Abstract
Context:
Prediabetes is a heterogeneous disorder classified on the basis of fasting glucose concentrations and 2-hour glucose tolerance.
Objective:
We sought to determine the relative contributions of insulin secretion and action to the pathogenesis of isolated impaired glucose tolerance (IGT).
Design:
The study consisted of an oral glucose tolerance test and a euglycemic clamp performed in two cohorts matched for anthropometric characteristics and fasting glucose but discordant for glucose tolerance.
Setting:
An inpatient clinical research unit at an academic medical center.
Patients or Other Participants:
Twenty-five subjects who had normal fasting glucose (NFG) and normal glucose tolerance (NGT) and 19 NFG/IGT subjects participated in this study.
Intervention(s):
Subjects underwent a seven-sample oral glucose tolerance test and a 4-hour euglycemic, hyperinsulinemic clamp on separate occasions. Glucose turnover during the clamp was measured using tracers, and endogenous hormone secretion was inhibited by somatostatin.
Main Outcome Measures:
We sought to determine whether hepatic glucose metabolism, specifically the contribution of gluconeogenesis to endogenous glucose production, differed between subjects with NFG/NGT and those with NFG/IGT.
Results:
Endogenous glucose production did not differ between groups before or during the clamp. Insulin-stimulated glucose disappearance was lower in NFG/IGT (24.6 ± 2.2 vs 35.0 ± 3.6 μmol/kg/min; P = .03). The disposition index was decreased in NFG/IGT (681 ± 102 vs 2231 ± 413 × 10−14 dL/kg/min2 per pmol/L; P < .001).
Conclusions:
We conclude that innate defects in the regulation of glycogenolysis and gluconeogenesis do not contribute to NFG/IGT. However, insulin-stimulated glucose disposal is impaired, exacerbating defects in β-cell function.
The current experiment examines isolated impaired glucose tolerance. In isolated IGT defects in peripheral, but not hepatic, insulin action are accompanied by impaired î2-cell function.
Type 2 diabetes is a complex and heterogeneous metabolic disease characterized by defects in insulin secretion and action, which are accompanied by changes in carbohydrate, protein, and lipid metabolism (1). The transition from normal glucose metabolism to diabetes occurs through an intermediate phase characterized by impaired glucose tolerance (IGT) and/or impaired fasting glucose (IFG) and often referred to as “prediabetes” (2). This is a condition of considerable interest in terms of understanding the mechanisms underlying the progression to diabetes (3). Indeed, the transition from prediabetes to diabetes is variable so that “only” approximately 40% of subjects with a fasting glucose ≥ 110 mg/dL develop diabetes in the subsequent 10-year period (4). Moreover, affected subjects have a higher risk of developing cardiovascular disease (5, 6).
Quantitative measurement of β-cell function is typically achieved by using model-based measures of insulin secretion and action to derive a “disposition index” that expresses insulin secretion as a function of prevailing insulin action (7). Subjects with prediabetes exhibit significant heterogeneity in β-cell function (3, 8). Indeed, subjects with isolated IFG (ie, with normal glucose tolerance [NGT]) have quantitative indices of β-cell function that do not differ from subjects with normal fasting glucose (NFG) and NGT (8). On the other hand, subjects with isolated IGT (ie, NFG/IGT) exhibit lower indices than those observed in other categories of prediabetes. However, such model-based estimates derived from cross-sectional studies cannot determine the temporal contribution of insulin resistance or defective insulin secretion to IGT. More importantly, in the absence of tracer-based methodology, these studies cannot discern whether defective insulin action affects all insulin-sensitive tissues equally or whether defects affect the liver or skeletal muscle disproportionately (9).
Whereas fasting glucose concentrations are determined by the rate of endogenous glucose production (EGP) and the rate of disappearance (Rd) of peripheral glucose, postprandial glucose concentrations represent the net sum of the systemic appearance of ingested glucose together with the EGP and Rd. Rising postprandial glucose and insulin concentrations suppress EGP and stimulate Rd (9). Prior experiments using a labeled mixed meal have suggested that postprandial suppression of EGP is not impaired in prediabetes (8). On the other hand, using a euglycemic, hyperinsulinemic clamp, Basu et al (10) demonstrated that in isolated IFG and in those with IFG/IGT, increased fasting EGP and impaired suppression of EGP by insulin (IFG/IGT) contribute to the hyperglycemia observed in affected subjects. The contribution of abnormal hepatic glucose metabolism to the pathogenesis of isolated IGT (NFG/IGT) remains uncertain.
The current experiment examines the pathogenesis of isolated NFG/IGT. To do so, we measured insulin secretion and hepatic and extrahepatic insulin action using the oral minimal model and a hyperinsulinemic, euglycemic clamp. We compared results observed in subjects with NFG/IGT to age-, weight-, and gender-matched subjects with normal glucose metabolism (NFG/NGT). We measured β-cell function using an oral glucose challenge together with hepatic glucose metabolism using a euglycemic, hyperinsulinemic clamp with [3-3H]glucose and the deuterated water method after correction for the transaldolase exchange (11, 12). We report that subjects with NFG/IGT have both impaired β-cell function and impaired insulin-induced stimulation of glucose uptake in peripheral tissues. However, both fasting and insulin-induced suppression of EGP, gluconeogenesis, and glycogenolysis do not differ from those observed in subjects with NFG/NGT.
Subjects and Methods
Subjects
Individuals who participated in a prior study (11) were invited to participate in a subsequent experiment measuring hepatic glucose metabolism after an overnight fast and during a euglycemic clamp (12). Participants were initially identified from a sample of 4000 subjects in the Mayo Clinic Biobank on the basis of their genotype at rs7903146, and of these, 120 had previously participated in a study examining the effect of TCF7L2 genotype on β-cell function in response to acute insulin resistance. Fifty-eight of the 120 subjects gave written, informed consent to participate in the subsequent study (12). Subjects underwent a screening examination together with a 75-g oral glucose tolerance test (OGTT) to determine their glucose tolerance status. We were able to identify 25 NFG/NGT subjects and 19 NFG/IGT who were otherwise matched for baseline characteristics and genotype (Table 1). Body composition was measured by dual-energy x-ray absorptiometry as before.
Table 1.
Baseline Characteristics at the Time of Screening in Each Group
| NFG/NGT | NFG/IGT | P Value | |
|---|---|---|---|
| N (males/females) | 25 (9/16) | 19 (5/14) | |
| rs7903146 genotype, TT/CC | 13/12 | 10/9 | .97a |
| Age, y | 48 ± 2 | 50 ± 3 | .65 |
| Body weight, kg | 79 ± 3 | 84 ± 4 | .25 |
| BMI, kg/m2 | 27.3 ± 0.7 | 29.0 ± 0.9 | .12 |
| LBM, kg | 48.6 ± 2.4 | 48.0 ± 1.9 | .84 |
| Screening 75-g 2-h OGTT | |||
| Fasting glucose, mmol/L | 4.8 ± 0.1 | 4.9 ± 0.1 | .69 |
| Peak glucose, mmol/L | 9.0 ± 0.2 | 10.4 ± 0.3 | 4.1 × 10E-5 |
| AAB glucose, mmol/2 h | 266 ± 20 | 435 ± 28 | 1.1 × 10E-5 |
| Fasting insulin, pmol/L | 25 ± 5 | 29 ± 4 | .46 |
| Peak insulin, pmol/L | 289 ± 36 | 328 ± 32 | .43 |
| AAB insulin, nmol/2 h | 19.0 ± 2.6 | 24.2 ± 2.7 | .19 |
| Fasting C-peptide, nmol/L | 0.59 ± 0.03 | 0.77 ± 0.06 | .01 |
| Peak C-peptide, nmol/L | 3.15 ± 0.18 | 3.85 ± 0.27 | .03 |
| AAB C-peptide, nmol per 2 h | 212 ± 14 | 245 ± 19 | .17 |
| Fasting glucagon, ng/L | 81 ± 3 | 88 ± 5 | .24 |
| Nadir glucagon, ng/L | 59 ± 3 | 63 ± 4 | .37 |
| AAB glucagon, ng per 2 h | −2313 ± 249 | −2483 ± 370 | .70 |
Abbreviations: BMI, body mass index; LBM, lean body mass. Values are expressed as means ± SEM. P value reports the results of an unpaired, two-tailed t test.
The result of a χ2 test.
Experimental design—OGTT
An 18-gauge cannula was inserted in a retrograde fashion into a dorsal hand vein of the nondominant arm. The hand was placed in a heated box (55°C) to enable sampling of arterialized venous blood. Blood was drawn at 0 (baseline), 10, 20, 30, 60, 90, and 120 minutes for the measurement of glucose, insulin, and C-peptide concentrations. After the baseline blood draw, subjects ingested 75 g of glucose over a period of 5 minutes.
Net insulin action (Si) was measured using the oral minimal model (13). β-Cell responsivity indices were determined using the oral C-peptide minimal model (14), incorporating age-associated changes in C-peptide kinetics (15). The model assumes that insulin secretion comprises static and dynamic components. The parameter φd defines the dynamic responsivity index and is proportional to the rate of increase of glucose concentrations. φs represents the provision of new insulin to the releasable pool. An index of total β-cell responsivity to glucose (Φ) is then derived from both indices (16). The disposition index was subsequently derived from these indices as previously described (17). The area above basal (AAB) was calculated using the trapezoidal rule.
Experimental design—euglycemic clamp
Participants were admitted to the Clinical Research Unit at 5 pm the day before the study. After a standard 10 kcal/kg caffeine-free meal, blood was sampled for baseline enrichment, and the subjects fasted overnight; 1.67 g/kg body weight of deuterated water (2H2O) was then given in three divided doses at 10 pm, midnight, and 2 am.
At 6 am the following morning, a dorsal hand vein was cannulated, placed in a heated Plexiglas box, and maintained at 55°C to allow sampling of arterialized venous blood. The contralateral forearm vein was cannulated for tracer, glucose, and hormone infusions. At 6:30 am (−180 minutes), a primed, continuous infusion of [3-3H]glucose (12 μCi prime, 0.12 μCi/min continuous) and [1-13C]acetate (2.5 μmol/kg/min) was started and continued for the duration of the experiment. At 9:30 am (0 minutes), an infusion of somatostatin (60 ng/kg/min), glucagon (0.65 ng/kg/min), and GH (0.25 ng/kg/min) was started and was maintained for the duration of study. Insulin was also infused at 0.30 mU/kg/min. At this time, a variable infusion of 50% dextrose containing [3-3H]glucose was commenced with the infusion rate varied to maintain glucose at approximately 5.5 mmol/L over the period of study. Arterialized venous blood samples were collected to allow measurement of hormone, tracer, and substrate concentrations.
Analytical techniques
All blood was immediately placed on ice, centrifuged at 4°C, separated, and stored at −80°C until assay. Glucose concentrations were measured using a glucose oxidase method (Yellow Springs Instruments). Plasma insulin was measured using a chemiluminescence assay (Access Assay; Beckman). Plasma glucagon and C-peptide were measured by RIA (Linco Research). Plasma [3-3H]glucose specific activity was measured by liquid scintillation counting. 2H nuclear magnetic resonance spectroscopy was used to measure and analyze deuterium enrichment on the second and fifth carbons of plasma glucose after derivatization to monoacetone glucose (18). To correct for errors introduced by transaldolase exchange (19), estimation of the transaldolase exchange was accomplished by measurement of [3-13C]glucose and [4-13C]glucose enrichment by nuclear magnetic resonance spectroscopy, as previously described (20).
Calculations
Glucose appearance and disappearance were calculated using the steady-state equations of Steele et al (21), where the actual tracer infusion rate was utilized. The volume of distribution of glucose was assumed to be 200 mL/kg with a pool correction factor equal to 0.65. EGP was calculated by subtracting the glucose infusion rate from the tracer-determined rate of glucose appearance. Fasting and clamp rates represent the mean of the −30 to 0- and 210 to 240-minute values, respectively. All rates of infusion and turnover were expressed per kilogram of lean body mass.
The rate of gluconeogenesis was calculated by multiplying the C5/C2 enrichment ratio by the respective EGP. The C5 enrichment was corrected for transaldolase exchange using the following equation: C5corrected = C5observed − {([3-13C]glucose/[4-13C]glucose) × C5observed}, as previously described. Glycogenolysis was then calculated by subtracting the rate of gluconeogenesis from EGP (12).
Statistical analysis
Data in the text and in figures are expressed as means ± SEM. All rates are expressed per kilogram of lean body mass. An unpaired, two-tailed Student's t test (or a Mann-Whitney test for values that were not normally distributed) was used to test differences between NGT and IGT groups. The statistical analysis was undertaken in Primer 5 (GraphPad Software). A P value < .05 was considered statistically significant.
Results
Volunteer characteristics (Table 1)
Twenty-five subjects had NFG and NGT, and 19 subjects had NFG and IGT. Their characteristics at the time of screening are summarized in Table 1.
Glucose, insulin, C-peptide, and glucagon concentrations during a 75-g OGTT (Figure 1)
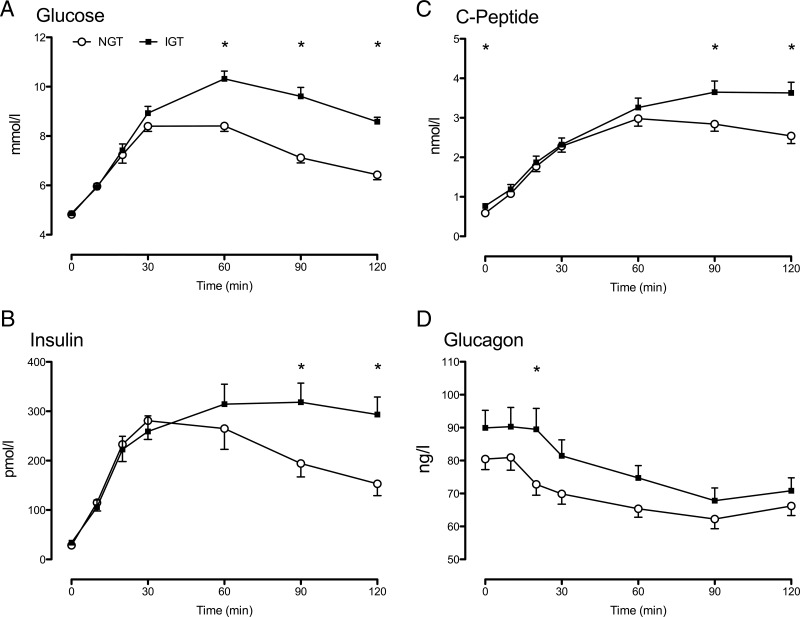
Figure 1.
Glucose (A), insulin (B), C-peptide (C), and glucagon (D) in response to a 75-g oral glucose challenge in subjects with NGT (open circles) and IGT (solid squares). Values plotted are means ± SEM. *, P < .05 for a post hoc unpaired, two-tailed t test.
Although fasting glucose concentrations did not differ, peak (10.4 ± 0.3 vs 9.0 ± 0.2 mmol/L; P < .01) and integrated (435 ± 28 vs 266 ± 20 mmol/2 h; P < .01) glucose concentrations were higher in the NFG/IGT group compared to the NFG/NGT group, respectively (Figure 1A and Table 1). Similarly, peak and integrated concentrations of insulin (Figure 1B) and C-peptide (Figure 1C) were higher in the IGT group. Fasting and integrated glucagon concentrations (Figure 1D and Table 1) did not differ significantly between groups.
Net insulin action, β-cell responsivity, and disposition index in NFG/IGT and NFG/NGT subjects (Figure 2)
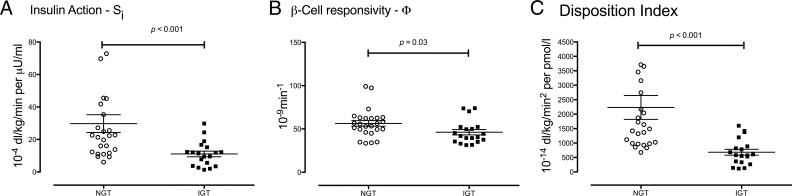
Figure 2.
Insulin action (Si; A), β-cell responsivity (Φ; B), and disposition index (C) in subjects with NGT (open circles) and IGT (solid squares) in response to a 75-g oral glucose challenge. The P values represent the results of a nonparametric, two-tailed, Mann-Whitney test.
In response to 75-g oral glucose, insulin action (Si, Figure 2A) was lower in the NFG/IGT group compared to the NFG/NGT group (11 ± 2 vs 30 ± 6; P < .001). In addition, β-cell responsivity to glucose (Φ, Figure 2B; 46 ± 3 vs 56 ± 3 × 10−9min−1; P = .03) was also impaired in the NFG/IGT group. To determine whether insulin secretion was appropriate for the prevailing level of insulin action, the product of individual values of Si and Φ was used to calculate a disposition index for each subject, as previously described (17). The disposition index was significantly decreased in the NFG/IGT group compared to the NFG/NGT group (681 ± 102 vs 2231 ± 413 × 10−14 dL/kg/min2 per pmol/L; P < .001; Figure 2C).
Glucose, insulin, C-peptide, and glucagon concentrations during the euglycemic clamp (Figure 3)
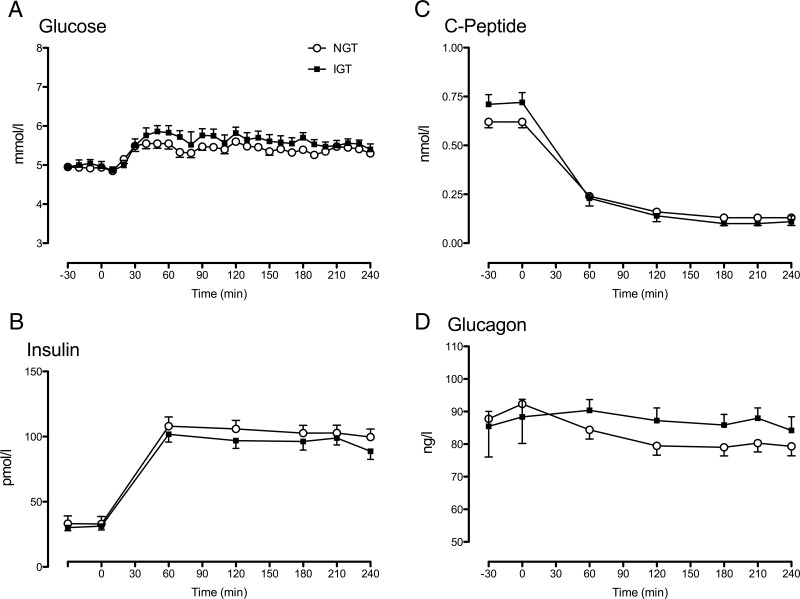
Figure 3.
Glucose (A), insulin (B), C-peptide (C), and glucagon (D) during a euglycemic clamp infusion in subjects with NGT (open circles) and IGT (solid squares). Values plotted are means ± SEM. *, P < .05 for a post hoc unpaired, two-tailed t test.
Glucose concentrations (Figure 3A) did not differ between groups either before or during the clamp. Similarly, insulin concentrations (Figure 3B), by design, did not differ between the two groups either before or during the clamp.
Fasting C-peptide concentrations before the clamp study (Figure 3C; 0.71 ± 0.05 vs 0.62 ± 0.03 nmol/L; P = .10) did not differ between groups. During the clamp, C-peptide concentrations were equally suppressed by somatostatin in both groups. Fasting glucagon (Figure 3D) concentrations did not differ between groups before the clamp and remained equal during the somatostatin/glucagon infusion.
EGP, glucose disappearance, glucose infusion rate, and specific activity during the euglycemic clamp (Figure 4)
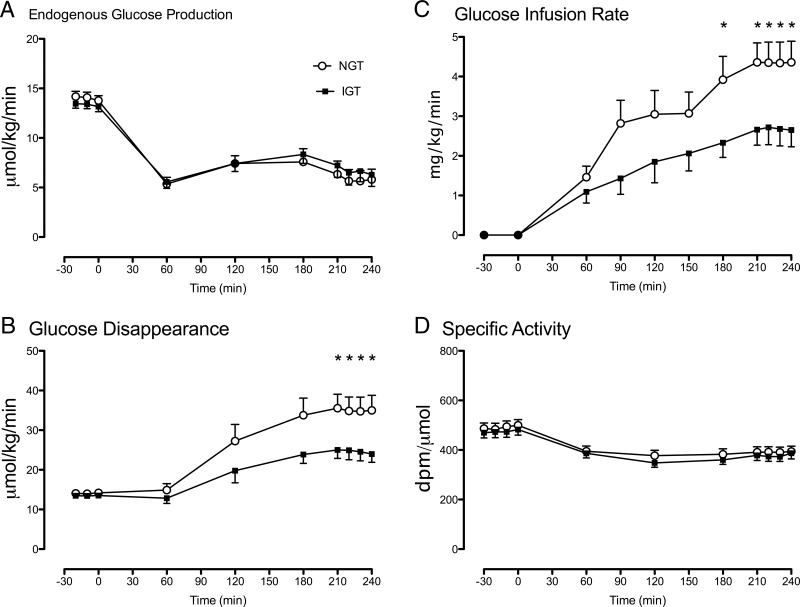
Figure 4.
EGP (A), glucose disappearance (B), glucose infusion rate (C), and the specific activity of labeled/unlabeled glucose (D) during a euglycemic clamp infusion in subjects with NGT (open circles) and IGT (solid squares). Values plotted are means ± SEM. *, P < .05 for a post hoc unpaired, two-tailed t test.
EGP (Figure 4A) did not differ between groups either before or during the clamp. Fasting glucose disappearance also did not differ between groups. However, during the clamp, insulin-stimulated glucose disappearance (Figure 4B) was significantly lower in the NFG/IGT group compared to the NFG/NGT group (24.6 ± 2.2 vs 35.0 ± 3.6 μmol/kg/min; P = .03). The glucose infusion rate necessary to maintain euglycemia (Figure 4C) during the final 30 minutes of the clamp was also significantly lower (2.7 ± 0.4 vs 4.4 ± 0.5 mg/kg/min; P = .02) in the NFG/IGT group than in the NFG/NGT group. The glucose specific activity (Figure 4D) did not differ between groups either before or during the clamp.
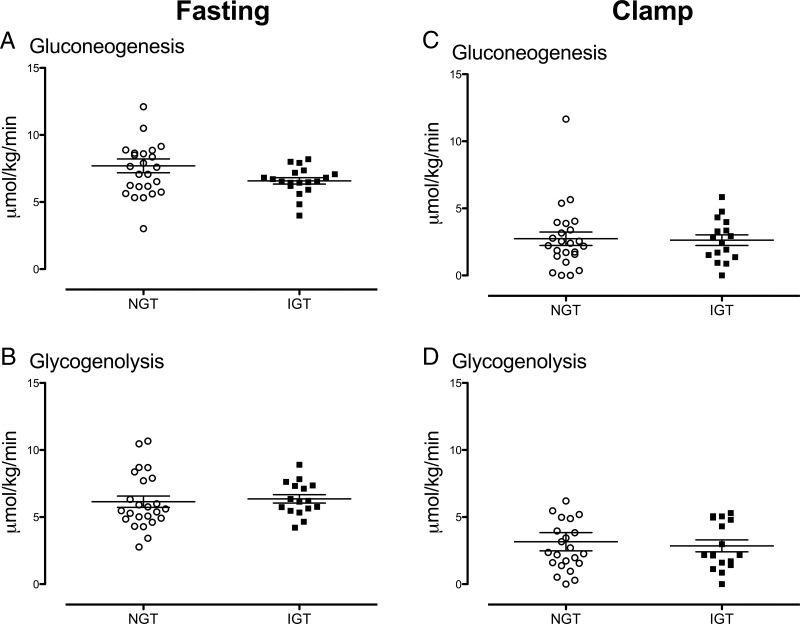
Rates of gluconeogenesis and glycogenolysis during fasting and during the euglycemic clamp (Figure 5)
Figure 5.
Rates of gluconeogenesis (A) and glycogenolysis (B) while fasting, together with rates of gluconeogenesis (C) and glycogenolysis (D) during the clamp in individual subjects with NGT (open circles) and IGT (solid squares). Values plotted are means ± SEM. *, P < .05 for a post hoc unpaired, two-tailed t test.
Rates of gluconeogenesis did not differ between NFG/IGT and NFG/NGT groups either before (Figure 5A; 6.6 ± 0.2 vs 7.7 ± 0.5 μmol/kg/min; P = .15) or during (Figure 5C; 2.6 ± 0.4 vs 2.7 ± 0.5 μmol/kg/min; P = .81) the clamp. Similarly, glycogenolysis did not differ between groups, either before (Figure 5B; 6.4 ± 0.3 vs 6.1 ± 0.4 μmol/kg/min; P = .38) or during (Figure 5D; 2.9 ± 0.4 vs 3.2 ± 0.7 μmol/kg/min; P = .99) the clamp in the NFG/IGT and NFG/NGT subjects.
Discussion
The present data indicate that people with isolated IGT have defects in both insulin secretion and extrahepatic insulin action. In contrast, fasting EGP, gluconeogenesis, and glycogenolysis data did not differ from those observed in subjects with NFG/NGT. Insulin-induced suppression of EGP, gluconeogenesis, and glycogenolysis also did not differ, implying that defects in hepatic glucose metabolism and hepatic insulin action do not play a role in isolated IGT (NFG/IGT). On the other hand, insulin action in the extrahepatic tissues was impaired in NFG/IGT, as evidenced by decreased rates of glucose disappearance presumably representing decreased rates of glucose uptake by peripheral tissues. Insulin secretion is also impaired; β-cell responsivity to glucose was decreased in the NFG/IGT group compared to the NFG/NGT group and was inappropriate for the prevailing level of insulin action, resulting in a lower disposition index in subjects with NFG/IGT when compared to subjects with NFG/NGT. These data indicate that subjects with isolated IGT have defective insulin action in extrahepatic tissues and an inability of β-cell function to overcome these defects.
It is notable that subjects with NFG/IGT, in response to an oral challenge, exhibited higher peak and integrated glucose concentrations compatible with defects in both insulin secretion and insulin action, respectively (22). Although an accompanying delay in the suppression of glucagon secretion would contribute to postprandial hyperglycemia in situations where insulin secretion is deficient (23, 24), this was not the case in this series of experiments. In addition, subjects with NFG/IGT, in both the current and prior studies (8, 25), tend to have higher fasting C-peptide concentrations for a given fasting glucose; if expressed as a ratio using qualitative measures of insulin action, this would suggest hepatic insulin resistance. However, when directly measured, both fasting and insulin-induced suppression of EGP were normal, indicating normal hepatic insulin action.
Because EGP is the sum of gluconeogenesis and glycogenolysis, reciprocal differences in these fluxes between IFG and NFG/NGT groups could exist in the setting of identical EGP rates. The present data indicate that this is not the case. We utilized a refinement of the deuterated water method to eliminate any error introduced by transaldolase-mediated exchange in the measurement of gluconeogenesis (12). Using this methodology, there was no evidence of abnormal hepatic glucose metabolism in people with IGT, in contrast with previously reported abnormalities in isolated IFG and IFG/IGT (10). Taken together, these data are consistent with the notion that increased fasting glucose concentrations are due to increased EGP, whereas EGP is normal when fasting glucose concentrations are normal (as in the present study).
These findings complement those reported by Laakso et al (26) in the EUGENE2 consortium where subjects with isolated IGT exhibited insulin secretory responses to an iv glucose tolerance test that did not differ from subjects with NGT, whereas results from an OGTT demonstrated higher insulin and glucose concentrations in IGT within 30 minutes of an oral challenge. As measured by a euglycemic clamp, subjects with IGT exhibited insulin resistance compared to NGT; however, in the absence of a tracer, the experimental design (unlike in our current experiment) could not distinguish hepatic vs extrahepatic contributions to the defect in insulin action.
The relationship of insulin concentration to response differs between the liver and skeletal muscle (27). Typically, higher insulin concentrations are required to maximally stimulate peripheral glucose uptake than are required to completely suppress EGP. However, at insulin concentrations designed to suppress EGP by approximately 50%, the present data clearly indicate a defect in extrahepatic insulin action in people with NFG/IGT. Consistent with the clamp data, the oral minimal model indicated a defect in net insulin action. This is in keeping with previous data suggesting a good correlation between clamp-derived and net insulin action and the net insulin action calculated by the oral minimal model (28).
Despite qualitatively increased insulin secretion compared to NFG/NGT subjects, in response to the higher glucose concentrations present during an oral challenge in subjects with NFG/IGT, this was inappropriate for the prevailing insulin action as quantified by the disposition index. Of note, the same pattern of decreased disposition index was observed when the same subjects were studied as part of a separate series of experiments examining the effect of free fatty acid-induced insulin resistance on β-cell function approximately 1 year earlier (Supplemental Figures 1 and 2).
Previously, a graded glucose infusion was used to demonstrate a delayed insulin secretory response to rising glucose concentrations in subjects with IGT implying a defect in β-cell secretion (29). Unfortunately, because insulin action was not measured, the appropriateness of the secretory response for the prevailing degree of insulin resistance could not be quantified. However, Festa et al (30) also reported that NFG/IGT is characterized by defects in insulin secretion and action, although in that cohort affected subjects were older and heavier than subjects with NGT—demographic differences that exhibit their own effects on insulin secretion and action. In contrast, Ferrannini et al (31) reported that compared to matched obese subjects with NGT, subjects with IGT are less insulin sensitive but exhibit similar β-cell secretory indices. Of note, in this relatively large cross-sectional study, insulin secretion and action declined in concert with rising fasting glucose concentrations and with 2-hour postchallenge glucose concentrations implying that, as in our current and prior studies (3), defects in both insulin secretion and action are required for abnormalities of glucose tolerance to develop. Unfortunately, as with the current study, this was a cross-sectional study and therefore cannot provide insight as to the temporal development of insulin secretion and action in the pathogenesis of IGT.
In conclusion, subjects with isolated NFG/IGT have defects in peripheral, but not hepatic, insulin action. Although insulin-induced stimulation of glucose disappearance is impaired in people with NFG/IGT, insulin-induced suppression of EGP, gluconeogenesis, and glycogenolysis is normal. This defect in extrahepatic insulin action is accompanied by an inability of β-cells to secrete sufficient insulin to overcome this defect, thereby causing glucose intolerance. These data suggest that agents that both enhance β-cell function and improve insulin-induced stimulation of glucose uptake will be of value for the prevention of NFG/IGT to overt type 2 diabetes.
Acknowledgments
This study was supported by funds from the Mayo Clinic General Clinical Research Center (UL1 TR000135) and the National Institutes of Health (DK78646). R.T.V. is supported by training grant 5T32DK007352–37 from the National Institutes of Health. Structural funding for the Center for Neurosciences and the UC-NMR facility is supported in part by FEDER–European Regional Development Fund through the COMPETE 2020 Programme and the Portuguese Foundation for Science and Technology through grants EXCL/DTP-PIC/0069/2012, UID/NEU/04539/2013, REEQ/481/QUI/2006, RECI/QEQ-QFI/0168/2012, CENTRO-07-CT62-FEDER-002012, and Rede Nacional de Ressonância Magnética Nuclear.
R.T.V. researched data and ran the studies. C.D.M. measured β-cell function. A.S. researched data and assisted with the studies. I.V. measured rates of gluconeogenesis. C.B. measured rates of gluconeogenesis. C.M. measured rates of gluconeogenesis. M.S. researched data and assisted with the studies. J.M.M. measured free fatty acid concentrations during induction of acute insulin resistance. R.A.R contributed to the discussion and reviewed/edited the manuscript, J.G.J. oversaw the measurement of gluconeogenesis, contributed to the discussion, and reviewed/edited the manuscript. C.C. oversaw the measurement of β-cell function, contributed to the discussion, and reviewed/edited the manuscript. A.V. designed the study, oversaw its conduct, researched data, and wrote the manuscript. A.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: A.V. is an investigator in multicenter studies sponsored by Novartis and GI Dynamics, respectively. He has consulted for XOMA, Sanofi-Aventis, Novartis, and Bristol-Myers Squibb in the past 5 years. None of the other authors have relevant disclosures.
Footnotes
- AAB
- area above basal
- EGP
- endogenous glucose production
- IFG
- impaired fasting glucose
- IGT
- impaired glucose tolerance
- NFG
- normal fasting glucose
- NGT
- normal glucose tolerance
- OGTT
- oral glucose tolerance test
- Rd
- rate of disappearance.
Reference
- 1. McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363:2339–2350. [DOI] [PubMed] [Google Scholar]
- 2. Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. J Pediatr. 2012;161:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sathananthan A, Dalla Man C, Zinsmeister AR, et al. A concerted decline in insulin secretion and action occurs across the spectrum of fasting and postchallenge glucose concentrations. Clin Endocrinol (Oxf). 2012;76:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dinneen SF, Maldonado D, 3rd, Leibson CL, et al. Effects of changing diagnostic criteria on the risk of developing diabetes. Diabetes Care. 1998;21:1408–1413. [DOI] [PubMed] [Google Scholar]
- 5. Meigs JB, Nathan DM, D'Agostino RB, Sr, Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845–1850. [DOI] [PubMed] [Google Scholar]
- 6. DECODE. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. [DOI] [PubMed] [Google Scholar]
- 7. Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014;63:1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bock G, Dalla Man C, Campioni M, et al. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55:3536–3549. [DOI] [PubMed] [Google Scholar]
- 9. Basu R, Di Camillo B, Toffolo G, et al. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E55–E69. [DOI] [PubMed] [Google Scholar]
- 10. Basu R, Barosa C, Jones J, et al. Pathogenesis of prediabetes: role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. J Clin Endocrinol Metab. 2013;98:E409–E417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah M, Varghese RT, Miles JM, et al. TCF7L2 genotype and α-cell function in humans without diabetes. Diabetes. 2016;65:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varghese RT, Viegas I, Barosa C, et al. Diabetes-associated variation in TCF7L2 is not associated with hepatic or extrahepatic insulin resistance. Diabetes 2016;65:887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab. 2004;287:E637–E643. [DOI] [PubMed] [Google Scholar]
- 14. Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of β-cell function and insulin sensitivity. Diabetes. 2001;50:150–158. [DOI] [PubMed] [Google Scholar]
- 15. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368–377. [DOI] [PubMed] [Google Scholar]
- 16. Cobelli C, Man CD, Sparacino G, Magni L, De Nicolao G, Kovatchev BP. Diabetes: models, signals, and control. IEEE Rev Biomed Eng. 2009;2:54–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dalla Man C, Bock G, Giesler PD, et al. Dipeptidyl peptidase-4 inhibition by vildagliptin and the effect on insulin secretion and action in response to meal ingestion in type 2 diabetes. Diabetes Care. 2009;32:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones JG, Barosa C, Gomes F, et al. NMR derivatives for quantification of 2H and 13C-enrichment of human glucuronide from metabolic tracers. J Carbohydr Chem. 2006;25:203–217. [Google Scholar]
- 19. Bock G, Schumann WC, Basu R, et al. Evidence that processes other than gluconeogenesis may influence the ratio of deuterium on the fifth and third carbons of glucose: implications for the use of 2H2O to measure gluconeogenesis in humans. Diabetes. 2008;57:50–55. [DOI] [PubMed] [Google Scholar]
- 20. Basu R, Barosa C, Basu A, et al. Transaldolase exchange and its effects on measurements of gluconeogenesis in humans. Am J Physiol Endocrinol Metab. 2011;300:E296–E303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187:15–24. [DOI] [PubMed] [Google Scholar]
- 22. Basu A, Alzaid A, Dinneen S, Caumo A, Cobelli C, Rizza RA. Effects of a change in the pattern of insulin delivery on carbohydrate tolerance in diabetic and nondiabetic humans in the presence of differing degrees of insulin resistance. J Clin Invest. 1996;97:2351–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol. 1999;277:E283–E290. [DOI] [PubMed] [Google Scholar]
- 24. Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:4053–4059. [DOI] [PubMed] [Google Scholar]
- 25. Bock G, Chittilapilly E, Basu R, et al. Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes. 2007;56:1703–1711. [DOI] [PubMed] [Google Scholar]
- 26. Laakso M, Zilinskaite J, Hansen T, et al. Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia. 2008;51:502–511. [DOI] [PubMed] [Google Scholar]
- 27. Vella A, Shah P, Reed AS, Adkins AS, Basu R, Rizza RA. Lack of effect of exendin-4 and glucagon-like peptide-1-(7,36)-amide on insulin action in non-diabetic humans. Diabetologia. 2002;45:1410–1415. [DOI] [PubMed] [Google Scholar]
- 28. Dalla Man C, Piccinini F, Basu R, Basu A, Rizza RA, Cobelli C. Modeling hepatic insulin sensitivity during a meal: validation against the euglycemic hyperinsulinemic clamp. Am J Physiol Endocrinol Metab. 2013;304:E819–E825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polonsky KS, Sturis J, Bell GI. Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulin-dependent diabetes mellitus - a genetically programmed failure of the β cell to compensate for insulin resistance. N Engl J Med. 1996;334:777–783. [DOI] [PubMed] [Google Scholar]
- 30. Festa A, D'Agostino R, Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM. Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes. 2004;53:1549–1555. [DOI] [PubMed] [Google Scholar]
- 31. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90:493–500. [DOI] [PubMed] [Google Scholar]