Abstract
Objective:
Lipodystrophy syndromes are extremely rare disorders of deficient body fat associated with potentially serious metabolic complications, including diabetes, hypertriglyceridemia, and steatohepatitis. Due to their rarity, most clinicians are not familiar with their diagnosis and management. This practice guideline summarizes the diagnosis and management of lipodystrophy syndromes not associated with HIV or injectable drugs.
Participants:
Seventeen participants were nominated by worldwide endocrine societies or selected by the committee as content experts. Funding was via an unrestricted educational grant from Astra Zeneca to the Pediatric Endocrine Society. Meetings were not open to the general public.
Evidence:
A literature review was conducted by the committee. Recommendations of the committee were graded using the system of the American Heart Association. Expert opinion was used when published data were unavailable or scarce.
Consensus Process:
The guideline was drafted by committee members and reviewed, revised, and approved by the entire committee during group meetings. Contributing societies reviewed the document and provided approval.
Conclusions:
Lipodystrophy syndromes are heterogeneous and are diagnosed by clinical phenotype, supplemented by genetic testing in certain forms. Patients with most lipodystrophy syndromes should be screened for diabetes, dyslipidemia, and liver, kidney, and heart disease annually. Diet is essential for the management of metabolic complications of lipodystrophy. Metreleptin therapy is effective for metabolic complications in hypoleptinemic patients with generalized lipodystrophy and selected patients with partial lipodystrophy. Other treatments not specific for lipodystrophy may be helpful as well (eg, metformin for diabetes, and statins or fibrates for hyperlipidemia). Oral estrogens are contraindicated.
Multiple worldwide endocrine societies developed practice guidelines for diagnosis and management of lipodystrophy syndromes based on current evidence.
The lipodystrophy syndromes are a heterogeneous group of rare disorders that have in common selective deficiency of adipose tissue in the absence of nutritional deprivation or catabolic state (Figure 1). Lipodystrophies are categorized based on etiology (genetic or acquired) and distribution of lost adipose tissue, affecting the entire body (generalized) or only regions (partial). This yields four major categories: congenital generalized lipodystrophy (CGL), familial partial lipodystrophy (FPLD), acquired generalized lipodystrophy (AGL), and acquired partial lipodystrophy (APL) (Figure 1). Additional subtypes include progeroid disorders, autoinflammatory disorders, and others (Table 1). This practice guideline will not discuss lipodystrophy in HIV infected patients or localized lipodystrophy (eg, from injectable drugs).
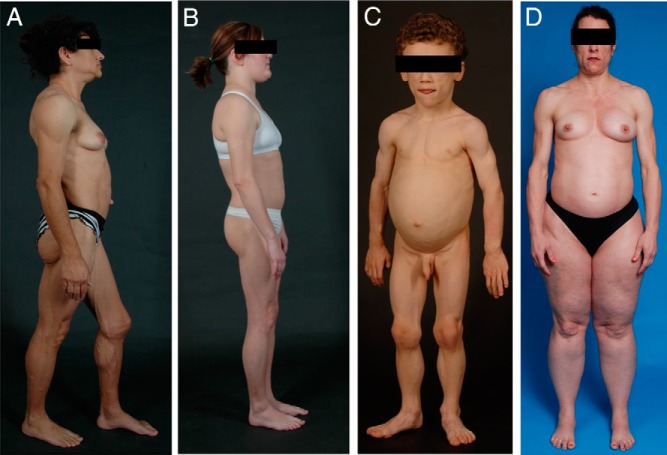
Figure 1.
Physical appearance of patients with the four main subtypes of lipodystrophy syndromes. A, Lateral view of a 33-year-old Hispanic female with congenital generalized lipodystrophy (also known as Berardinelli-Seip congenital lipodystrophy), type 1 due to homozygous c.589–2A>G; p.(Val197Glufs*32) mutation in the AGPAT2 gene. The patient had generalized loss of subcutaneous (sc) fat with acanthosis nigricans in the axillae and neck. She has umbilical prominence and acromegaloid features (enlarged mandible, hands, and feet). B, Lateral view of a 26-year-old female with familial partial lipodystrophy of the Dunnigan variety due to heterozygous c.575A>T; p.(Asp192Val) mutation in the LMNA gene. She had marked loss of sc fat from the upper and lower extremities and accumulation of sc fat in the face and chin. C, Anterior view of an 8-year-old German boy with acquired generalized lipodystrophy. He had severe generalized loss of sc fat with marked acanthosis nigricans in the neck, axillae, and groin. D, Anterior view of a 45-year-old Caucasian female with acquired partial lipodystrophy (Barraquer-Simons syndrome). She had marked loss of sc fat from the face, neck, upper extremities, and chest but had lipodystrophy on localized regions on the anterior thighs. She had increased sc fat deposition in the lower extremities.
Table 1.
Subtypes and Inheritance of Lipodystrophy
| Inheritance Pattern | Subtype | Lipodystrophy Phenotype | Genes Involved | Refs. |
|---|---|---|---|---|
| Autosomal recessive | CGL | Near total absence of body fat, generalized muscularity, metabolic complications | AGPAT2, BSCL2, CAV1, PTRF, PCYT1A, PPARγ | 11, 84–88 |
| Progeroid syndromes | Partial or generalized absence of body fat, progeroid features, variable metabolic complications | LMNA, ZMPSTE24, SPRTN, WRN, BANF1 | 89–93 | |
| FPLD | Absence of fat in limbs, metabolic complications | CIDEC, LIPE, PCYT1A | 87, 92, 94–96 | |
| Autoinflammatory | Variable absence of fat, variable metabolic complications | PSMB8 | 97 | |
| Autosomal dominant | FPLD | Absence of fat from the limbs, metabolic complications | LMNA, PPARG, AKT2, PLIN1 | 98–103 |
| Progeroid syndromes | Partial or generalized absence of body fat, progeroid features, variable metabolic complications | LMNA, FBN1, CAV1, POLD1, KCNJ6 | 104–109 | |
| SHORT syndrome | Variable loss of body fat, metabolic complications | PIK3R1 | 110 | |
| Acquired | AGL | Near total absence of body fat, metabolic complications | None | 4 |
| APL | Absence of fat in upper body with increased fat in lower body, mild or no metabolic complications | None | 17 |
Lipodystrophy syndromes are frequently associated with hormonal and metabolic derangements resulting in severe comorbidities (Table 2) that depend on the subtype, extent of fat loss, age, and gender. Many complications of lipodystrophy are secondary to deficient adipose mass, resulting in ectopic lipid storage in the liver, muscle, and other organs and causing insulin resistance. Insulin resistance leads to diabetes, hypertriglyceridemia, polycystic ovarian syndrome (PCOS), and nonalcoholic fatty liver disease (NAFLD) (1).
Table 2.
Major Comorbidities and Complications of Lipodystrophy
| Complication | Affected Subtypes | Refs. |
|---|---|---|
| Hyperphagia | AGL, CGL, ±FPLD | 4, 10, 111 |
| Dyslipidemia (high triglycerides, low HDL-cholesterol, acute pancreatitis, eruptive xanthomas) | AGL, CGL, FPLD | 4, 5, 7, 9, 13, 21, 30 |
| Insulin resistance/diabetes, acanthosis nigricans (and diabetes complications) | AGL, CGL, FPLD | 4, 5, 7, 9, 13, 17, 20, 21, 69 |
| Reproductive dysfunction (PCOS, oligomenorrhea, reduced fertility, hirsutism, preeclampsia, miscarriage, macrosomia) | AGL, CGL, FPLD | 4, 5, 7, 10, 14, 20, 55, 112 |
| NAFLD (ranging from simple steatosis to cirrhosis) | AGL, CGL, FPLD, ±APL | 4, 7, 10, 17, 19, 49, 51, 69, 113 |
| Renal dysfunction (proteinuria, MPGN, FSGS, diabetic nephropathy) | AGL, CGL, FPLD, APL | 17, 34, 114 |
| Heart disease (hypertension, cardiomyopathy, arrhythmias, conduction abnormalities, CAD) | AGL, CGL, FPLD | 3–5, 9, 13, 15, 25 |
| Autoimmune disease | AGL, APL | 4, 10, 17, 19, 20 |
Abbreviations: CAD, coronary artery disease; FSGS, focal segmental glomerulosclerosis. Many of these features are also found in other forms of lipodystrophy, including progeroid disorders.
Major causes of mortality include heart disease (cardiomyopathy, heart failure, myocardial infarction, arrhythmia) (2–5), liver disease (liver failure, gastrointestinal hemorrhage, hepatocellular carcinoma) (6, 7), kidney failure (6), acute pancreatitis (7), and sepsis.
Due to the rarity of lipodystrophy syndromes, many clinicians are unfamiliar with their diagnosis and management. In December 2015, an expert panel including representatives from endocrine societies around the world convened to generate this practice guideline. Evidence was rated using the system of the American Heart Association (Supplemental Table 1) (8). Details of the literature review, consensus, and endorsement process are provided in the Supplemental Data.
Overview of Lipodystrophy Syndromes
This section reviews major categories of lipodystrophy. Details on individual subtypes are in Supplemental Table 2.
Congenital generalized lipodystrophy (Berardinelli-Seip syndrome)
CGL is an autosomal recessive disorder characterized by near-complete lack of fat starting at birth or infancy, prominent muscles, phlebomegaly, acanthosis nigricans, hepatomegaly, umbilical prominence, and voracious appetite in childhood (9, 10). Multiple genetic causes have been identified, each with unique clinical features (11–13). Metabolic complications are frequent and may be severe. Cardiomyopathy or rhythm disturbances may occur.
Familial partial lipodystrophy
FPLD is a group of usually autosomal dominant disorders characterized by loss of fat affecting the limbs, buttocks, and hips (10). Regional excess fat accumulation is frequent, varies by subtype, and may result in a Cushingoid appearance. Fat distribution is typically normal in early childhood, with loss of fat occurring around puberty. Muscular hypertrophy is common. Metabolic complications are common in adulthood (14), with increased risk of coronary heart disease (15) and occasionally early cardiomyopathy.
Acquired generalized lipodystrophy (Lawrence syndrome)
AGL is more common in females (females:males, 3:1) and appears usually before adolescence (but may develop at any time in life) with progressive loss of fat affecting the whole body including palms and soles (4). Some fat accumulation can appear in the face, neck, or axillae. Metabolic complications are frequent and may be severe. AGL is often associated with autoimmune diseases (4, 16).
Acquired partial lipodystrophy (Barraquer-Simons syndrome)
APL is more frequent in females (females:males, 4:1) and usually begins in childhood or adolescence. Loss of fat follows a cranio-caudal trend, progressively affecting the face, neck, shoulders, arms, and trunk. Fat accumulation can appear in the hips, buttocks, and legs (17). APL is associated with autoimmune diseases, especially membranoproliferative glomerulonephritis (MPGN) in approximately 20% (17). Most patients have low serum complement 3 (C3) levels, and some have presence of C3 nephritic factor. Metabolic complications are uncommon (17).
Diagnosis of Lipodystrophy
Diagnosis of lipodystrophy is based on history, physical examination, body composition, and metabolic status. (Class I, Level B)
There are no defined serum leptin levels that establish or rule out the diagnosis of lipodystrophy. (Class IIa, Level C)
Confirmatory genetic testing is helpful in suspected familial lipodystrophies. (Class I, Level A)
Genetic testing should be considered in at-risk family members. (Class IIa, Level C)
Serum complement levels and autoantibodies may support diagnosis of acquired lipodystrophy syndromes. (Class IIa, Level B)
Firm diagnostic criteria for lipodystrophy have not been established. Figure 2 shows a suggested diagnostic approach.
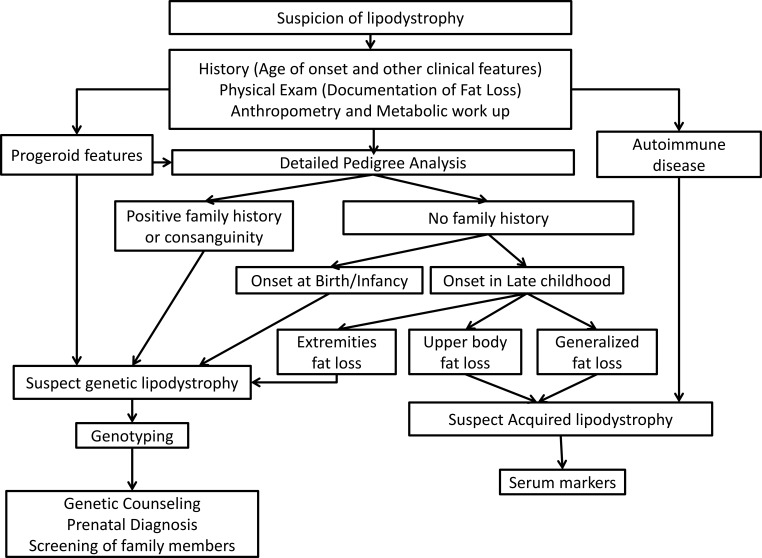
Figure 2.
Diagnostic approach to lipodystrophy syndromes. Lipodystrophy should be suspected in patients with regional or generalized lack of adipose tissue. History should assess age of onset of fat loss and comorbidities. Physical examination should determine distribution of sc fat loss and presence of prominent muscles, phlebomegaly, acanthosis nigricans, hepatomegaly, xanthomas, and acromegaloid or progeroid appearance. All patients should undergo a metabolic workup for insulin resistance, diabetes, dyslipidemia, and fatty liver disease. Conventional anthropometry including skinfold thickness measurements, ± dual energy x-ray absorptiometry, and whole-body magnetic resonance imaging (if available) should be performed to confirm the pattern of fat loss. Common genetic lipodystrophies include CGL, FPLD, and progeroid lipodystrophies. They require genotyping to confirm the diagnosis, followed by genetic counseling and screening of family members. Patients with progeroid lipodystrophies have progeroid features like bird-like facies, high-pitched voice, skin atrophy and pigmentation, alopecia, and nail dysplasia. Patients with FPLD have fat loss of the extremities typically occurring around puberty and can have a positive family history. Patients with CGL have near-complete lack of fat starting at birth or infancy. Acquired lipodystrophies have fat loss typically in late childhood. Patients with AGL have generalized loss of sc fat and often have associated autoimmune diseases. Patients with APL have cranio-caudal fat loss affecting the face, neck, shoulders, arms, and upper trunk, and most patients have low serum C3 levels.
Establishing the presence of lipodystrophy
Lipodystrophy should be suspected in patients with regional or generalized lack of adipose tissue outside of the normal range by physical examination, which can be supported by anthropometry, dual energy x-ray absorptiometry, and whole-body magnetic resonance imaging (Supplemental Table 3) (18). Recognizing the loss of sc fat is particularly challenging in partial lipodystrophy and especially in men, in whom low body fat overlaps with normal variation and metabolic manifestations of lipodystrophy are less severe. In both genetic and acquired lipodystrophies, the loss of fat may be gradual, delaying diagnosis.
Physical, historical, and comorbid features that increase the suspicion of lipodystrophy (18) are shown in Table 3.
Table 3.
Clinical Features That Increase the Suspicion of Lipodystrophy
| Essential feature |
| Generalized or regional absence of body fat |
| Physical features |
| Failure to thrive (infants and children) |
| Prominent muscles |
| Prominent veins (phlebomegaly) |
| Severe acanthosis nigricans |
| Eruptive xanthomata |
| Cushingoid appearance |
| Acromegaloid appearance |
| Progeroid (premature aging) appearance |
| Comorbid conditions |
| Diabetes mellitus with high insulin requirements |
| ≥200 U/d |
| ≥2 U/kg/d |
| Requiring U-500 insulin |
| Severe hypertriglyceridemia |
| ≥500 mg/dL with or without therapy |
| ≥250 mg/dL despite diet and medical therapy |
| History of acute pancreatitis secondary to hypertriglyceridemia |
| Non-alcoholic steatohepatitis in a non-obese individual |
| Early-onset cardiomyopathy |
| PCOS |
| Other historical clues |
| Autosomal dominant or recessive pattern of similar physical features or metabolic complications |
| Significant hyperphagia (may manifest as irritability/aggression in infants/children) |
Adapted from Ref. 18.
Because serum leptin assays are not standardized and leptin concentrations in patients with lipodystrophy (especially partial forms) overlap the general population, leptin levels do not help in diagnosis but may help with the choice of therapies.
Differential diagnosis
Differential diagnosis should include conditions presenting with severe weight loss (malnutrition, anorexia nervosa, uncontrolled diabetes mellitus, thyrotoxicosis, adrenocortical insufficiency, cancer cachexia, HIV-associated wasting, chronic infections). Especially difficult is differentiating lipodystrophy from uncontrolled diabetes because both may have extreme hypertriglyceridemia. However, restoring glycemic control in patients with nonlipodystrophic diabetes leads to a regain of body fat. Generalized lipodystrophies can be confused with mutations of the insulin receptor or acromegaly/gigantism, and FPLD with Cushing's syndrome, truncal obesity, and multiple symmetric lipomatosis.
Establishing the subtype of lipodystrophy
Pattern of fat loss
Although the pattern of body fat loss in patients with a particular subtype of genetic lipodystrophy is quite characteristic, heterogeneity occurs in the onset, severity, and pattern of fat loss, even within families.
Distinguishing genetic from acquired lipodystrophy
Pedigree analysis can suggest genetic vs acquired lipodystrophy. Review of photographs from infancy may distinguish CGL from AGL because infants typically show absent fat in CGL and normal fat in AGL. However, there have been cases of AGL with loss of fat during the first few months of life (4). Patients with AGL lack family history but can be confused with any type of genetic lipodystrophy, especially de novo mutations.
The presence of autoimmune diseases (myositis, type 1 diabetes, autoimmune hepatitis, and others) (4, 10, 16, 17, 19, 20) increases the suspicion of acquired lipodystrophy. In APL, low serum C3, C3 nephritic factor, proteinuria, or biopsy-proven MPGN support the diagnosis.
Genetic testing
Genotyping may include limited candidate gene sequencing, a panel of candidate genes, or whole-exome/whole-genome sequencing. The website www.genetests.org lists clinical and research laboratories conducting genetic testing for lipodystrophy syndromes. Because there is strong evidence for additional loci for genetic lipodystrophies, negative tests do not rule out a genetic condition.
Genetic counseling and screening of family members
Genetic counseling must take into consideration that the current understanding of the natural history of genetic lipodystrophies is incomplete. In affected pedigrees, premarital counseling with genetic testing to detect carrier status can be considered.
Clinical diagnosis of lipodystrophy may be difficult in men (21), and some genotypes are associated with mild lipodystrophy phenotypes (22, 23). Genetic screening of family members may help identify individuals with subtle phenotypes. Genetic screening may be particularly important for families with specific LMNA mutations associated with cardiomyopathy and arrhythmia.
Screening for Comorbidities
All patients should be screened for diabetes, dyslipidemia, NAFLD, and cardiovascular and reproductive dysfunction. Because patients with APL are at low risk for metabolic complications, clinical judgment should guide follow-up screening. Screening for comorbidities specific to individual lipodystrophy subtypes is not extensively discussed here.
Diabetes mellitus
Diabetes screening should be performed annually. (Class IIa, Level C)
Diabetes screening should follow the guidelines of the American Diabetes Association (fasting plasma glucose, oral glucose tolerance test, or glycosylated hemoglobin [HbA1c]). Patients with AGL may develop type 1 diabetes in addition to insulin resistance (24); measurement of autoantibodies may clarify the diagnosis.
Dyslipidemia
Triglycerides should be measured at least annually and with occurrence of abdominal pain or xanthomata. (Class I, Level C)
Fasting lipid panel (total cholesterol, low-density lipoprotein [LDL]-cholesterol, high-density lipoprotein [HDL]-cholesterol, triglycerides) should be measured at diagnosis and annually after age 10 years. (Class IIa, Level C)
Liver disease
Alanine aminotransferase and aspartate aminotransferase should be measured annually. (Class IIa, Level C)
Liver ultrasound should be performed at diagnosis, then as clinically indicated. (Class IIa, Level C)
Liver biopsy should be performed as clinically indicated. (Class IIa, Level C)
In addition to physical examination, ultrasound and elastography are useful to estimate liver and spleen size, severity of steatosis and fibrosis, and existence of portal hypertension. Patients with CGL2 are at high risk for early cirrhosis, and those with AGL may have autoimmune hepatitis in addition to NAFLD (19).
Reproductive dysfunction
Gonadal steroids, gonadotropins, and pelvic ultrasonography should be performed as clinically indicated. (Class IIa, Level C)
Pubertal staging should be performed annually in children. (Class IIa, Level C)
Early adrenarche, true precocious puberty, or central hypogonadism may occur in children with generalized lipodystrophy. Oligo/amenorrhea, decreased fertility, and PCOS are common in women.
Cardiac disease
Blood pressure should be measured at least annually. (Class I, Level C)
Electrocardiogram and echocardiogram should be performed annually in CGL and progeroid disorders, at diagnosis, and as clinically indicated in FPLD and AGL. (Class IIa, Level C)
Evaluation for ischemia and rhythm monitoring should be considered in patients with progeroid disorders and FPLD2 with cardiomyopathy. (Class IIa, Level C)
Hypertension is common (25), even in children. In patients with CGL4, atypical progeroid syndromes, and FPLD2 due to LMNA mutations, cardiac abnormalities including ischemic heart disease, cardiomyopathy, arrhythmias, and sudden death are reported (3, 23, 26–33).
Kidney disease
Urine protein should be measured annually using 24-hour urine collection or spot urine protein-to-creatinine ratio. (Class IIa, Level C)
Proteinuria is common (34). Kidney biopsy should be performed as clinically indicated, and pathology may include diabetic nephropathy, focal segmental glomerulosclerosis (especially in CGL) (34) or MPGN (especially in APL) (17).
Malignancy
Lymphomas, particularly peripheral T-cell lymphoma, occur in AGL, with a prevalence of approximately 7% (4, 35). Appropriate screening has not been established but would reasonably include annual skin and lymph node examination. Generalized lipodystrophy has been reported as a paraneoplastic manifestation of pilocytic astrocytoma in three children who regained body fat after cancer therapy (36). Clinicians should consider screening for brain tumors in children who present with idiopathic AGL or atypical CGL. Specific progeroid syndromes (eg, Bloom and Werner syndromes) are associated with increased malignancy risk (Supplemental Table 2).
Treatment of Lipodystrophy Syndromes
Current therapies prevent or ameliorate the comorbidities of lipodystrophy syndromes. There is no cure for lipodystrophy and no treatment that can regrow adipose tissue.
Diet
Most patients should follow diets with balanced macronutrient composition. (Class IIa, Level C)
Energy-restricted diets improve metabolic abnormalities and may be appropriate in adults. (Class I, Level C)
Very-low-fat diets should be used in chylomicronemia-induced acute pancreatitis. (Class I, Level C)
A dietician should be consulted for specialized dietary needs, especially in infants and young children.Overfeeding should be avoided. (Class IIa, Level C)
Medium-chain triglyceride oil formulas can provide energy and reduce triglycerides in infants. (Class IIa, Level C)
The cornerstone of therapy for metabolic complications of lipodystrophy is diet. Studies of specific diets in lipodystrophy are lacking, and recommendations rely on sparse literature and clinical experience.
Patients with lipodystrophy, especially generalized forms, are typically hyperphagic due to leptin deficiency. Energy-restricted diets in adolescents and adults lower triglycerides and glucose (37), but dietary restriction is challenging to achieve. Food restriction to control metabolic complications must be balanced by requirements for growth in children. Overfeeding to achieve normal weight may worsen metabolic complications and hepatic steatosis. Assessment of weight-for-length and body mass index by comparison to reference growth data is not appropriate because body composition is atypical. Low weight-for-length or body mass index is acceptable provided linear growth is maintained.
Patients should follow a 50–60% carbohydrate, 20–30% fat, and approximately 20% protein diet. Simple sugars should be restricted in preference for high-fiber complex carbohydrates, distributed evenly among meals and snacks and consumed in combination with protein or fat. Dietary fat should be primarily cis-mono-unsaturated fats and long-chain omega-3 fatty acids. In extremely hypertriglyceridemic infants, medium-chain triglyceride-based formula may help (38, 39). During acute pancreatitis, bowel rest followed by a very-low-fat (<20 g) diet should be used.
Exercise
Patients with lipodystrophy should be encouraged to exercise in the absence of specific contraindications. (Class IIa, Level C)
Patients with subtypes of lipodystrophy predisposed to cardiomyopathy should undergo cardiac evaluation before initiating an exercise regimen. (Class III, Level C)
Individuals with lipodystrophy engaged in intense exercise have amelioration of metabolic complications. Most patients should be encouraged to be physically active. However, strenuous exercise should be avoided in patients with cardiomyopathy. Contact sports should be avoided in patients with severe hepatosplenomegaly and CGL patients with lytic bone lesions.
Metreleptin
In generalized lipodystrophy, metreleptin (with diet) is a first-line treatment for metabolic and endocrine abnormalities (Class I, Level B) and may be considered for prevention of these comorbidities in children. (Class IIb, Level C)
Metreleptin may be considered for hypoleptinemic (leptin <4 ng/mL) patients with partial lipodystrophy and severe metabolic derangements (HbA1c >8% and/or triglycerides >500 mg/dL). (Class IIb, Level B)
Currently, metreleptin (recombinant human methionyl leptin) is the only drug approved specifically for lipodystrophy. It is approved in the United States as an adjunct to diet for treatment of metabolic complications in patients with generalized lipodystrophy (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm387060.htm). In Japan, it is approved for both generalized and partial lipodystrophy (http://www.shionogi.co.jp/en/company/news/2013/pmrltj0000000ufd-att/e_130325.pdf). It is available in other parts of the world (eg, Europe) through compassionate use programs. There is no age limit for initiation of metreleptin; children as young as 6 months have been treated. A dosing algorithm is provided in Supplemental Table 4 (40). Dose adjustments should be made in response to metabolic parameters and weight change, with clinical and laboratory assessment performed every 3–6 months.
Metreleptin in generalized lipodystrophy
Metreleptin decreases hyperphagia (41–45), frequently leading to weight loss. Reduced food intake is at least partially responsible for many of the metabolic improvements. If excessive weight loss occurs, the dose of metreleptin should be reduced (Supplemental Table 4) (40).
Metreleptin markedly improved fasting glucose as early as the first week (42) and lowered HbA1c by 2% after 1 year (46). To reduce the risk of hypoglycemia, frequent glucose monitoring is recommended. Providers should consider reducing insulin doses by approximately 50% on initiation of metreleptin in patients with well-controlled diabetes. Many young patients with CGL are able to discontinue insulin (46).
Metreleptin lowered triglycerides within 1 week (42), reaching 60% reduction at 1 year (46). Metreleptin also decreased LDL- and total cholesterol but did not change HDL-cholesterol (47, 48). Acute pancreatitis due to hypertriglyceridemia has occurred in patients who acutely discontinued or reduced metreleptin (47).
Metreleptin reduced hepatic steatosis, serum transaminases, and NASH scores within 6 to 12 months (42, 49–51). In one case, metreleptin ameliorated recurrence of severe hepatic steatosis after liver transplantation (52).
Metreleptin decreased proteinuria in most patients (34, 42). However, four patients had worsened renal disease during metreleptin treatment, so renal function should be monitored closely with preexisting renal disease (34).
In females, metreleptin normalized gonadotropin secretion, leading to normal progression of puberty, normalization of menstrual periods (42, 45, 53, 54), and improved fertility (1). Metreleptin decreased T in women but did not alter ovarian morphology (45, 53, 55). In males, metreleptin increased T (45).
Metreleptin in partial lipodystrophy
The response to metreleptin in partial lipodystrophy is less robust than in generalized lipodystrophy. In one study, metreleptin reduced hypertriglyceridemia and improved glycemia in severely hypoleptinemic patients with partial lipodystrophy and severe metabolic derangements (baseline HbA1c >8%, triglycerides >500 mg/dL, leptin <4 ng/mL) (46). In a second study, metreleptin improved triglycerides and indices of insulin sensitivity and secretion in FPLD2 patients with moderate to severe hypoleptinemia (56). However, in a third study, no glycemic improvement was observed in FPLD2 patients with serum leptin <7 ng/mL (57). Metreleptin is only available to patients with partial lipodystrophy through clinical trials, compassionate use programs, and in Japan.
Side effects of metreleptin
Approximately 30% of patients experience side effects (47). The most clinically important are hypoglycemia (in patients receiving concomitant insulin) and infrequent injection-site reactions (erythema, urticaria).
In vivo neutralizing antibody activity to leptin has been reported (58, 59). The clinical implications remain unclear, but may include treatment failure and sepsis (59). Additional serious adverse events occurring during metreleptin treatment are likely related to the underlying lipodystrophy syndrome, rather than metreleptin. These include T-cell lymphoma in patients with AGL (35), pancreatitis (47), and worsening of liver (47) and kidney (34) disease.
Additional treatments for specific comorbidities
Diabetes
Metformin is a first-line agent for diabetes and insulin resistance. (Class IIa, Level C)
Insulin is effective for hyperglycemia. In some patients, concentrated preparations and high-doses may be required. (Class IIa, Level C)
Thiazolidinediones may improve metabolic complications in partial lipodystrophy but should only be used with caution in generalized lipodystrophy. (Class IIb, Level B)
Among the oral hypoglycemic agents, metformin is used most frequently. In patients with partial lipodystrophy, thiazolidinediones improved HbA1c, triglycerides, hepatic volume, and steatosis but may increase regional fat excess (Supplemental Table 5) (60, 61). In patients with high insulin requirements, concentrated insulins should be considered (62). Insulin glargine and degludec kinetics may be altered when injected in lipodystrophic areas because their long duration of action requires sc fat (63, 64). Patients with generalized lipodystrophy may have to take insulin by im routes for the lack of sc fat. Many other hypoglycemic agents have been used in lipodystrophy, but their efficacy has not been studied.
Dyslipidemia
Statins should be used concomitantly with lifestyle modification (after consideration of age, reproductive status, and tolerance). (Class 1, Level C)
Fibrates and/or long-chain omega-3 fatty acids should be used for triglycerides >500 mg/dL and may be considered for triglycerides >200 mg/dL. (Class IIb, Level C)
Lipids should be managed in accordance with U.S. and European guidelines for the general population, with statins as first-line therapy (65–67). Statins and fibrates should be used with caution due to increased risk of myopathy, especially in the presence of known myositis or muscular dystrophy (68). Because cardiovascular risk may be enhanced in lipodystrophic syndromes independent of other risk factors, clinicians may consider applying stricter lipid targets (eg, LDL-cholesterol <100 mg/dL, non-HDL-cholesterol <130 mg/dL, triglycerides <200 mg/dL), even in patients without diabetes. In addition to diet, fibrates and long-chain omega-3-fatty acids from fish oils have wide clinical use to avoid acute complications of severe hypertriglyceridemia (46) but have not been formally studied. Plasmapheresis has been used in extreme hypertriglyceridemia, but must be repeated frequently (69). Additional lipid-lowering drugs have not been studied in patients with lipodystrophy.
Hypertension
Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are first-line treatments for hypertension in patients with diabetes. (Class IIa, Level C)
As in other patients with diabetes, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers should be used for hypertension (70).
Liver disease
Cholic acid did not reduce hepatic steatosis in patients with FPLD in a double-blind, placebo-controlled crossover study (71). In NAFLD not associated with lipodystrophy, diet and exercise are first-line treatments (72), and among pharmacological treatments, vitamin E (in children and adults) (73, 74) and pioglitazone (in adults) (73, 75) have shown the most consistent benefit for liver histopathology. However, these treatments have not been studied in patients with lipodystrophy and are not approved for NAFLD.
Cosmetic treatment
Patients should be assessed for distress related to lipodystrophy and referred as necessary to mental health professionals and/or plastic surgeons. (Class IIa, Level C)
Changes in physical appearance from lipodystrophy can cause psychological distress and physical discomfort (eg, from absent fat pads in feet or buttocks). Data regarding cosmetic surgery are limited. For facial lipoatrophy, autologous fat transfer (in APL), dermal fillers (7, 76), or muscle grafts (77) may be used. Excess fat from the head, neck, or vulva may be surgically reduced or ameliorated by liposuction (7). Breast implants are helpful in some women (78, 79). Acanthosis nigricans is improved through successful treatment of insulin resistance (80, 81). Management of hirsutism is reviewed elsewhere (82).
Contraception and hormone replacement therapy
Oral estrogens are contraindicated. (Class IIa, Level C)
If contraception is needed, progestin-only or nonhormonal contraceptives should be considered. (Class IIa, Level C)
If estrogen replacement is needed, transdermal estrogen should be used. (Class IIa, Level C)
Oral estrogens are contraindicated in lipodystrophy syndromes due to the risk of severe hypertriglyceridemia and acute pancreatitis. Transdermal estrogens may be safer due to lesser hepatic exposure (83). There is clinical experience in the safe use of oral progestins and progestin-containing intrauterine devices.
Pregnancy
Pregnant patients should receive prenatal care from an obstetrician experienced in managing diabetes and a physician experienced in managing lipodystrophy. (Class IIa, level C)
Should a patient become pregnant while taking metreleptin, clinicians may consider continuing metreleptin if withdrawal would harm the mother and fetus and the patient understands that the effects of metreleptin in pregnancy are unknown (FDA category C), and wishes to continue. (Class IIc, level C)
In patients with lipodystrophy with extreme insulin resistance, worsening insulin resistance during pregnancy may make diabetes management difficult, with attendant fetal risks. Furthermore, metreleptin withdrawal has been associated with rebound hypertriglyceridemia (41), placing patients at risk for pancreatitis, endangering both mother and fetus.
Conclusions
Lipodystrophy syndromes are heterogeneous with diverse pathophysiology. For diagnosis, clinical recognition and physical examination are critical. In management efforts, attention should be paid to metabolic derangements and to many other facets of these syndromes affecting multiple organs and quality of life.
Acknowledgments
We thank the staff of the Pediatric Endocrine Society for their help in sponsoring and organizing this practice guideline, the patients from around the world whose contributions to research allowed the development of these guidelines, and Elaine Cochran for providing the guideline for metreleptin dosing.
This practice guideline was sponsored and organized by the Pediatric Endocrine Society via an unrestricted education grant from AstraZeneca. Individual authors were supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health Grants RO1-DK088114, RO1-DK105448, and RO1-DK101941; the Sopha Fund for Lipodystrophy Research at the University of Michigan, and Grant 14-35-00026 of the Russian Science Foundation.
Endorsing Societies include: Pediatric Endocrine Society, American Diabetes Association, American Association of Clinical Endocrinologists, Endocrine Society, Japanese Society for Pediatric Endocrinology, Australasian Pediatric Endocrine Group, European Society for Pediatric Endocrinology, Asia Pacific Pediatric Endocrine Society, African Society for Pediatric and Adolescent Endocrinology, and International Society for Pediatric and Adolescent Diabetes.
Disclosure Summary: R.J.B., P.T.C., D.D., M.J., L.M., N.P., K.R., J.v.S., E.S., T.S., M.W., R.W., and T.Y. have nothing to declare. A.G. consults for Aegerion Pharmaceuticals, Ionis, and Amgen; previously consulted for Biomedical Insights, Clearview Healthcare, Gerson Lehrman, and Smithsolve; and received grant support from Aegerion Pharmaceuticals, Pfizer (2013–2016), and Ionis Pharmaceuticals (2015–2017). D.A.-V. has consulted for Bristol-Myers Squibb and AstraZeneca. E.A.O. received past grant/drug support from Bristol-Myers Squibb, AstraZeneca, and Amylin Pharmaceuticals; current research/drug support from Aegerion Pharmaceuticals and Ionis Pharmaceuticals; previously consulted for all of the previously listed companies; and is a current consultant to AstraZeneca, Aegerion, and Ionis Pharmaceuticals. C.V. has consulted for AstraZeneca and Aegerion Pharmaceuticals.
Footnotes
- AGL
- acquired generalized lipodystrophy
- APL
- acquired partial lipodystrophy
- C3
- complement 3
- CGL
- congenital generalized lipodystrophy
- FPLD
- familial partial lipodystrophy
- HbA1c
- glycosylated hemoglobin
- HDL
- high-density lipoprotein
- LDL
- low-density lipoprotein
- MPGN
- membranoproliferative glomerulonephritis
- NAFLD
- nonalcoholic fatty liver disease
- PCOS
- polycystic ovary syndrome.
Reference
- 1. Brown RJ, Gorden P. Leptin therapy in patients with lipodystrophy and syndromic insulin resistance. In: Dagogo-Jack S, ed. Leptin: Regulation and Clinical Applications. Cham, Switzerland: Springer International Publishing; 2015:225–236. [Google Scholar]
- 2. PG, Semb BK, Trygstad O, Seip M. Echocardiographic assessment of cardiac function and morphology in patients with generalised lipodystrophy. Eur J Pediatr. 1985;144(4):355–359. [DOI] [PubMed] [Google Scholar]
- 3. Lupsa BC, Sachdev V, Lungu AO, Rosing DR, Gorden P. Cardiomyopathy in congenital and acquired generalized lipodystrophy: a clinical assessment. Medicine (Baltimore). 2010;89(4):245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore). 2003;82(2):129–146. [DOI] [PubMed] [Google Scholar]
- 5. Jackson SN, Howlett TA, McNally PG, O'Rahilly S, Trembath RC. Dunnigan-Kobberling syndrome: an autosomal dominant form of partial lipodystrophy. QJM. 1997;90(1):27–36. [DOI] [PubMed] [Google Scholar]
- 6. Seip M. Generalized lipodystrophy. In: Frick P, von Harnack GA, Muller AF, Prader A, Schoen R, Wolff HP, eds. Ergebnisse der Inneren Medizin und Kinderheilkunde. Berlin, Germany: Springer; 1971:59–95. [DOI] [PubMed] [Google Scholar]
- 7. Garg A. Clinical review: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gibbons RJ, Smith S, Antman E, et al. American College of Cardiology/American Heart Association clinical practice guidelines. Part I: where do they come from? Circulation. 2003;107(23):2979–2986. [DOI] [PubMed] [Google Scholar]
- 9. Agarwal AK, Simha V, Oral EA, et al. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88(10):4840–4847. [DOI] [PubMed] [Google Scholar]
- 10. Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–1234. [DOI] [PubMed] [Google Scholar]
- 11. Hayashi YK, Matsuda C, Ogawa M, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119(9):2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simha V, Garg A. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or seipin genes. J Clin Endocrinol Metab. 2003;88(11):5433–5437. [DOI] [PubMed] [Google Scholar]
- 13. Van Maldergem L, J, Khallouf TE, et al. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet. 2002;39(10):722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vantyghem MC, Vincent-Desplanques D, Defrance-Faivre F, et al. Fertility and obstetrical complications in women with LMNA-related familial partial lipodystrophy. J Clin Endocrinol Metab. 2008;93(6):2223–2229. [DOI] [PubMed] [Google Scholar]
- 15. Hegele RA. Premature atherosclerosis associated with monogenic insulin resistance. Circulation. 2001;103(18):2225–2229. [DOI] [PubMed] [Google Scholar]
- 16. Savage DB, Semple RK, Clatworthy MR, et al. Complement abnormalities in acquired lipodystrophy revisited. J Clin Endocrinol Metab. 2009;94(1):10–16. [DOI] [PubMed] [Google Scholar]
- 17. Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore). 2004;83(1):18–34. [DOI] [PubMed] [Google Scholar]
- 18. Handelsman Y, Oral EA, Bloomgarden ZT, et al. The clinical approach to the detection of lipodystrophy - an AACE consensus statement. Endocr Pract. 2013;19(1):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Safar Zadeh E, Lungu AO, Cochran EK, et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol. 2013;59(1):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pope E, Janson A, Khambalia A, Feldman B. Childhood acquired lipodystrophy: a retrospective study. J Am Acad Dermatol. 2006;55(6):947–950. [DOI] [PubMed] [Google Scholar]
- 21. Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab. 2000;85(5):1776–1782. [DOI] [PubMed] [Google Scholar]
- 22. Savage DB, Soos MA, Powlson A, et al. Familial partial lipodystrophy associated with compound heterozygosity for novel mutations in the LMNA gene. Diabetologia. 2004;47(4):753–756. [DOI] [PubMed] [Google Scholar]
- 23. Decaudain A, Vantyghem MC, Guerci B, et al. New metabolic phenotypes in laminopathies: LMNA mutations in patients with severe metabolic syndrome. J Clin Endocrinol Metab. 2007;92(12):4835–4844. [DOI] [PubMed] [Google Scholar]
- 24. Park JY, Chong AY, Cochran EK, et al. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. J Clin Endocrinol Metab. 2008;93(1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown RJ, Meehan CA, Gorden P. Leptin does not mediate hypertension associated with human obesity. Cell. 2015;162(3):465–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ben Turkia H, Tebib N, Azzouz H, et al. [Congenital generalized lipodystrophy: a case report with neurological involvement [in French]. Arch Pediatr. 2009(1);16:27–31. [DOI] [PubMed] [Google Scholar]
- 27. Debray FG, Baguette C, Colinet S, Van Maldergem L, Verellen-Dumouin C. Early infantile cardiomyopathy and liver disease: a multisystemic disorder caused by congenital lipodystrophy. Mol Genet Metab. 2013;109(2):227–229. [DOI] [PubMed] [Google Scholar]
- 28. A-Vilar D, Lado-Abeal J, Palos-Paz F, et al. A novel phenotypic expression associated with a new mutation in LMNA gene, characterized by partial lipodystrophy, insulin resistance, aortic stenosis and hypertrophic cardiomyopathy. Clin Endocrinol (Oxf). 2008;69(1):61–68. [DOI] [PubMed] [Google Scholar]
- 29. Bhayana S, Siu VM, Joubert GI, Clarson CL, Cao H, Hegele RA. Cardiomyopathy in congenital complete lipodystrophy. Clin Genet. 2002;61(4):283–287. [DOI] [PubMed] [Google Scholar]
- 30. Caux F, Dubosclard E, Lascols O, et al. A new clinical condition linked to a novel mutation in lamins A and C with generalized lipoatrophy, insulin-resistant diabetes, disseminated leukomelanodermic papules, liver steatosis, and cardiomyopathy. J Clin Endocrinol Metab. 2003;88(3):1006–1013. [DOI] [PubMed] [Google Scholar]
- 31. Khalife WI, Mourtada MC, Khalil J. Dilated cardiomyopathy and myocardial infarction secondary to congenital generalized lipodystrophy. Tex Heart Inst J. 2008;35(2):196–199. [PMC free article] [PubMed] [Google Scholar]
- 32. Rheuban KS, Blizzard RM, Parker MA, Carter T, Wilson T, Gutgesell HP. Hypertrophic cardiomyopathy in total lipodystrophy. J Pediatr. 1986;109(2):301–302. [DOI] [PubMed] [Google Scholar]
- 33. Andre P, Schneebeli S, Vigouroux C, Lascols O, Schaaf M, Chevalier P. Metabolic and cardiac phenotype characterization in 37 atypical Dunnigan patients with nonfarnesylated mutated prelamin A. Am Heart J. 2015;169(4):587–593. [DOI] [PubMed] [Google Scholar]
- 34. Javor ED, Moran SA, Young JR, et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J Clin Endocrinol Metab. 2004;89(7):3199–3207. [DOI] [PubMed] [Google Scholar]
- 35. Brown RJ, Chan JL, Jaffe ES, et al. Lymphoma in acquired generalized lipodystrophy. Leuk Lymphoma. 2016;57(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patni N, Alves C, von Schnurbein J, et al. A novel syndrome of generalized lipodystrophy associated with pilocytic astrocytoma. J Clin Endocrinol Metab. 2015;100(10):3603–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robbins DC, Danforth E, Jr, Horton ES, Burse RL, Goldman RF, Sims EA. The effect of diet on thermogenesis in acquired lipodystrophy. Metabolism. 1979;28(9):908–916. [DOI] [PubMed] [Google Scholar]
- 38. Glueck CJ, Mellies MJ, Tsang RC, Kashyap ML, Steiner PM. Familial hypertriglyceridemia in children: dietary management. Pediatr Res. 1977;11(9 Pt 1):953–957. [DOI] [PubMed] [Google Scholar]
- 39. Wilson DE, Chan IF, Stevenson KB, Horton SC, Schipke C. Eucaloric substitution of medium chain triglycerides for dietary long chain fatty acids in acquired total lipodystrophy: effects on hyperlipoproteinemia and endogenous insulin resistance. J Clin Endocrinol Metab. 1983;57(3):517–523. [DOI] [PubMed] [Google Scholar]
- 40. Meehan CA, Cochran E, Kassai A, Brown RJ, Gorden P. Metreleptin for injection to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy. Expert Rev Clin Pharmacol. 2016;9(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–578. [DOI] [PubMed] [Google Scholar]
- 42. Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92(2):532–541. [DOI] [PubMed] [Google Scholar]
- 43. Moran SA, Patten N, Young JR, et al. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism. 2004;53(4):513–519. [DOI] [PubMed] [Google Scholar]
- 44. McDuffie JR, Riggs PA, Calis KA, et al. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. J Clin Endocrinol Metab. 2004;89(9):4258–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Musso C, Cochran E, Javor E, Young J, Depaoli AM, Gorden P. The long-term effect of recombinant methionyl human leptin therapy on hyperandrogenism and menstrual function in female and pituitary function in male and female hypoleptinemic lipodystrophic patients. Metabolism. 2005;54(2):255–263. [DOI] [PubMed] [Google Scholar]
- 46. Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17(6):922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53(1):27–35. [DOI] [PubMed] [Google Scholar]
- 49. Javor ED, Ghany MG, Cochran EK, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41(4):753–760. [DOI] [PubMed] [Google Scholar]
- 50. Simha V, Szczepaniak LS, Wagner AJ, DePaoli AM, Garg A. Effect of leptin replacement on intrahepatic and intramyocellular lipid content in patients with generalized lipodystrophy. Diabetes Care. 2003;26(1):30–35. [DOI] [PubMed] [Google Scholar]
- 51. Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109(10):1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Casey SP, Lokan J, Testro A, et al. Post-liver transplant leptin results in resolution of severe recurrence of lipodystrophy-associated nonalcoholic steatohepatitis. Am J Transplant. 2013(11);13:3031–3034. [DOI] [PubMed] [Google Scholar]
- 53. Oral EA, Ruiz E, Andewelt A, et al. Effect of leptin replacement on pituitary hormone regulation in patients with severe lipodystrophy. J Clin Endocrinol Metab. 2002;87(7):3110–3117. [DOI] [PubMed] [Google Scholar]
- 54. Abel BS, Muniyappa R, Stratton P, Skarulis MC, Gorden P, Brown RJ. Effects of recombinant human leptin (metreleptin) on nocturnal secretion in lipodystrophy patients. Neuroendocrinology. 2016;103(3–4):402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lungu AO, Zadeh ES, Goodling A, Cochran E, Gorden P. Insulin resistance is a sufficient basis for hyperandrogenism in lipodystrophic women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2012;97(2):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vatier C, Fetita S, Boudou P, et al. One-year metreleptin improves insulin secretion in patients with diabetes linked to genetic lipodystrophic syndromes. Diabetes Obes Metab. 2016;18(7):693–697. [DOI] [PubMed] [Google Scholar]
- 57. Simha V, Subramanyam L, Szczepaniak L, et al. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. J Clin Endocrinol Metab. 2012;97(3):785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Beltrand J, Lahlou N, Le Charpentier T, et al. Resistance to leptin-replacement therapy in Berardinelli-Seip congenital lipodystrophy: an immunological origin. Eur J Endocrinol. 2010;162(6):1083–1091. [DOI] [PubMed] [Google Scholar]
- 59. Chan JL, Koda J, Heilig JS, et al. Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin Endocrinol (Oxf). 2016;85(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arioglu E, Duncan-Morin J, Sebring N, et al. Efficacy and safety of troglitazone in the treatment of lipodystrophy syndromes. Ann Intern Med. 2000;133(4):263–274. [DOI] [PubMed] [Google Scholar]
- 61. Luedtke A, Boschmann M, Colpe C, et al. Thiazolidinedione response in familial lipodystrophy patients with LMNA mutations: a case series. Horm Metab Res. 2012;44(4):306–311. [DOI] [PubMed] [Google Scholar]
- 62. Lane WS, Cochran EK, Jackson JA, et al. High-dose insulin therapy: is it time for U-500 insulin? Endocr Pract. 2009;15(1):71–79. [DOI] [PubMed] [Google Scholar]
- 63. Karges B, Boehm BO, Karges W. Early hypoglycaemia after accidental intramuscular injection of insulin glargine. Diabet Med. 2005;22(10):1444–1445. [DOI] [PubMed] [Google Scholar]
- 64. Bolli GB, Owens DR. Insulin glargine. Lancet. 2000;356(9228):443–445. [DOI] [PubMed] [Google Scholar]
- 65. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 66. Catapano AL, Chapman J, Wiklund O, Taskinen MR. The new joint EAS/ESC guidelines for the management of dyslipidaemias. Atherosclerosis. 2011;217(1):1. [DOI] [PubMed] [Google Scholar]
- 67. Jellinger PS, Smith DA, Mehta AE, et al. American Association of Clinical Endocrinologists' guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr Pract. 2012;18(suppl 1):1–78. [DOI] [PubMed] [Google Scholar]
- 68. Settergren J, Eiermann B, Mannheimer B. Adherence to drug label recommendations for avoiding drug interactions causing statin-induced myopathy–a nationwide register study. PLoS One. 2013;8(8):e69545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bolan C, Oral EA, Gorden P, Taylor S, Leitman SF. Intensive, long-term plasma exchange therapy for severe hypertriglyceridemia in acquired generalized lipoatrophy. J Clin Endocrinol Metab. 2002;87(1):380–384. [DOI] [PubMed] [Google Scholar]
- 70. American Diabetes Association. Standards of medical care in diabetes-2016 abridged for primary care providers. Clin Diabetes. 2016;34(1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ahmad Z, Subramanyam L, Szczepaniak L, Simha V, Adams-Huet B, Garg A. Cholic acid for hepatic steatosis in patients with lipodystrophy: a randomized, controlled trial. Eur J Endocrinol. 2013;168(5):771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mitchel EB, Lavine JE. Review article: the management of paediatric nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40(10):1155–1170. [DOI] [PubMed] [Google Scholar]
- 73. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305(16):1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Graivier MH, Bass LS, Busso M, Jasin ME, Narins RS, Tzikas TL. Calcium hydroxylapatite (Radiesse) for correction of the mid- and lower face: consensus recommendations. Plast Reconstr Surg. 2007;120(6 suppl):55S–66S. [DOI] [PubMed] [Google Scholar]
- 77. Hurwitz PJ, Sarel R. Facial reconstruction in partial lipodystrophy. Ann Plast Surg. 198(3)2;8:253–257. [DOI] [PubMed] [Google Scholar]
- 78. Calderoni DR, Ramos TM, de Castro JR, Kharmandayan P. Surgical management of phenotypic alterations related to the Dunnigan variety of familial partial lipodystrophy. J Plast Reconstr Aesthet Surg. 2011;64(9):1248–1250. [DOI] [PubMed] [Google Scholar]
- 79. Hughes JM, Stephen C, Johnson AB, Wilson S. Breast augmentation in familial partial lipodystrophy: a case report. J Plast Reconstr Aesthet Surg. 2011;64(5):e121–e124. [DOI] [PubMed] [Google Scholar]
- 80. Araujo-Vilar D, -Amarelle C, et al. Recombinant human leptin treatment in genetic lipodystrophic syndromes: the long-term Spanish experience. Endocrine. 2015;49(1):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Eberting CL, Javor E, Gorden P, Turner ML, Cowen EW. Insulin resistance, acanthosis nigricans, and hypertriglyceridemia. J Am Acad Dermatol. 2005;52(2):341–344. [DOI] [PubMed] [Google Scholar]
- 82. Loriaux DL. An approach to the patient with hirsutism. J Clin Endocrinol Metab. 2012;97(9):2957–2968. [DOI] [PubMed] [Google Scholar]
- 83. Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325(17):1196–1204. [DOI] [PubMed] [Google Scholar]
- 84. Agarwal AK, Arioglu E, De Almeida S, et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31(1):21–23. [DOI] [PubMed] [Google Scholar]
- 85. M, Khallouf E, et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28(4):365–370. [DOI] [PubMed] [Google Scholar]
- 86. Kim CA, Delpine M, Boutet E, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93(4):1129–1134. [DOI] [PubMed] [Google Scholar]
- 87. Payne F, Lim K, Girousse A, et al. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc Natl Acad Sci USA. 2014;111(24):8901–8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dyment DA, Gibson WT, Huang L, Bassyouni H, Hegele RA, Innes AM. Biallelic mutations at PPARG cause a congenital, generalized lipodystrophy similar to the Berardinelli-Seip syndrome. Eur J Med Genet. 2014;57(9):524–526. [DOI] [PubMed] [Google Scholar]
- 89. Novelli G, Muchir A, Sangiuolo F, et al. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet. 2002;71(2):426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12(16):1995–2001. [DOI] [PubMed] [Google Scholar]
- 91. Lessel D, Vaz B, Halder S, et al. Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat Genet. 2014;46(11):1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Donadille B, D'Anella P, Auclair M, et al. Partial lipodystrophy with severe insulin resistance and adult progeria Werner syndrome. Orphanet J Rare Dis. 2013;8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cabanillas R, Cadianos J, Villameytide JA, et al. Nstor-Guillermo progeria syndrome: a novel premature aging condition with early onset and chronic development caused by BANF1 mutations. Am J Med Genet A. 2011;155A(11):2617–2625. [DOI] [PubMed] [Google Scholar]
- 94. Rubio-Cabezas O, Puri V, Murano I, et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 2009;1(5):280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Albert JS, Yerges-Armstrong LM, Horenstein RB, et al. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N Engl J Med. 2014;370(24):2307–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Farhan SM, Robinson JF, McIntyre AD, et al. A novel LIPE nonsense mutation found using exome sequencing in siblings with late-onset familial partial lipodystrophy. Can J Cardiol. 2014;30(12):1649–1654. [DOI] [PubMed] [Google Scholar]
- 97. Agarwal AK, Xing C, DeMartino GN, et al. PSMB8 encoding the β5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet. 2010;87(6):866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Herbst KL, Tannock LR, Deeb SS, Purnell JQ, Brunzell JD, Chait A. Kbberling type of familial partial lipodystrophy: an underrecognized syndrome. Diabetes Care. 2003;26(6):1819–1824. [DOI] [PubMed] [Google Scholar]
- 99. Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9(1):109–112. [DOI] [PubMed] [Google Scholar]
- 100. Shackleton S, Lloyd DJ, Jackson SN, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24(2):153–156. [DOI] [PubMed] [Google Scholar]
- 101. Barroso I, Gurnell M, Crowley VE, et al. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402(6764):880–883. [DOI] [PubMed] [Google Scholar]
- 102. George S, Rochford JJ, Wolfrum C, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304(5675):1325–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gandotra S, Le Dour C, Bottomley W, et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364(8):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. De Sandre-Giovannoli A, Bernard R, Cau P, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300(5628):2055. [DOI] [PubMed] [Google Scholar]
- 106. Graul-Neumann LM, Kienitz T, Robinson PN, et al. Marfan syndrome with neonatal progeroid syndrome-like lipodystrophy associated with a novel frameshift mutation at the 3′ terminus of the FBN1-gene. Am J Med Genet A. 2010;152A(11):2749–2755. [DOI] [PubMed] [Google Scholar]
- 107. Garg A, Kircher M, Del Campo M, et al. Whole exome sequencing identifies de novo heterozygous CAV1 mutations associated with a novel neonatal onset lipodystrophy syndrome. Am J Med Genet A. 2015;167A(8):1796–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Weedon MN, Ellard S, Prindle MJ, et al. An in-frame deletion at the polymerase active site of POLD1 causes a multisystem disorder with lipodystrophy. Nat Genet. 2013;45(8):947–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Masotti A, Uva P, Davis-Keppen L, et al. Keppen-Lubinsky syndrome is caused by mutations in the inwardly rectifying K+ channel encoded by KCNJ6. Am J Hum Genet. 2015;96(2):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Thauvin-Robinet C, Auclair M, Duplomb L, et al. PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am J Hum Genet. 2013;93(1):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Aotani D, Ebihara K, Sawamoto N, et al. Functional magnetic resonance imaging analysis of food-related brain activity in patients with lipodystrophy undergoing leptin replacement therapy. J Clin Endocrinol Metab. 2012;97(10):3663–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Panchal R, Bosio P, Waugh J. Familial partial lipodystrophy complicated by pre-eclampsia. J Obstet Gynaecol. 2005;25(2):196–197. [DOI] [PubMed] [Google Scholar]
- 113. Ldtke A, Genschel J, Brabant G, et al. Hepatic steatosis in Dunnigan-type familial partial lipodystrophy. Am J Gastroenterol. 2005;100(10):2218–2224. [DOI] [PubMed] [Google Scholar]
- 114. Peters DK, Charlesworth JA, Sissons JG, et al. Mesangiocapillary nephritis, partial lipodystrophy, and hypocomplementaemia. Lancet. 1973;2(7828):535–538. [DOI] [PubMed] [Google Scholar]