Abstract
Context:
Growth of short children in puberty is limited by the effect of estrogen on epiphyseal fusion.
Objectives:
To compare: 1) the efficacy and safety of aromatase inhibitors (AIs) vs GH vs AI/GH on increasing adult height potential in pubertal boys with severe idiopathic short stature (ISS); and 2) differences in body composition among groups.
Design:
Randomized three-arm open-label comparator.
Setting:
Outpatient clinical research.
Patients:
Seventy-six pubertal boys [mean (SE) age, 14.1 (0.1) years] with ISS [height SD score (SDS), −2.3 (0.0)].
Intervention:
Daily AIs (anastrozole or letrozole), GH, or AI/GH for 24–36 months.
Outcomes:
Anthropometry, bone ages, dual x-ray absorptiometry, spine x-rays, hormones, safety labs.
Results:
Height gain [mean (SE)] at 24 months was: AI, +14.0 (0.8) cm; GH, +17.1 (0.9) cm; AI/GH, +18.9 (0.8) cm (P < .0006, analysis of covariance). Height SDS was: AI, −1.73 (0.12); GH, −1.43 (0.14); AI/GH, −1.25 (0.12) (P < .0012). Those treated through 36 months grew more. Regardless of treatment duration, height SDS at near-final height [n = 71; age, 17.4 (0.2) years; bone age, 15.3 (0.1) years; height achieved, ∼97.6%] was: AI, −1.4 (0.1); GH, −1.4 (0.2); AI/GH, −1.0 (0.1) (P = .06). Absolute height change was: AI, +18.2 (1.6) cm; GH, +20.6 (1.5) cm; AI/GH, +22.5 (1.4) cm (P = .01) (expected height gain at −2.0 height SDS, +13.0 cm). AI/GH had higher fat free mass accrual. Measures of bone health, safety labs, and adverse events were similar in all groups. Letrozole caused higher T and lower estradiol than anastrozole.
Conclusions:
Combination therapy with AI/GH increases height potential in pubertal boys with ISS more than GH and AI alone treated for 24–36 months with a strong safety profile.
76 boys with idiopathic short stature were randomized to aromatase inhibitors (AI), GH, or AI/GH for 24–36 months. All groups grew taller, those on AI/GH had greatest increase in height potential.
Increasing height potential in growth-retarded children during puberty is often complicated by the inexorable tempo of epiphyseal fusion caused by pubertal sex steroids, greatly limiting the time available for growth. High-dose GH (1) or GnRH analogs (GnRHa) combined with GH have been used with positive, albeit variable, results (2–6). Although GnRHa treatment is effective in delaying epiphyseal fusion, it renders youngsters hypogonadal at a critical time of development. Studies of males with mutations in the estrogen receptor gene (7) or the aromatase enzyme gene (8, 9), and animal data (10) have shown that estrogen is the principal regulator of epiphyseal fusion in both genders. Estrogen decreases progenitor cells in resting state chondrocytes and increases structural senescence (11), mostly through an estrogen receptor α-mediated mechanism. Hence, more selective suppression of estrogen production or action can promote linear growth while allowing continued sexual maturation in males.
Aromatase inhibitors (AIs) block the conversion of androgens to estrogens with significant selectivity and potency and are approved by the Food and Drug Administration (FDA) in postmenopausal women with breast cancer. AIs in young males have similar pharmacokinetics as those reported in women (12, 13). We observed that GnRHa treatment had significant catabolic effects in males, diminishing rates of whole-body protein synthesis, increasing urinary calcium excretion, and increasing adiposity (14, 15), effects not seen with aromatase blockade at least for the time window of the studies (16).
Studies in boys with constitutional growth delay (17), idiopathic short stature (ISS) (18), and GH deficiency (19) treated with AIs alone (18) or combined with T (17) or with GH (19) have shown promising results, with treatment enhancing height potential by delaying epiphyseal fusion while promoting linear growth. In adolescent boys with GH deficiency treated with GH, addition of anastrozole increased height potential by +4.5 cm after 24 months and by +6.7 cm after 36 months of combined treatment, vs +1.0-cm gain with placebo and GH at the same time points (19).
We designed these studies to better assess the impact of AIs (both anastrozole and letrozole), vs GH, vs combination AI/GH on increasing adult height potential in adolescent boys with ISS. We also aimed to assess bone density and morphology and lean body mass accrual with treatments. As secondary aims, we investigated the degree of aromatase suppression using letrozole vs anastrozole using highly sensitive assays.
Subjects and Methods
Study subjects
Studies were conducted at the Pediatric Endocrine Clinics at Nemours Children's Health System in Jacksonville, Florida; and Philadelphia, Pennsylvania; and at the University of Chile after institutional review board approvals. Informed written consent was obtained from participants, parents, and children as appropriate. Inclusion criteria included boys ages ≥12 and <18 years with ISS and residual height potential (bone age ≤14 ½ years) who were in puberty. ISS was defined as a height SD score (SDS) ≤−2.0 and no other hormonal, skeletal, or systemic pathology identified. Subjects had GH stimulation tests with peak GH responses of ≥5 ng/mL and/or normal IGF-1 and IGF binding protein-3 before study entry. All were naive to treatment and had normal birth weight. Studies were registered at clinicaltrials.gov (NCT01248416).
Study design
At baseline all subjects had a physical examination and pubertal staging using the standards of Tanner (20). Anthropometric measures were obtained using Harpenden stadiometers and digital scales. A left hand and wrist x-ray was obtained for bone age determination, and dual x-ray absorptiometry (DXA) of the lumbar spine (anteroposterior and lateral) and whole body was performed. Blood and urine samples were collected in the early morning. Subjects were then randomized to treatment with an AI (anastrozole or letrozole—balanced 1:1), somatropin (GH), or combination treatment (AI/GH) for the next 24 months. The protocol was amended to continue treatment for another 12 months (36 months total) if the subject had residual height potential and he and his family wished to continue on an active drug. Protocol milestones were at 0, 3, 6, 9, 12, 18, and 24 months, and if treatment continued, at 30 and 36 months also. When possible, subjects were followed for at least another 12 months after discontinuation of treatment, and several were followed beyond this timeline if they continued growing. All adverse events (AEs) and serious AEs (SAEs) were carefully recorded and were reported quarterly to the study's data safety management board. An abbreviated bone questionnaire was used to assess bone discomfort or pain throughout the study (21).
Study drugs
An investigational new drug number was assigned by the FDA. Drug supply agreements were provided in kind for anastrozole (Arimidex; AstraZeneca), letrozole (Femara; Novartis), and GH (Nutropin AQ, Genentech; and Genotropin, Pfizer). Depending on randomization, daily doses were: anastrozole, 1 mg orally; letrozole, 2.5 mg orally; and GH, approximately 42 μg/kg/d sc.
Assays
T and estradiol concentrations were measured by tandem liquid chromatography mass spectrometry (Agilent Technologies, Inc) at Mayo Clinic laboratories. Plasma 17 β-estradiol was extracted with methylene chloride and derivatized with dansyl chloride, followed by high-pressure liquid chromatography and tandem liquid chromatography mass spectrometry. Intra-assay coefficients of variation (CVs) were 6.0/1.6% at 0.74 and 35 pg/mL, respectively; interassay CVs were 6.9/5.1% at 0.77 and 32 pg/mL; lower assay sensitivity was 0.3 pg/mL. T intra-assay CVs were 2.3–0.9% and interassay CVs were 3.5%. IGF-1 was measured at the Nemours Biochemical Analysis Laboratory using ELISA (R&D Systems), with a 4.0% intra-assay CV. General chemistries and plasma lipids were measured by automated chemistry analyzers.
X-rays and DXA
Left hand and wrist x-rays for bone age were centrally read by a single reader at Fels Institute (Ohio) (22) and predicted adult height calculated using Bayley Pinneau tables (23). DXA of the lumbar spine (anteroposterior, lateral) measured L1–L4, and whole-body DXA was performed using either a Hologic (Discovery or Horizon) or Lunar densitometer; the same software/instrument was used per subject throughout the trial. If not available through DXA, a lateral thoracic plain x-ray was obtained to assess bone morphology. A single radiologist (D.M.) who was blinded to treatment reviewed and scored all spine films for vertebral changes including: disc space narrowing, wedging, compression, and irregularity. Z-scores were corrected for height (24).
Statistical analysis
Descriptive statistics, mean (± SE) were used as appropriate. These studies were not powered to sort out efficacy by type of AI on anthropometric and body composition metrics. Hence, data were grouped by randomization arm regardless of AI type, either anastrozole or letrozole for analysis of covariance (ANCOVA) or repeated measures ANOVA, to compare changes between treatment groups at 24 and 36 months and near-final height within and between groups. When comparing within and between treatment group changes in mean responses over time involving more than two time points (eg, DXA bone mineral density [BMD] Z-scores at 0, 12, and 24 months), we performed mixed-effects repeated-measures ANOVA. Two-factor ANCOVA using type and length of treatment was used to compare means changes in near-final height. Models were adjusted for baseline values as appropriate, and assumptions were checked. Nonparametric Kruskal-Wallis tests were used for comparisons when appropriate. Whenever possible, subjects were measured until growth velocity was <2 cm/y and/or bone age was ≥16 years. If the subject was not located or was unable to be seen, the last measurement was used as the last height. Significance was established at P < .05. The only subanalysis performed by type of AI was the degree of suppression of aromatase based on changes in T and estradiol concentrations. The statistical software SAS version 9.3 (SAS Institute. Inc) was used for analysis.
Results
Seventy-six adolescent boys were recruited in three treatment groups (AI, GH, and AI/GH) and were well-matched for age, height, BMI, and midparental height (Table 1). Of those, 72 completed all procedures at 12 months, 68 at 18 months, and 65 at 24 months. If subjects had residual height potential at 24 months (n = 54), they could choose to continue (n = 19) or discontinue medication (n = 35); all 54 had procedures completed by 36 months. Three subjects changed treatment at 24 months; two on AI-only added GH, one on AI/GH discontinued AI, and their data were excluded from the post 24-month analysis. Regardless of treatment, 71 subjects have been followed to near-final height (Supplemental Figure 1).
Table 1.
Clinical Characteristics of Study Subjects at Baseline
| All | AI | GH | AI/GH | |
|---|---|---|---|---|
| n | 76 | 25 | 25 | 26 |
| Age, y | 14.1 ± 0.1 | 14.2 ± 0.2 | 14.1 ± 0.2 | 14.0 ± 0.2 |
| Height, cm | 144.8 ± 0.7 | 145.7 ± 1.1 | 144.2 ± 1.4 | 144.5 ± 1.3 |
| Height SDS | −2.3 ± 0.0 | −2.2 ± 0.1 | −2.4 ± 0.1 | −2.3 ± 0.1 |
| BMI, kg/m2 | 18.7 ± 0.3 | 18.4 ± 0.4 | 18.4 ± 0.6 | 19.2 ± 0.5 |
| Bone age, y | 12.8 ± 0.1 | 12.8 ± 0.3 | 12.9 ± 0.3 | 12.7 ± 0.2 |
| MPH, cm | 171.1 ± 0.6 | 171.8 ± 0.8 | 170.1 ± 1.3 | 171.6 ± 0.9 |
| IGF-1, ng/mL | 161 ± 10 | 146 ± 12 | 154 ± 15 | 181 ± 23 |
| T, ng/dL | 223 ± 22 | 205 ± 37 | 244 ± 39 | 222 ± 37 |
Abbreviation: MPH, midparental height. Data are expressed as mean ± SE.
Growth parameters and bone age
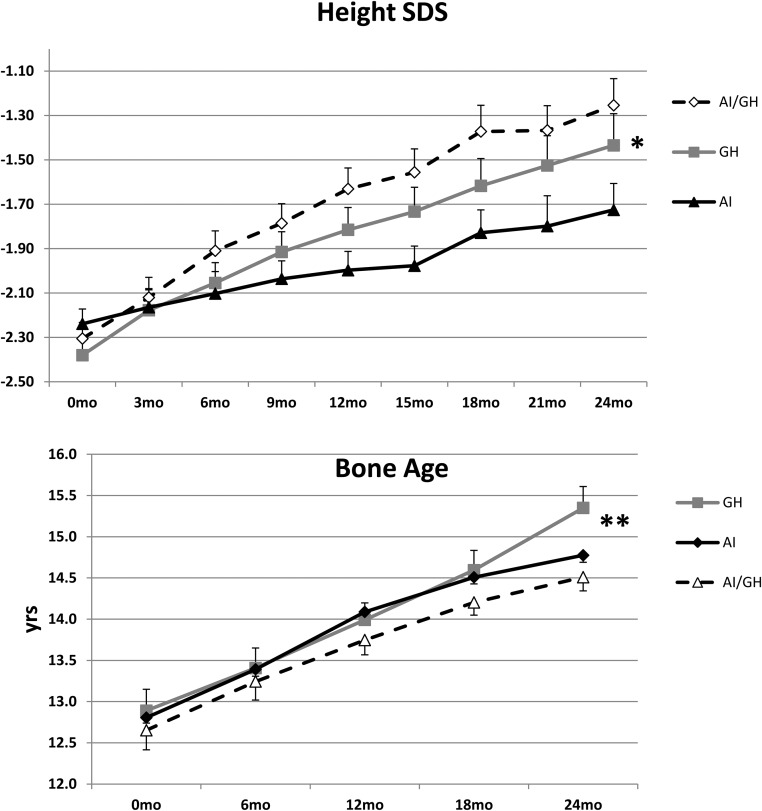
All patients grew during the initial 24 months of treatment; hence, all within-group changes are highly significant (between-group comparisons are shown in parentheses): mean change (SE)—AI, +14.0 (0.8) cm; GH, +17.1 (0.9) cm; AI/GH, +18.9 (0.8) cm (P < .0006 between groups) (P value represents the probability of difference in mean changes between groups for all parameters. ANCOVA was used and model adjusted for baseline height.). The expected height gain in boys with height SDS of −2.0 is +10.1 cm in the same age period (25). At 36 months, those who continued treatments grew more (between 24 and 36 months) than those who discontinued: continued—AI, +4.9 (1.0) cm; GH, +6.8 (0.4) cm; AI/GH, +7.8 (0.5) cm (P = .032) (P value represents the probability of difference in mean changes between groups for all parameters. ANCOVA was used and model adjusted for baseline height.); discontinued—AI, +4.1 (1.1) cm; GH, +2.1 (0.6) cm; AI/GH, 3.0 (0.5) cm (P = .103) (P value represents the probability of difference in mean changes between groups for all parameters. ANCOVA was used and model adjusted for baseline height.) (P = < .001) [P value of overall difference in mean changes (from 24 mo to 36 mo) between continued and discontinued group]. Mean absolute height SDS in all three groups was comparable at baseline (−2.2 to −2.4 SDS) and improved at 24 months: AI, −1.73 (0.12) SDS; GH, −1.43 (0.14) SDS; AI/GH, −1.25 (0.12) SDS (P < .0012) (P value represents the probability of difference in mean changes between groups for all parameters. ANCOVA was used and model adjusted for baseline height.) (Figure 1A). The change in bone age in 24 months from baseline was: AI, +2.1 (0.3) years; GH, +2.5 (0.1) years; AI/GH, +1.9 (0.2) years (P = .002) (P value represents the probability of difference in mean changes between groups for all parameters. ANCOVA was used and model adjusted for baseline height.), larger change for the GH group (Figure 1B). Height SDS adjusted for bone age at 24 months was: AI, −1.06 (0.14); GH, −1.11 (0.20); AI/GH, −0.41 (0.13) (P = .0002) (P value represents the probability of difference in mean changes between groups for all parameters. ANCOVA was used and model adjusted for baseline height.).
Figure 1.
Changes in mean (SE) height SDS (top panel) and bone age (bottom panel) over 24 months in the groups treated with AIs, GH, and AI/GH. *, P < .0012 (top panel); and **, P = .002 (bottom panel) represent the probability of difference in mean changes between groups for all parameters (ANCOVA). n = 76 (baseline), 72 (12 months), and 65 (24 months).
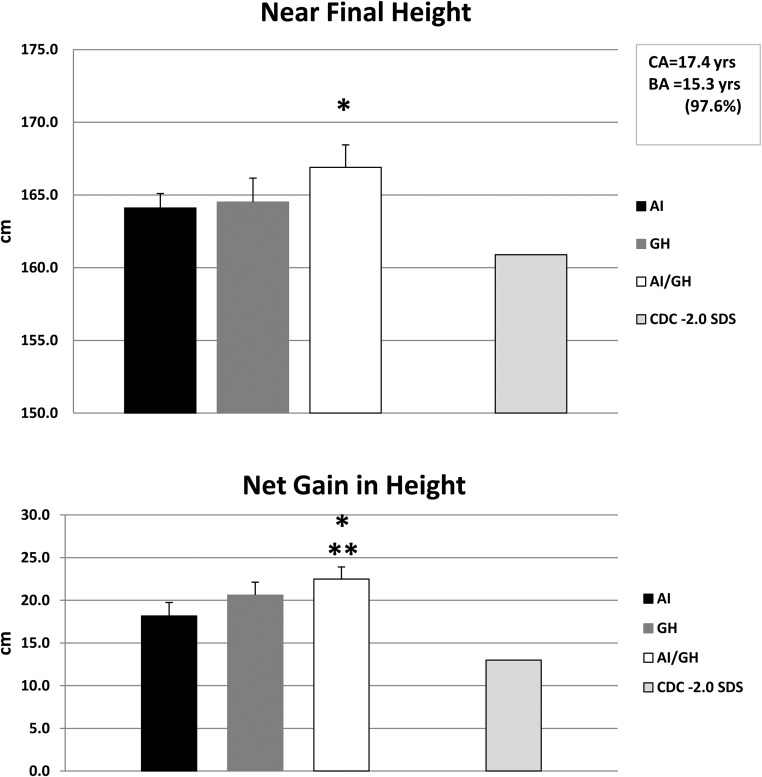
Except for three subjects who switched treatment arms after 24 months and two lost to follow-up, all available subjects (n = 71) have been followed as long as possible to near-final height, with mean age of 17.4 (0.2) years and bone age of 15.3 (0.1) years, which corresponds to 97.6% of the height achieved. Using intent-to-treat analysis, regardless of whether or not they continued on treatment past 24 months, the last measured mean absolute height was as follows: AI, 164.1 (1.6) cm; GH, 164.8 (1.6) cm; AI/GH, 166.9 (1.5) cm (P = .19 among groups); whereas adult height at −2.0 SDS is 160.9 cm (25) (Figure 2A). Height SDS at near-final height was: AI, −1.4 (0.1); GH, −1.4 (0.2); AI/GH, −1.0 (0.1) (P = .06) (P value represents the probability of difference in mean changes between groups for all parameters. ANCOVA was used and model adjusted for baseline height.). The absolute change in height from baseline at near-final height was highly significant within groups (P < .0001 each): AI, +18.2 (1.6) cm; GH, +20.6 (1.5) cm; AI/GH, +22.5 (1.4) cm (P = .01 between all groups (P value represents the probability of difference in mean changes between all groups using 2 factor ANOVA model including treatment type and duration), P = .002 between AI and AI/GH groups); the expected height gain in boys with height SDS of −2.0 is +13.0 cm (25) (Figure 2B); our subjects were even shorter (height SDS, −2.2 to −2.4). When data of subjects who continued their medications through 36 months were separated from data of those who discontinued at 24 months, the overall net gain in height at near-final height from baseline was greater in those who continued (P < .0001): continued treatment—AI, +23.8 (2.3) cm; GH, +26.7 (2.0) cm; AI/GH, +30.7 (1.1) cm (P = .06 between treatment groups) (P value represents the probability of difference in mean changes between groups for all parameters. ANCOVA was used and model adjusted for baseline height.); discontinued treatment—AI, +14.7 (1.5) cm; GH, + 17.8 (1.6) cm; AI/GH, 19.9 (1.4) cm (P = .12 between groups) (P value represents the probability of difference in mean changes between groups for all parameters. ANCOVA was used and model adjusted for baseline height.).
Figure 2.
Top panel shows mean (SE) differences in near-final height (cm) (n = 71) in the AI, GH, and AI/GH groups regardless of length of treatment (n = 21, 25, and 25, respectively) (P < .001 within groups; *, P = .19 among groups). Bottom panel shows net gain in height (cm) in the same three groups (*, P = .01 among groups; **, P = .002 between AI and AI/GH groups). Average height and net gain in height of young men of similar ages with height SDS −2.0 are shown for comparison on the far right bars (CDC data).
Mean relative differences between estimated target (midparental) height and near-final height were: AI, −7.8 ± 1.6 cm (10% of subjects were taller than target height); GH, −5.3 ± 1.3 cm (24% of subjects were taller); AI/GH, −4.5 ± 1.4 cm (32% of subjects were taller) (P = .27 among groups). Those who continued treatment had lesser differences between near-final and target height.
Bone assessments (Table 2)
Table 2.
Bone Assessments
| Months | Vertebral Findings |
DXA BMD Z Score Corrected |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disc Space Narrowing |
Wedging |
Compression |
Irregularity |
Lumbara |
Whole Bodyb |
|||||||||||||
| AI | GH | AI/GH | AI | GH | AI/GH | AI | GH | AI/GH | AI | GH | AI/GH | AI | GH | AI/GH | AI | GH | AI/GH | |
| 0 | 2 | 3 | 4 | 2 | 0 | 1 | 0 | 0 | 4 | 1 | 1 | 0 | 0.372 ± .187 | 0.488 ± .320 | 0.569 ± .309 | −0.328 ± .232 | −0.464 ± .312 | −0.062 ± .252 |
| 12 | 5 | 3 | 6 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0.043 ± .181 | 0.243 ± .353 | 0.216 ± .312 | −0.583 ± .195 | −0.700 ± .309 | −0.448 ± .281 |
| 24 | 3 | 2 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | −0.328 ± .227 | 0.243 ± .328 | 0.067 ± .306 | −1.061 ± .313 | −0.586 ± .244 | −0.605 ± .257 |
P value of mean difference among groups for lumbar spine: 0 months, .99; 12 months, .52; 24 months, .03. Within-group changes over time: AI, < .001; GH, .04; AI/GH, .001. Baseline, n = 76; 12 months, n = 72; 24 months, n = 62.
P value of mean difference among groups: 0 months, .93; 12 months, .46; 24 months, .906. Within-group changes over time: AI, .003; GH, .06; AI/GH, <.001.
Lumbar and whole-body BMD Z-scores were low in the entire cohort throughout the study. However, when BMD Z-scores were adjusted for height (24), lumbar spine scores (reflective mostly of trabecular bone) were normal at baseline and decreased in all groups on treatment, but still within the normal range. BMD Z-scores were mildly low at the whole-body level (reflective of mostly cortical bone), diminishing in all groups, particularly the AI group, but still remaining within the normal range (Table 2). Lateral thoracic spine x-rays showed an array of vertebral findings including disc space narrowing, wedging, compression, and irregularities, many present at baseline and comparable in all three arms. Bone-specific alkaline phosphatase, a marker of bone formation, was similar in all three groups throughout 24 months of treatment (Supplemental Table 1).
Body composition
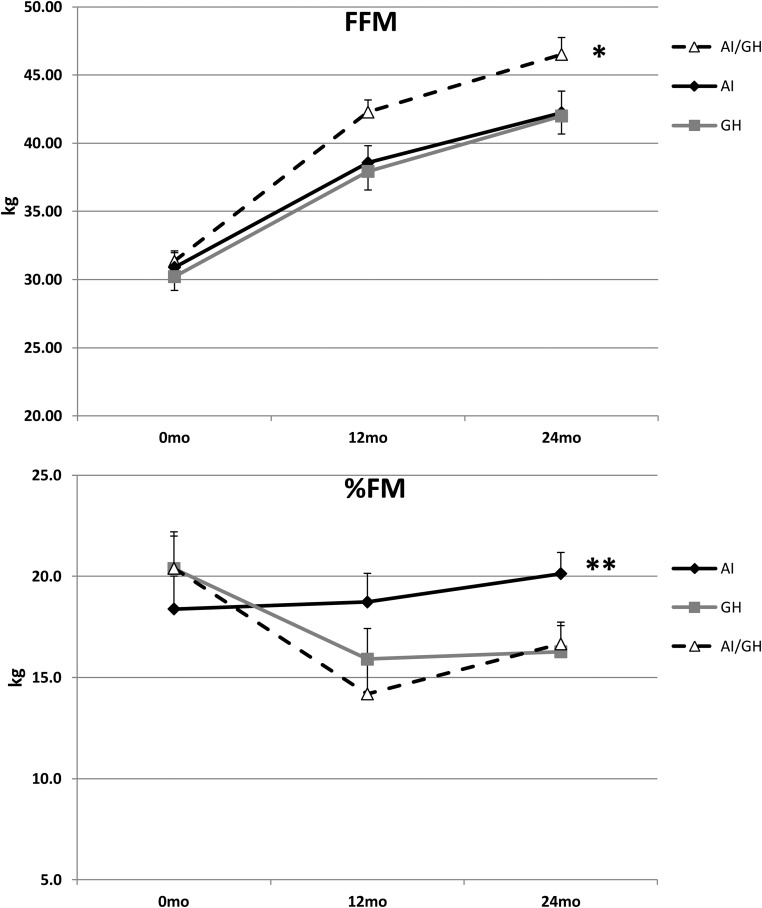
There were differential responses in body composition as boys progressed through puberty, depending on treatment arm. Those on AI or GH accrued fat free mass (FFM) similarly over 24 months (DXA), whereas those on combined AI/GH accrued more. FFM values at 0, 12, and 24 months, respectively, were: AI, 30.9 (1.1), 38.6 (1.2), and 42.2 (1.6) kg (P ≤ .0001); GH, 30.2 (1.0), 37.9 (1.4), and 42.0 (1.3) kg (P ≤ .0001); AI/GH, 31.3 (0.8), 42.3 (0.9), and 46.5 (1.2) kg (P ≤ .0001) (P = .015 among groups). Percentage fat mass was lower in subjects on GH and AI/GH compared to AI during treatment; values at 0, 12, and 24 months, respectively, were: AI, 18.4 (1.6); 18.7 (1.4); and 20.1 (1.0) (P = .50); GH, 20.4 (1.6), 15.9 (1.5), and 16.3 (1.3) (P = .0012); AI/GH, 20.4 (1.8), 14.2 (1.5), and 16.7 (1.1) (P < .0001) (P = .003 among groups) (Figure 3).
Figure 3.
Changes in FFM (top panel) and percentage fat mass (%FM) (bottom panel) over 24 months in the AI, GH, and AI/GH groups by DXA [mean (SE)]. FFM, P < .001 within each group; *, P = .015 among groups; %FM, P = .50. AI, 0.0012; GH, < .0001; AI/GH, **, P = .003 among groups. n = 76 (baseline), 72 (12 months), 63 (24 months).
IGF-1 concentrations
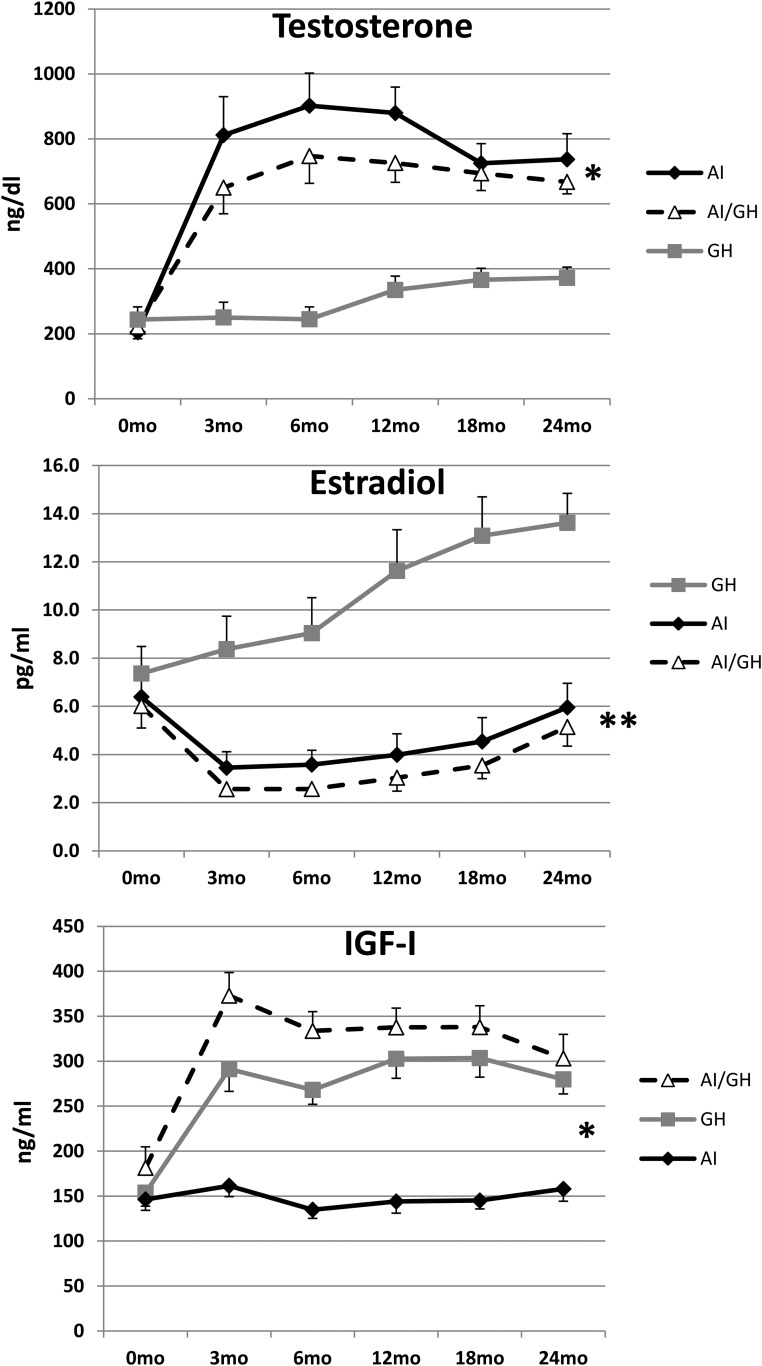
IGF-1 concentrations remained constant during 24 months of treatment in the AI group and increased in the GH and AI/GH groups; values at 0, 12, and 24 months, respectively, were: AI, 146 (12), 144 (13), and 158 (14) ng/mL (P = .70); GH, 154 (15), 303 (22), and 280 (16) ng/mL (P < .001); and AI/GH, 181 (23), 338 (21), and 303 (27) ng/mL (P < .001) (P < .0001 among groups) (Figure 4).
Figure 4.
Changes in mean (SE) concentrations of T (top panel), estradiol (middle panel), and IGF-1 (bottom panel) in the AI, GH, and AI/GH groups (*, P < .0001 among groups for T and IGF-1; **, P < .001 for estradiol). n = 76 (baseline), 72 (12 months), 65 (24 months).
Puberty progression and sex steroids
By design, study subjects were recruited in puberty, with the average genital Tanner stage of 2–3 at study entry, 3–4 by 12 months, and 4–5 by 24 months in all three groups. Testicular volumes were symmetrical and remained so throughout the study: AI, 10, 20, and 25 mL; GH, 10, 15, and 20 mL; and AI/GH, 10, 20, and 25 mL at 0, 12, and 24 months, respectively.
Aromatase blockade caused a significant and comparable increase in T concentrations with AI alone or AI/GH, compared to GH alone, although still within normal range. At 0, 12, and 24 months, respectively, T concentrations were: AI, 205 [to convert to T (ng/dl) to nmol/L multiply by 0.0347] (37), 880 (80), and 737 (79) ng/dL (P ≤ .0001 within group); GH, 244 (39), 335 (43), and 372 (33) ng/dL (P = .007); and AI/GH, 222 (37), 726 (60), and 668 (37) ng/dL (P ≤ .0001) (P < .0001 between groups). Estradiol concentrations at 0, 12, and 24 months were: AI, 6.4 [estradiol (pg/ml) to pmol/L multiply by 3.67] (1.0), 4.0 (0.9), and 6.0 (2.5) pg/mL (P = .009); GH, 7.4 (1.1), 11.6 (1.7), and 13.6 (1.2) pg/mL (P < .001); and AI/GH, 6.0 (0.9), 3.0 (0.6), and 5.1 (0.8) pg/mL (P = .003) (P < .001 between groups) (Figure 4).
We characterized the degree of aromatase blockade by the type of inhibitor used, based on changes on sex steroid concentrations. Data on those taking AI alone or AI/GH combined were grouped by type, either anastrozole or letrozole. There were significant differences in the levels of T by AI at 0, 12, and 24 months, respectively: anastrozole, 140 (37), 550 (64), and 509 (74) ng/dL; letrozole, 256 (56), 1068 (87), 920 (97) ng/dL (P = .0002; P value of differences between anastrozole/letrozole over time). There was a reciprocal greater decrease in estradiol after letrozole compared to anastrozole at 0, 12, and 24 months: anastrozole, 5 (1), 6 (2), and 8 (1) pg/mL; letrozole, 8 (1), 3 (1), and 4 (1) pg/mL (P = .0003) (P value of differences between anastrozole/letrozole over time) (Supplemental Figure 2).
Chemistries
Liver function tests and plasma lipids were measured throughout the initial 24 months with no significant changes over time (Supplemental Table 1).
Safety
All documented AEs are included in Supplemental Table 2. In 36 months, there were 382 AEs in the entire cohort, 118 in AI group, 114 GH group, and 150 AI/GH. The most common AEs were musculoskeletal, mostly related to physical activity and sports injuries. There were eight SAEs requiring hospitalization: three on AIs (concussion after falling from a tree, testicular torsion with bell clapper congenital deformity, and vertebra compression fracture after flipping on a four-wheeler), two on GH (pneumonia, and self-cutting episode), and three on AI/GH (upper tibial fracture during soccer trauma, vascular headaches present before study, and slipped capital femoral epiphyses [SCFE]). SCFE was thought to be related to the study drug (GH) and to the subject's increased BMI (90th percentile).
Discussion
Management of significant short stature in adolescence is challenging, particularly in those naive to treatment, because the time window for growth is limited. Differences between chronological age and bone age are also eliminated as puberty progresses. In this three-arm comparator study using AIs, GH, and combination AI/GH, AIs performed well, increasing linear growth when used for 2–3 years, particularly when combined with GH. For the first 24 months, patients showed a significant net gain in height from baseline on AI/GH (+18.9 [0.8] cm) > GH alone (+17.1 [0.9] cm) > AI alone (+14.0 [0.8] cm). This translated into a taller height SDS at 24 months for AI/GH (−1.25 [0.12]) > GH (−1.43 [0.14]) > AI (−1.73 [0.12]) SDS. Height SDS corrected for bone age was even taller because the AI groups had slower bone age progression. This growth compares favorably with an expected average net gain in height of +10.1 cm in boys the same age with an SDS of −2.0 cm; our subjects' height SDS was even shorter at −2.2 to −2.4. These results are remarkable, considering that boys were older (average age, 14.1 years) at study entry and quite short (height SDS, −2.3) despite being well in the midst of puberty (initial T, 223 ng/dL). Our data are congruent with recently published positive results using AI/GH vs GH alone for 11–19 months in a small cohort (n = 24) of 15-year-old boys with ISS treated at the end of puberty (26).
Whenever possible, we followed these young men to near-adult height for 1–2 years after discontinuation of all study drugs, with a mean age of 17.4 years and bone age of 15.3 years when approximately 97.6% of adult height had been achieved (23). In aggregate, regardless of the length of treatment, absolute height changes from baseline to near-final height were: AI, +18.2 (1.6) cm; GH, +20.6 (1.5) cm; and AI/GH, +22.5 (1.4) cm (P = .01 between groups). Height gains were greater if treatments were continued through 36 months compared to baseline: AI, +23.8 (2.3) cm; GH, +26.7 (2.0) cm; and AI/GH, +30.7 (1.1) cm. These gains in height also compare favorably with the expected height gain from 14.1 to 17.4 years of +13.0 cm for boys with a height SDS of −2.0 (our subjects, −2.2 to −2.4).
Calculated differences between estimated target (midparental) height and near-final height showed modest group differences of −7.8, −5.3, and −4.5 cm shorter than the target for AI, GH, and AI/GH, respectively. This likely overestimates the differences between near-final and target height because often we could not measure the height of both parents, and adults often tend to overestimate their own height. However, these results underscore the positive impact of these growth-promoting therapies, even when initiated in the midst of puberty. Multiple subjects ended up taller than the target height.
We carefully assessed bone health during these interventions. Bone density of the lumbar spine—which reflects trabecular bone—was normal once corrected for the subjects' height (24) compared to age appropriate normative data. It remained within the normal range with all interventions for 24 months, although with AI alone it was less (corrected Z-score, −1.06). BMD Z-scores corrected for height were mildly low in all groups at the whole-body level—which reflects mostly cortical bone—and remained constant with interventions after 24 months of treatment. This is comparable to the lack of change in BMD in our previous reports in GH-deficient boys treated with GH and anastrozole (19) and in ISS boys treated with letrozole (18). Bone-specific alkaline phosphatase, a marker of bone formation, was the same regardless of treatment arm.
AIs were previously reported to be associated with vertebral irregularities in a group of boys with ISS or constitutional growth delay treated with letrozole (17, 18, 27). We therefore carefully assessed vertebral changes focusing on the thoracic spine. We found no differences in the three groups for disc space narrowing, wedging, compression, and overall vertebral irregularities during the 24-month treatment. Actually, some of these findings were present at baseline, and some were no longer detected (such as compression) as treatment progressed. The extent of these abnormalities was indeed very mild and was similar to those commonly seen in short adolescents (28, 29). Bone pain questionnaires did not reveal any differences between groups (data not shown). Overall, the use of AIs, either alone or in combination, was not detrimental to bone health when used for up to 3 years.
A secondary outcome of these studies was to assess differential effects of AIs on body composition compared to GH (alone or in combination) in adolescents with ISS. Youngsters with short stature not due to GH deficiency are often skinny and have poor muscle mass (30). AIs and GH, when given alone, had a comparable positive impact on FFM accrual (AI, +11.5 [1.1] kg; GH, +12.1 [1.1] kg), but combination treatment had a clearly greater effect on FFM (+15.4 [1.4] kg) after 24 months. This is likely secondary to the potent protein-anabolic effects of the increase in androgens (14, 31) plus GH (32). The GH-alone and AI/GH groups had lower percentages of fat mass than the AI-alone group. In aggregate, combination AI/GH had a positive effect on body composition, increasing FFM and decreasing adiposity in these adolescents.
Plasma IGF-1 concentrations remained comparable throughout the first 24 months of the study in the AI-alone group, whereas they increased in the GH and AI/GH groups. This is congruent with the well-established effect of estrogen enhancing GH, and hence IGF-1 production (33, 34). The increase in growth in the AI-only group, despite a lack of increase in IGF-1, is similar to previously reported effects using letrozole in ISS boys (18) and to the increased growth observed with oxandrolone, a nonaromatizable androgen (34–36). Although mechanisms of growth increase without an IGF-1 increase are not fully characterized, this suggests a direct effect of androgens on the epiphyseal growth plate, likely mediated via the androgen receptor (37–39).
As expected, aromatase blockade caused a significant increase in circulating T concentrations and a reciprocal decline in estradiol whether administered alone or in combination with GH. We examined the relative impact of aromatase blockade on sex steroids depending on AI used, and letrozole caused greater T and lesser estradiol concentrations than anastrozole. In postmenopausal women, an 88% vs 85% tissue aromatase blockade has been reported for letrozole vs anastrozole (P = not significant) (40), with mean residual estradiol concentrations of 10.1% for anastrozole and 5.9% for letrozole (41), findings also confirmed by others (40) and congruent with our findings. There is, however, no difference in breast cancer survival using anastrozole vs letrozole (40, 41). Our study was not powered to detect differences in any of the principal clinical outcomes such as growth or body composition by AI, but letrozole will likely increase T more than anastrozole, necessitating closer monitoring of sex steroid levels. Any differential effects on growth would await further study.
The use of AIs, alone or with GH, was well tolerated and was safe overall. The incidence of AEs was comparable, and SAEs were not likely related to study drugs, except for one occurrence of SCFE. All chemistries, including liver function and plasma lipids, remained within normal limits throughout the study.
In summary, the use of combination AI/GH improved linear growth > GH alone and AIs alone in adolescent boys with ISS naive to treatment who were started on treatment in adolescence and treated for 24 months. Linear growth was improved further with more prolonged (36 months total) treatment in those with residual growth potential. Regardless of the length of treatment, near-final height gains were: AI, +18.2 (1.6) cm; GH, +20.6 (1.5) cm; and AI/GH, +22.5 (1.4) cm; resulting in the following near-adult height SDS: AI, −1.4 (0.1) cm; GH, −1.4 (0.2) cm; and AI/GH, −1.0 (0.1) cm. FFM accrual was greater in the AI/GH group. Measures of bone health showed no detrimental effects with AIs. Anastrozole and letrozole had differential effects on sex steroid concentrations, with greater T and lesser estradiol in those treated with letrozole vs anastrozole. AIs alone and in combination with GH were well tolerated and safe for up to 3 years. In conclusion, AIs are an alternative treatment to enhance linear growth in adolescent boys with ISS, particularly in combination with GH.
Acknowledgments
The authors are grateful for the technical support of Shawn Sweeten in the Biochemical Analysis Laboratory at the Nemours Children's Health System in Jacksonville, Florida, as well as the technical support at Dr. Ravinder Singh's laboratory at the Mayo Clinic, Rochester, Minnesota. We are grateful to Karen Kowal, physician assistant at the Nemours Clinic at Jefferson University and Dupont Hospital for Children, for her excellent care of these patients; to Sylvia Kyle, Nemours librarian, for her outstanding assistance; to Genentech, Pfizer, Novartis, and AstraZeneca for providing drug supplies for these studies; and for a generous grant from Mr. W. J. Wadsworth and the Thrasher Research Fund, who funded these studies. Our thanks go to the data safety management board, including Edward Reiter, MD; Janet Silverstein, MD; and Pamela Arn, MD. We are also grateful to the physicians who referred patients and all the adolescent boys and their parents who participated in these studies.
This work was supported by grants from the Thrasher Research Fund (to N.M.), National Institutes of Health Grant UL1 TR000135 from the National Center for Advancing Translational Sciences (to R.S.), a generous gift from W. J. Wadsworth (to N.M.), and drug supply agreements from AstraZeneca, Novartis, Genentech, and Pfizer.
Clinical Trial Registration No.: NCT01248416.
Disclosure Summary: N.M. has received research support in drug supply agreements from Genentech, Pfizer, Novartis and AstraZeneca; and consulted for Opko. J.R. receives research support from Versartis and Novo Nordisk and consults for Novo Nordisk. All other authors have nothing to declare.
Footnotes
- AE
- adverse event
- AI
- aromatase inhibitor
- ANCOVA
- analysis of covariance
- BMD
- bone mineral density
- BMI
- body mass index
- CV
- coefficient of variation
- DXA
- dual x-ray absorptiometry
- FFM
- fat free mass
- GnRHa
- GnRH analog
- ISS
- idiopathic short stature
- SAE
- serious AE
- SCFE
- slipped capital femoral epiphyses
- SDS
- SD score.
Reference
- 1. Mauras N, Attie KM, Reiter EO, Saenger P, Baptista J. High dose recombinant human growth hormone (GH) treatment of GH-deficient patients in puberty increases near-final height: a randomized, multicenter trial. Genentech, Inc., Cooperative Study Group. J Clin Endocrinol Metab. 2000;85(10):3653–3660. [DOI] [PubMed] [Google Scholar]
- 2. Mericq MV, Eggers M, Avila A, Cutler GB, Jr, Cassorla F. Near final height in pubertal growth hormone (GH)-deficient patients treated with GH alone or in combination with luteinizing hormone-releasing hormone analog: results of a prospective, randomized trial. J Clin Endocrinol Metab. 2000;85(2):569–573. [DOI] [PubMed] [Google Scholar]
- 3. Pucarelli I, Segni M, Ortore M, Arcadi E, Pasquino AM. Effects of combined gonadotropin-releasing hormone agonist and growth hormone therapy on adult height in precocious puberty: a further contribution. J Pediatr Endocrinol Metab. 2003;16(7):1005–1010. [DOI] [PubMed] [Google Scholar]
- 4. Reiter EO. A brief review of the addition of gonadotropin-releasing hormone agonists (GnRH-Ag) to growth hormone (GH) treatment of children with idiopathic growth hormone deficiency: previously published studies from America. Mol Cell Endocrinol. 2006;254–255:221–225. [DOI] [PubMed] [Google Scholar]
- 5. Scalco RC, Melo SS, Pugliese-Pires PN, et al. Effectiveness of the combined recombinant human growth hormone and gonadotropin-releasing hormone analog therapy in pubertal patients with short stature due to SHOX deficiency. J Clin Endocrinol Metab. 2010;95(1):328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lem AJ, van der Kaay DC, de Ridder MA, et al. Adult height in short children born SGA treated with growth hormone and gonadotropin releasing hormone analog: results of a randomized, dose-response GH trial. J Clin Endocrinol Metab. 2012;97(11):4096–4105. [DOI] [PubMed] [Google Scholar]
- 7. Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. [DOI] [PubMed] [Google Scholar]
- 8. Carani C, Qin K, Simoni M, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337(2):91–95. [DOI] [PubMed] [Google Scholar]
- 9. Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80(12):3689–3698. [DOI] [PubMed] [Google Scholar]
- 10. Gunther DF, Calikoglu AS, Underwood LE. The effects of the estrogen receptor blocker, Faslodex (ICI 182,780), on estrogen-accelerated bone maturation in mice. Pediatr Res. 1999;46(3):269–273. [DOI] [PubMed] [Google Scholar]
- 11. Nilsson O, Weise M, Landman EB, Meyers JL, Barnes KM, Baron J. Evidence that estrogen hastens epiphyseal fusion and cessation of longitudinal bone growth by irreversibly depleting the number of resting zone progenitor cells in female rabbits. Endocrinology. 2014;155(8):2892–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mauras N, Lima J, Patel D, et al. Pharmacokinetics and dose finding of a potent aromatase inhibitor, aromasin (exemestane), in young males. J Clin Endocrinol Metab. 2003;88(12):5951–5956. [DOI] [PubMed] [Google Scholar]
- 13. Mauras N, Bishop K, Merinbaum D, Emeribe U, Agbo F, Lowe E. Pharmacokinetics and pharmacodynamics of anastrozole in pubertal boys with recent-onset gynecomastia. J Clin Endocrinol Metab. 2009;94(8):2975–2978. [DOI] [PubMed] [Google Scholar]
- 14. Mauras N, Hayes V, Welch S, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83(6):1886–1892. [DOI] [PubMed] [Google Scholar]
- 15. Mauras N, Hayes VY, Vieira NE, Yergey AL, O'Brien KO. Profound hypogonadism has significant negative effects on calcium balance in males: a calcium kinetic study. J Bone Miner Res. 1999;14(4):577–582. [DOI] [PubMed] [Google Scholar]
- 16. Mauras N, O'Brien KO, Klein KO, Hayes V. Estrogen suppression in males: metabolic effects. J Clin Endocrinol Metab. 2000;85(7):2370–2377. [DOI] [PubMed] [Google Scholar]
- 17. Wickman S, Sipil I, Ankarberg-Lindgren C, Norjavaara E, Dunkel L. A specific aromatase inhibitor and potential increase in adult height in boys with delayed puberty: a randomised controlled trial. Lancet. 2001;357(9270):1743–1748. [DOI] [PubMed] [Google Scholar]
- 18. Hero M, Norjavaara E, Dunkel L. Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90(12):6396–6402. [DOI] [PubMed] [Google Scholar]
- 19. Mauras N, Gonzalez de Pijem L, Hsiang HY, et al. Anastrozole increases predicted adult height of short adolescent males treated with growth hormone: a randomized, placebo-controlled, multicenter trial for one to three years. J Clin Endocrinol Metab. 2008;93(3):823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanner J. Growth at Adolescence. 2nd ed Oxford, UK: Blackwell Scientific Publications; 1962. [Google Scholar]
- 21. Ettinger B, Black DM, Nevitt MC, et al. Contribution of vertebral deformities to chronic back pain and disability. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1992;7(4):449–456. [DOI] [PubMed] [Google Scholar]
- 22. Roche AF, Chumlea WC, Thissen D. Assessing the Skeletal Maturity of the Hand-Wrist: Fels Method. Springfield, IL: Charles C. Thomas; 1988. [DOI] [PubMed] [Google Scholar]
- 23. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40(4):423–441. [DOI] [PubMed] [Google Scholar]
- 24. Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the Bone Mineral Density in Childhood Study. J Clin Endocrinol Metab. 2011;96(10):3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. National Center for Growth Statistics. CDC Growth charts. Z-score data tables. http://www.cdc.gov/growthcharts/zscore.htm Accessed July 12, 2016.
- 26. Rothenbuhler A, Linglart A, Bougnres P. A randomized pilot trial of growth hormone with anastrozole versus growth hormone alone, starting at the very end of puberty in adolescents with idiopathic short stature. Int J Pediatr Endocrinol. 2015(1);2015:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hero M, Toiviainen-Salo S, Wickman S, Mitie O, Dunkel L. Vertebral morphology in aromatase inhibitor-treated males with idiopathic short stature or constitutional delay of puberty. J Bone Miner Res. 2010;25(7):1536–1543. [DOI] [PubMed] [Google Scholar]
- 28. Ramadorai U, Hire J, DeVine JG, Brodt ED, Dettori JR. Incidental findings on magnetic resonance imaging of the spine in the asymptomatic pediatric population: a systematic review. Evid Based Spine Care J. 2014;5(2):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jaremko JL, Siminoski K, Firth GB, et al. Common normal variants of pediatric vertebral development that mimic fractures: a pictorial review from a national longitudinal bone health study. Pediatr Radiol. 2015;45(4):593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han JC, Balagopal P, Sweeten S, Darmaun D, Mauras N. Evidence for hypermetabolism in boys with constitutional delay of growth and maturation. J Clin Endocrinol Metab. 2006;91(6):2081–2086. [DOI] [PubMed] [Google Scholar]
- 31. Mauras N, Haymond MW, Darmaun D, Vieira NE, Abrams SA, Yergey AL. Calcium and protein kinetics in prepubertal boys. Positive effects of testosterone. J Clin Invest. 1994;93(3):1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mauras N, Haymond MW. Are the metabolic effects of GH and IGF-I separable? Growth Horm IGF Res. 2005;15(1):19–27. [DOI] [PubMed] [Google Scholar]
- 33. Veldhuis JD, Roemmich JN, Richmond EJ, et al. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26(1):114–146. [DOI] [PubMed] [Google Scholar]
- 34. Veldhuis JD, Metzger DL, Martha PM, Jr, et al. Estrogen and testosterone, but not a nonaromatizable androgen, direct network integration of the hypothalamo-somatotrope (growth hormone)-insulin-like growth factor I axis in the human: evidence from pubertal pathophysiology and sex-steroid hormone replacement. J Clin Endocrinol Metab. 1997;82(10):3414–3420. [DOI] [PubMed] [Google Scholar]
- 35. Sas TC, Gault EJ, Bardsley MZ, et al. Safety and efficacy of oxandrolone in growth hormone-treated girls with Turner syndrome: evidence from recent studies and recommendations for use. Horm Res Paediatr. 2014;81(5):289–297. [DOI] [PubMed] [Google Scholar]
- 36. Vottero A, Pedori S, Verna M, et al. Final height in girls with central idiopathic precocious puberty treated with gonadotropin-releasing hormone analog and oxandrolone. J Clin Endocrinol Metab. 2006;91(4):1284–1287. [DOI] [PubMed] [Google Scholar]
- 37. Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE. The localization of androgen receptors in human bone. J Clin Endocrinol Metab. 1997;82(10):3493–3497. [DOI] [PubMed] [Google Scholar]
- 38. van der Eerden BC, van Til NP, Brinkmann AO, Lowik CW, Wit JM, Karperien M. Gender differences in expression of androgen receptor in tibial growth plate and metaphyseal bone of the rat. Bone. 2002;30(6):891–896. [DOI] [PubMed] [Google Scholar]
- 39. Nilsson O, Chrysis D, Pajulo O, et al. Localization of estrogen receptors-alpha and -beta and androgen receptor in the human growth plate at different pubertal stages. J Endocrinol. 2003;177(2):319–326. [DOI] [PubMed] [Google Scholar]
- 40. Sendur MA, Aksoy S, Zengin N, Altundag K. Comparative efficacy study of 5-year letrozole or anastrozole in postmenopausal hormone receptor-positive early breast cancer. J BUON. 2013;18(4):838–844. [PubMed] [Google Scholar]
- 41. Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol. 2011;29(17):2342–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]