsylvania State University College of Medicine Hershey Center for Applied Research facility in ventilated high-efficiency particulate air filtered cages with ad libitum access to sterile food and water (Harlan 2918 diet, irradiated). Following a 1-week acclimation period after arrival to the facility, male mice were sacrificed by decapitation. Estrous cycle staging for all female mice was performed by daily vaginal lavage for 3–4 weeks, and animals were sacrificed during diestrus. Brainstem, cerebellar, cortical, hippocampal, and retinal tissue were rapidly dissected and flash frozen in liquid nitrogen for subsequent biochemical analysis. For immunohistochemical localization studies, animals were perfused with 1× phosphate-buffered saline at sacrifice.
Gene Expression Analysis
Gene expression was assessed by OpenArray quantitative polymerase chain reaction (qPCR) technology (Thermo Fisher Scientific, Waltham, MA) with n = 7–8/group (age/sex/region). OpenArray plates contain 3,072 33 nL wells in 48 subarrays, each subarray containing 64 wells with preloaded TaqMan gene expression qPCR assays (Supplementary Table 2) within each well. For this study, an 18 (3×) assay × 48 sample array format was utilized allowing all samples to be assayed in technical triplicates. OpenArray experiments were conducted according to manufacturer’s protocols.
Immunohistochemistry Analysis
Mice used for immunohistochemical analysis were transcardially perfused with 1× phosphate-buffered saline followed by 4% paraformaldehyde buffered in 0.1-M sodium phosphate buffer (pH 7.4). Brains were postfixed in 4% paraformaldehyde. All immunohistochemical experiments [n = 3/group (age/region/sex) were performed as previously described (18,27), and respective antibodies are listed in Supplementary Table 3.
DNA Methylation Analysis
Cytosine methylation in the promoter regions of H2-D1 and H2-K1 was determined by bisulfite amplicon sequencing (28) with n = 3–4/group (age/sex/region). Genomic DNA was isolated, quantified, and then bisulfite converted. Promoter regions for H2-D1 and H2-K1 were then PCR amplified using the bisulfite-converted DNA and primers (graciously provided by Dr. Sean D. Fouse) listed in Supplementary Table 4. PCR products were cleaned of primers, enzymes, and dNTPs using the QIAquick PCR purification system (QIAGEN) and quantified by fluorometric Picogreen assay (Invitrogen).
Dual-indexed sequencing libraries were generated using Nextera XT library preparation, sized by capillary electrophoresis and quantified by qPCR as previously described (28). Sequencing was performed using the Illumina MiSeq benchtop sequencer as previously described (28) and was conducted by paired-end, dual-indexing with 151 cycles per read. FASTQ files were mapped and aligned with Bismark (29)/Bowtie2 (30) against genomic regions of the H2-K1 and H2-D1 loci of mm10/GRCm38 (31). Bismark methylation extractor (29) was used to produce text files containing the location of each C, in all three contexts (CG, CHG, and CHH) the percent methylation and coverage at that site. This workflow was also repeated using alignment and quantitation in the CLC Genomics Workbench Bisulfite Sequencing Plugin and returned comparable results.
Statistics
All data were analyzed using SigmaStat 3.5 (SyStat Software, San Jose, CA) and GeneSpring 13.1 (Agilent Technologies). A two-tiered statistical strategy was applied to the gene expression data. First, a three-way ANOVA was performed, with the factors of region, sex, and age. As region was, unsurprisingly, a significant factor for every gene examined, statistical analyses were then split by region. Regional transcript expression differences were analyzed by One-way ANOVA with SNK post hoc testing with α < 0.05. Sex and age differences in gene expression and their interactions were analyzed by two-way ANOVA with SNK post hoc testing with α < 0.05. If data failed normality testing (Shapiro–Wilk), a Kruskal–Wallis test was performed with a Dunn’s post hoc test. A comprehensive multiple testing approach was used to control for Type I errors. ANOVA tests were subjected to BHMTC to control for Type I false positive errors (32). The number of tests was defined as the number of genes examined for the ANOVA comparisons. For the correlation testing, the number of tests was the number of pairwise correlations in a brain region. Assessment of gene expression correlations was performed using Pearson correlations with α < 0.05 and presented through cytoscape visualizations (33).
Cytosine methylation was analyzed similar to the gene expression data. Across region differences were analyzed by one-way ANOVA, and sex and age differences were analyzed by two-way ANOVA with SNK post hoc testing with α < 0.05. Across region correlation of gene expression to site-specific levels of methylation was performed by Pearson correlation with only with α < 0.05 and a cutoff of r > |0.25|.
Human Microarray Meta-analysis
All human microarray experimental data were obtained from NCBI’s GEO database of publicly available microarray experiments that included the brain regions of interest and age-related keywords as terms. Unambiguously identified samples were available in sufficient numbers for only hippocampus and frontal cortex. A number of samples were not coded for sex, and therefore sex-specific analyses were not possible. Entries were then manually screened to include only noncancerous samples. Experimental data sets were then downloaded and probes were collapsed to genes by mean value using the probe-gene mappings provided by the AILUN database (34). Thus, the Z-score provided for each gene is the average Z-score of probes that map to the gene. Experiments were normalized both on the sample level (to create a range of values that could be compared across experiments) and on the experimental level (ie, by GSE identifier, to eliminate batch effects). Negative (glyceraldehyde 3-phosphate dehydrogenase) and positive controls (glial fibrillary acidic protein [GFAP], CD52, and CD74) were also examined.
Results
To examine localization, expression, and regulation of CNS-specific MHCI, male and female C56BL/6 mice aged 3 months (young), 12 months (adult), and 24 months (aged) were compared. Estrous cycle staging of female mice was monitored daily for 3–4 weeks, and all female mice were sacrificed during diestrus. Aged female mice were confirmed to be in reproductive senescence (permanent diestrus).
Cellular Localization of MHCI Protein in the Male and Female CNS
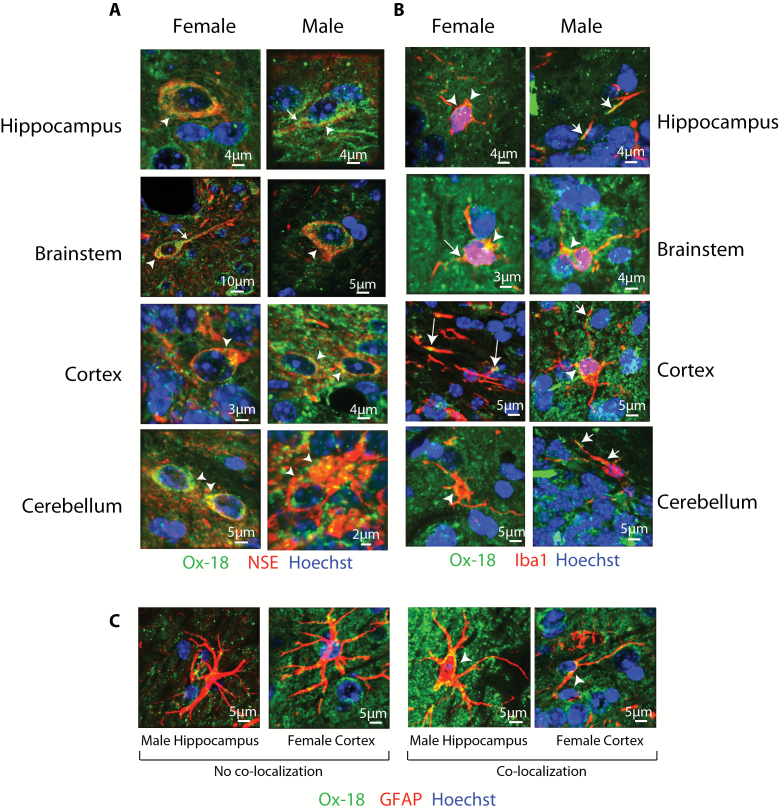
Previously, MHCI has been localized in the CNS to neurons, microglia, and astrocytes, but cellular localization has not been examined across brain regions, the life span, and sexes in a single animal model. The cellular localization of MHCI in the brainstem, cerebellum, cortex, and hippocampus was examined by colocalizing pan-MHCI (Ox-18) with cell-type–specific markers for astrocytes (GFAP), microglia (Iba1), and neurons (NSE) in both the aged (24 months) male and aged female mouse brain. Although not comprehensive of all brain structures, these tissues represent highly distinct regions with different cellular populations and functions. Ox-18 recognizes all classical forms of MHCI (H2-K, H2-D, H2-L) and has been used in previous examinations of MHCI expression in the rodent CNS (15,18,35). Immunoreactivity of Ox-18 in KbDb −/− mice is also greatly diminished, demonstrating specificity for classical MHCI (36). Ox-18 immunoreactivity was evident throughout the CNS and showed qualitatively identical patterns in both males and females. In the brainstem, cerebellum, cortex, and hippocampus colocalization of Ox-18 and the neuron-specific marker NSE demonstrated distinct cytosolic expression, often concentrated in the axon hillock region of neurons, with staining also apparent in neuronal processes (Figure 1A).
Figure 1.
Cellular localization of MHCI in aged neurons, microglia, and astrocytes. (A) Neuronal localization of MHCI protein was observed in all tissues examined (brainstem, cerebellum, cortex, and hippocampus) in the aged (24 month) male and female brain. Ox-18 immunoreactivity was seen in neuronal cell bodies (arrowheads) and processes (arrows). Red NSE, green MHCI, blue Hoechst, yellow NSE/MHCI colocalization. (B) Lower levels of microglial localization of MHCI were evident in both the aged male and female brain. Microglial cell bodies are noted as arrowheads whereas processes are noted as arrows. Red Iba1, green MHCI, blue Hoechst, yellow Iba1/MHCI colocalization. (C) Astrocytic localization of MHCI across the brain in both aged male and female mice was rare and inconsistent. Red GFAP, green MHCI, blue Hoechst, yellow GFAP/MHCI colocalization.
Colocalization of MHCI, with the microglia-specific marker Iba1, was also observed in both males and females and in all brain regions examined (Figure 1B). Sparse MHCI colocalization with the astrocyte-specific marker, GFAP, was observed across astrocyte cell bodies. The few instances of colocalization are presented in Figure 1C.
MHCI Pathway Expression Across CNS Regions
Four classes of genes (i) MHCI components: classical MHCI isoforms, H2-D1 and H2-K1, and the invariant cofactor β2M; (ii) MHCI receptors: the T-cell receptor component CD247 (CD3ζ), the natural killer cell receptor Klra2 (Ly49b), and the leukocyte immunoglobulin-like receptor, Lilrb3 (PirB); (iii) antigen-processing components: Tap1, Tap2, and Tapbp (components of the TAP complex); and (iv) inflammatory factors: the interleukins IL1α, IL1β, and IL6, and the proinflammatory cytokine TNFα were assessed for differences in expression between CNS regions (brainstem, cerebellum, cortex, hippocampus, and retina) in male and female young (3 months), adult (12 months), and aged (24 months) mice (n = 7–8/brain region/sex/age, n = 47 animals, N = 230 samples). To permit sufficient powered analyses and interoperability of the data not possible with other methods, a high-throughput qPCR approach was utilized (37) in which more than 3,000 nanovolume wells per PCR plate are assayed. Data were first analyzed with a three-way analysis of variance (ANOVA) design with region, age, and sex as factors. Not surprisingly, region was found to be a significant factor for every gene examined. To clarify the comparisons, regional comparisons were subsequently analyzed separately from the age and sex comparisons.
The expression of MHCI complex components (H2-D1, H2-K1, and β2M) differed significantly between brain regions examined [one-way ANOVA, Benjamini–Hochberg multiple testing correction (BHMTC), within age and sex groups] (Supplementary Figure 1A). This finding of differential gene expression across CNS regions can be expected given regional differences in cellular makeup and functionality (38). An interesting finding is that genes encoding the MHCI signaling complex (H2-D1 and H2-K1) demonstrated differential patterns of expression from proposed neuronal MHCI receptors including CD247 (7), Klra2 (39), and Lilrb3 (40) (Supplementary Figure 1B), indicating that MHCI signaling processes may vary by brain region. Regional differences in TAP complex and inflammatory factors were also observed (Supplementary Figure 2A and B).
Age- and Sex-related Differences in MHCI Pathway Expression
Age- and sex-related differences between young, adult, and aged male and female samples were determined next. A conservative statistical approach of a two-way ANOVA with the factors of age and sex within each CNS region (BHMTC) was used to determine differences. Significant age and sex differences were observed in all brain regions examined (Supplementary Table 1). Statistically significant interactions of the factors (sex and age) were also observed in retina, cerebellum, and hippocampus for selected genes.
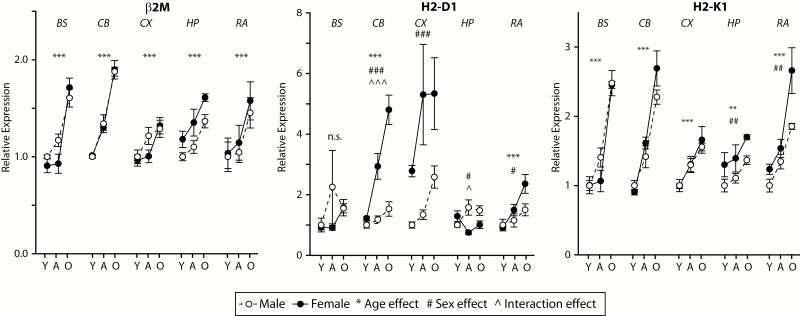
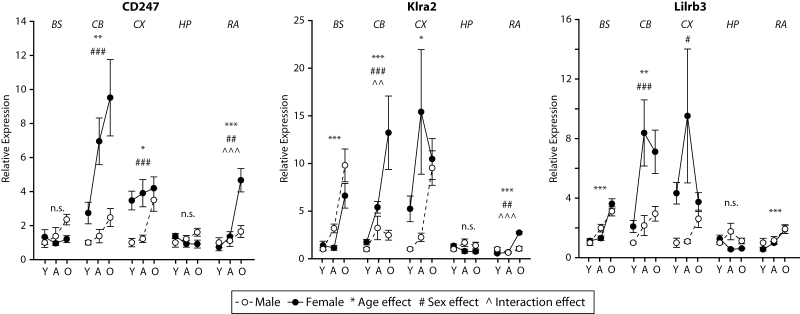
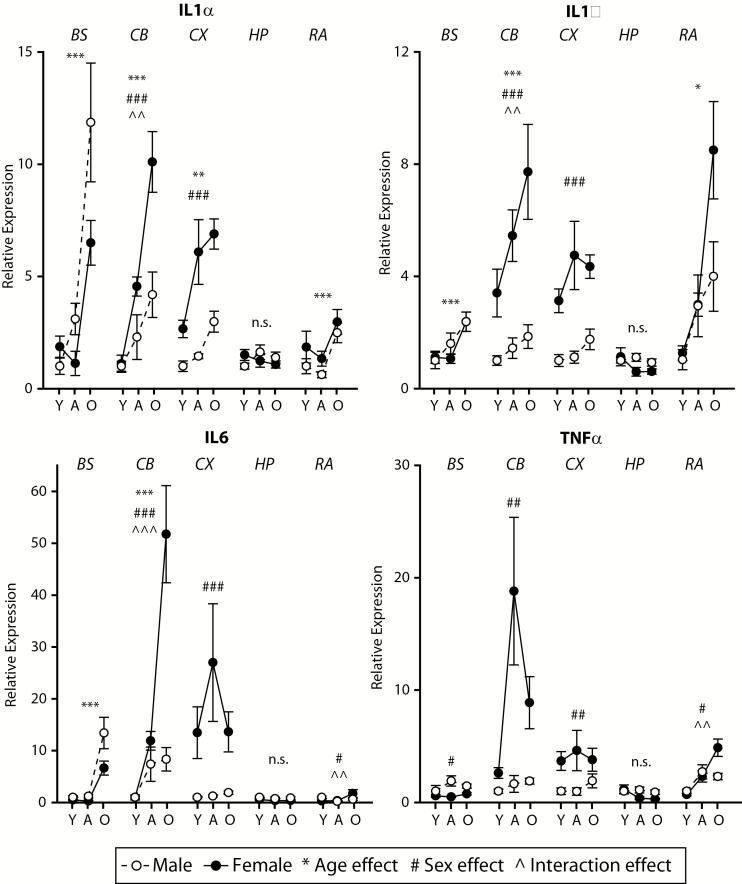
Age-related induction of MHCI components was observed in all brain regions examined, though the set of genes reaching statistical significance differed by brain region. Increased expression of specific MHCI components (Figure 2) and receptors (Figure 3), antigen-processing machinery (Supplementary Figure 3) and inflammatory factor genes (Figure 4) were observed in aged mice as compared with both young and adult mice. In some cases, there was also higher expression observed in adult as compared with young mice. The specific genes and patterns of increased expression varied to some degree between brain regions examined, though an age-related increase in expression of H2-K1 was observed in all brain regions.
Figure 2.
MHCI complex expression increases with aging across the CNS. The invariant chain of MHCI (Beta-2 microglobulin, β2M) as well as the classical MHCI isoforms (H2-D1 and H2-K1) increase in expression with age across the CNS regions examined. While β2M did not show any sex effects, H2-D1 and H2-K1 demonstrate sex and interaction effects in a number of the brain regions examined. Y=Young, A=Adult, O=Old/Aged. Two-way analysis of variance (Age × Sex), **p < .01, ***p < .001 Age effect, ##p < .01, ###p < .001 Sex effect, ^p < .05, ^^^p < .001 interaction, Benjamini–Hochberg Multiple Testing Correction, n.s. not significant, n = 7–8/group.
Figure 3.
MHCI receptor induction with aging and sex differences across CNS regions is distinct. Expression of the MHCI receptors, CD247, Klra2, and Lilrb3 are induced with aging in most of the brain regions examined with the exception of the hippocampus. In some regions, for example, cortex and cerebellum, significantly higher expression was evident in females. Y=Young, A=Adult, O=Old/Aged. Two-way analysis of variance (Age × Sex), **p < .01, ***p < .001 Age effect, ## p < .01, ### p < .001 Sex effect, ^p < .05, ^^^p < .001 interaction, Benjamini–Hochberg Multiple Testing Correction, n.s. not significant, n = 7–8/group (age/sex/region).
Figure 4.
Sexually dimorphic inductions of inflammatory gene expression with advanced age across neural tissues. The inflammatory factors IL1α, IL1β, IL6, and TNFα demonstrated high levels of induction with aging, in many cases much higher in females than males. Y=Young, A=Adult, O=Old/Aged. Two-way analysis of variance (Age × Sex), **p < .01, ***p < .001 Age effect, ## p < .01, ### p < .001 Sex effect, ^p < .05, ^^^p < .001 interaction, Benjamini–Hochberg Multiple Testing Correction, n.s. not significant, n = 7–8/group (age/sex/region).
Sex differences were observed in all brain regions examined. In the cortex, cerebellum, hippocampus, and retina, higher levels of MHCI components, antigen-processing genes, MHCI receptors, and inflammatory factors were observed in females as compared with males, though the specific genes in which this pattern was evident varied by region. Of note is that these sex differences were, in pairwise comparisons, only evident in adult or aged animals demonstrating that the sexual dimorphism of MHCI component expression develops with aging and is not present in young animals. Tap1, Tap2, IL6, and TNFα expression in the brainstem demonstrated an exception to this pattern with higher expression in aged males than in aged females.
Age-related Induction of MHCI Components in the Human CNS
With the data presented here and in our previous report (18), we have demonstrated an age-related induction of MHCI in both the mouse and rat CNS. To test whether the age-related induction of MHCI pathway transcript expression (HLA-B, HLA-C, and β2M) in the CNS also occurs in humans, we performed a meta-analysis of all publically available Gene Expression Omnibus (GEO) data sets for human brain tissue. Only data sets for which a postbirth age was available were used, and only the hippocampus (n = 59) and frontal cortex (n = 645) had sufficient numbers of data sets for correlations. Experiments were normalized at both the sample and experimental level, and the resultant Z scores were then assessed for age-related correlations (Pearson’s, BHMTC). HLA-B and HLA-C, which share the greatest sequence homology with H2-D and H2-K, respectively, as well as β2M, were examined in the correlations. In both the frontal cortex and hippocampus, HLA-B, HLA-C, and β2M were positively correlated to age (Table 1), that is, increased expression level with aging. β-actin, used as an endogenous control in the mouse PCR experiments, was used a negative control for the human data and did not demonstrate any correlations with age in the human data sets.
Table 1.
Correlations of Gene Expression Data with Aging in Human Data Sets
| Frontal Cortex (n = 645) | Hippocampus (n = 59) | |
|---|---|---|
| Gene | Pearson correlation with age | Pearson Correlation with age |
| [R, p value (BHMTC)] | [R, p value (BHMTC)] | |
| HLA-B | 0.283*** | 0.324*** |
| HLA-C | 0.28*** | 0.375*** |
| β2M | 0.285*** | 0.397* |
| β-Actin | −0.035 | 0.023 |
Notes: BHMTC = Benjamini–Hochberg multiple testing correction.
*p < 0.05. ***p < 0.001.
Differences in H2-D1 and H2-K1 Promoter Methylation Across the CNS
Of particular interest were the observed differences in H2-D1 and H2-K1 gene expression between brain regions, ages, and sexes. As epigenetic regulation has been proposed to be a driver of age-related gene expression changes (41), cytosine methylation levels in the promoter regions of H2-D1 and H2-K1 were determined using a focused, base-specific, next-generation sequencing approach (28) in a subset of the same young and aged mice (n = 3–4 per group) used in the gene expression study.
In total, 81 CG and 233 CH sites were examined in the H2-K1 promoter region from −496 upstream of the transcription start site to +521. For H2-D1, 13 CG sites and 106 CH sites were examined in the promoter ranging from −537 to −81 upstream of the transcription start site (average coverage per C 22,940±14,105). Similar to the gene expression analysis, a three-way ANOVA with region, sex, and age as factors determined that brain region was a significant factor for a large number of sites. To clarify the comparisons, regional differences were subsequently analyzed separately by one-way ANOVA, and age and sex differences were analyzed by two-way ANOVA and post hoc Student–Newman–Keuls (SNK), with only sites passing post hoc testing being retained.
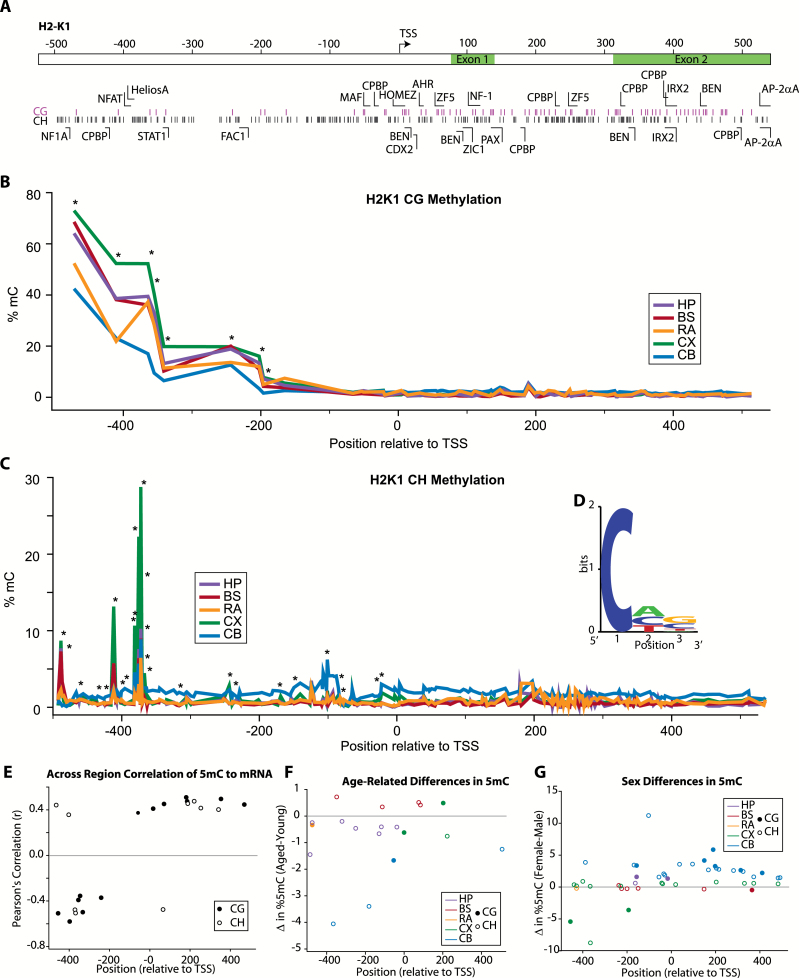
H2-K1 cytosine methylation was analyzed across a region extending from the promoter into the second exon (Figure 5A). Topographically, CG methylation was highest in the distal promoter, decreasing to low levels closer to the transcription start site and intragenically (Figure 5B). Non-CG methylation was low across the region examined with the exception of a few peaks demonstrating methylation levels greater than 5% (Figure 5C). Previous reports have suggested that non-CG methylation is concentrated in CAC trinucleotide motifs where the first C is the methylated cytosine (42–44). Taking only CH sites with greater than median methylation, there was a moderate preference for A and C in the second position and G and C in the third position (Figure 5D). Examining differences in methylation levels between brain regions, differences in CG and CH methylation levels were observed in the promoter region but not intragenically (asterisks, Figure 5B and C). Methylation levels at each cytosine were correlated to gene expression across the brain regions examined (Pearson’s, r > |0.25|) in young animals (leaving out the aged animals to eliminate age-related changes in gene expression). Negative correlations were generally observed in the promoter region with positive correlations observed intragenically (Figure 5E). Age-related differences were examined within each brain region, and statistically significant CG and CH differences were observed throughout the assayed region for all brain regions examined though the specific sites varied (Figure 5F). The majority of changes were decreases in methylation with aging as could be expected with the increased expression of H2-K1 in aged as compared with young animals. Sex differences in base-specific methylation were also observed with a predominance of increased methylation in females for the sites passing statistical criteria (Figure 5G).
Figure 5.
CG and non-CG methylation of H2-K1 promoter and intragenic regions. (A) Schematic of region analyzed, locations of CG and non-CG sites, and transcription factor binding domains. (B) Topography of site-specific CG methylation level in H2-K1 for each of the CNS regions analyzed. Average methylation at each site is presented. Sites with differential methylation between regions (one-way ANOVA, *p < .05) are noted. (C) Low levels of non-CG methylation were detected with the exception of specific peaks of higher (>5%) methylation. Average methylation at each site is presented. Sites with differential methylation between regions (one-way ANOVA, *p < .05) are noted. (D) Non-CG methylation has been proposed to be enriched in specific motifs, and the enrichment of specific nucleotides was examined for the non-CG sites with the highest (>average for the region examined) methylation levels. (E) H2-K1 expression across CNS regions was correlated to paired methylation levels from the same animals at specific CG and non-CG sites. Only young animals were analyzed to control for age-related differences in gene expression. Among sites with significant correlations (Pearson correlation α > 0.05 r > |0.25|), methylation at CG and CH sites in the promoter region was overall negatively correlated with gene expression, whereas methylation at intragenic CG and CH sites was positively correlated. (F) Age- and (G) sex-related differences in CG and non-CG methylation levels were evident across the H2-K1 region examined. Overall, observed age-specific differences were primarily decreases in methylation with increased age. The majority of differences in methylation observed between sexes were increases in methylation in females vs. males. Only sites with statistically significant differences are shown [two-way ANOVA (Age × Sex), Student–Newman–Keuls post hoc p < .05 within each CNS region], sites are color coded by brain region examined (HP-hippocampus, BS-brainstem, RA-retina, CX-cortex, CB-cerebellum) and whether the site is a CG (filled circle) or non-CG/CH (open circle) is noted. n = 3–4/group (age/sex/region).
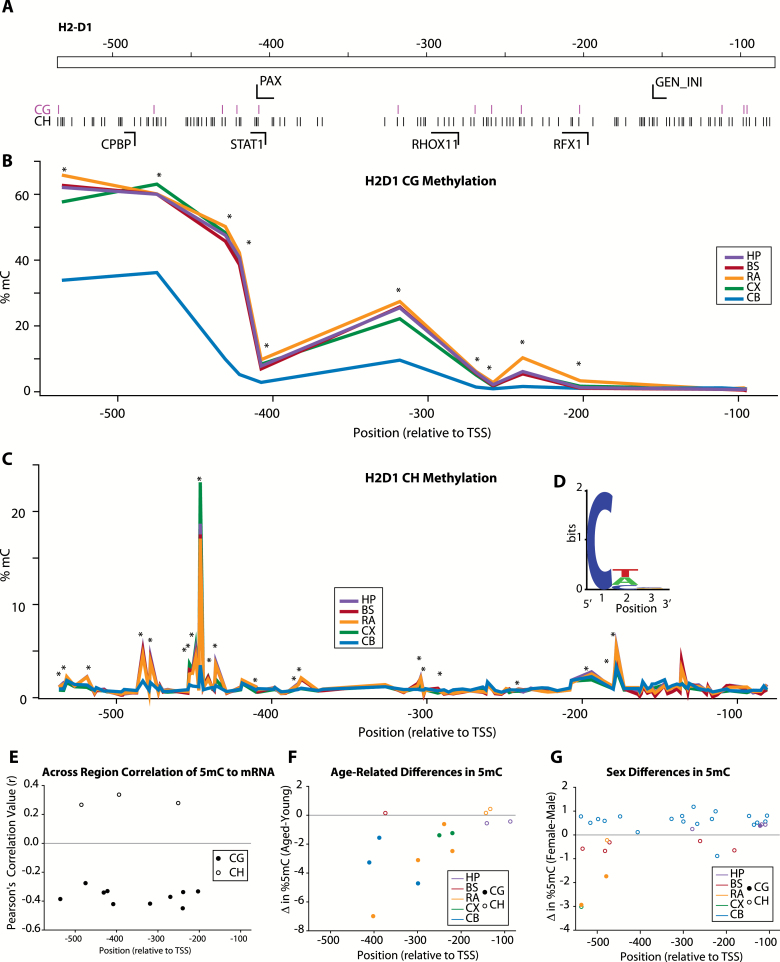
Within the promoter region of H2-D1 (Figure 6A) [bisulfite amplicon sequencing primers designed against the beginning of the intragenic region performed poorly and were not included in the analysis (data not shown)], a similar profile of CG methylation was observed with higher methylation levels in the distal promoter, declining to low levels near the transcription start site (Figure 6B). Peaks of non-CG methylation were observed across the region examined (Figure 6C). The motif of greater than median CH methylation demonstrated only a slight preference for T and A in the second position with no preference at the third position (Figure 6D). Differential levels of CG and CH methylation across the brain regions examined were observed across the promoter (asterisks, Figure 6B and C). Intriguingly, when methylation levels were correlated to gene expression (Pearson’s, r > |0.25|) in young animals across the brain regions examined, negative correlations were observed for CG sites, but CH sites that met criteria were positively correlated to H2-D1 gene expression (Figure 6E). Similar to the analysis of the H2-K1 promoter, age-related differences in CG and CH methylation in the H2-D1 promoter were predominantly decreases in methylation with age (Figure 6F), whereas sex differences demonstrated both higher and lower levels in females as compared with males (Figure 6G).
Figure 6.
CG and non-CG methylation of H2-D1 promoter region. (A) Schematic of region analyzed, locations of CG and non-CG sites, and transcription factor binding domains. (B) Topography of site-specific CG methylation levels in H2-D1 for each of the CNS regions analyzed. Average methylation at each site is presented. Sites with differential methylation between regions [one-way ANOVA, *p < .05] are noted. (C) Low levels of non-CG methylation were detected with the exception of specific peaks of higher (>5%) methylation. Average methylation at each site is presented. Sites with differential methylation between regions (one-way ANOVA, *p < .05) are noted. (D) Non-CG methylation has been proposed to be enriched in specific motifs, and the enrichment of specific nucleotides was examined for the non-CG sites with the highest (>average for the region examined) methylation levels. (E) H2-D1 expression across CNS regions was correlated to paired methylation levels from the same animals at specific CG and non-CG sites. Only young animals were analyzed to control for age-related differences in gene expression. Among sites with significant correlations (Pearson correlation α > 0.05 r > |0.25|), methylation at CG and CH sites in the promoter region was overall negatively correlated with gene expression, whereas methylation at intragenic CG and CH sites was positively correlated. (F) Age and (G) sex-related differences in CG and non-CG methylation levels were evident across the H2-D1 region examined. Overall, observed age-specific differences were primarily decreases in methylation with increased age. The majority of differences in methylation observed between sexes were increases in methylation in females vs. males. Only sites with statistically significant differences are shown [two-way ANOVA (Age × Sex), Student–Newman–Keuls post hoc p < .05 within each CNS region], sites are color coded by brain region examined (HP-hippocampus, BS-brainstem, RA-retina, CX-cortex, CB-cerebellum) and whether the site is a CG (filled circle) or non-CG/CH (open circle) is noted. n = 3–4/group (age/sex/region).
Examination of Potential MHCI Pathway: Inflammatory Factor Expression Correlations
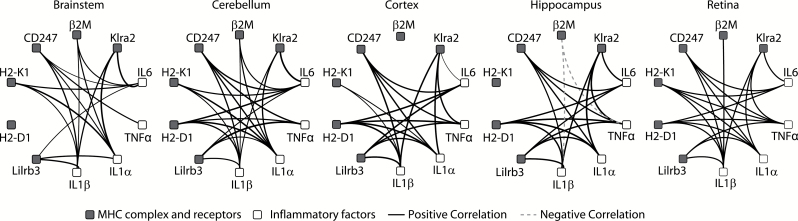
In addition to the epigenetic differences observed, the age- and sex-dependent changes in MHCI pathway expression could be regulated through inflammatory factor signaling, which as described earlier is also induced with age. With the large number of animals examined in this study, it is possible to examine the correlation of the MHCI processing and presentation pathway with expression of inflammatory factors that may regulate MHCI gene expression. Paired (ie, from the same animal and brain region) expression values of MHCI processing and presentation pathway genes (β2M, H2-D1, H2-K1, CD247, Klra2, and Lilrb3) and inflammatory genes (IL1α, IL1β, IL6, and TNFα) within each animal were correlated (Pearson’s, BHMTC) (Figure 7). MHCI complex and receptor genes were not correlated to each other in this analysis. A large number of significant positive correlations between inflammatory factors and MHCI processing and presentation pathway elements were observed in all brain regions. This suggests a potential regulatory role for these inflammatory factors, or they may share common upstream regulators with the MHCI processing and presentation pathway. The only exception to this pattern was a negative relationship between β2M, IL1β, and TNFα in the hippocampus. A number of transcription factor binding sites (eg, CPBP, STAT1) for inflammatory signaling are present in the promoter regions for H2-D1 and H2-K1 (see Figures 5A and 6A), suggesting that induction in inflammatory processes may result in increased MHCI expression. Also, inflammatory factors could work synergistically to increase expression of these target genes (45).
Figure 7.
Correlated gene expression between MHCI components, receptors, and inflammatory factors. (A) Significant correlations between expression of MHCI components and receptors (gray) and inflammatory mediators (white). (B) Significant correlations between expression of MHCI components (dark gray) and receptors (white). All black lines represent positive significant correlations (Pearson’s p < .05), and all dashed lines represent negative significant correlations, following multiple testing correction (Benjamini–Hochberg multiple testing correction), and line width is proportional to correlation coefficient values.
Discussion
In this study, we examined, in young (3 months), adult (12 months), and aged (24 months) male and female mice MHCI cellular localization, expression of the MHCI pathway and associated inflammatory genes, and the potential regulation of MHCI through epigenetic and proinflammatory mechanisms.
Cellular Localization and Regional Expression of MHCI
Our data demonstrate that in the aged female and male mouse, MHCI protein is expressed by both neurons and microglia across the hippocampus, brainstem, cortex, and cerebellum. Previous studies of MHCI localization in the CNS have examined rodent models at early developmental ages, in a limited number of structures, or did not quantitatively compare regions (7,35). Neuronal and glial MHCI protein expression were observed across all brain regions examined. MHCI protein expression by endogenous microglia has been previously described by us and others [for examples see (18,46)], but it should be noted that not all studies have observed microglial expression (15). The observed neuronal expression of MHCI agrees with previous studies, for example, of the hippocampus (18) and cortex (35) in rats, and lateral geniculate nucleus in mice (47). We observed cytoplasmic MHCI expression as well as localization to cellular processes in both neurons and microglia. Cytosolic localization has been reported previously in mouse, rat, and human neuronal cell bodies (18,48–50). This expression pattern may be a result of detecting MHCI protein being shuttled to the cell surface or being returned from the surface for degradation during MHCI processing (5). It should be noted that finer resolution studies also conclusively demonstrate membrane-localized MHCI in the CNS (35). The expression of MHCI by both neurons and microglia opens the possibility to a variety of regulatory and functional effects of MHCI depending on the cell type presenting and whether signaling is in trans (ie, intercellular communication) to other cells and/or signals in cis (ie, intra-cellular communication) (51). Additional studies using quantitative imaging techniques with greater resolution, like those previously applied (35), are needed to characterize distribution in different cells and parts of the CNS and to determine whether there are differences in the subcellular distribution of MHCI with aging. Additionally, detailed characterization of MHCI expression by neuronal cell type and microglial activation state is needed to provide a more complete landscape of CNS-specific MHCI (and MHCI receptor) expression.
The lack of expression in astrocytes concurs with previous findings that astrocytes do not express high levels of MHCI in vivo (15), even if in vitro expression has been observed (52). Studies in PirB knockout suggest that PirB signaling is necessary for astrocyte reactivation following experimental stroke (15), and thus astrocytes may express MHCI receptors such as PirB but lack robust MHCI expression. This would allow astrocytes to detect alterations in MHCI expression on both neurons and microglia and react accordingly. This may be either in response to inflammation, cell stress leading to reactivation, or in response to minute changes in synaptic activity resulting in alterations in metabolism of neurotransmitters by astrocytes. The present study does not assess cellular localization of PirB or other MHCI receptors, but this is a critical next step for understanding the functions of MHCI signaling. Given that large differences in abundance of MHCI receptors were observed across brain regions and aging, these future studies will need to be conducted across the CNS, life span, and between sexes.
The previous understanding of restricted MHCI distribution has been replaced with an understanding that MHCI is widely expressed (5). However, it has only been recently suggested that MHCI performs functionally pleiotropic roles outside of immunity in the CNS (24). The expression of MHCI components and putative receptors has not previously been quantitatively compared between brain regions. Significant expression differences in MHCI components (H2-K1, H2-D1, and β2M) and putative MHCI receptors (CD247, Klra2, and Lilrb3) were observed across the brain regions examined, in males and females and at all ages. Although this phenomenon of differential gene expression across neural regions is not specific to MHCI and often occurs with other genes, these findings demonstrate brain region–specific expression patterns of the MHCI complex and receptors. This could result in different MHCI signaling functions across the CNS depending on the complement of MHCI components and receptors present. This is supported by recent findings detailing the specific functions of H2-D1 in the CNS (9). Additionally, the potential for nonreceptor– and non-β2M–mediated MHCI signaling (also known as open conformers) exists (51). Observed MHCI expression may also be derived from antigen-presenting endothelial cells that are necessary for CD8+ T-cell entry across the blood brain barrier (53,54). Importantly, stimulus-induced immune cell infiltration into the brain parenchyma has been shown to increase with age (55,56) and may be a result of enhanced cerebrovascular expression of MHCI. Together, the gene expression analysis presented here demonstrates that all MHCI pathway genes examined are expressed across a number of CNS regions. However, patterns and relative levels of MHCI components and receptors vary by CNS structure.
Age and Sex differences in Cellular Localization and Regional Expression of MHCI
MHCI expression has been demonstrated to be induced with aging in the rat hippocampus (18), and examination of published transcriptomic data sets from mice (20), rats (19), and humans (57,58) supports the potential for CNS MHCI induction with aging to be conserved. Additionally, recent evidence suggests that enhanced levels of circulating β2M found in aged animals are related to increased cognitive dysfunction and decreased neurogenesis (21), further suggesting that the MHCI pathway is involved in cognitive aging. Our findings demonstrate that not only is local expression of MHCI induced with aging in another rodent species, but also that MHCI expression is increased, to varying degrees, across several regions of the murine CNS. The magnitude and exact MHCI components and receptors induced differ according to brain region. For the first time, we also demonstrate that the increased expression of MHCI components, TAP complex members, and MHCI receptors with aging, in many cases, is greater in females than in males. Importantly, these are not inherent sex differences but are only evident in adult and aged mice. It has been suggested that CNS MHCI expression declines with aging (16), but this is likely a matter of the ages examined, as previously reported adult ages are the same age or younger than the young animals in this study (6,35,48). As such, MHCI expression may be elevated postnatally, decline into early adulthood, and increase with advanced age. Nonetheless, MHCI pathway induction with aging in the CNS is conserved across species, including humans as demonstrated in our meta-analysis of publically available data, and warrants further studies into the regulation of MHCI and the functional effects of increased expression.
The age-related induction of MHCI components and receptors is of interest due to the emerging role of MHCI in synapse elimination and inhibiting synaptic plasticity (16), both hallmarks of normative brain aging (2). Specifically, in vitro and in vivo inhibition of MHCI increases synapse density, and in vitro MHCI overexpression expression decreases synapse density (6). The roles and mechanisms of MHCI in synaptic plasticity are still being delineated, but clear evidence in vivo shows that inhibiting MHCI lowers the threshold for establishing long-term potentiation (7). The regulation of synaptic strength by MHCI may be through modulation of NMDAR (N-methyl-D-aspartate receptor) function and AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor) trafficking (6,8). More recent work suggests that these processes may be more dependent on H2-D than on H2-K (9), but the receptor, or nonreceptor (51), signaling processes remain unclear with both evidence for paired Ig-like receptors (40), killer cell–like receptors (59), and T-cell receptor components (CD247/CD3ζ) (7,60). The upregulation of these receptors with advanced aging in concert with MHCI components in the CNS strengthens the rationale for functional studies of MHCI signaling and effects on synaptic morphology and plasticity with aging. Previous reports have demonstrated decreased dendritic spine density and spine length in specific areas of the brain and subpopulations of dendrites with advanced age in C57BL/6 mice (22,26). Additionally, synaptic plasticity decreases with advanced age and can be rescued with the introduction of young blood in C57BL/6 mice (21,22). Together, this suggests that increased MHCI expression with advanced age in these mice may alter dendritic spine and synapse morphology, which in turn may cause diminished synaptic plasticity.
The greater induction of MHCI components and receptors in females versus males is of note. Limited research into sex differences with brain aging, especially in neuroinflammatory processes, has been reported. Increased expression of MHCI components and receptors is evident in specific areas of the brain in aged humans suggesting that this sexually dimorphic phenomenon occurs in multiple species (58). Clinically, some studies have suggested a higher age-matched risk for women to develop cognitive impairment and Alzheimer’s disease (61), but this finding is not consistent across all similar studies (62). Preclinically, aged female mice have been reported to have greater numbers of microglia as compared with age-matched males (63) which could be a source of increased MHCI expression.
With these sex differences only occurring with advanced age, sex hormones may play a role in the regulation of MHCI processing and presentation, the induction of inflammation, and clinically, the manifestation of age-associated cognitive impairments. Specifically, estrogen has been found to be neuroprotective against inflammation and cognitive aging (64) and has been shown to positively regulate synapse density in the female hippocampus both during natural and experimental manipulations of estrogen concentrations (65). Changes in circulating sex hormones with age could also underlie resistance to age-associated brain volume loss observed in females (66), though this finding is a matter of some disagreement (67). Together, these data suggest that sex hormones may influence synapse dynamics. Importantly, women experience a decline in circulating estrogen with age, suggesting that decreased circulating estrogen may cause an induction in neuroinflammation and ultimately reduced synapse density and plasticity. The findings presented here highlight a need to further examine effects of sex hormones on the expression of the MHCI pathway and inflammatory mediators and how these changes may directly affect synapse dynamics.
Regulation of MHCI Expression
To address the regulatory mechanisms underlying regional, age, and sex differences, we examined promoter methylation levels and coexpression of proinflammatory mediators. Examinations of the epigenetic regulation of MHCI in the brain have not been previously reported. We found that DNA methylation levels of CG and non-CG sites in the promoter regions of H2-K1 and H2-D1 significantly differ between brain regions. Additionally, within each brain region, methylation levels of CG and non-CG sites are altered with age and also differ between sexes. Expression set points between brain regions have been demonstrated to be controlled through DNA methylation (68). H2-K1 cytosine methylation at a number of distal promoter sites is inversely correlated to gene expression in the same sample, as would be expected from a generally repressive nature of methylation on gene expression, whereas intragenic CG and non-CG methylation was positively correlated with expression. A large number of highly methylated non-CG methylation sites were found in both the H2-K1 and H2-D1 promoters confirming previous findings that CH methylation is enriched in CNS tissue and present in mature neurons (42–44,69,70). Cerebellar methylation was found to be significantly different from other brain regions demonstrating higher non-CG methylation levels specifically in the distal H2-K1 promoter region. Methylation profiles of genes from cerebellar tissue have been shown previously to be distinct from other brain regions, likely because of the cellular makeup (68,71). The trinucleotide motifs of non-CG methylation across brain regions in the H2-K1 gene demonstrate a slight preference for CA methylation, which has been previously reported (42–44). H2-D1 CG methylation also displayed a negative correlation to gene expression, whereas interestingly non-CG methylation was positively correlated across the distal promoter. Trinucleotide motifs of non-CG methylation at the H2-D1 promoter differ from H2-K1, but there is a slight preference for CA methylation, again confirming previous findings. These differences in non-CG methylation motifs from previous findings are not surprising given that the motifs seen in the literature are averaged across the entire genome, whereas our results are from single gene loci. Regional differences suggest that the set point of H2-K1 and H2-D1 in different brain regions may be regulated by promoter methylation. It should be noted that this likely reflects, at some level, the differences in cellular populations between the regions examined. However, alterations in both the methylation of CG and non-CG sites in both the H2-K1 and H2-D1 promoters with age and between sexes being present at or near transcription factor binding sites potentially regulate MHCI gene expression as has been shown for other factors both in vitro and in vivo (42,70,72). Although the sample numbers examined (n = 3–4/group, N = 55) were sufficient to detect a number of changes, as the epigenomics field evolves, methods such as bisulfite amplicon sequencing will need to further increase in sample throughput capabilities to accommodate more samples. Promoter methylation did not, however, appear to be the sole regulator of sex- and age-related differences. Future studies addressing the mechanism of MHCI promoter regulation will be helpful in identifying the role of epigenetic regulation of MHCI expression with age. From a technical standpoint, these data demonstrate the power of focused, high read-depth, base-specific quantitation of genomic regions of interest.
The age-related (and sexually dimorphic) increase in proinflammatory mediators, IL1α, IL1β, IL6, and TNFα, all of which can directly or indirectly drive NFkB and/or STAT signaling and induce MHCI expression (73,74) may work in concert with epigenetic changes to drive increased MHCI pathway gene expression. This positive correlation between proinflammatory factors, that in many cases exhibit increased expression with aging and/or higher expression in females, suggests that these factors could be regulating (or share common regulation with) MHCI pathway expression, which is supported by previous studies [for review see (75)]. In many cases, these proinflammatory signals were induced with aging and displayed sex differences where expression was higher in females than in males, bolstering the evidence for their potential positive regulation of the MHCI pathway.
Conclusion
In this study, we demonstrate that MHCI protein is expressed by neurons and microglia in the mouse CNS and that transcript expression of MHCI components and receptors are induced across the CNS with aging. Furthermore, these data provide the first detailed evidence for sexually dimorphic expression of the MHCI pathway with aging. The functional implications of increased MHCI signaling remain to be determined, but given the many roles for MHCI in synapse regulation and synaptic plasticity that coincide with known age-related synaptic dysfunction, additional studies of MHCI in brain aging are warranted. Further studies of sex differences in the regulation of and functional effects of CNS MHCI expression are needed to address many of the questions raised by this data, highlighting the importance of including females in preclinical studies (76).
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported the Donald W. Reynolds Foundation, the National Institute on Aging (R01AG026607, P30AG050911, F31AG038285), National Eye Institute (R01EY021716, R21EY024520), and Oklahoma Center for Advancement of Science and Technology (HR14-174).
Supplementary Material
Acknowledgments
The authors thank Robert Brucklacher in the Genome Sciences Facility at the Penn State Hershey College of Medicine for quantitative PCR assistance, Wendy Holtry for helping execute all perfusion protocols, and Dr. Shaun Fouse for generously providing the primer sequences used in the methylation studies, Paul Kemp for assistance with figure preparation, and the OUHSC and OMRF next-generation sequencing facilities for equipment access. OpenArray experiments were conducted with the assistance of Roxann Ashworth using the Life Technologies QuantStudio 12K Flex machine located at the Genetic Resources Core Facility at the Johns Hopkins Institute of Genetic Medicine, Baltimore, MD. The authors declare no financial conflicts of interest.
C.A.M. designed the studies with W.M.F. and W.E.S. and in conjunction with D.R.M., G.V.B., and M.M.F. performed the animal studies, molecular and biochemical experiments. D.R.S. and A.P. performed bioinformatic analyses. C.B.G. and J.D.W. performed the meta-analysis of human transcriptome data. All authors contributed to writing of the manuscript.
References
- 1. Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi:10.1016/j.neuron.2009.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi:10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- 3. Joly E, Mucke L, Oldstone MB. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. [DOI] [PubMed] [Google Scholar]
- 4. Neuwelt EA, Clark WK. Unique aspects of central nervous system immunology. Neurosurgery. 1978;3:419–430. [DOI] [PubMed] [Google Scholar]
- 5. Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–836. doi:10.1038/nri3084 [DOI] [PubMed] [Google Scholar]
- 6. Glynn MW, Elmer BM, Garay PA, et al. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci. 2011;14:442–451. doi:10.1038/nn.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fourgeaud L, Davenport CM, Tyler CM, Cheng TT, Spencer MB, Boulanger LM. MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proc Natl Acad Sci U S A. 2010;107:22278–22283. doi:10.1073/pnas.0914064107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee H, Brott BK, Kirkby LA, et al. Synapse elimination and learning rules co-regulated by MHC class I H2-D. Nature. 2014;509:195–200. doi:10.1038/nature13154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu ZP, Washburn L, Bilousova TV, et al. Enhanced neuronal expression of major histocompatibility complex class I leads to aberrations in neurodevelopment and neurorepair. J Neuroimmunol. 2011;232:8–16. doi:10.1016/j.jneuroim.2010.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu ZP, Bilousova T, Escande-Beillard N, et al. Major histocompatibility complex class I-mediated inhibition of neurite outgrowth from peripheral nerves. Immunol Lett. 2011;135:118–123. doi:10.1016/j.imlet.2010.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bilousova T, Dang H, Xu W, et al. Major histocompatibility complex class I molecules modulate embryonic neuritogenesis and neuronal polarization. J Neuroimmunol. 2012;247:1–8. doi:10.1016/j.jneuroim.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Washburn LR, Zekzer D, Eitan S, et al. A potential role for shed soluble major histocompatibility class I molecules as modulators of neurite outgrowth. PLoS One. 2011;6:e18439. doi:10.1371/journal.pone.0018439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dixon-Salazar TJ, Fourgeaud L, Tyler CM, Poole JR, Park JJ, Boulanger LM. MHC class I limits hippocampal synapse density by inhibiting neuronal insulin receptor signaling. J Neurosci. 2014;34:11844–11856. doi:10.1523/JNEUROSCI.4642-12.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adelson JD, Barreto GE, Xu L, et al. Neuroprotection from stroke in the absence of MHCI or PirB. Neuron. 2012;73:1100–1107. doi:10.1016/j.neuron.2012.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McAllister AK. Major histocompatibility complex I in brain development and schizophrenia. Biol Psychiatry. 2014;75:262–268. doi:10.1016/j.biopsych.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Needleman LA, McAllister AK. The major histocompatibility complex and autism spectrum disorder. Dev Neurobiol. 2012;72:1288–1301. doi:10.1002/dneu.22046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. VanGuilder Starkey HD, Van Kirk CA, Bixler GV, et al. Neuroglial expression of the MHCI pathway and PirB receptor is upregulated in the hippocampus with advanced aging. J Mol Neurosci. 2012;48:111–126. doi:10.1007/s12031-012-9783-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blalock EM, Chen KC, Sharrow K, et al. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weindruch R, Kayo T, Lee CK, Prolla TA. Gene expression profiling of aging using DNA microarrays. Mech Ageing Dev. 2002;123:177–193. [DOI] [PubMed] [Google Scholar]
- 21. Smith LK, He Y, Park JS, et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi:10.1038/nm.3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boulanger LM. MHC class I in activity-dependent structural and functional plasticity. Neuron Glia Biol. 2004;1:283–289. doi:10.1017/S1740925X05000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi:10.1016/j.neuron.2009.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McConnell MJ, Huang YH, Datwani A, Shatz CJ. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci U S A. 2009;106:6784–6789. doi:10.1073/pnas.0902018106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res. 2006;83:525–531. doi:10.1002/jnr.20759 [DOI] [PubMed] [Google Scholar]
- 27. Vanguilder HD, Bixler GV, Sonntag WE, Freeman WM. Hippocampal expression of myelin-associated inhibitors is induced with age-related cognitive decline and correlates with deficits of spatial learning and memory. J Neurochem. 2012;121:77–98. doi:10.1111/j.1471-4159.2012.07671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Masser DR, Berg AS, Freeman WM. Focused, high accuracy 5-methylcytosine quantitation with base resolution by benchtop next-generation sequencing. Epigenetics Chromatin. 2013;6:33. doi:10.1186/1756-8935-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–1572. doi:10.1093/bioinformatics/btr167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi:10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenbloom KR, Armstrong J, Barber GP, et al. The UCSC Genome Browser database: 2015 update. Nucleic Acids Res. 2015;43:D670–681. doi:10.1093/nar/gku1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Meth. 1995;57:289–300. doi:S0166-4328(01)00297-2 [pii] [Google Scholar]
- 33. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi:10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen R, Li L, Butte AJ. AILUN: reannotating gene expression data automatically. Nat Methods. 2007;4:879. doi:10.1038/nmeth1107-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Needleman LA, Liu XB, El-Sabeawy F, Jones EG, McAllister AK. MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood. Proc Natl Acad Sci U S A. 2010;107:16999–17004. doi:10.1073/pnas.1006087107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Datwani A, McConnell MJ, Kanold PO, et al. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi:10.1016/j.neuron.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morrison T, Hurley J, Garcia J, et al. Nanoliter high throughput quantitative PCR. Nucleic Acids Res. 2006;34:e123. doi:10.1093/nar/gkl639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kasukawa T, Masumoto KH, Nikaido I, et al. Quantitative expression profile of distinct functional regions in the adult mouse brain. PLoS One. 2011;6:e23228. doi:10.1371/journal.pone.0023228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scarpellino L, Oeschger F, Guillaume P, et al. Interactions of Ly49 family receptors with MHC class I ligands in trans and cis. J Immunol. 2007;178:1277–1284. doi:10.4049/jimmunol.178.3.1277 [DOI] [PubMed] [Google Scholar]
- 40. Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi:10.1126/science.1128232 [DOI] [PubMed] [Google Scholar]
- 41. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi:10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo JU, Su Y, Shin JH, et al. Distribution, recognition and regulation of non-CG methylation in the adult mammalian brain. Nat Neurosci. 2014;17:215–222. doi:10.1038/nn.3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Varley KE, Gertz J, Bowling KM, et al. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res. 2013;23:555–567. doi:10.1101/gr.147942.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kozlenkov A, Roussos P, Timashpolsky A, et al. Differences in DNA methylation between human neuronal and glial cells are concentrated in enhancers and non-CG sites. Nucleic Acids Res. 2014;42:109–127. doi:10.1093/nar/gkt838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Y, Lei CQ, Hu YH, et al. Kruppel-like factor 6 is a co-activator of NF-kappaB that mediates p65-dependent transcription of selected downstream genes. J Biol Chem. 2014;289:12876–12885. doi:10.1074/jbc.M113.535831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Streit WJ, Graeber MB, Kreutzberg GW. Peripheral nerve lesion produces increased levels of major histocompatibility complex antigens in the central nervous system. J Neuroimmunol. 1989;21:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. [DOI] [PubMed] [Google Scholar]
- 48. Chacon MA, Boulanger LM. MHC class I protein is expressed by neurons and neural progenitors in mid-gestation mouse brain. Mol Cell Neurosci. 2013;52:117–127. doi:10.1016/j.mcn.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 49. Zhang A, Yu H, Shen Y, et al. The expression patterns of MHC class I molecules in the developmental human visual system. Neurochem Res. 2013;38:273–281. doi:10.1007/s11064-012-0916-9 [DOI] [PubMed] [Google Scholar]
- 50. Zhang A, Yu H, He Y, et al. Developmental expression and localization of MHC class I molecules in the human central nervous system. Exp Brain Res. 2015;233:2733–2743. doi:10.1007/s00221-015-4345-2 [DOI] [PubMed] [Google Scholar]
- 51. Arosa FA, Santos SG, Powis SJ. Open conformers: the hidden face of MHC-I molecules. Trends Immunol. 2007;28:115–123. [DOI] [PubMed] [Google Scholar]
- 52. Wong GH, Bartlett PF, Clark-Lewis I, Battye F, Schrader JW. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984;310:688–691. [DOI] [PubMed] [Google Scholar]
- 53. Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells–conditional innate immune cells. J Hematol Oncol. 2013;6:61. doi:10.1186/1756-8722-6-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Galea I, Bernardes-Silva M, Forse PA, van Rooijen N, Liblau RS, Perry VH. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med. 2007;204:2023–2030. doi:10.1084/jem.20070064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gemechu JM, Bentivoglio M. T cell recruitment in the brain during normal aging. Front Cell Neurosci. 2012;6:38. doi:10.3389/fncel.2012.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu YZ, Nygård M, Kristensson K, Bentivoglio M. Regulation of cytokine signaling and T-cell recruitment in the aging mouse brain in response to central inflammatory challenge. Brain Behav Immun. 2010;24:138–152. doi:10.1016/j.bbi.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 57. Cribbs DH, Berchtold NC, Perreau V, et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179. doi:10.1186/1742-2094-9-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Berchtold NC, Cribbs DH, Coleman PD, et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci U S A. 2008;105:15605–15610. doi:10.1073/pnas.0806883105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zohar O, Reiter Y, Bennink JR, et al. Cutting edge: MHC class I-Ly49 interaction regulates neuronal function. J Immunol. 2008;180:6447–6451. doi:10.4049/jimmunol.180.10.6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baudouin SJ, Angibaud J, Loussouarn G, et al. The signaling adaptor protein CD3zeta is a negative regulator of dendrite development in young neurons. Mol Biol Cell. 2008;19:2444–2456. doi:10.1091/mbc.E07-09-0947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008;71:337–343. doi:10.1212/01.wnl.0000310773.65918.cd [DOI] [PubMed] [Google Scholar]
- 62. Katz MJ, Lipton RB, Hall CB, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi:10.1097/WAD.0b013e31823dbcfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mouton PR, Long JM, Lei DL, et al. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–35. doi:10.1016/S0006-8993(02)03475-3 [DOI] [PubMed] [Google Scholar]
- 64. Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33:105–115. doi:10.1016/j.yfrne.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Murphy DG, DeCarli C, McIntosh AR, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. [DOI] [PubMed] [Google Scholar]
- 67. Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi:10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- 68. Ladd-Acosta C, Pevsner J, Sabunciyan S, et al. DNA methylation signatures within the human brain. Am J Hum Genet. 2007;81:1304–1315. doi:10.1086/524110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Farré P, Jones MJ, Meaney MJ, Emberly E, Turecki G, Kobor MS. Concordant and discordant DNA methylation signatures of aging in human blood and brain. Epigenetics Chromatin. 2015;8:19. doi:10.1186/s13072-015-0011-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen L, Chen K, Lavery LA, et al. MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome. Proc Natl Acad Sci U S A. 2015;112:5509–5514. doi:10.1073/pnas.1505909112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xin Y, Chanrion B, Liu MM, et al. Genome-wide divergence of DNA methylation marks in cerebral and cerebellar cortices. PLoS One. 2010;5:e11357. doi:10.1073/pnas.1505909112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Barres R, Osler ME, Yan J, et al. Non-CG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab. 2009;10:189–198. doi:10.1016/j.cmet.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 73. van den Elsen PJ. Expression regulation of major histocompatibility complex class I and class II encoding genes. Front Immunol. 2011;2:48. doi:10.3389/fimmu.2011.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chevrel G, Granet C, Miossec P. Contribution of tumour necrosis factor alpha and interleukin (IL) 1beta to IL6 production, NF-kappaB nuclear translocation, and class I MHC expression in muscle cells: in vitro regulation with specific cytokine inhibitors. Ann Rheum Dis. 2005;64:1257–1262. doi:10.1136/ard.2004.032359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kobayashi KS, van den Elsen PJ. NLRC5: a key regulator of MHC class I-dependent immune responses. Nat Rev Immunol. 2012;12:813–820. doi:10.1038/nri3339 [DOI] [PubMed] [Google Scholar]
- 76. Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi:10.1038/509282a [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.