Abstract
Background:
More than 40% of patients with paragangliomas (PGLs) harbor a germline mutation of the known PGL susceptibility genes, mainly in the SDHB or SDHD genes.
Objective:
The objective of the study was to characterize the genetic background of the French Canadian (FC) patients with PGLs and provide new clinical and paraclinical insights on SDHC-related PGLs.
Methods:
Genetic testing has been offered to FC patients affected with PGLs followed up at the adrenal genetics clinic at Centre hospitalier de l'Université de Montréal. After genetic counseling, 29 FC patients consented for PGL genetic testing.
Results:
Thirteen of 29 patients (44.8%) carried a germline mutation. The same heterozygous nonsense mutation at codon 133 of exon 5 of the SDHC gene (c.397C>T, p.[Arg133Ter]) was found in nine patients, representing 69.2% of the patients having a germline mutation. Seventy percent of these patients had head and neck PGLs. Twenty percent had multiple and 30% had malignant PGLs. We traced back the ascending genealogy of 10 index cases (nine patients from our cohort and one patient referred to us) and found that this mutation was most probably introduced in Nouvelle France by a couple of French settlers who established themselves in the 17th century.
Conclusions:
We found that 31% of the PGLs in the French Canadian can be explained by the SDHC mutation (c.397C>T, p.[Arg133Ter]). The dominance of the SDHC mutation is unique to the FCs and is most likely due to a French founder effect. SDHC gene analysis should be prioritized in FC patients with PGL.
The genetic of the French Canadian patients with paragangliomas was characterized. 31% of the patients carry the same SDHC mutation suggesting a French founder effectin this population.
Paragangliomas (PGLs) and pheochromocytomas (PCCs) are rare neuroendocrine tumors with an incidence estimated to be less than 1 per 300 000 persons per year. PGLs are tumors derived from the paragangliomic system and may be localized from the skull base to the pelvic floor in contrast to PCCs that are located at the level of the adrenal glands. They can be sporadic or part of a genetic or familial syndrome. More than 40% of cases with PCCs and PGLs harbor a germline mutation in one of the known susceptibility genes (1).
Genes associated with an increased risk of developing PGLs and PCCs include VHL, RET, NF1, the genes encoding the four subunits of the mitochondrial enzyme succinate dehydrogenase: SDHD, SDHB, SDHC, and SDHA, the assembly factor SDHAF2, and, recently, TMEM127, MAX, FH, EPAS1, and MDH2 genes (1). Mutations in SDHB and SDHD genes are usually the most common. SDHC mutations are rare, encompassing only 7% of hereditary PGLs/PCCs (2).
There is a well-documented founder effect in the French-Canadian (FC) population of the province of Québec. The genetic status of the PGL/PCC population has never been studied in the FC population. We investigated the genetic profile of FC subjects with PGLs. Surprisingly, the same SDHC mutation was identified in 10 apparently unrelated patients. We provide detailed clinical and paraclinical parameters of these 10 patients and three of their relatives. Then we performed a genealogical reconstruction of the 10 index cases reported to evaluate genetic contribution of ancestors of these apparently unrelated cases. We found that the index cases are more related than the control group and that their common ancestors are immigrants or parents of immigrants from France, supporting that the SDHC mutation identified in FC patients is originating from France.
Materials and Methods
FC patients with PGLs
Genetic testing has been offered to patients affected by PGLs since 2005 seen at our adrenal genetics clinic at Centre hospitalier de l'Université de Montréal (CHUM). The clinical details of the investigated patients are given in Table 1. One previously reported patient was referred to us from McGill University and was included in the genealogical reconstruction study described below (3). Written informed consent for genetic testing was obtained from each patient. The study was approved by the Institutional Review Board of the CHUM (Montréal, Canada).
Table 1.
Clinical Details of the Investigated FC Patients With the SDHC [c.397C>T, p.(Arg133Ter]) Mutation and PGLs
| Index Cases | Age, y | Sex | Family History | Parental Transmission | Initial Localization | Isolated or Multiple | Malignant | Tumor Size, cm | Secretion | U Norepi (N < 440 nmol/d) | U Epi (N < 110 nmol/d) | U Dopa (N < 2570 nmol/d) | U Normeta (N 240) | U Meta (N < 275 nmol/d) | MIBG | Octreo-scan | PET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC1 | 37 | M | No | Unknown | Retrocardiac left paraaortic | Multiple | No | 5 × 3.2 × 2.5 3.5 × 3 × 1.5 4.7 × 3 × 2 | S | 2145 | <33 | 1168 | 2277 | 172 | No | No | Yes |
| IC2 | 52 | F | No | Paternal obligatory carrier | HN R carotid body | Isolated | No | 4 × 3.5 × 2,3 | S | 855 | 151 | 3787a | 605 | 83 | No | Yes | NA |
| IC3 | 23 | F | Yes | Paternal | Intrapericardial | Isolated | No | 3.5 × 3 × 2 | S | 10 800 | <12 | 28 704 | 559 | No | Yes | Yes | |
| IC4 | 66 | F | No | Unknown | Anterior and mean mediastinal with extension in the left atrial | Isolated | Malignant multiple bone metastases | 8.5 × 10 × 10 | NS | 326 | 41 | 2097 | 176 | 49 | Yes | NA | Yes |
| IC5 | 32 | M | Yes | Maternal obligatory carrier | HN L carotid | Isolated | No | 4.5 × 4.2 × 2.2 | ND | NA | NA | NA | NA | NA | NA | NA | NA |
| IC6 | 41 | F | No | Maternal | HN R jugulare | Isolated | Malignant; Locally invasive | 3,7 × 2,1 | S | 3363 | 116 | 6104 | 1539 | 226 | NA | NA | NA |
| IC7 | 49 | M | No | Paternal | HN L: vagal R: carotid | Multiple | No | L: 7.2 × 3.8 × 4.6 R: 1.1 × 1.4 | NS | N | N | N | N | N | NA | NA | NA |
| IC8 | 54 | F | No | Unknown | HN L tympanojugular | Isolated | No | 1.5 × 1 × 0.7 | NS | 141 | 12 | 168 | 117 | 71 | NA | NA | NA |
| IC9 | 34 | F | No | Paternal obligatory carrier | HN R carotid | Isolated | Malignant multiple bone metastases | 3 × 2 × 2 | S | 784 | 56 | 61 371b | 412 | 107 | No | Yes | Yes |
| IC10 | 57 | M | No | Unknown | HN carotid-jugulare space | Isolated | No | 6 × 3.5 × 2.8 | S | 428 | 56 | 44 15c | 180 | 179 | NA | NA | Yes |
| R IC3 | 40 | M | Yes | Paternal obligatory carrier | HN carotid-jugulare space | Isolated | No | 3.9 × 3.5 × 2.2 | NS | N | N | N | N | N | NA | NA | NA |
| R IC5 | 47 | M | Yes | Paternal | HN R vagal | Isolated | No | 6.5 × 2.5 × 1.5 | NS | 415 | 31 | 3508 | 319 | 185 | NA | NA | NA |
| Father R IC5 | 81 | M | Yes | Unknown | HN L carotid | Isolated | No | 0.8 | NS | N | N | N | N | N | NA | NA | Yes |
Abbreviations: F, female; HN, head and neck; IC, index case; L, left; M, male; MIBG, (123I)metaiodobenzylguanidine; N, normal; NA, not available; ND, not determined; NS, nonsecreting; R, right; R IC, related of index case; S, secreting; U, urinary.
Dosage of 9 948 nmol/d urinary methoxytyramine N < 1450.
Plasma methoxytyramine: 12.4 nmol/L N < 0.17.
Plasma methoxytyramine: 1.6 nmol/liter N < 0.17.
Bold indicates abnormal values.
Genetic analysis of PGL-related genes
After genetic counseling, 29 FC patients with PGLs asked for PGL genetic testing and signed informed consent. After blood sampling, germline DNA was extracted from leukocytes and then sent to the Genetic department of Hôpital Européen Georges Pompidou (Paris, France). The genetic testing was done in that expert laboratory by direct sequencing, quantitative multiplex PCR of short fluorescent fragments, and specific multiplex ligation-dependent probe amplification as previously described (2, 4, 5). Over the years, several PPGL-related genes were analyzed for each patient; however, in the last few years, we observed the high prevalence of the SDHC mutation in our cohort of FC patients; thus, the SDHC gene analysis was prioritized in our population using the sequential specific genetic testing approach.
Genealogical reconstruction
To get a better understanding of the origins of the SDHC mutation, we carried out a genealogical reconstruction study. All index cases carrying the SDHC mutation accepted to participate in the genealogical reconstruction study and signed a specific informed consent. Each index case provided the following information required for maternal and paternal lineage reconstruction: name, date of birth, and dates and parishes of marriages of maternal and paternal ancestors. Then the ascending genealogies were reconstructed for each individual to the ancestors who settled in Nouvelle France. This was performed at the University of Québec in Chicoutimi by Project BALSAC using the BALSAC population. The BALSAC population register and its peripheral databases contain linked genealogical information on nearly 340 000 individuals married in the province of Québec between the beginning of the 17th century and the present day (Vézina H, Projet BALSAC-Rapport annual report, 2013–2014; Chicoutimi [Québec] [http://balsac.uqac.ca/]). Reconstruction was performed for the first immigrants to Canada (10 generations ago). Each case was matched to three different controls based on place and year of marriage of their parents. Thirty controls were selected in the BALSAC-RETRO database for comparison. The goal was to determine whether the genealogical characteristics of individuals carrying the SDHC mutation were related to their status or whether they merely depicted the underlying population structure, and the genealogies were analyzed using various software programs developed at the University of Québec in Chicoutimi.
Kinship coefficient and genetic contribution
The kinship coefficient and genetic contribution between probands were calculated as previously described (6). The kinship coefficient between two individuals (B1 and B2) represents the probability that one allele from individual B1 is identical by descent to an allele from individual B2 picked at random at the same locus and is calculated as follows:
where A is a common ancestor of B1 and B2; C is the number of genealogical paths between B1 and B2; m(A, C) is the number of generations separating the founder A from the individual B1 for each path; n(A, C) is the number of generations separating the founder A from the individual B2 for each path; and F(A) is the inbreeding coefficient of the founder A. The mean kinship coefficient for a group of individuals is the kinship coefficient divided by the total number of pairs of individuals.
The genetic contribution of an ancestor represents the sum of transmission probabilities of one gene to each individual of a given group and their genetic contribution to the carriers' group and to the three control groups. Calculation of the genetic contribution of an ancestor to a set of probands S is calculated as follows:
where s is the number of individuals in a given group genealogically related to the ancestor; p is the number of genealogical paths between the ancestor and the individual; and g is the number of generations separating the ancestor from the individual. The mean genetic contribution per individual is the genetic contribution divided by the number of individuals.
Results
Genetic characterization of FC patients with PGLs
Twenty-nine FC patients with histologically proven PGLs seen at our adrenal genetics clinic at CHUM (IB) accepted, after information, to perform genetic analysis of PGL susceptibility genes. Thirteen of 29 patients (44.8%) carried a germline mutation. The same heterozygous nonsense mutation at codon 133 of exon 5 of the SDHC gene (c.397C>T, p.[Arg133Ter]) was found in nine patients representing 69.2% of the patients having a germline mutation and 31% of all FC patients evaluated for PGL genetic testing. The SDHC nonsense mutation (c.397C>T, p.[Arg133Ter]) leads to an amino acid changed from arginine to a stop codon, causing premature truncation of the protein. Among the four other FC patients with PGLs carrying a germline mutation, there were two patients with a SDHD mutation (c.112C>T, p.[Arg38Ter] and c.119_125del, p.[Ile240AsnfsX44]), one patient with a SDHB mutation (c.137G>A, p.[Arg46Gln]), and one patient with PCCs and PGLs due to a FH mutation (c.268-2A>G, p.[?]). This last patient has been reported previously (7).
Clinical characteristics of FC patients carrying a SDHC mutation
Clinical and paraclinical characteristics of the nine index cases of our cohort of FC patients, in addition of the case referred to us from McGill University in Montreal (IC7) (3) carrying the (c.397C>T, p.[Arg133Ter]) SDHC mutation, are summarized in Table 1. The average age at the first diagnosis was 44.5 years (ranging from 23 to 66 y). PGLs were isolated and localized in the head and neck (HNPGL) (n:7), in the thorax (n:2), in the pericardium (n:1), and in the mediastinum (n:1). One patient had multiple PGLs located in retrocardiac position and in the abdomen. A 49-year-old male had a bilateral HNPGL. Three of seven HNPGLs were considered as malignant: a PGL originating from the glomus jugulare that was locally invasive in the mastoid bone (IC6) and two PGLs (IC4 and IC9) with multiple bone metastases. IC4 was metastatic at the initial work-up, and IC9 who has been operated on a carotid PGL at 34 years old was diagnosed 12 years later with multiple bone metastases associated with elevated dopamine levels. Urine noradrenaline, normetanephrines, and/or dopamine levels were elevated in six patients including three patients with HNPGLs, whereas HNPGLs are usually reported to be associated with a catecholamine secretion in less than 5% of cases. Plasma methoxytyramine levels were available for two patients and were in both cases highly elevated (IC9, 12.4 nmol/L [N < 0.17]; and IC10, 1.6 nmol/L). (123I)metaiodobenzylguanidine was positive in one of five cases, 111In-labeled pentetreotide scan (OctreoScan) in three of four cases, and FDG-PET in five of five patients.
Mode of transmission and penetrance of the disease
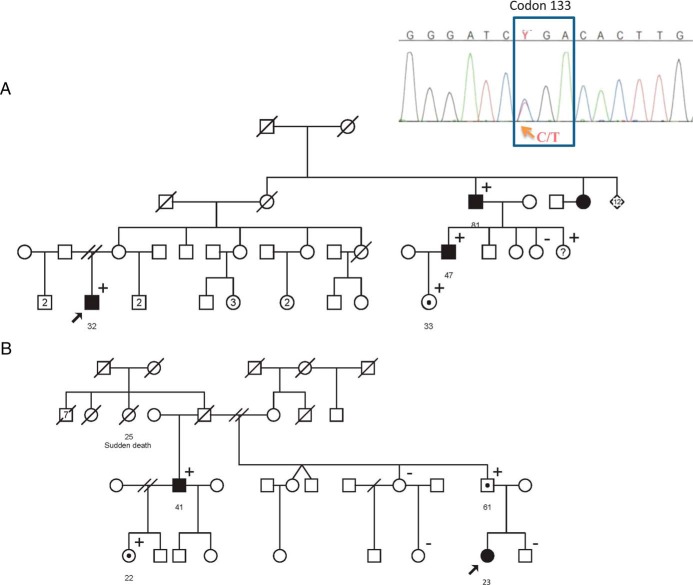
Within the 10 index cases, three individuals had a family history of PGLs. Patient IC5 had a familial history of HNPGLs for the sister of his maternal grandmother (Figure 1A) and the son of a brother of his maternal grandmother (RIC5). Patient IC8 had a sister with a HNPGL, and one son of this sister was known to suffer a PGL (unknown location). At the initial interview, patient IC3 did not report any familial history of PGL but a sudden death event in a sister of her paternal grandfather. Then the half-brother of her father developed a HNPGL at 41 years of age (Figure 1B). Within these three families, the disease, as well as the SDHC mutation, both follows an autosomal mode of transmission.
Figure 1.
Familial pedigrees of patients IC5 (A) and IC3 (B). The arrow shows the index patient with paraganglioma carrying the SDHC mutation (c.397C>T). Squares, men; circles, women; black figures, individuals with PGL; white figures with a black circle in the middle, asymptomatic carriers; +, carrier of the SDHC mutation; −, no carrier of the SDHC mutation.
Parental transmission has been confirmed by genetic analysis in four index cases: paternal branch (n:3) and maternal branch (n:1). In addition, the fathers were obligatory carriers in two cases and the mother in one case. Both maternal and paternal transmission was observed in the family of IC5; moreover, IC3 developed an intrapericardial PGL, whereas her paternal uncle developed a HNPGL, suggesting that the SDHC mutation may be associated with variable expressivity within a same family.
Familial screening of first-degree family members of the index cases with (c.397C>T, p.[Arg133Ter]) SDHC mutation
First-degree relatives of index cases were offered a predictive genetic testing for the presence of germline SDHC mutation. Three parents (two fathers and one mother) were determined as being carriers of the mutation. Clinical investigations including plasmatic metanephrines, normetanephrines, and chromogranin A levels and urinary catecholamines in addition to head and neck, thoracic, and abdominal imaging were negative for two of them. An 8-mm nonsecreting left carotid HNPGL had been found 2 years before the genetic testing in the father of IC5 and was then confirmed to be a unique tumor by 18F-fluorodeoxyglucose-PET. The SDHC mutation was identified in six of nine children of index cases, and in all cases no tumor has been diagnosed. All mutation carriers are currently followed up with biochemical investigation annually and imaging every 2–3 years.
Ascending genealogical reconstruction
Up to the third generation, there were no common ancestors between probands. However, a geographical concentration was revealed. Actually 60% of the parents, 55% of the grandparents, and 50% of the great-grandparents of the 10 index cases were married in des Bois Francs region in the province of Québec followed by the Lanaudière region where 10% of the parents, 10% of the grandparents, and 10% of the great-grandparents were married.
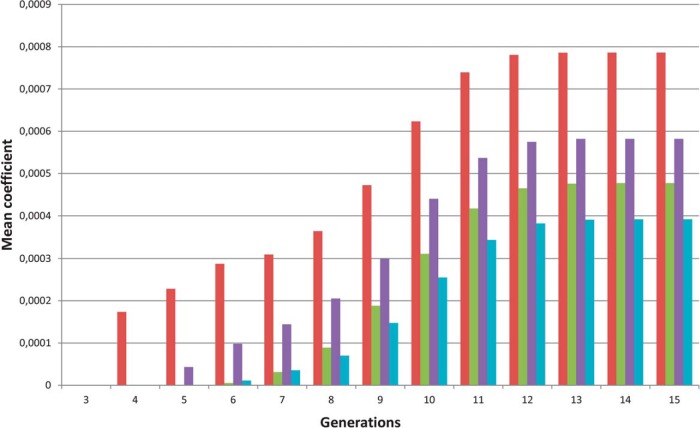
Starting at generation 4, the index cases are more related than the control groups as shown in Figure 2, in which kinship coefficient are higher among index cases than among each of the three control groups, indicating that the probability for index individuals to share an allele identical by descent at the same locus is greater than that of controls.
Figure 2.
Mean kinship coefficients for the SDHC carriers (red bars) and for the three groups of controls (controls 1, green bars; controls 2, purple bars; controls 3, blue bars). Starting at generation 4, the kinship coefficients were higher in the group of SDHC carriers compared with the three control groups, indicating that the index cases are more related than the control groups.
We found 22 common ancestors to the 10 index cases, starting from the eighth generation. They are immigrants or parents of immigrants from France at the beginning of the 17th century, supporting that the SDHC mutation (c.397C>T, p.[Arg133Ter]) is originating from France. These 22 common ancestors are regrouped in four large families including couples with their parents and grandparents. The four more contemporary couples are described in Table 2.
Table 2.
Mean Genetic Contribution of the Common Ancestors in the 10 Index cases (IC) Carrying the SDHC Mutation and the 3 Control Genealogies
| Ancestors |
Mean Genetic Contribution per Individuals |
Genetic Contribution IC/Mean of Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Couple | Gender | Place of Marriage | Date of Marriage | Origin | Migration State | IC | C1 | C2 | C3 | |
| D | M | Montréal, Canada | 1654 | France | Immigrant | 0.003 540 | 0.003 247 | 0.001 428 | 0,000 720 | 1.97 |
| F | France | Immigrant | 0.003 540 | 0.003 247 | 0.001 428 | 0,000 720 | 1.97 | |||
| E | M | France | 1628 | France | Immigrant | 0.003 882 | 0.002 917 | 0.002 948 | 0,002 759 | 1.35 |
| F | France | Immigrant | 0.003 882 | 0.002 917 | 0.002 948 | 0,002 759 | 1.35 | |||
| F | M | France | 1622 | France | Immigrant | 0.002 570 | 0.002 856 | 0.001 337 | 0,002 191 | 1.21 |
| F | France | France | 0.002 570 | 0.002 856 | 0.001 337 | 0,002 191 | 1.21 | |||
| A | M | Québec, Canada | 1653 | France | Immigrant | 0.000 964 | 0.000 610 | 0.000 537 | 0,000 464 | 1.80 |
| F | France | Immigrant | 0.001 990 | 0.002 002 | 0.002 148 | 0,002 710 | 0.87 | |||
Abbreviations: C1, C2, C3, control genealogies; F, female; IC, index case; M, male. The mean genetic contribution of couple D to each index case is of 0.003 540. This means that the probability that the index case had received the mutated SDHC allele from this ancestor is of 0.003 540, which suggest that each index case has a chance on 282 to have received the SDHC mutated gene from this ancestor.
Then, to determine which couple most likely represented the common ancestor to each proband heterozygous for the SDHC mutation (c.397C>T, p.[Arg133Ter]), we compared the genetic contribution of those four couples with three control genealogies (Table 2). Ancestors of couples A, D, E, and F showed a higher mean genetic contribution in the index cases compared with the control groups except for the female of couple A, who showed a higher genetic contribution in the controls.
All individuals of these four couples were born in France. Two couples were married in France, and two others were married in Nouvelle France (one in Québec City in 1654 and one in Montréal City in 1653) after immigration. The French origin of the SDHC mutation (c.397C>T, p.[Arg133Ter]) has been confirmed by the genetics laboratory of Hôpital Européen Georges Pompidou that previously identified the SDHC mutation (c.397C>T, p.[Arg133Ter]) in five French affected cases (four unrelated): four males and one female aged of 35, 36, 38, 66, and 72 years at diagnosis. All developed an HNPGL. A 35-year-old male (patient 2) had a locally invasive tumor as found in IC6 (Table 3).
Table 3.
Clinical Details of the Investigated French Patients With the SDHC (c.397C>T, p.[Arg133Ter]) Mutation With Paragangliomas
| Cases Department of Birth | Age at Diagnosis, y | Sex | Family History | Parental Transmission | Initial Localization | Unique or Multiple | Malignant | Location | Tumor Size, cm | Secretion |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 Loiret | 36 | M | No | Paternal | HN | Unique | N | Vagal | 5 | NS |
| Patient 2 Seine-Maritime | 35 | M | No | Maternal | HN | Unique | Locally invasive | Jugulocarotid | 5 | NS |
| Patient 3 Paris | 38 | M | No | Maternal | HN | Multiple | N | Tympanojugulare | L:4 R:1,7 | NS |
| Patient 10 Landes | 72 | F | No | Unknown | HN | Unique | Locally invasive | Tympanojugulare | NA | NA |
| Patient 12 Eure-et-Loir | 66 | M | Yes Father of patient 1 | Unknown | HN | Unique | N | Vagale | 4 | NS |
Abbreviations: F, female; HN, head and neck; L, left; M, male; NA, not available; NS, nonsecreting; R, right.
Discussion
We report here the clinical characteristics of a cohort of 18 patients (Tables 1 and 3) affected by PGLs caused by a SDHC mutation, including 10 FC index cases and three affected relatives of those, and four French index cases with one of their relatives. All are carrying the SDHC mutation (c.397C>T, p.[Arg133Ter]). The SDHC mutation related to PGLs, which is referred to as paraganglioma type 3, was first described in 2000 by Niemann and Muller (8). In the last decade, SDHC mutations were believed to be associated mainly with head and neck PGLs (9). In 2014, Else et al (10) described a cohort of eight patients with PGLs caused by various SDHC mutations. Three of those patients had mediastinal PGLs. In the cohort reported here, 3 of 10 index cases have mediastinal PGLs located close to the heart in all cases; one was intrapericardial, one retrocardiac, and one originating from the mediastinum with an extension to the left atrial. In our entire cohort of 18 patients, in contrast to the cohort of Else et al, in which there were no cases of malignancy, 5 of 18 cases (28%) had malignant disease including three patients with locally invasive HNPGLs, a 34-year-old woman who was diagnosed with HNPGLs and developed secondary multiples bone metastases that were diagnosed 12 years later and a 66-year-old female with mediastinal PGLs that showed multiple bone metastases at diagnosis. These cases of malignant disease represent 28% of our large cohort of patients, indicating for the first time that SDHC-related PGLs may be frequently malignant and underlying that these patients should be followed up all their lives. Whether the malignant phenotype is specific to the SDHC mutation (c.397C>T, p.[Arg133Ter]) or to all SDHC mutations will have to be determined.
SDHC mutations are infrequent worldwide in patients with PGLs, and this is contrasting with the high prevalence of SDHC mutation found in 31% of our FC cohort of PGLs. To our knowledge, only six other cases carrying the SDHC mutation (c.397C>T, p.[Arg133Ter]) associated with PGLs have been described so far. Three unrelated patients were described previously by a German team (11) and three from the United States into three different reports (10, 12, 13) (Table 4). The ethnical origin of these cases was not described. Within these six described cases, there were five patients with HNPGLs (10–13) including a 66-year-old male with an aggressive invasive temporal bone PGL (13) and one patient in the study by Bickmann et al (11), similar to our patient IC4 with a large mediastinal PGL infiltrating the left ventricular myocardium with bone metastases. One HNPGL case was reported by Zbuk et al (12), who described a woman with epithelial thyroid cancer and carotid PGL who carried in addition to the SDHC mutation a germline PTEN mutation. Four of six HNPGLs cases were secreting tumors, which is much higher than the former admitted level of 5% secreting HNPGLs: two secreted mainly noradrenaline and normetanephrine and two others secreted very high levels of dopamine and methoxytyramine. These findings understress that all individual diagnosed with SDHC-related HNPGLs should have a biochemical evaluation including methoxytyramine and/or dopamine levels. Although dopamine does not lead to high blood pressure and does not necessitate antihypertensive medications prior to surgery, it may become a tumor marker in the follow-up of these patients.
Table 4.
Clinical Details of Six Unrelated Patients (Three From Germany and Three From the United States) Who Were Described Previously Carrying the SDHC (c.397C>T, p.[Arg133Ter]) Mutation
| Reference | Country | Age at Diagnosis y | Sex | Family History | Parental Transmission | Initial Localization | Isolated or Multiple | Malignant | Tumor Size, cm | Secretion | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bickmann et al, 2014 (11) | Germany | 51 | F | No | NA | Mediastinal PGL | Isolated | Y bone metastases | NA | NS | |
| Bickmann et al, 2014 (11) | Germany | 57 | M | No | NA | HN R jugular | Isolated | No | NA | NA | Suspicion of a left adrenal pheochromocytoma |
| Bickmann et al, 2014 (11) | Germany | 45 | F | No | NA | HN L carotid bifurcation | Isolated | No | NA | NA | |
| Zbuk et al, 2007 (12) | United States | 37, 39 | F | No | NA | HN L carotid and R carotid | Multiple | No | 3 cm and 3 × 1.5 cm | NS | Germline PTEN mutation papillary thyroid cancer |
| Else et al, 2014 (10) | United States | 30 | F | No | NA | HN jugular | Isolated | No (local recurrence) | NA | NA | |
| Isaacson et al, 2015 (13) | United States | 66 | M | No | NA | HN L temporal bone | Isolated | Yes locally invasive | 4.8 × 5.3 × 6.2 cm | Yes, NE and NM elevated | Primary hyperparathyroidism (adenoma) |
Abbreviations: F, female; HN, head and neck; L, left; M, male; NA, not available; NE, norepinephrines; NM, normetanephrines; NS, nonsecreting; R, right.
The fact that the SDHC (c.397C>T, p.[Arg133Ter]) mutation was recurrent in our FC cohort led us to hypothesize that this mutation might have a single origin. Using a genealogical ascending reconstruction study, we found that the apparently unrelated 10 index cases were more genetically related than the control groups. The SDHC (c.397C>T, p.[Arg133Ter]) mutation was most likely introduced by a couple of settlers from France in the 17th century and has been enriched to the FC population as a result of a founder effect. The limited number of founders, which is estimated during the peak of French immigration of approximately 3380 pioneers settled permanently in the St Lawrence River Valley in La Nouvelle France, constitutes the basis for the unique genetic background of the FC population leading to FC founder mutations in various diseases and now in PGLs.
Some evidence for the founder effect in SDHx genes have been reported in other populations; founder mutations in the SDHAF2, SDHB, and SDHD genes were described in The Netherlands (14, 15) and in the SDHD gene in an area of central Italy (16) and Austria (17). However, to our knowledge we report here the first founder effect in the SDHC gene.
The prevalence of PGLs is unknown in the Province of Québec, but the early introduction of the SDHC (c.397C>T, p.[Arg133Ter]) mutation in the Québec settlement process could imply that this mutation has a high prevalence in the FC population. The fact that 7 of 10 index cases with founder SDHC mutation present without a family history of PGLs suggests a low penetrance of the SDHC mutation, which is supporting by that among nine carriers of SDHC mutation, we found no PGLs except for a 80-year-old man who was diagnosed with a small HNPGL 2 years prior the genetic screening.
Genetic screening in the FC population should take into account the unusually high prevalence of the SDHC founder mutation. This study should significantly simplify the molecular testing and clinical management of FC patients with PGLs and provide new clinical and paraclinical data on the rare SDHC-related PGLs.
Acknowledgments
We acknowledge the following physicians for addressing patients to our adrenal genetics clinic and for this study: Dr Nahla Aris-Jilwan, Dr Catherine Beauregard, Dr Jean-Hugues Brossard, Dr William Foulkes, Dr Nader Khaouam, Dr Maude Lefebvre, Dr André Lacroix, Dr Rébecca Leboeuf, Dr Isabel Quintin, and Dr Hélène Villeneuve. We thank Michèle Jomphe and Eve-Marie Lavoie and the Réseau de Médecine Génétique Appliquée du Fonds de Recherche du Québec-Santé Support BALSAC, who performed the data analysis of the Genealogical Reconstruction study. We also thank INSERM for their support to the French registry for SDH-related paraganglioma (PGL.R) and Isabelle Lévesque for her assistance. We also gratefully thank all the patients and their families whose participation made this work possible.
This work was supported in part by a salary grant from the Fonds de Recherche du Québec-Santé (to I.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CHUM
- Centre Hospitalier de l'Université de Montréal
- FC
- French Canadian
- HNPGL
- PGLs localized in the head and neck
- PCC
- pheochromocytoma
- PET
- positron emission tomography
- PGL
- paraganglioma.
Reference
- 1. Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101–111. [DOI] [PubMed] [Google Scholar]
- 2. Burnichon N, Rohmer V, Amar L, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Clin Endocrinol Metab. 2009;94(8):2817–2827. [DOI] [PubMed] [Google Scholar]
- 3. Lefebvre M, Foulkes WD. Pheochromocytoma and paraganglioma syndromes: genetics and management update. Curr Oncol. 2014;21(1):e8–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buffet A, Venisse A, Nau V, et al. A decade (2001–2010) of genetic testing for pheochromocytoma and paraganglioma. Horm Metab Res. 2012;44(5):359–366. [DOI] [PubMed] [Google Scholar]
- 5. Amar L, Bertherat J, Baudin E, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005;23(34):8812–8818. [DOI] [PubMed] [Google Scholar]
- 6. Vezina H, Durocher F, Dumont M, et al. Molecular and genealogical characterization of the R1443X BRCA1 mutation in high-risk French-Canadian breast/ovarian cancer families. Hum Genet. 2005;117(2–3):119–132. [DOI] [PubMed] [Google Scholar]
- 7. Castro-Vega LJ, Buffet A, De Cubas AA, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23(9):2440–2446. [DOI] [PubMed] [Google Scholar]
- 8. Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26(3):268–270. [DOI] [PubMed] [Google Scholar]
- 9. Schiavi F, Boedeker CC, Bausch B, et al. Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. JAMA. 2005;294(16):2057–2063. [DOI] [PubMed] [Google Scholar]
- 10. Else T, Marvin ML, Everett JN, et al. The clinical phenotype of SDHC-associated hereditary paraganglioma syndrome (PGL3). J Clin Endocrinol Metab. 2014;99(8):E1482–E1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bickmann JK, Sollfrank S, Schad A, et al. Phenotypic variability and risk of malignancy in SDHC-linked paragangliomas: lessons from three unrelated cases with an identical germline mutation (p.Arg133*). J Clin Endocrinol Metab. 2014;99(3):E489–E496. [DOI] [PubMed] [Google Scholar]
- 12. Zbuk KM, Patocs A, Shealy A, Sylvester H, Miesfeldt S, Eng C. Germline mutations in PTEN and SDHC in a woman with epithelial thyroid cancer and carotid paraganglioma. Nat Clin Pract Oncol. 2007;4(10):608–612. [DOI] [PubMed] [Google Scholar]
- 13. Isaacson B, Bullova P, Frone M, et al. An aggressive temporal bone Sdhc paraganglioma associated with increased Hif-2a signaling. Endocr Pract. 2016;22(2):190–195. [DOI] [PubMed] [Google Scholar]
- 14. Bayley JP, Grimbergen AE, van Bunderen PA, et al. The first Dutch SDHB founder deletion in paraganglioma-pheochromocytoma patients. BMC Med Genet 2009;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hensen EF, van Duinen N, Jansen JC, et al. High prevalence of founder mutations of the succinate dehydrogenase genes in the Netherlands. Clin Genet. 2012;81(3):284–288. [DOI] [PubMed] [Google Scholar]
- 16. Simi L, Sestini R, Ferruzzi P, et al. Phenotype variability of neural crest derived tumours in six Italian families segregating the same founder SDHD mutation Q109X. J Med Genet. 2005;42(8):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janecke AR, Willett-Brozick JE, Karas C, Hasipek M, Loeffler-Ragg J, Baysal BE. Identification of a 4.9-kilobase-pair Alu-mediated founder SDHD deletion in two extended paraganglioma families from Austria. J Hum Genet. 2010;55(3):182–185. [DOI] [PubMed] [Google Scholar]