Introduction
It is estimated that between 2.2 and 3.2 million people are infected with hepatitis C virus (HCV) in the United States.1 For more than 20 years, the mainstay of HCV treatment has been interferon (IFN) alfa, a nonspecific immunomodulatory antiviral cytokine, and ribavirin (RBV). This regimen had limited efficacy and was poorly tolerated. Because of this, therapy was not widely deployed and the majority of patients with HCV infection remained untreated.2 In December 2013, sofosbuvir (SOF), an inhibitor of the HCV NS5B RNA polymerase, was approved by the U.S. Food and Drug Administration, and thus began an era of all-oral IFN-free HCV therapies. Currently approved direct-acting antiviral (DAA) therapies target 1 of 3 viral proteins, the NS5B polymerase, the NS3/4A protease, or the NS5A protein (Table 1). These agents profoundly inhibit viral replication and, when used in combination, can produce viral clearance without dependence on IFN.3
Table 1.
Currently FDA-approved direct-acting antiviral agents
| NS5B polymerase inhibitors | NS3/4A protease inhibitors | NS5A inhibitors |
|---|---|---|
| Sofosbuvir | Simeprevir | Daclatasvir |
| Dasabuvir | Paritaprevir Grazoprevir |
Ledipasvir Ombitasvir Elbasvir |
FDA, U.S Food and Drug Administration.
DAA regimens offer significantly increased efficacy, shorter duration of therapy, and dramatically improved side effect profiles. Real-world data demonstrate cure rates of more than 90% with combination therapy consisting of SOF and simeprevir for genotype 1 infections, which is similar to the results from the phase III clinical trials.4, 5, 6 The IFN-free DAA combinations of SOF plus simeprevir and SOF plus RBV are extremely well tolerated both in clinical trials and in real-world patient experiences, with the most common side effects being headache, fatigue, and nausea.6 Mild anemia was observed only in regimens containing RBV.
Autoimmune phenomena are well described sequela of IFN-based therapies for HCV infection7, 8, 9; IFN may aggravate preexisting autoimmunity, unmask previously silent autoimmune processes, or even cause the emergence of de novo autoimmune disease.8, 10 The mechanism is thought to be mediated by the effect of IFN on modulation of the host’s immune system or as a result of molecular mimicry.11 However, autoimmune syndromes are not currently described in patients receiving all-oral IFN-free DAA therapies. These regimens directly target viral replication and therefore have not been considered “immunomodulatory.” In this series we report on 3 cases of patients undergoing HCV therapy with SOF-based DAA therapy in whom a biopsy-proven lupus-like immune complex–mediated glomerulonephritis developed.
Case Presentation
Case 1
The first patient is a 51-year-old white female with a medical history significant for HCV genotype 3a infection complicated by cirrhosis with a Model for End-Stage Liver Disease (MELD) score of 10, Child-Pugh class A, who was referred for treatment of HCV infection (Figure 1a and Tables 2 and 3). Her baseline kidney function was normal; her serum creatinine level was 0.72 mg/dl, and her estimated glomerular filtration rate was >60 ml/min per 1.73 m2. Cirrhosis was diagnosed on the basis of a liver biopsy and previously failed attempts to eradicate HCV with pegylated IFN and RBV in 2002. The patient began antiviral therapy with SOF, 400 mg daily, and weight-based RBV (1000 mg daily) for a total of 24 weeks. Her other medications were spironolactone, 50 mg daily, and furosemide, 20 mg taken as needed for edema. During the course of antiviral therapy, she experienced mild nausea and diarrhea. Otherwise, no other adverse effects were noted. Twelve weeks after treatment she achieved sustained virological response with an undetectable viral load. Three months after completing treatment, she presented to her primary doctor with complaints of rash and pruritus of the arms, abdomen, and thighs. The rash was morbilliform with papules involving both thighs. There were no pustules, vesicles, or ulcerations noted on physical examination. Her liver function test results were elevated, with an alanine aminotransferase level of 1194 U/l, aspartate aminotransferase level of 1608 U/l, alkaline phosphatase level of 179 U/l, and total bilirubin of 6.0 U/l. She was admitted to the hospital and a liver biopsy was performed; it demonstrated panlobular hepatitis against a background of cirrhosis. The differential diagnosis of this histopathologic lesion included acute viral injury, immune-mediated drug reaction, and autoimmune hepatitis. Her serologic work-up results were abnormal, with a positive antinuclear antibody test result (a titer of 1:320 titer) and positive smooth muscle antibody test result (a titer of 1:80). The results of all available serological tests before and after treatment are shown in Table 3. Tests for hepatitis A virus, hepatitis B virus, hepatitis E virus, cytomegalovirus, Epstein–Barr virus and HCV RNA were performed and all the results were negative. The episode of acute hepatitis began to resolve spontaneously. While she was hospitalized, however, her serum creatinine level began to rise, with 3+ proteinuria detected by urinary dipstick. She had hematuria with dysmorphic red blood cells and cellular casts containing leukocytes and erythrocytes on microscopic examination of her urine.
Figure 1.
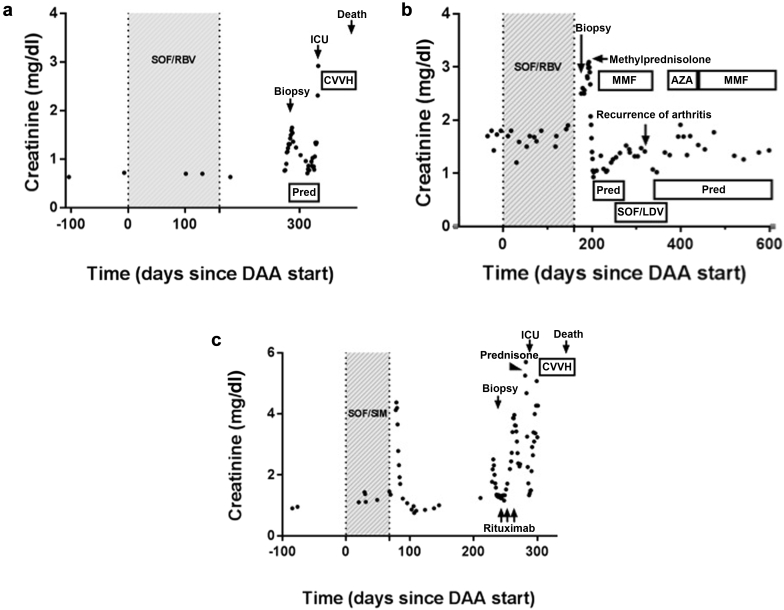
Creatinine trend over time in cases 1, 2, and 3. The clinical course of the first (a), second (b), and third (c) cases with immune complex–mediated glomerulonephritis are shown. The time during which the patient was receiving the initial course of direct-acting antiviral (DAA) is shown in shaded gray. AZA, azathioprine; CVVH, continuous venovenous hemofiltration; ICU, intensive care unit; LDV, ledipasvir; MMF, mycophenolate mofetil; Pred, prednisone; RBV, ribavirin; SIM, simeprevir; SOF, sofosbuvir.
Table 2.
Summary of cases of lupus-like immune complex glomerulonephritis
| Case | Patient characteristics | Clinical presentation | Immunosuppression | Outcome |
|---|---|---|---|---|
| 1 |
|
|
|
|
| 2 |
|
|
|
|
| 3 |
|
|
|
|
d, day(s); HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFNα, interferon alfa; LT, liver transplant; MMF, mycophenolate mofetil; PEG-IFN, pegylated interferon; mo, month(s); RBV, ribavirin; SVR12, sustained virological response at 12 weeks; wk, week(s).
Table 3.
Comparison of autoimmune serologic findings before and after DAA treatment
| Case | Baseline serologic findings | Post-DAA serologic findings |
|---|---|---|
| 1 | Cryoglobulin: negative Remainder not available |
ANA: 1:320 Ds-DNA: negative Anti-histone: ND Anti-RNP: negative Anti-Smith: negative Complement levels C3: 74 (low) C4: 14 Cryoglobulin: negative Rheumatoid factor: Negative Anti–smooth muscle Ab: 1:80 ANCA: Negative |
| 2 | ANA: 1:640 in 2007 Cryoglobulin: negative Rheumatoid factor: Positive in 2007 Remainder not available |
ANA: >1:5120 Ds-DNA: Positive 1:80 Anti-histone: >7.0 Anti-Ro/La: negative Anti-Smith: negative Complements levels C3: 54 C4: 9 (low) Cryoglobulin: negative Rheumatoid factor: negative |
| 3 | ANA: 1:160 Ds-DNA: negative Anti Ro/La: negative Anti-Smith: negative Cryoglobulin level: 5% Complement levels C3: 87 C4: 20 (normal) Rheumatoid factor: negative Remainder not available |
ANA: 1:160 Ds-DNA: Negative Anti-histone: 1.5 Units Anti Ro/La: positive Anti-Smith: Negative Cryoglobulin level: 1% Complement levels C3: 42 (low) C4: 7 (low) Rheumatoid factor: negative |
Ab, antibody; ANCA, antineutrophil cytoplasmic antibody; ANA, antinuclear antibody; DAA, direct-acting antiviral; Ds-DNA, double-stranded DNA; ND, not done; RNP, ribonucleoprotein.
Normal range for complements levels: C3, 81–157 mg/dl; C4, 12–39 mg/dl. Normal range for antihistone antibody, <1.0 U.
Her blood urea nitrogen level peaked at 59 mg/dl and her serum creatinine level peaked at 1.65 mg/dl. The results of cryocrit, double-stranded DNA, and rheumatoid factor tests were all negative. Her complement C3 level was 74 mg/dl (reference range 81–157 mg/dl), and her C4 level was 14 mg/dl (reference range 12–39 mg/dl).
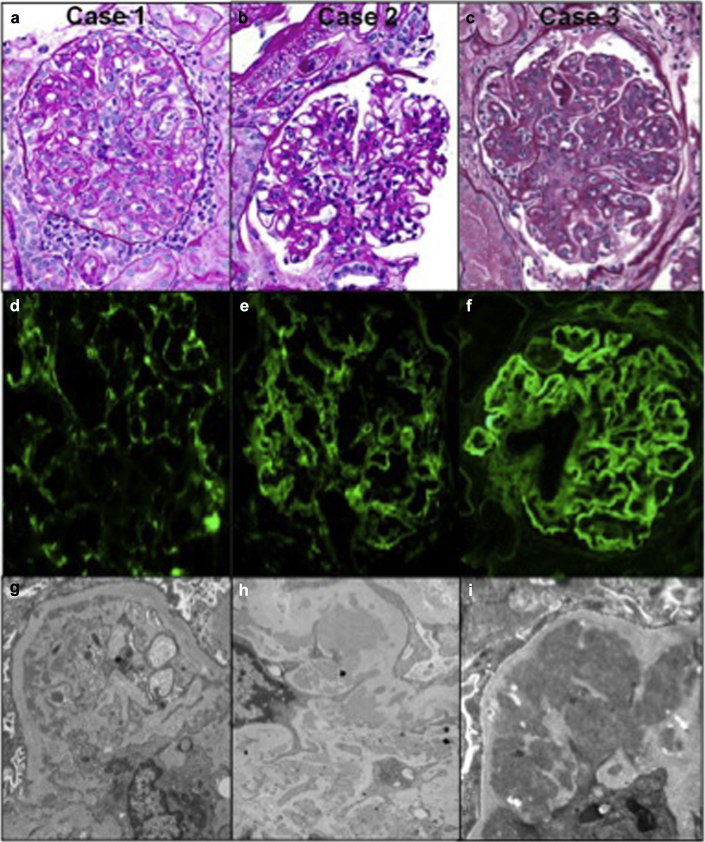
She underwent an ultrasound-guided renal biopsy that showed 19 nonsclerotic glomeruli, all showing marked endocapillary hypercellularity characterized by endothelial cell swelling, intracapillary neutrophils and macrophages, apoptotic debris, and mild mesangial hypercellularity. A single pseudothrombus was seen in 1 glomerulus. This was associated with moderate tubulointerstitial nephritis. Rare red cell casts were seen. Focal vasculitis was present. There was no glomerulosclerosis or chronic vascular disease. Interstitial fibrosis and tubular atrophy were minimal. Immunofluorescence showed granular mesangial and glomerular basement membrane (GBM) staining for IgG, IgA, IgM, C3, C1q, and kappa and lambda light chains. Immunoflourescent staining of the tubular basement membranes and vessels were negative. Many mesangial, paramesangial, and subendothelial electron-dense deposits without substructure were present. Tubuloreticular inclusions were seen. These features were consistent with an acute immune complex glomerulonephritis with a lupus-like pattern of immune complex deposition (Table 4 and Figure 2a, d, and g).
Table 4.
Summary of pathologic characteristics
| Parameter | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Light microscopy | |||
| Number of glomeruli | 19 | 9 | 33 |
| Global glomerulosclerosis | 0% | 67% | 9% |
| Segmental glomerulosclerosis | 0% | 0% | 0% |
| Endocapillary hypercellularity | +++ | ++ | +++ |
| Pseudothrombi | + | – | – |
| Mesangial hypercellularity | + | ++ | ++ |
| Segmental GBM duplication | + | + | +++ |
| Crescents | – | – | – |
| Fibrosis and tubular atrophy | <5% | ∼50% | 20% |
| Interstitial inflammation | + | + | ± |
| Tubular injury | ± | + | + |
| Vascular disease | ± | ± | ++ |
| Vasculitis | + hilar arteriole | – | – |
| Immunofluorescence microscopy results | |||
| Glomeruli | |||
| IgG | ++ | ++ | +++ |
| IgA | ++ | +++ | ++++ |
| IgM | + | ++ | ++ |
| C3 | +++ | ++ | +++ |
| C1q | ++ | +++ | ± |
| Fibrin | – | – | +++ |
| Kappa light chain | ++ | +++ | +++ |
| Lambda light chain | ++ | +++ | ++++ |
| Tubules | – | Focal, broad linear IgG and granular C3 | – |
| Interstitium | – | – | – |
| Vessels | – | focal C3 | focal C3 |
| Electron microscopy | |||
| Foot process effacement | segmental | global | segmental |
| Electron-dense deposits | mesangial, paramesangial, subendothelial | mesangial, paramesangial, subendothelial, intramembranous | mesangial, subendothelial, and intramembranous |
| Substructure | – | – | – |
| Tubuloreticular inclusions | + | +++ | – |
| GBM duplication | ± | + | +++ |
| Tubular basement membrane deposits | – | – | – |
GBM, glomerular basement membrane.
In case 3, ± staining for C1q indicates present but minimal staining.
Figure 2.
Light microscopy of the 3 cases of immune complex–mediated glomerulonephritis. The cases show varying degrees of glomerular activity from (a) global endocapillary hypercellularity with endothelial cell swelling, intracapillary mononuclear cells, neutrophils, and apoptotic debris (case 1) and (b) segmental endocapillary hypercellularity and moderate mesangial hypercellularity (case 2) to (c) global endocapillary and mesangial hypercellularity with double contours (case 3). A full house immunofluorescence pattern was present in all cases (case 3 had only minimal C1q). (d) Granular glomerular basement membrane (GBM) and segmental mesangial staining for C3 (case 1). (e) Granular mesangial and segmental GBM staining for IgG (case 2). (f) Global granular mesangial and GBM staining for IgG (case 3). Electron microscopy showing (g) subendothelial and mesangial electron-dense deposits and activated endothelium in a glomerular capillary loop (case 1) and (h,i) mesangial and paramesangial deposits (cases 2 and 3, respectively).
She began receiving on i.v. methylprednisolone, 500 mg daily for 3 days, and was then transitioned to prednisone 60 mg (1 mg/kg). This resulted in the normalization of her serum creatinine level (0.8 mg/dl) over the next 3 weeks. At the same time, however, she indicated that she had begun feeling confused and anxious; she was found to have a plasma ammonia level of 165 μmol/l and was admitted to the hospital for treatment of hepatic decompensation with lactulose and rifaximin, resulting in resolution of encephalopathy. During this hospitalization, ascites and a urinary tract infection were diagnosed, and the patient was subsequently treated with ceftriaxone and discharged to a rehabilitation facility. Over the next 4 days leukocytosis, hyponatremia, decreased urinary output, abdominal pain, and rising serum creatinine level (a rise from 0.77 mg/dl to 0.95 mg/dl) developed, and she was transferred back to the hospital. She was again given ceftriaxone for presumed urinary tract infection due to hematuria and leukocyturia, although urine cultures demonstrated no growth. During her hospital stay, she had persistent leukocytosis and a chest radiograph revealed a right lower lobe opacity for which she began treatment with i.v. vancomycin and piperacillin-tazobactam; prednisone was rapidly tapered. Her serum creatinine level began to rise and peaked at 1.3 mg/dl. Vancomycin was stopped on account of elevated trough values (37μg/ml [upper limit of normal 20 μg/ml]), and her creatinine level stabilized. She was discharged to a rehabilitation hospital. However, 48 hours later she was readmitted to the medical intensive care unit with hypoxic reparatory failure and septic shock due to pneumonia with empyema and oliguric acute kidney injury with a serum creatinine level of 2.34 mg/dl secondary to presumed acute tubular necrosis. She was intubated and placed on continuous venovenous hemofiltration on account of acidosis and worsening volume overload. Cultures from a bronchial alveolar lavage and of the pleural fluid revealed growth of Aspergillus fumigatus, and amphotericin was started; however, she died the following day.
Case 2
The second patient is a 56-year-old white female with HCV genotype 1b infection who had received a liver transplant because of hepatocellular carcinoma in 2009 (Figure 1b and Tables 2 and 3). Before receiving the transplant, she was treated with pegylated IFN and RBV with an initial response followed by a relapse after completion of therapy. Cirrhosis developed in the transplanted liver and was complicated by chronic ascites and hepatic encephalopathy that were controlled medically. Her Model for End-Stage Liver Disease score was 17, Child-Pugh class B. At baseline she had chronic kidney disease stage 3 with a serum creatinine ranging from 1.4 to 1.7 mg/dl and an estimated glomerular filtration rate of 31 to 38 ml/min per 1.73m2 without significant proteinuria, hematuria, or leukocyturia detected on pretreatment urinalysis. Her renal insufficiency had been attributed to chronic calcineurin use and decompensated cirrhosis (hepatorenal syndrome type II), although a biopsy had not been performed. She began therapy with SOF, 400 mg daily, and RBV, 200 mg twice daily (dose reduced for her renal dysfunction), for 24 weeks. Her other medications included allopurinol 100, mg twice daily; trimethoprim/sulfamethoxazole, single-strength tablet once daily; bumetanide, 2 mg twice daily; colchicine; 0.6 mg daily; cyclosporine, 25 mg twice daily; erythropoietin, 2000 units weekly; labetalol. 200 mg 3 times per day; lactulose, 30 ml 3 times per day; magnesium oxide, 800 mg twice daily; metolazone, 5 mg daily; omeprazole, 20 mg twice daily; potassium chloride extended release, 40 milliequivalents daily; and rifaximin, 550 mg twice per day.
Her viral load was undetectable by week 4 of treatment and remained undetected for the duration of therapy. Her serum creatinine fluctuated between 1.2 and 1.9 mg/dl throughout therapy. Her RBV was dose reduced to 200 mg once daily because of development of anemia. While receiving treatment she complained of new onset of joint pain involving her hands and wrists, decreased mobility, and worsening lower extremity edema and ascites. Two weeks after completing therapy, she was referred to a nephrologist when it was discovered that her serum creatinine level had risen to 2.6 mg/dl and her urinalysis showed leukocytosis and trace proteinuria on dipstick. Urine microscopy was notable for the presence of white blood cell casts. A serologic profile was notable for a positive antinuclear antibody test result with a titer >1:5120 and a histone antibody test result positive at >7.0 Units and double-stranded DNA antibody positive at a 1:10 titer. Her complement levels were low; her C3 level was 54 (reference range 81–157 mg/dl) and her C4 level was was 9 (reference range 12–39 mg/dl). Cryoglobulins were not detected in her serum. The results of all available serological tests before and after treatment are shown in Table 3.
She underwent a renal biopsy that showed mild endocapillary hypercellularity characterized by intracapillary mononuclear cells and swollen endothelial cells and moderate mesangial hypercellularity. Focal GBM duplication was present. There was moderate tubulointerstitial inflammation. Leukocyte and granular casts were seen. There was no vasculitis. Immunofluorescence showed granular mesangial and GBM staining for IgG, IgA, IgM, C3, C1q, and kappa and lambda light chains. Mesangial, paramesangial, subendothelial, and intramembranous electron-dense deposits without substructure were present. Many tubuloreticular inclusions were seen. These findings, along with the clinical features, supported an active immune complex–mediated glomerulonephritis with a lupus-like pattern of immune complex deposition. There was also a background of moderate glomerular and tubulointerstitial scarring, possibly from chronic injury from hepatorenal syndrome (Table 4 and Figure 2b, e, and h).
She was treated with methylprednisolone 250 mg i.v. for 3 days, followed by prednisone, 60 mg daily (1 mg/kg), and mycophenolate mofetil, 500 mg twice daily. Her renal function rapidly improved and serum creatinine decreased to 1.40 mg/dl within 3 days. Significant delirium and agitation developed, and the dose of prednisone was rapidly decreased to 20 mg daily and then tapered by 5 mg/wk. Because of the tubulointerstitial inflammation, trimethoprim/sulfamethoxazole and allopurinol were permanently discontinued. At time of discharge 1 week after kidney biopsy, the serum creatinine level improved to 1.0 mg/dl and then returned to 1.4 mg/dl once diuretics were reintroduced. Delirium resolved and the patient reported improvement in joint pain and increased mobility. Unfortunately, the HCV viral load became detectable 4 weeks after treatment, indicating relapse. Three months later, she began receiving on SOF, 400 mg, plus ledipasvir, 90 mg, fixed-dose combination therapy with the addition of RBV, 200 mg, every alternate day for 12 weeks. However, approximately 8 weeks into therapy, her joint pain relapsed with prominent synovitis in the bilateral wrists, metacarpal joints, and knees, and she required a course of prednisone therapy starting at 40 mg and tapered by 10 mg every 2 weeks. Mycophenolate mofetil was discontinued. Azathioprine was substituted as a steroid-sparing agent; however, her joint pain continued, so she was switched back to mycophenolate mofetil (Figure 2b). By 12 weeks after treatment, her arthritis improved and there was no evidence of relapse of glomerulonephritis. Her viral load was negative, indicating cure of HCV infection. She continues to receive mycophenolate mofetil, 500 mg twice daily, and low-dose prednisone that is slowly being tapered. She is without joint pain or worsening renal function.
Case 3
The third patient is a 48-year-old Hispanic female with a medical history of HCV genotype 1 infection and cirrhosis who was initiated on a 12-week course of DAA therapy with SOF, 400 mg ,and simeprevir, 150 mg daily (Figure 1c and Tables 2 and 3). She had contracted HCV infection at age 18 and her medical history included idiopathic thrombocytopenic purpura treated by splenectomy in her mid-20s and systemic lupus erythematous diagnosed in 1998 (at age 31) that had been precipitated by IFN alfa use for HCV infection. Lupus manifestations included hemolytic anemia; arthritis of the hands, knees, and ankles; and central nervous system manifestations, including headache, vertigo, and diplopia. Initially, she had multiple flares and had been treated with azathioprine, hydroxychloroquine, and prednisone until 2009. At that point, because of disease quiescence, prednisone and azathioprine were discontinued and she continued to receive hydroxychloroquine, 200 mg daily. She continued to have quiescent disease and did not experience a lupus flare between 2009 and when DAAs were started in 2014. A diagnosis of cirrhosis was made clinically on the basis of recent imaging showing a nodular liver. She was listed for a liver transplant and had a Model for End-Stage Liver Disease score of 11, Child-Pugh class B, before beginning DAA therapy. Her baseline serum creatinine level was 0.95 mg/dl, which corresponded to an estimated glomerular filtration rate of 67 ml/min per 1.73m2. Her other medications included aspirin, 81 mg daily, clonidine, 0.1 mg twice per day, folic acid, 1 mg daily, furosemide, 80 mg daily, hydroxychloroquine, 200 mg daily, spironolactone, 50 mg daily, and valsartan 320, mg daily.
Two weeks into DAA treatment a triangular dark red plaque on the upper chest with hemorrhagic small bulla on the inferior edge, and erythema and erosions around the nose and cheeks developed along with erythematous and edematous plaques on the lateral part of the wrists and thumbs after a brief exposure to the sun, and she was treated with topical steroids. During the fourth week of DAA treatment, she complained of several days of increased somnolence, fatigue, lower abdominal and back discomfort, and dark urine. Laboratory studies demonstrated a rise in serum creatinine level to 1.43 mg/dl and her urinalysis showed red blood cells, white blood cells, and hyaline casts. She was referred to the emergency department, where she was treated empirically with i.v. hydration and ciprofloxacin for presumed pyelonephritis, although the results of a urine culture were later negative. Her serum creatinine level decreased from 1.43 mg/dl to 1.11 mg/dl and she was discharged with a 10-day prescription of ciprofloxacin. Furosemide and spironolactone were temporarily discontinued. Four weeks later, after a total of 9 weeks of DAA therapy she again presented to the hepatologist with back pain and dark urine. Laboratory studies were notable for hyperbilirubinemia (5.4 mg/dl), prolonged prothrombin time (international normalized ratio 1.7), hypoalbuminemia (1.8 g/dl), and a serum creatinine level of 1.45 mg/dl. SOF and simeprevir were discontinued at this time and she was treated with 2 additional courses of antibiotics for possible urinary tract infection. Because of persistent elevation of her serum creatinine level, hematuria, and proteinuria, she underwent renal biopsy.
The renal biopsy showed enlarged, lobular glomeruli with marked endocapillary with intracapillary mononuclear cells and marked mesangial hypercellularity and expansion. The GBMs were thickened and showed double contours. There was no tubulointerstitial inflammation and no vasculitis. Immunofluorescence microscopy showed granular mesangial and GBMs staining for IgG, IgA, IgM, C3, C1q, kappa and lambda light chains, and fibrinogen. Numerous large and confluent subendothelial, mesangial, paramesangial and intramembranous electron dense deposits were present and did not show substructures. These findings were consistent with a severe active and chronic immune complex–mediated glomerulonephritis resembling a diffuse proliferative lupus nephritis (Table 4 and Figure 2c, f, and i).
Her serologic work-up results were notable for a positive antinuclear antibody test result at a titer of 1:160, her histone antibody level was 1.5 U, her cryoglobulin percentage was 1%, and she had low serum complement (C3 and C4) levels (Table 3). Serum HCV RNA was undetectable more than 3 months after completion of therapy, indicating that HCV had been eradicated despite the fact that her regimen had been shortened to 9 weeks. Three weeks after the renal biopsy, after completing the antibiotic course prescribed to treat a positive urine culture demonstrating Klebsiella infection, she was given a pulse dose of methylprednisolone, 250 mg i.v. for 3 days, and then transitioned to prednisone 60 mg daily (1 mg/kg) followed by weekly rituximab infusions for 3 doses. Her serum creatinine stabilized at 2.3 mg/dl and she was discharged.
However, 1 week later she presented to the hospital with lethargy and back pain and was admitted to the medical intensive care unit with septic shock from Serratia bacteremia with pancolitis seen on imaging. Oliguric acute kidney injury developed, and she required continuous venovenous hemofiltration. She had progressive respiratory failure from Stenotrophomonas pneumonia and required intubation. Her liver function worsened, disseminated intravascular coagulation developed, and she died on day 30 of hospitalization.
Discussion
Autoimmune phenomena are well-described complications of IFN-based therapies for HCV infection. Here we have described 3 cases of lupus-like immune complex glomerulonephritis with “full house” immunofluorescence in patients with cirrhosis who were treated with IFN-free all-oral DAA regimens. It is possible that this autoimmune phenomenon represents an immune reconstitution syndrome. In the first 2 cases, these patients had no known autoimmune history, nor suggestive symptoms before initiation of DAAs. Case 2 had a prior positive antinuclear antibody test result (Table 3) that was performed as a part of routine evaluation before liver transplantation and was not due to suggestive clinical symptoms. In the third case, DAA therapy rapidly aggravated the patient's preexisting IFN alfa–induced lupus that had been diagnosed 17 years prior. In this case, the timing of symptom onset and the fact that her disease had been clinically quiescent for more than 5 years suggest that clearance of her HCV aggravated preexisting autoimmunity. In this case C1q was minimal and tubuloreticular inclusions were not detected, in contrast to the majority of cases of lupus nephritis.12 Lupus nephritis is generally characterized by immune deposits that stain dominantly or codominantly for IgG. In this series, although IgG staining was strong, it was not dominant (Table 4). We note that IgA was codominant in case 2 and 3 and that this may be due to known association of mesangial IgA deposition in advanced liver disease.13, 14, 15
Two patients received SOF paired with RBV and 1 received SOF and simeprevir; the fact that this occurred with the use of agents that purely target steps in the HCV life cycle raises the possibility that an autoimmune diathesis was unmasked with removal of HCV, even in the absence of IFN or RBV use. It is tempting to speculate, given the known effects of chronic HCV on suppression of host IFN responses, that removal of viral antigen in otherwise predisposed patients may elicit sensitivity to endogenous IFN effects and resultant unmasking of autoimmunity.16, 17 Two of the 3 patients had antihistone antibody titers determined and in both cases this autoantibody was detected in the serum (Table 3). Although a drug-induced lupus may be an alternative explanation in these patients with presence of antihistone antibodies, in 1 of the cases with a positive antihistone antibody test result, systemic lupus erythematous had been a long-standing condition. However, drug-induced lupus is only rarely associated with glomerulonephritis. In case 2, the recurrence of severe joint pain during retreatment with SOF/ledipasvir and RBV strengthens the relationship between treatment and emergence of lupus-like symptoms. Finally, all patients had remote previous exposures to IFN; it is possible that a smoldering lupus syndrome existed but was not clinically detected; however, the dramatic emergence of symptomatic lupus with both renal and nonrenal manifestations in all cases (Table 2) argues for the relationship between IFN-free DAA treatment and disease.
Although the pattern of glomerular injury seen in lupus nephritis and mixed cryoglobulinemia can result in identical morphological changes, the lack of substructures in the deposits by electron microscopy in all cases, absence of large intracapillary deposits in the glomeruli (pseudothrombi), and negative HCV RNA test result at the time of biopsy for all cases favors a lupus-like nephritis over an HCV-associated immune complex disease. Furthermore, HCV-associated renal disease typically improves with HCV eradication.18, 19, 20, 21, 22
All 3 patients experienced rapid improvement in serum creatinine level with the initiation of high-dose (1 mg/kg) corticosteroids with or without the addition of other immunosuppressive agents (Figure 1a–c). All 3 experienced normalization of serum creatinine level within 1 to 3 weeks. Unfortunately, 2 of the 3 patients died of infectious complications while receiving high-dose steroids. The lone surviving patient had been rapidly tapered off steroids early in her course on account of delirium and was maintained on low-dose mycophenolate for 3 to 6 months, which suggests that this glomerular lesion may respond to a short course of steroids; it is unclear whether or not maintenance therapy is needed. The lone surviving patient continues to receive mycophenolate mofetil to manage joint pains and to prevent relapse of glomerulonephritis. Thus, a key take-away point from this series is that these patients, who each had baseline cirrhosis in addition to new-onset glomerulonephritis, are susceptible to adverse outcomes from immunosuppression. Recent evidence in the literature highlights the occurrence of severe infections after treatment with rituximab in patients with kidney disease.23, 24, 25 Thus, aggressive immunosuppressive therapy should be used in these cases with extreme caution, particularly if cirrhosis is present.
We have reported 3 cases of immune complex–mediated glomerulonephritis with full house immunofluorescence occurring in patients being treated for HCV with novel all-oral direct-acting antiviral therapies who presented with joint pain or rash in addition to hematuria, pyuria, and rising creatinine level. This phenomenon was not reported in phase II/III studies and appears to have affected a very small minority of the patients treated in our health care system. Although this possible reaction is rare, it is important that HCV providers and nephrologists be aware of it and consider kidney biopsy in any patient with worsening renal function, proteinuria, or development of active urine sediment during or after treatment with DAAs. Further study is needed to determine the mechanism of autoimmunity in patients treated with IFN-free regimens.
Disclosures
MES received a research grant from Gilead Sciences and is an Abbvie advisory board member. ALL is a consultant to Genzyme. RIT is a Merck advisory board member. RTC has received research grant support from Gilead, Merck, Abbvie, BMS, Janssen, and Mass Biologics. All the other authors declared no competing interests.
Acknowledgments
RTC was supported by a National Institutes of Health grant (DK078772). JAH was supported by an American Association for the Study of Liver Diseases grant, a National Institutes of Health Opportunity Fund Grant AI082630, and a National Health and Medical Research Council Early Career Fellowship. RIT was supported by a National Institutes of Health grant (DK094872-04).
References
- 1.Denniston M.M., Jiles R.B., Drobeniuc J. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gogela N.A., Lin M.V., Wisocky J.L., Chung R.T. Enhancing our understanding of current therapies for hepatitis C virus (HCV) Curr HIV/AIDS Rep. 2015;12:68–78. doi: 10.1007/s11904-014-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feeney E.R., Chung R.T. Antiviral treatment of hepatitis C. BMJ. 2014;348:g3308. doi: 10.1136/bmj.g3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulkowski M.S., Vargas H.E., Di Bisceglie A.M. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology. 2016;150:419–429. doi: 10.1053/j.gastro.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modi A.A., Nazario H., Trotter J.F. Safety and efficacy of simeprevirplus sofosbuvir with or without ribavirin in patients with decompensated genotype 1 hepatitis C cirrhosis. Liver Transpl. 2016;22:281–286. doi: 10.1002/lt.24324. [DOI] [PubMed] [Google Scholar]
- 6.Aqel B.A., Pungpapong S., Leise M. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 in patients with cirrhosis. Hepatology. 2015;62:1004–1012. doi: 10.1002/hep.27937. [DOI] [PubMed] [Google Scholar]
- 7.Markowitz G.S., Nasr S.H., Stokes M.B., D'Agati V.D. Treatment with IFN-{alpha}, -{beta}, or -{gamma} is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:607–615. doi: 10.2215/CJN.07311009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronnblom L.E., Alm G.V., Oberg K.E. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med. 1991;115:178–183. doi: 10.7326/0003-4819-115-3-178. [DOI] [PubMed] [Google Scholar]
- 9.Wilson L.E., Widman D., Dikman S.H., Gorevic P.D. Autoimmune disease complicating antiviral therapy for hepatitis C virus infection. Semin Arthritis Rheum. 2002;32:163–173. doi: 10.1053/sarh.2002.37277. [DOI] [PubMed] [Google Scholar]
- 10.Ho V., McLean A., Terry S. Severe systemic lupus erythematosus induced by antiviral treatment for hepatitis C. J Clin Rheumatol. 2008;14:166–168. doi: 10.1097/RHU.0b013e3181775e80. [DOI] [PubMed] [Google Scholar]
- 11.Gregorio G.V., Choudhuri K., Ma Y. Mimicry between the hepatitis C virus polyprotein and antigenic targets of nuclear and smooth muscle antibodies in chronic hepatitis C virus infection. Clin Exp Immunol. 2003;133:404–413. doi: 10.1046/j.1365-2249.2003.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennette J.C., Iskandar S.S., Dalldorf F.G. Pathologic differentiation between lupus and nonlupus membranous glomerulopathy. Kidney Int. 1983;24:377–385. doi: 10.1038/ki.1983.170. [DOI] [PubMed] [Google Scholar]
- 13.Terzi A., Ozdemir B.H., Taslica F.Z., Ozdemir F.N., Kirnap M., Haberal M. Clinicopathologic study of kidney biopsies in patients before or after liver transplant. Exp Clin Transplant. 2014;12(suppl 1):129–135. [PubMed] [Google Scholar]
- 14.McGuire B.M., Julian B.A., Bynon J.S., Jr. Brief communication: glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med. 2006;144:735–741. doi: 10.7326/0003-4819-144-10-200605160-00007. [DOI] [PubMed] [Google Scholar]
- 15.Pouria S., Feehally J. Glomerular IgA deposition in liver disease. Nephrol Dial Transplant. 1999;14:2279–2282. doi: 10.1093/ndt/14.10.2279. [DOI] [PubMed] [Google Scholar]
- 16.Tsuge M., Fujimoto Y., Hiraga N. Hepatitis C virus infection suppresses the interferon response in the liver of the human hepatocyte chimeric mouse. PloS One. 2011;6:e23856. doi: 10.1371/journal.pone.0023856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe T., Kaname Y., Hamamoto I. Hepatitis C virus nonstructural protein 5A modulates the toll-like receptor-MyD88-dependent signaling pathway in macrophage cell lines. J Virol. 2007;81:8953–8966. doi: 10.1128/JVI.00649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sise M.E., Bloom A.K., Wisocky J. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with sofosbuvir-based direct-acting antiviral agents. Hepatology. 2016;63:408–417. doi: 10.1002/hep.28297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gragnani L., Fognani E., Piluso A. Long-term effect of HCV eradication in patients with mixed cryoglobulinemia: a prospective, controlled, open-label, cohort study. Hepatology. 2014;61:1145–1163. doi: 10.1002/hep.27623. [DOI] [PubMed] [Google Scholar]
- 20.Johnson R.J., Gretch D.R., Yamabe H. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328:465–470. doi: 10.1056/NEJM199302183280703. [DOI] [PubMed] [Google Scholar]
- 21.Rossi P., Bertani T., Baio P. Hepatitis C virus-related cryoglobulinemic glomerulonephritis: long-term remission after antiviral therapy. Kidney Int. 2003;63:2236–2241. doi: 10.1046/j.1523-1755.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 22.Alric L., Plaisier E., Thebault S. Influence of antiviral therapy in hepatitis C virus-associated cryoglobulinemic MPGN. Am J Kidney Dis. 2004;43:617–623. doi: 10.1053/j.ajkd.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Fabrizi F., Cresseri D., Fogazzi G.B. Rituximab therapy for primary glomerulonephritis: report on two cases. World J Clin Cases. 2015;3:736–742. doi: 10.12998/wjcc.v3.i8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besada E., Koldingsnes W., Nossent J.C. Long-term efficacy and safety of pre-emptive maintenance therapy with rituximab in granulomatosis with polyangiitis: results from a single centre. Rheumatology (Oxford) 2013;52:2041–2047. doi: 10.1093/rheumatology/ket257. [DOI] [PubMed] [Google Scholar]
- 25.Roberts D.M., Jones R.B., Smith R.M. Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun. 2015;57:60–65. doi: 10.1016/j.jaut.2014.11.009. [DOI] [PubMed] [Google Scholar]