Abstract
Context:
Treatment of patients with adrenocortical carcinomas (ACC) with mitotane and/or chemotherapy is often associated with toxicity and poor tumor response. New therapeutic options are urgently needed.
Objective:
The objectives of the study were to evaluate the therapeutic possibilities of temozolomide (TMZ) in ACC cells and to assess the potential predictive role of the DNA repair gene O6-Methylguanine-DNA methyltransferase (MGMT) in adrenocortical tumors.
Methods:
Three human ACC cell lines and eight primary ACC cultures were used to assess effects of TMZ in vitro. In the cell lines, 11 normal adrenals, 16 adrenocortical adenomas, and 29 ACC, MGMT promoter methylation and expression were determined.
Results:
IC50 values of TMZ on cell growth were 39 μM, 38 μM, and 44 μM for H295R, HAC15, and SW13, respectively. TMZ induced apoptosis and provoked cytotoxic and cytostatic effects by reducing the surviving fraction of ACC colonies and the colony size. TMZ thereby induced cell cycle arrests in ACC cell lines. TMZ and mitotane both inhibited growth of ACC cells cultured as three-dimensional spheroids. TMZ inhibited cell amount in five of eight primary ACC cultures and induced apoptosis in seven of eight primary ACC cultures. In ACC cell lines and adrenal tissues, MGMT promoter methylation was low. In ACCs, methylation was inversely correlated with MGMT mRNA expression. MGMT protein expression was not correlated with MGMT methylation.
Conclusions:
For the first time, we show the therapeutic potential of temozolomide for ACC, offering an urgently needed potential alternative for patients not responding to mitotane alone or with etoposide, doxorubicin, and cisplatin. (Pre-)clinical studies are warranted to assess efficacy in vivo.
For the first time we show the therapeutic efficacy of temozolomide for ACC, potentially offering an urgently needed alternative for patients not responding to mitotane alone or with chemotherapy.
Adrenocortical carcinoma (ACC) includes a diverse group of tumors, with a generally poor prognosis (1, 2). Frequently patients present with advanced or metastasized tumors, in which mitotane is the standard therapy. However, mitotane is effective in only a subset of these patients (25%–30% response) and often manifests with severe toxicity (3–6). In case of progression, mitotane can be combined with cytotoxic drugs like etoposide, doxorubicin, and cisplatin (7). The median overall survival for this regimen was still only 14.8 months (7). Several targeted therapies have been proposed and clinically tested but to date with discouraging results (6). Therefore, better therapeutic options are urgently needed.
Temozolomide (TMZ), a DNA-alkylating agent, is used as cytostatic drug incorporated in the standard care for patients with malignant gliomas (8). TMZ is an oral formulation of the first metabolite of dacarbazine but less toxic. TMZ has shown efficacy in 17 of 25 patients with poorly differentiated endocrine carcinomas and in various other tumors (9, 10). Cytotoxicity and antiproliferative activity are primarily thought to act by alkylation of specific sites on especially the O6 position of guanine, which mispairs with thymine during the next DNA replication cycle (11). The methyl group in O6-methylguanine can be removed by the O6-methylguanine-DNA methyltransferase (MGMT) gene, leading to impaired efficacy of TMZ, one of the possible explanations for drug resistance (12). Epigenetic marks regulating MGMT expression are now used as a predictive marker for response to TMZ in glioblastoma patients (13).
In this study we investigated the therapeutic possibilities of TMZ in ACCs by investigating the in vitro effects of TMZ on three ACC cell lines and eight primary ACC cultures. We also determined MGMT methylation and expression and the potential predictive role of the MGMT gene in adrenal tumors.
Materials and Methods
Adrenocortical tissues
Adrenocortical tissues were obtained between May 1995 and October 2015 at the Department of Surgery, Erasmus Medical Center (Rotterdam, The Netherlands). Directly after resection, adrenal tissues were embedded in Tissue-Tek and stored at −80°C. For eight ACCs, a tissue part was used to obtain primary cultures. Diagnosis was confirmed using the Weiss score or Van Slooten index (14, 15). Patient and tumor characteristics were obtained from electronic patient records. The study was conducted under guidelines that were approved by the Medical Ethics Committee of the Erasmus Medical Center. Informed consent was obtained from all patients.
Cell culture and compounds
Three available human ACC cell lines were used: H295R, HAC15, and SW13, obtained from the American Type Culture Collection, ECACC, and from Dr W. Rainey (as a kind gift), respectively. Short tandem repeat profiling using a Powerplex kit (Promega) of NCI-H295R and SW13 gave results consistent with the ATCC database, confirming the identity of both cell lines. Short tandem repeat profiling of HAC15 showed a genetic profile identical to H295R, which is consistent with a previous report by Wang and Rainey (16) that HAC15 is a clone of H295R. Cells were cultured as previously described (17). TMZ, mitotane, and the demethylating drug 5′-AZA-2′-deoxycytidine (AZA) stock solutions (10 mM), prepared in 100% dimethylsulfoxide, absolute EtOH, and H2O, respectively (Sigma-Aldrich), were stored at −20°C. After trypsinization, cells were plated at the appropriate density to obtain 80% confluency at the end of the experiment. The next day, incubations were started in quadruplicate. Control cells were vehicle treated. Cell culture experiments were carried out at least twice, except primary cultures, due to the limited number of cells obtained from the specimens. Primary cultures were obtained as previously described (18). Cortisol was measured in the supernatant of cortisol producing ACCs using a chemiluminescence immunoassay system (Immulite 2000XPi).
DNA amount (as a measure of cell amount) and apoptosis measurement
Effects of TMZ (1–100 μM) and/or mitotane (1–50 μM), on cell growth in ACC cell lines was assessed as previously described (19). In primary cultures, DNA amounts were measured using the Quant-iT PicoGreen double-stranded DNA assay kit (Thermo Fisher Scientific), an ultrasensitive method for DNA measurement. Apoptosis was assessed using the cell death detection ELISAPlus kit (Roche Diagnostics).
Colony-forming assay
The colony-forming assay is the gold standard for measurement of effects of cytotoxic agents on cancer cells in vitro. Effects on colony size and surviving fraction of colonies were assessed at 25 μM (H295R and HAC15) or 50 μM (SW13) TMZ. Plates were coated with 1 mL poly-L-lysine (10 μg/mL), in which afterward 1500, 1250, or 250 cells were plated for H295R, HAC15, and SW13, respectively. After 1 day, drug treatment was initiated. Media were removed after 1 or 3 days and refreshed without TMZ. When colonies contained at least 50 cells (3, 4, and 2 wk for H295R, HAC15, and SW13, respectively), the cells were washed and fixed. Cells were colored with hematoxylin, and amounts and sizes of colonies were measured using MultiImage light cabinet (Alpha Innotech). Surviving fraction was calculated as previously described (20).
Cell cycle analysis
For cell cycle analysis, ACC cells were treated with 12.5–100 μM TMZ. After 3 and 7 days, cells were harvested, washed with NaCl, fixed with ice-cold 70% EtOH, and stored at −20°C until analysis. Analyses were performed using the Muse cell cycle assay kit using a Muse cell analyzer (Merck Millipore).
Three-dimensional (3D) multicellular spheroid cultures
For 3D spheroid cultures, 10 000 cells (H295R and HAC15) or 1000 cells (SW13) were plated in 24-well culture plates with cell-repellent Surface (Greiner Bio-One). The plates were rotated at 100 rpm for 72 hours. Seven days after plating, treatments were initiated (TMZ and mitotane 1–100 μM). Photomicrographs were taken at ×50 magnification at days 0, 3, and 7 from treatment initiation. Image J software (National Institutes of Health, Bethesda, Maryland) was used to measure the pixel number of the area occupied by the spheroid. Spheroids were measured only when a clear outer boundary of the spheroid was visible. Growth rate was used to compare control and treated spheroids.
Bisulfite conversion and pyrosequencing
DNA isolation from ACC cells (control, 1 μM AZA treated) and adrenal tissues, bisulfite conversion, PCR (hybrid temperature 57°C), and pyrosequencing was performed as previously described (21) but with the use of the MGMT primer. The primer was designed based on previous studies using Pyromark assay design (22, 23) (Supplemental Table 1). High and low methylated DNA (QIAGEN, Benelux) was used as internal control.
MGMT mRNA expression analysis
MGMT mRNA expression was assessed in ACC cells (control, TMZ 12.5 μM, and/or mitotane 5 μM, AZA 1 μM) and in adrenal tissues. RNA isolation, cDNA synthesis, and RT-PCR were performed as previously described but using other primers (Supplemental Table 2; Sigma-Aldrich) (21). Three housekeeping genes were used: hypoxanthine-guanine phosphoribosyl transferase 1 (HPRT; Sigma-Aldrich), β-actin (B-actin; Thermo Fisher Scientific), and glucuronidase-β (GUSB; Thermo Fisher Scientific). Reliable housekeeping genes were determined for cell culture experiments, and relative MGMT expression was calculated using the comparative cycle threshold method 2-δδCt. For accurate RT-PCR expression profiling in adrenal tissues, HPRT, GUSB, and B-actin were determined to normalize mRNA levels using the method of Vandesompele et al (24).
MGMT immunohistochemistry
Two tissue microarrays (TMAs) comprising specimens from seven normal adrenals (NAs), 15 adrenocortical adenomas (ACAs), and 23 ACCs were constructed, as previously described using the automated TMA constructor (ATA-27; Beecher Instruments) available at the Department of Pathology, Erasmus Medical Center (25). For an optimal representation, an expert pathologist identified three areas per specimen in hematoxylin and eosin-stained slides. Cores with a diameter of 1 mm were extracted from donor block and brought into the recipient paraffin block at predefined coordinates. In addition, formalin-fixed, paraffin-embedded whole sections were used from three cases not included in the TMA. Five-micrometer sections were cut and used for immunohistochemistry as previously described (26), with the adjustments that Tris-EDTA buffer (pH 6.0) and the mouse monoclonal MGMT antibody (MT 3.1 dilution 1:20; Thermo Fisher Scientific) were used. Sections were independently and blinded for the tissue type scored by two investigators (S.G.C., L.J.H.) using a semiquantitative, well-established immunoreactivity score (IRS), calculated by the product of the percentage of positive cells (4, > 80%; 3, > 51%–80%; 2, > 10%; 1, 0) and intensity of staining (3, strong; 2, moderate; 2, mild; 0, no staining) (27).
Statistical analysis
GraphPad Prism version 3.0 (GraphPad Software) and SPSS Statistics version 21 (SPSS 21.0; SPSS Inc) were used. For the cell culture experiments, the ANOVA test (followed by a Tukey's test) or a Student's t test was used for comparisons among treatment groups. A Kruskal-Wallis test or ANOVA, when applicable, was used to assess differences between adrenal tissues. Correlations were tested using Spearman's rank coefficient. Values of P < .05 were considered significant. Data are indicated as mean ± SEM, unless specified otherwise.
Results
Patient characteristics
Eleven NAs, obtained during nephrectomy, 16 ACAs, and 29 ACCs were enrolled. MGMT mRNA expression and promoter methylation were assessed in 42 adrenal specimens (seven NAs, 12 ACAs, 23 ACCs), whereas MGMT immunohistochemistry was performed in 46 tissues (seven NAs, 13 ACAs, 26 ACCs). Patient and tumor characteristics are listed in Table 1.
Table 1.
Patient and Tumor Characteristics of Patients Included in This Study
| ACCs (n = 29) | ACAs (n = 16) | |
|---|---|---|
| Mean age at diagnosis, y | 53 (range 9–82) | 46 (range 26–61) |
| Mean follow-up, mo | 39 (range 3–187) | 34 (range 1–83) |
| Male, % | 11 (38%) | 3 (19%) |
| Mean tumor size, cm (SD) | 14.45 (SD 5.7) | 3.17 (SD 1.95) |
| Secretion | ||
| Androgens | 9 (31%) | 1 (6%) |
| Glucocorticoids | 15 (52%) | 6 (38%) |
| Mineralocorticoids | 0 (0%) | 5 (31%) |
| Precursors | 3 (10%) | 0 (0%) |
| Estradiol | 4 (14%) | 0 (0%) |
| Nonsecreting | 12 (41%) | 4 (25%) |
| Weiss score (SD) | 6.0 (SD 1.31) | 0.25 (SD 0.45) |
| Van Slooten Index | 21.1 (SD 4.8) (n = 26) | 1.56 (SD 0.869) |
| ENSAT | ||
| I | 0 (0%) | 14 (88%) |
| II | 15 (52%) | 2 (13%) |
| III | 3 (10%) | 0 (0%) |
| IV | 11 (37%) | 0 (0%) |
Van Slooten Index was not available for all patients, dependent on the year of diagnosis.
Effects of temozolomide on ACC cell lines
Cell growth and apoptosis
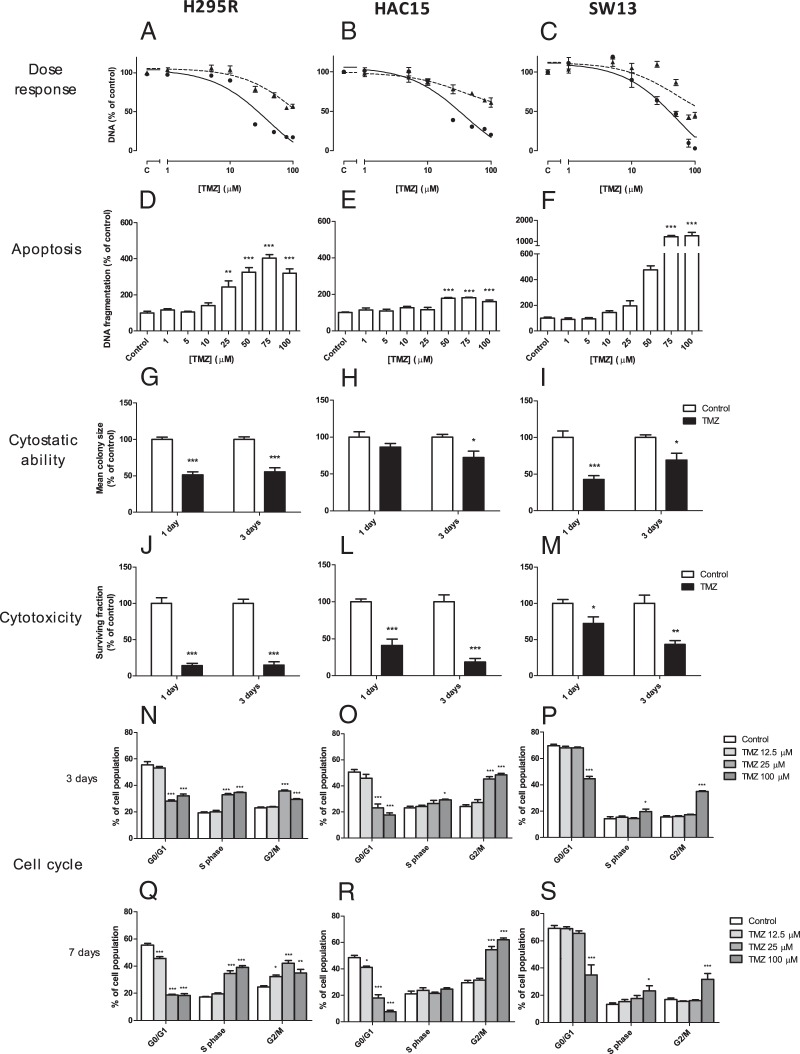
TMZ strongly inhibited cell growth, measured as DNA amount per well, in H295R, HAC15, and SW13 (Figure 1, A–C), in a time- and dose-dependent manner. IC50 values were lower for H295R (38.6 μM; 95% confidence interval [CI] 25.5–58.3) and HAC15 (37.9 μM; 95% CI 23.9–60.1), compared with SW13 (43.5 μM; 95% CI 27.6–68.6; P = .0007 and P = .0011, respectively). Maximum inhibition of cell growth was 82% in H295R, 80% in HAC15, and 97% in SW13, which was significantly higher in SW13 (P < .0004). In H295R and SW13, there was a dose-dependent induction of apoptosis after TMZ, with 220% and 1169% induction at 100 μM TMZ (Figure 1, D and F; both P < .001 vs control), respectively. A less strong induction of apoptosis (61%) was present in HAC15 (Figure 1E).
Figure 1.
Effects of TMZ treatment on H295R, HAC15, and SW13 cells on cell amount expressed as DNA content (A–C), apoptosis expressed as DNA fragmentation (D–F), cytostatic ability expressed as mean colony size (G–I), cytotoxicity expressed as surviving fraction of colonies (J–L), and effect on cell cycle analysis after 3 (M–O) and 7 days (P–R) of treatment with TMZ. In panels A–C, dotted and solid lines represent 3 and 7 days of treatment, respectively, and panel C on the y-axis represents the vehicle-treated control. Apoptosis was assessed after 3 days. For the colony-forming assay, 25 μM was used for H295R and HAC15 and 50 μM TMZ for SW13. Values represent mean ± SEM and are shown as a percentage of control. *, P < .05, **, P < .01, ***, P < .001 vs control. C, control.
Colony-forming assay
In H295R and SW13, the mean colony size significantly decreased after 1 day (both P < .001) and 3 days (Figure 1, G and I; H295R, P < .001; SW13, P < .05) of TMZ treatment. In HAC15, we observed an effect on colony size only after 3 days of treatment (Figure 1H; P < .05). Cytotoxicity analysis showed a significantly lower surviving fraction of colonies after 1 and 3 days of TMZ in all cell lines (Figure 1, J–M; P < .05).
Cell cycle analysis
Cell cycle analysis showed an accumulation of H295R and HAC15 cells in the G2/M phase after TMZ treatment in a dose-dependent manner (both P < .001), with maximum increase of 42% in H295R and 109% in HAC15. In H295R, the G2/M phase accumulation was associated with the gradual appearance of cells in the S phase (P < .001) and a decrease of cells in the G0/G1 phase (P < .001). In HAC15, we observed a time- and dose-dependent decrease of cells in the G0/G1 phase (P < .001) but without an increased S-phase population. In SW13, treatment with TMZ caused a minimal accumulation of cells in the S phase (P < .05) and G2/M phase (P < .001) and a minimal decrease in the G0/G1 phase (Figure 1, P and S; P < .001).
Three-dimensional multicellular spheroids
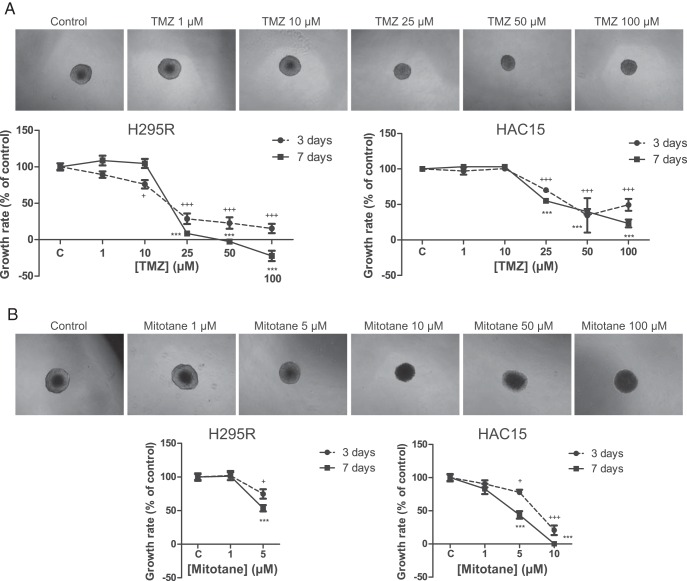
SW13 cells did not form 3D multicellular spheroids, and the effect of the drugs could thus not be studied in this cell line. Visually, TMZ and mitotane seemed to exert inhibition of spheroid growth differently because only the mitotane-treated spheroids lacked a clear outer boundary in the highest treatment concentrations (Figure 2). It was therefore not possible to reliably measure the spheroids treated with the highest concentrations of mitotane (H295R, 10–100 μM; HAC15, 50–100 μM) (Figure 2B). A dose-dependent inhibitory effect was seen on growth rate of the spheroids by both TMZ and mitotane. Already at 25 μM, spheroid growth rate was inhibited by 91% and 45% in H295R and HAC15, respectively. TMZ 100 μM decreased the initial size (size at start of drug treatment) of the spheroids in H295R after 7 days (P < .05) (Figure 2).
Figure 2.
Effect of TMZ and mitotane on ACC cells cultured as 3D multicellular spheroids. Effects of different concentrations of TMZ (A) and mitotane (B) on H295R (left panel) and HAC15 (right panel) spheroid growth rate compared with control. Photographs are taken after 7 days of treatment. After mitotane treatment, it was not possible to measure the spheroids in the highest concentrations (H295R, 10, 50, and 100 μM; HAC15, 50 and 100 μM) because there was no clear outer boundary of the spheroids visible. Negative numbers indicate shrinking of the spheroid. Values represent mean ± SEM. *, P < .05, **, P < .01, ***, P < .001 vs control. C, control.
Effects of combination of TMZ and mitotane on cell number and MGMT mRNA expression
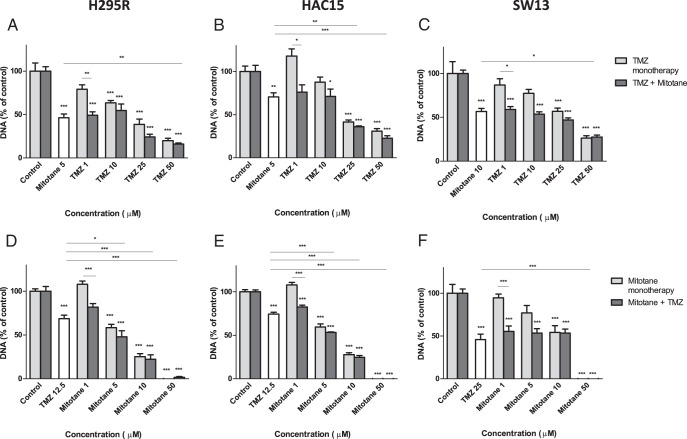
Combination therapy of TMZ and mitotane showed no additive effect on cell growth (Figure 3). In the lowest concentration of TMZ, in which no effect on cell amount was seen in monotherapy, we observed the expected effect of mitotane (Figure 3, A–C; P < .05). The same trend was seen when a low dose of mitotane was given with TMZ (Figure 3, D–F; P < .001). When we compared the effects of mono- or combination therapy of TMZ and mitotane on ACC cells in the other concentrations, no differences were observed. There was no effect on MGMT mRNA expression when H295R cells were treated with 12.5 μM TMZ, 5 μM mitotane, or the combination (data not shown).
Figure 3.
Combined effect of TMZ and mitotane on cell number in H295R, HAC15, and SW13. Effect of different concentrations of TMZ with or without a fixed concentration of mitotane (5 or 10 μM) in H295R (A), HAC15 (B), and SW13 (C) is shown. Effect of different concentrations of mitotane with or without a fixed concentration of TMZ (12.5 or 25 μM) in H295R (D), HAC15 (E), and SW13 (F) is also shown. Cell number is depicted as total DNA amounts. Values are represented as mean ± SEM and are shown as a percentage of control. *, P < .05, **, P < .01, ***, P < .001 vs own control or as stated by the lines.
Effects of TMZ on primary cultures of ACCs
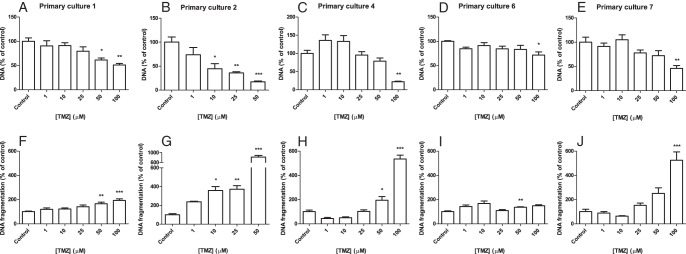
The effect of TMZ in vitro was evaluated in eight primary ACC cultures (Supplemental Table 3). A statistically significant inhibitory effect on the cell amount was seen in five of the eight primary ACC cultures after 7 days of TMZ treatment, varying from 29% to 83% inhibition (Figure 4, A–E; primary culture numbers 1, 2, 4, 6, and 7). Apoptosis induction was seen in the same five primary ACC cultures, ranging from 37% to 716% increase (Figure 4, F–J). In two other primary cultures, apoptosis was also induced with 42% and 127% at TMZ 100 μM. TMZ inhibited cortisol production (corrected for cell amount) by 97% in one of three primary cultures of cortisol-producing ACCs (number 5). In one (number 4) of the six primary ACC cultures (numbers 1–4, 6, and 7) in which TMZ and mitotane were combined, an additive inhibitory effect on the cell amount was observed (Supplemental Figure 1D). Primary culture 1 responded to the combined treatment only with mitotane (50 μM) and TMZ (25 μM) but not to the monotherapies (Supplemental Figure 1B). In three of six primary ACC cultures, mitotane 50 μM strongly inhibited the cell number, limiting the possibility to investigate the additive effects of TMZ. One of these six primary cultures did not respond to either TMZ or mitotane (number 3).
Figure 4.
Effects of TMZ on five of the eight primary ACC cultures that responded to TMZ by an inhibition of cell number (A–E) after 7 days of treatment and an induction of apoptosis (F–J). Cell number is depicted as amount of DNA and apoptosis as amount of DNA fragmentation. Two additional primary cultures showed induction of apoptosis but no inhibitory effect on cell number. One primary culture did not respond to TMZ by either a change in cell amount or apoptosis. Values represent mean ± SEM and are shown as percentage of control. *, P < .05, **, P < .01, ***, P < .001 vs control.
MGMT promoter methylation status and mRNA expression in ACC cell lines and adrenal tissues
Mean MGMT promoter methylation percentages were low and amounted 2.9% ± 0.03%, 2.8% ± 0.09%, and 2.4% ± 0.41% for H295R, HAC15, and SW13, respectively (Supplemental Figure 2). MGMT mRNA expression was significantly higher in SW13 compared with H295R and HAC15 (both P < .001). Treatment with AZA decreased MGMT methylation on average with 24%, whereas MGMT mRNA expression only slightly decreased in H295R (Supplemental Figure 2; P < .05).
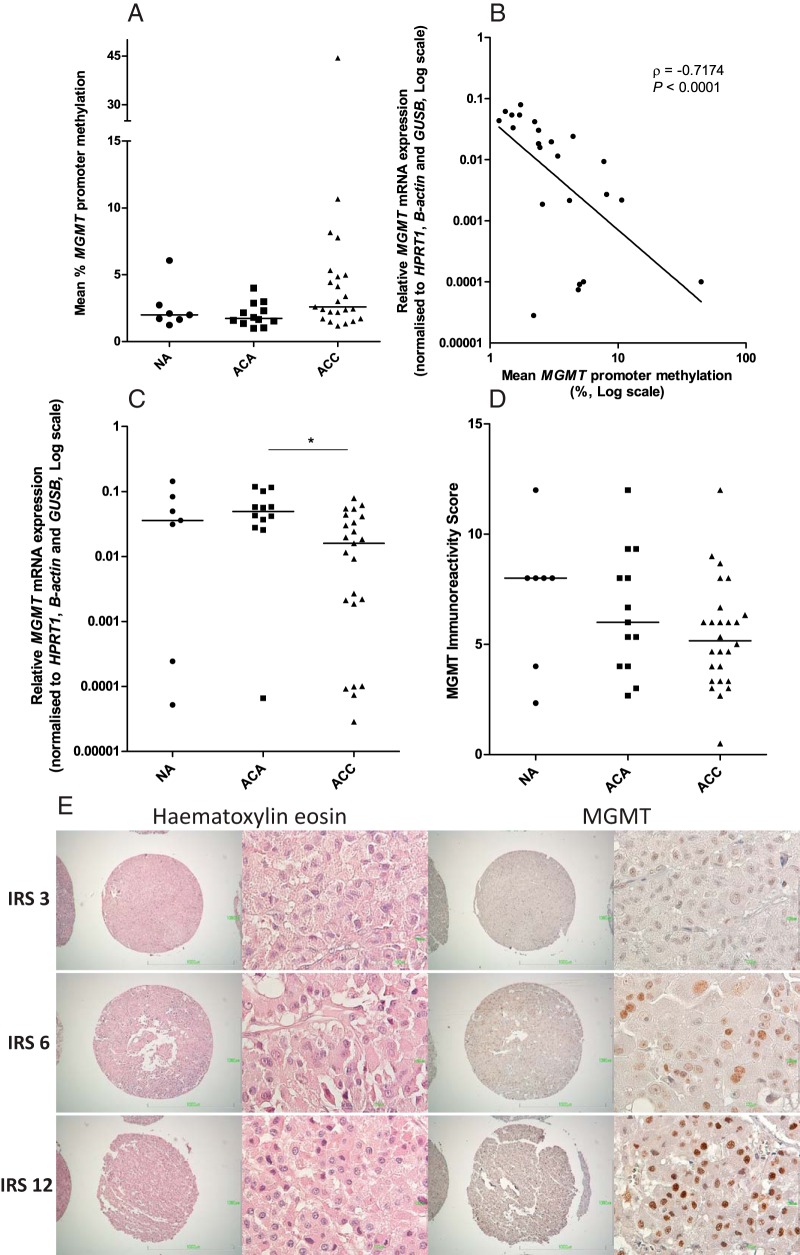
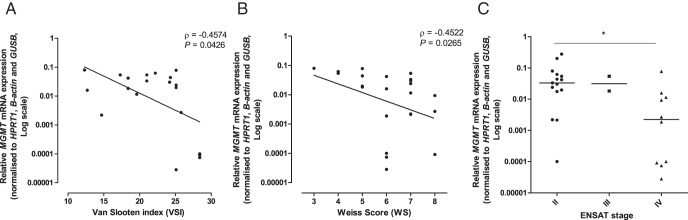
MGMT promoter methylation was low in NAs (2.5% ± 1.6%), ACAs (2.0% ± 0.89%), and ACCs (5.4% ± 8.8%), with no statistically significant differences between groups (all mean ± SD; Figure 5A). MGMT mRNA expression was lower in ACCs compared with ACAs (Figure 5C; P < .05). In ACCs, an inverse relationship of MGMT methylation and mRNA expression was found (Figure 5B; ρ = −0.7174, P < .0001), although within a small range of methylation. Mean MGMT IRS was, respectively, 7.2 ± 3.2, 6.4 ± 2.8, and 5.4 ± 2.4 for NAs, ACAs, and ACCs (all mean ± SD), with no significant differences between groups (Figure 5, D and E). MGMT protein expression was not correlated with MGMT methylation and mRNA expression (n = 20) in ACCs (ρ = −0.157, P = .509; ρ = −0.024, P = .919, respectively). MGMT mRNA expression was inversely correlated with the Van Slooten Index and the Weiss score (Figure 6, A and B; ρ = −0.4574, P = .043; ρ = −0.4522, P = .027, respectively) and was lower in the tumors with European Network for the Study of Adrenal Tumors (ENSAT) stage IV vs ENSAT stage II (Figure 6C; P < .05).
Figure 5.
Overview of involvement of the MGMT gene in the adrenal with promoter methylation status of the MGMT gene (A), MGMT mRNA (B), and MGMT protein expression (C) in adrenocortical tissues. D, Correlation of MGMT promoter methylation and mRNA expression in ACCs. E, Representative example of MGMT immunohistochemical staining in adrenocortical carcinomas as determined by immunohistochemistry. Antibodies against MGMT stain tumor cell nuclei. Sections were blinded and independently evaluated by two investigators (microscopic magnification, ×40 and ×400). Lines represent medians. ρ represents Spearman's rank correlation coefficient. **, P < .01. NA, normal adrenals; ACA, adrenocortical adenoma; ACC, adrenocortical carcinoma; IRS, Immunoreactivity Score; MGMT, O6-Methylguanine-DNA methyltransferase.
Figure 6.
Correlation of MGMT mRNA expression with the Van Slooten Index (A), Weiss score (B), and the ENSAT stage (C) in ACCs. ρ, Spearman correlation coefficient. *, P < .05.
In primary ACC cultures, a trend was observed towards a higher MGMT methylation in the cultures that responded to TMZ, compared with the three primary cultures that did not show a decrease in cell amount (mean 4.6% ± 1.0% vs 2.0% ± 0.30%; both mean ± SD; P = .0571). No correlation was observed with MGMT mRNA and protein expression.
Discussion
Due to a lack of effective therapies for patients with ACC, current research focuses on discovering new therapeutic targets. So far, chemotherapeutic drugs are effective in only a small subset of patients, and targeted therapies showed disappointing results (6). In this study, we showed the first evidence for a potential novel treatment option of ACC patients with temozolomide.
We show that TMZ exerts potent antitumor responses on ACC cells in vitro, including a strong inhibition of cell growth, induction of apoptosis, and cytotoxic and cytostatic effects as well as a cell cycle arrest. Importantly, the antitumor effects of TMZ shown in this study were present at concentrations of TMZ that can be achieved in the plasma of patients with advanced cancers (∼25–55 μM) (28–30). However, a relevant consideration is that these represent plasma concentrations and not intratumoral concentrations. From studies of TMZ in glioblastoma and melanoma patients and cell lines, TMZ is known to cause a G2/M arrest and to minimally induce apoptosis (31, 32). The induction of apoptosis was variable in ACC cell lines, with the strongest induction in SW13. H295R and HAC15 appear to respond to a greater extent by undergoing cell cycle arrest.
One of the challenges of basic and translational ACC research is the limited number of available human cell lines. To circumvent this limitation and assess efficacy of TMZ in a more representative model from the ACC cells of origin, we also investigated the inhibition of cell amount and induction of apoptosis in primary ACC cultures. Inhibition of the cell amount was demonstrated in five of eight cultures, whereas apoptosis induction was seen in an even larger number of primary ACC cultures, ie, seven of eight. Furthermore, we showed an efficacy of TMZ in 3D ACC spheroids, giving the opportunity to study the effects of TMZ on ACC cells in a more (patho)physiologically relevant context, harboring important cell-cell and cell-matrix interactions and availability of drugs to the inner layer of the spheroid. Interestingly, we visually observed a different treatment effect on the spheroid by TMZ and mitotane, suggesting a different mechanism of action. However, we did not see an additive or synergistic effect when we tested the combined effect of TMZ and mitotane in ACC cell lines. It does seem that the two drugs do not adversely affect each other. We ruled out a possible up-regulation of MGMT expression by the combination of mitotane and TMZ. In contrast, in one primary ACC culture, an additive effect was observed when TMZ was added to mitotane. This issue remains to be further investigated.
One of the aims of this study was to investigate the potential role of the DNA repair gene MGMT in ACC because research has shown that epigenetic silencing of MGMT sensitizes glioblastoma cells to TMZ. Comparison of the IC50 values of TMZ on ACC cell lines and glioma cell lines shows that the IC50 values of TMZ in the ACC cell lines are comparable with the highly sensitive glioma cell lines (IC50 38–44 μM vs 22.5–52.4 μM). These glioma cell lines are MGMT hypermethylated (33). In contrast, ACC cell lines appear to have a low MGMT promoter methylation, which may also explain why treatment with the demethylating drug AZA minimally effected MGMT mRNA expression. Importantly, we also found low methylation in almost all ACCs, with most the ACCs harboring MGMT mRNA expression in the same range as the ACC cell lines. This suggests that our cell lines are a representative model for ACCs regarding the MGMT gene. Additionally, we observed a trend toward a slightly higher MGMT methylation in the responsive primary ACC cultures, despite the fact that the methylation was less than 5% in all tissues. This issue requires further investigation in a larger group. Although in a small range, a strong significant inverse correlation was found between methylation and MGMT mRNA expression in ACCs. The inverse correlation of MGMT mRNA expression with histopathology and ENSAT stage may indicate that silencing of MGMT plays a role in the development or progression of ACC, like in other types of cancer (34). MGMT protein expression was not correlated with MGMT promoter methylation, which might be explained by confounding factors in the presence of sampling bias because DNA and RNA are isolated from frozen specimens and immunohistochemistry is performed on paraffin-embedded sections. These fragments may originate from different parts of the tumor. Other potential explanations include tumor heterogeneity or a variety of normal resident cells within the tumor. In addition to these possibilities, several studies in human glioblastomas also reported no correlation between MGMT immunohistochemistry and promoter methylation, and promoter methylation assessment is generally accepted as the method of choice for prediction (35, 36).
Future investigations in ACCs could focus on other potential predictive factors, like absence of a functional DNA mismatch repair system because in gliomas this is known to contribute to TMZ resistance as well (37). Furthermore, research can focus on the combination of TMZ with capecitabine, considering the in vitro synergism shown in neuroendocrine tumor carcinoid BON cells (38). Reasonably, because these results are in vitro, (pre)clinical studies are warranted to further evaluate the therapeutic possibilities of TMZ for treatment of ACC. However, because the safety, pharmacodynamics, and pharmacokinetic profile of TMZ is known in cancer patients, the clinical application is more easily accessible. In addition, in contrast to several other drugs, CYP450-mediated metabolism does not seem to contribute significantly to the plasma clearance of temozolomide (39). This might indicate that mitotane, which increases CYP3A4 expression (40), does not influence TMZ plasma levels. Thereby, TMZ does not need hepatic metabolism to be activated. These findings are important beneficial remarks for a potential clinical application of TMZ in ACC patients because many patients will be (co)treated with mitotane.
In conclusion, we hypothesize that, based on the low MGMT methylation and meanwhile the responsiveness to TMZ, efficacy of TMZ in ACC cells does not seem to be limited by low methylation of MGMT. This is in contrast to glioblastomas. We show for the first time the therapeutic potential of TMZ for ACC, potentially offering an urgently needed alternative for patients who do not respond to the conventional therapy with mitotane, etoposide, doxorubicin, and cisplatin.
Acknowledgments
This work was supported by Erasmus Medical Center Vriendenfonds and by a donation from Mr and Mrs Westra.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACA
- adrenocortical adenoma
- ACC
- adrenocortical carcinoma
- AZA
- 5′-AZA-2′-deoxycytidine
- CI
- confidence interval
- 3D
- three dimensional
- ENSAT
- European Network for the Study of Adrenal Tumors
- IRS
- immunoreactivity score
- MGMT
- O6-methylguanine-DNA methyltransferase
- NA
- normal adrenal
- TMA
- tissue microarray
- TMZ
- temozolomide.
Reference
- 1. Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(12):4551–4564. [DOI] [PubMed] [Google Scholar]
- 2. Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: Have we made progress? World J Surg. 2006;30(5):872–878. [DOI] [PubMed] [Google Scholar]
- 3. Hahner S, Fassnacht M. Mitotane for adrenocortical carcinoma treatment. Curr Opin Investig Drugs. 2005;6(4):386–394. [PubMed] [Google Scholar]
- 4. Baudin E, Pellegriti G, Bonnay M, et al. Impact of monitoring plasma 1,1-dichlorodiphenildichloroethane (o,p′DDD) levels on the treatment of patients with adrenocortical carcinoma. Cancer. 2001;92(6):1385–1392. [DOI] [PubMed] [Google Scholar]
- 5. Haak HR, Hermans J, van de Velde CJ, et al. Optimal treatment of adrenocortical carcinoma with mitotane: results in a consecutive series of 96 patients. Br J Cancer. 1994;69(5):947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Creemers SG, Hofland LJ, Korpershoek E, et al. Future directions in the diagnosis and medical treatment of adrenocortical carcinoma. Endocr Relat Cancer. 2016;23(1):R43–R69. [DOI] [PubMed] [Google Scholar]
- 7. Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. [DOI] [PubMed] [Google Scholar]
- 8. Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6(7):2585–2597. [PubMed] [Google Scholar]
- 9. Welin S, Sorbye H, Sebjornsen S, Knappskog S, Busch C, Oberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer. 2011;117(20):4617–4622. [DOI] [PubMed] [Google Scholar]
- 10. Stevens MF, Hickman JA, Langdon SP, et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M, B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987;47(22):5846–5852. [PubMed] [Google Scholar]
- 11. Denny BJ, Wheelhouse RT, Stevens MFG, Tsang LLH, Slack JA. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994;33(31):9045–9051. [DOI] [PubMed] [Google Scholar]
- 12. Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol. 2002;20(9):2388–2399. [DOI] [PubMed] [Google Scholar]
- 13. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 14. Lau SK, Weiss LM. The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum Pathol. 2009;40(6):757–768. [DOI] [PubMed] [Google Scholar]
- 15. van Slooten H, Schaberg A, Smeenk D, Moolenaar AJ. Morphologic characteristics of benign and malignant adrenocortical tumors. Cancer. 1985;55(4):766–773. [DOI] [PubMed] [Google Scholar]
- 16. Wang T, Rainey WE. Human adrenocortical carcinoma cell lines. Mol Cell Endocrinol. 2012;351(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Koetsveld PM, Vitale G, de Herder WW, et al. Potent inhibitory effects of type I interferons on human adrenocortical carcinoma cell growth. J Clin Endocrinol Metab. 2006;91(11):4537–4543. [DOI] [PubMed] [Google Scholar]
- 18. van Koetsveld PM, Vitale G, Feelders RA, et al. Interferon-β is a potent inhibitor of cell growth and cortisol production in vitro and sensitizes human adrenocortical carcinoma cells to mitotane. Endocr Relat Cancer. 2013;20(3):443–454. [DOI] [PubMed] [Google Scholar]
- 19. Hofland LJ, van Koetsveld PM, Lamberts SW. Percoll density gradient centrifugation of rat pituitary tumor cells: a study of functional heterogeneity within and between tumors with respect to growth rates, prolactin production and responsiveness to the somatostatin analog SMS 201–995. Eur J Cancer. 1990;26(1):37–44. [DOI] [PubMed] [Google Scholar]
- 20. Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–2319. [DOI] [PubMed] [Google Scholar]
- 21. Creemers SG, van Koetsveld PM, van Kemenade FJ, et al. Methylation of IGF2 regulatory regions to diagnose adrenocortical carcinoma. Endocr Relat Cancer. 2016;23(9):727–737. [DOI] [PubMed] [Google Scholar]
- 22. Christians A, Hartmann C, Benner A, et al. Prognostic value of three different methods of MGMT promoter methylation analysis in a prospective trial on newly diagnosed glioblastoma. PLoS One. 2012;7(3):e33449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59(4):793–797. [PubMed] [Google Scholar]
- 24. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papathomas TG, Oudijk L, Zwarthoff EC, et al. Telomerase reverse transcriptase promoter mutations in tumors originating from the adrenal gland and extra-adrenal paraganglia. Endocr Relat Cancer. 2014;21(4):653–661. [DOI] [PubMed] [Google Scholar]
- 26. Gatto F, Feelders RA, van der Pas R, et al. Immunoreactivity score using an anti-sst2A receptor monoclonal antibody strongly predicts the biochemical response to adjuvant treatment with somatostatin analogs in acromegaly. J Clin Endocr Metab. 2013;98(1):E66–E71. [DOI] [PubMed] [Google Scholar]
- 27. Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue] Vorschlag zur einheitlichen definition eines immunreaktiven score (IRS) fur den immunhistochemischen Ostrogenrezeptor-Nachweis (ER-ICA) im Mammakarzinomgewebe. Pathologe. 1987;8(3):138–140. [PubMed] [Google Scholar]
- 28. Baker SD, Wirth M, Statkevich P, et al. Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res. 1999;5(2):309–317. [PubMed] [Google Scholar]
- 29. Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15(22):7092–7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dhodapkar M, Rubin J, Reid JM, et al. Phase I trial of temozolomide (NSC 362856) in patients with advanced cancer. Clin Cancer Res. 1997;3(7):1093–1100. [PubMed] [Google Scholar]
- 31. Mhaidat NM, Zhang XD, Allen J, Avery-Kiejda KA, Scott RJ, Hersey P. Temozolomide induces senescence but not apoptosis in human melanoma cells. Br J Cancer. 2007;97(9):1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11(4):448–457. [DOI] [PubMed] [Google Scholar]
- 33. Yoshino A, Ogino A, Yachi K, et al. Gene expression profiling predicts response to temozolomide in malignant gliomas. Int J Oncol. 2010;36(6):1367–1377. [DOI] [PubMed] [Google Scholar]
- 34. Sharma S, Salehi F, Scheithauer BW, Rotondo F, Syro LV, Kovacs K. Role of MGMT in tumor development, progression, diagnosis, treatment and prognosis. Anticancer Res. 2009;29(10):3759–3768. [PubMed] [Google Scholar]
- 35. Uno M, Oba-Shinjo SM, Camargo AA, et al. Correlation of MGMT promoter methylation status with gene and protein expression levels in glioblastoma. Clinics (Sao Paulo). 2011;66(10):1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dullea A, Marignol L. MGMT testing allows for personalised therapy in the temozolomide era. Tumor Biol. 2016;37(1):87–96. [DOI] [PubMed] [Google Scholar]
- 37. Nguyen SA, Stechishin OD, Luchman HA, et al. Novel MSH6 mutations in treatment-naive glioblastoma and anaplastic oligodendroglioma contribute to temozolomide resistance independently of MGMT promoter methylation. Clin Cancer Res. 2014;20(18):4894–4903. [DOI] [PubMed] [Google Scholar]
- 38. Fine RL, Gulati AP, Krantz BA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: the Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71(3):663–670. [DOI] [PubMed] [Google Scholar]
- 39. British Columbia Cancer Agency. Temozolomide. 2001. http://www.bccancer.bc.ca/drug-database-site/Drug%20Index/temozolomide_monograph_1Jul2015.pdf Accessed July 1, 2015.
- 40. Kroiss M, Quinkler M, Lutz WK, Allolio B, Fassnacht M. Drug interactions with mitotane by induction of CYP3A4 metabolism in the clinical management of adrenocortical carcinoma. Clin Endocrinol (Oxf). 2011;75(5):585–591. [DOI] [PubMed] [Google Scholar]