Abstract
Human migration has been identified as a potential factor for increased Chagas disease risk and has transformed the disease from a Latin American problem to a global one. We conducted a systematic review of the scientific literature between 2004–2014 in order to: summarize current seroprevalence estimates of Chagas disease among Latin American migrants, in both endemic and non-endemic settings; compare seroprevalence estimates in migrants to countrywide prevalence estimates; and identify risk factors for Chagas disease among migrants. A total of 320 studies were screened and 23 studies were included. We found evidence that the prevalence of Chagas disease is higher than expected in some migrant groups and that reliance on blood donor screening prevalence underestimates the burden of disease. Overall there is a dearth of high quality epidemiologic studies on the prevalence of Chagas in migrants, especially among intra-regional migrants within Latin America. Given that the disease cannot likely be eradicated, improved surveillance and reporting is vital to continuing control efforts. More accurate health surveillance of both Latin American migrants and the Chagas disease burden will help countries appropriately scale up their response to this chronic disease. Overall, improved estimates of Chagas disease among migrants would likely serve to highlight the real need for better screening, diagnostics, and treatment of individuals living with the disease.
Keywords: Chagas disease, American trypanosomiasis, migration, immigration, neglected tropical disease, systematic review
Graphical abstract
1. Introduction
One of the current challenges in combating Chagas disease (American trypanosomiasis) is that human migration is changing the distribution of disease in both endemic and non-endemic countries (Gascon et al., 2010; Pinazo and Gascon, 2015). This shift is so great that Chagas disease is now co-classified as both a re-emerging infection and a neglected tropical disease (Hotez et al., 2008; Mackey and Liang, 2012; Mackey et al., 2014; World Health Organization, 2013). Human migration represents both a risk for the re-emergence of new infections in countries with the vector and for the expansion of the geographical distribution of chronic Chagas cases to non-endemic countries.
Caused by the protozoan parasite Trypanosoma cruzi (T. cruzi), Chagas disease results in the largest burden of disease in disability-adjusted-life-years of any parasitic disease in the Americas (Lee et al., 2013; World Health Organization, 2012). Depending on the region, 20–30% of patients chronically infected with Chagas disease go on to develop cardiac and/or gastrointestinal damage and an estimated 10,000 people will die from Chagas each year (World Health Organization, 2010). The morbidity and mortality associated with Chagas disease results in a staggering annual global economic burden of US$7.2 billion (Lee et al., 2013).
The main mode of transmission of T. cruzi to humans is vector borne, which occurs only in the Americas. Traditionally considered a disease of poverty, risk of Chagas disease has been associated with housing in rural areas, of poor construction quality (e.g., palm roof, cracks in the walls), and with domestic pets and livestock in or near the house (Enger et al., 2004; Molina-Garza et al., 2014; Ramsey et al., 2005; World Health Organization, 2002). Coordinated efforts by endemic countries in the 1990’s were instrumental in shrinking the domestic vector infestation and thus the population at risk for Chagas disease (Coura and Dias; Dias and Schofield, 1999; Dias et al., 2002).
Despite the success of the spraying campaigns, the disease persists through its alternate transmission mechanisms, primarily congenital and blood transfusion, and to a lesser extent oral ingestion (Alarcon de Noya et al., 2010; Carlier et al., 2015; Coura, 2015; Rassi Jr et al., 2010). Multiple transmission mechanisms, coupled with the chronic, often asymptomatic nature of the disease and lack of effective and accessible treatment for patients means the burden of disease continues to remain high (Pan American Health Organization, 2014).
Within Latin America, migration into urban areas and an increase in urban poverty has transformed this once rural disease to an urban disease as well (Briceno-Leon and Galvan, 2007). There are an estimated 127 million people living below the poverty line in urban and peri-urban communities in Latin America (Ault, 2007) and sub-standard housing conditions within these urban settings have facilitated the domiciliation of triatomines (Gürtler, 2009; Levy et al., 2006; Medrano-Mercado et al., 2008). A 2013 review of qualitative research on socio-cultural aspects of Chagas disease found that changes in land use may both drive human migration and provide new homes for the vector (Ventura-Garcia et al., 2013). For example, in Peru it was found that human settlement patterns created shantytowns with favorable conditions for triatomines and seasonal agricultural workers may have carried the vector to the communities through their work (Bayer et al., 2009). Agricultural workers may be at greater personal risk through exposure to the vector during outdoor labor (Ventura-Garcia et al., 2013; World Health Organization, 2010).
Migration has also changed the distribution of Chagas disease from a health problem only in Latin America, to a global one. As of 2013 there were an estimated 36.7 million people who had migrated out of Latin America and the Caribbean and were residing elsewhere in the world, predominantly in North America (United Nations Department of Economic and Social Affairs, 2013b).
Generally, migrants are at greater risk of infectious diseases because of the existing poverty driving them to migrate and the social and economic inequalities they often face once relocating (Cabieses et al., 2013; Carballo and Nerukar, 2001). A 2013 review of qualitative research uncovered migration as a socio-structural risk factor for Chagas disease (Ventura-Garcia et al., 2013). After migrating, structural barriers to diagnosis and treatment include cost, language, lack of insurance, fear of deportation (in the case of undocumented migrants), and stigma against migrants (Bayer et al., 2009; Jackson et al., 2012; Minneman et al., 2012; Ventura-Garcia et al., 2013).
Current prevalence estimates of Chagas disease in non-endemic countries are largely extrapolations of countrywide prevalence from endemic birth countries multiplied by the proportion of immigrants from that country (Basile et al., 2011; Bern and Montgomery, 2009; Gascon et al., 2010; Schmunis, 2007). While country prevalence estimates provide a good starting point for estimating the burden among migrant populations, there are some substantial drawbacks. First, regional variations in migration rates and Chagas disease burden may be masked by the use of a single prevalence estimate for an endemic country. Secondly, these estimates do not take into account characteristics specific to migrants, who tend to be demographically different from the average person in any given country of origin (Weeks, 2015). Migrants often come from rural areas, have a different age profile (i.e., are younger), and are of a different socioeconomic status than the general population (Bern and Montgomery, 2009).
Determining accurate prevalence estimates of Chagas disease among migrants may enable health systems to more precisely gauge the true burden of disease and target populations most at risk. Further, given the heterogeneity of Chagas disease distribution within countries, there remain questions as to whether migrants are at heightened personal risk for this disease.
Therefore the purpose of this review was to: summarize current seroprevalence estimates of Chagas disease among Latin American migrants, in both endemic and non-endemic settings and compare seroprevalence estimates in migrants to countrywide prevalence estimates. We also report on risk factors for Chagas disease among migrants.
2. Methods
We conducted a systematic review of the literature from January 2004 to July 2014 using the PubMed and Scopus databases. We also searched relevant grey literature from international and governmental organizations, including the Pan American Health Organization (PAHO), World Health Organization (WHO), and the U.S. Centers for Disease Control and Prevention. Search terms included combinations of the following keywords: 1) Chagas, American trypanosomiasis, or Trypanosoma cruzi; 2) migra*, mobil*, immigra*, or non-endemic; 3) epidemi*, prevalence, or seroprevalence. Searches were not restricted by language. Reference lists of selected articles were examined for additional citations.
Articles were included in the present analysis if they met the following criteria: (i) original data on the prevalence of Chagas disease among human migrants in endemic or non-endemic countries. “Migrants” were defined as individuals living in a country different than that of their birth or who moved from an area of endemicity to one without vector transmission. Articles were excluded if they: (i) had obvious selection bias (i.e., only included participants with known Chagas disease, or sampled tropical disease hospital or cardiac patients) (ii) did not have full text available (iii) did not include migration status of participants.
First, all duplicates and papers published prior to 2004 were removed. Next, titles and abstracts were screened for relevancy and papers that were off-topic, not original research (e.g., review articles, guidelines, case studies), and those that were not epidemiological studies on humans (e.g., drug development, vector studies). The full text for each of the remaining articles was screened again for inclusion/exclusion criteria.
The following data were abstracted for the included studies, if available: first author, year of publication, country of study, study design, study setting, population of interest, mean/median age of participants, number of migrants tested, prevalence, dates of data collection, and screening tests used.
2.1. Comparison Data
We took a two pronged approach to exploring whether migrants have a higher prevalence of Chagas disease than the general population. First, we compared whether current countrywide prevalence estimates for non-endemic countries were similar to studies of migrants living within non-endemic countries.
According to the UN, the top 10 destination countries for Latin American migrants are: the United States, Spain, Italy, Canada, Japan, Portugal, France, the United Kingdom, China, and Australia.(United Nations Department of Economic and Social Affairs, 2013a) Major destinations from the highest Chagas disease prevalence sending countries (Bolivia, Argentina, El Salvador, Honduras, and Paraguay) are the United States, Argentina, and Spain.(United Nations Department of Economic and Social Affairs, 2013a)
We used three sources for non-endemic countrywide prevalence estimates. Gascon, Bern & Pinazo (2010), Bern and Montgomery (2009), and Basile et al. (2011) were based on PAHO prevalence estimates for endemic countries and documented and undocumented immigrant populations by country of origin (Basile et al., 2011; Bern and Montgomery, 2009; Gascon et al., 2010). These three sources estimated countrywide prevalence for the top 10 Latin American migrant receiving countries (except for China), plus Belgium, Germany, the Netherlands, and Switzerland. Additional countries were reported in this analysis only if migrant-specific estimates in that country were found in the reviewed literature.
The second comparison was made by stratifying migrant seroprevalence estimates from the reviewed articles by their endemic country of origin, and comparing each to the countrywide prevalence estimates in endemic countries. Here we refer to endemic countries as any country within Central or South America and Mexico. Endemic countrywide prevalence estimates were from 2006 PAHO estimates, which was regarded as the best source of prevalence estimates during the time period most reviewed articles were conducted (Pan American Health Organization, 2006).
When authors did not directly report prevalence among migrants, but sufficient information was provided, prevalence was calculated and reported. To facilitate a descriptive comparison of prevalence estimates, we calculated 95% confidence intervals for each study using the Wilson interval method (Brown et al., 2001; Wilson, 1927). Study prevalence estimates within this range were considered to be similar to countrywide estimates, those outside the interval were classified as being above or below. For ease of comparison, forest charts were generated using Microsoft Excel 2013 for countries with more than five prevalence estimates.
3. Results
3.1. Description of Included and Excluded Studies
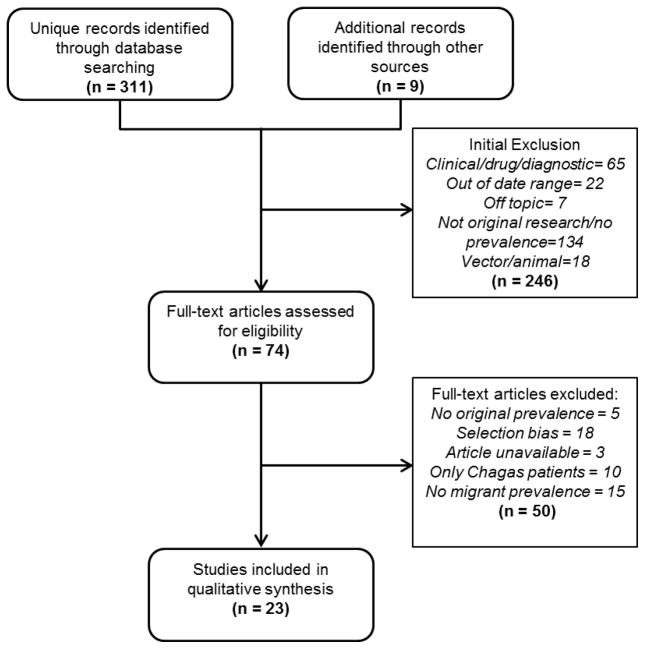
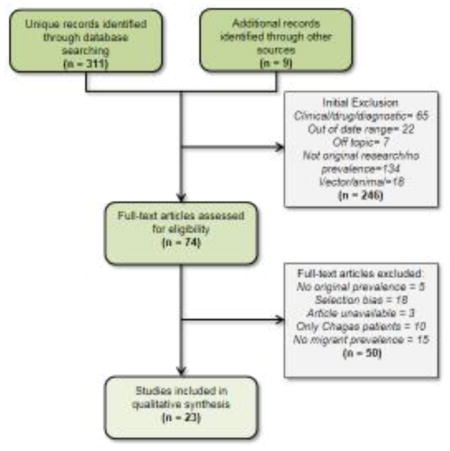
After duplicates were removed, the initial search identified a total of 311 original articles and an additional 9 references were identified through other sources. Of these records, 246 did not meet initial inclusion criteria (Figure 1). A total of 74 articles were included for full text review and 23 met the inclusion criteria for the final analysis. Among the excluded articles: 5 did not contain original prevalence estimates; 18 had selection bias (i.e., tropical disease hospital sample or cardiac patients); 3 did not have a full-text article available; 10 contained only Chagas disease patients; and 15 did not report prevalence among migrants. A full list of considered articles is available upon request.
Figure 1.
Flow chart of study selection process
All included studies were cross-sectional: 11 were among pregnant women, 5 were among blood donors, and 7 were among other populations of Latin American migrants (Table 1). Study size ranged from 72 to 31,615 participants. WHO guidelines recommend the use of ELISA for blood-bank screening and any current serological test for epidemiological surveys (World Health Organization, 2002). Two tests are recommended if the sensitivity of the test used is low and laboratory conditions are not ideal. Studies used a variety of serological tests, but the majority used ELISA. All but two studies (Jackson et al., 2009; Santiago et al., 2012) used at least one confirmatory test.
Table 1.
Summary of included studies between 2004–2014 reporting prevalence of Chagas disease among migrant populations
| Author (year) |
Country | Site | Population | Age of sample |
Migrants (N) |
Preval ence |
Diagnostic Tests | ||
|---|---|---|---|---|---|---|---|---|---|
| Dates | Initial | Confirm | |||||||
| Spain | |||||||||
| Avila-Arzanegui (2013) | Spain (Biscay) | Hospital, prenatal visit | Pregnant women born in LA | Mean 28.5 (+/−5.3) | 158 | 12.0 | 2008–2010 | IFA | ELISA (2) |
| Barona-Vilar (2012) | Spain (Valencia) | Primary care centers | Pregnant women born in LA | -- | 1,975 | 11.4 | 2009–2010 | ELISA (2) | IFA |
| Gimenez-Marti (2006) | Spain (Valencia) | Hospital microbiology lab* | Random sample serum from South American immigrants | -- | 432 | 3.7 | 2001 | ELISA | IP & IFA |
| Lucas (2009) | Spain (Valencia) | Hospital, gynecology visit | Pregnant women born in LA | Mean 25.9 (+/−5) | 383 | 9.7 | 2005–2007 | IP | IIF & ELISA |
| Munoz (2009) | Spain (Barcelona) | Hospital, maternity clinics | Pregnant women born in LA | Mean 31 (range: 18–43) | 1350 | 3.4 | 2005–2007 | ELISA (2) | ELISA & PCR |
| Munoz-Vilches (2012) | Spain (Almería) | Hospital, obstetrics visit | Pregnant women born in LA | -- | 261 | 1.5 | 2007–2011 | ELISA & IFA | none |
| Paricio-Talayero (2008) | Spain (Valencia) | Hospital, birth | Pregnant women born in LA | Mean 28.3 (SD: 5.8) | 624 | 4.6 | 2005–2007 | IP | IFI & PCR |
| Piron (2008) | Spain (Catalonia) | Blood bank | Blood donors born in LA | Mean 35 (SD: 10.7) | 1524 | 0.7 | 2005–2006 | PaGIA & ELISA | ELISA (2) |
| Ramos (2012a) | Spain (Elche) | Hospital, fetal pathophysiology unit | Pregnant women born in LA | Median 28.9 (range:16–45) | 545 | 1.3 | 2006–2010 | ELISA & IIF (x2) | PCR |
| Ramos (2012b) | Spain (Elche) | Community | Paraguayan and Bolivian immigrants | Median 30 | 73a 128b |
9.6a 4.7b |
2006–2010 | IP & ELISA | ELISA (2) & PCR |
| Roca (2011) | Spain (Barcelona) | Health center | LA patients >14yo | Without CD: 36.4 (SD:12.2) With: 39.86 (9.88) |
766 | 2.9 | 2007–2009 | ICT | ELISA (2) |
| Santiago (2012) | Spain (Madrid) | Tertiary hospital* | Pregnant women born in LA | -- | 265 | 4.9 | 2007–2008 | ChLIA | none |
| Soriano (2009) | Spain (Barcelona) | Health center, pediatric department | LA women 15–45yo | Mean 30 (range:14–45) | 116* | 4.3 | 2006–2007 | ICT | ELISA (2) |
| Other European Countries | |||||||||
| El Ghouzzi (2010) | France | Blood bank | Blood donors born in LA | -- | 972* | 0.3 | 2007–2008 | ELISA & bioELISA (x2) | IFA |
| Jackson (2010) | Switzerland (Geneva) | Primary care facility or 2 Latino churches | LA migrants (excluded <16yo, pregnant women) | Mean 37.2 (SD=11.3) | 1,012 | 12.8 | 2008 | ELISA (2) (x2) | ELISA & IFA external check |
| Jackson (2009) | Switzerland (Geneva) | Prenatal care visits* | Undocumented pregnant LA women | Median 30 (range 20–43) | 72 | 9.7 | 2006 | IFA | none |
| Lescure (2009) | France (Ile-de-France) | South American community | Volunteers | Median 33 (11–63) | 254 | 20.1 | 2008–2009 | IFA | ELISA (2–3x) & |
| Martinez de Tejada (2009) | Switzerland (Geneva) | Hospital, obstetrics visit | Pregnant women born in LA | Mean 30.4 (SD 5.7) | 305 | 2.0 | 2008 | IF | IF |
| Americas | |||||||||
| Custer (2012) | United States | BSI blood donation centers* | Blood donors born in LA | -- | 31,615 | 0.2 | 2007–2009 | ELISA (x2) | RIPA |
| Edwards (2014) | United States | Hospital, birth | LA pregnant women | -- | 3,090 | 0.3 | 2011–2012 | ELISA | IB & IFA |
| O’Brien (2013) | Canada | Canadian Blood Services* | Blood donors born in LA | -- | 3,675 | 0.3 | 2010–2011 | ChLIA | IB, PCR & RIPA |
| Steele (2007) | Canada | Community, clinics | LA refugees and immigrants | -- | 102 | 1.0 | -- | EIA (x2) | IFA |
| Luitgards-Moura (2005) | Brazil (Roraima) | Community and State blood bank* | Roraima agricultural settlers or blood donors | -- | 1,239 | 1.6 | 2000–2001 1997–2001 |
IIF or ELISA/IHA | IIF & ELISA |
Retrospective
Abbreviations: ChLIA: chemiluminescent immunoassay; EIA: enzyme immunoassay; ELISA: enzyme-linked immunosorbent assay; IC: immunochromatographic assay; IB: immunoblot; IF: immunofluorescence; IFA: indirect fluorescent antibody test; IHA: indirect hemagglutination; IIF: indirect immunofluorescence; IP: Immunopreciptation; LA: Latin American; PaGIA: particle gel immunoassay; PCR: polymerase chain reaction; RIPA: radioimmunoprecipitation; WB: western blot; (x2): test repeated if reactive or inconclusive; (2): two tests were run simultaneously
Bolivian subset
Paraguayan subset
3.2. Prevalence of Chagas Disease: Migrant vs. Non-Endemic Countrywide Prevalence Estimates
There were prevalence rates of Chagas disease reported for migrants from Chagas-endemic countries in Canada, France, Spain, Switzerland and the United States; of these 58% were similar (fell within the 95% confidence interval) to PAHO estimates and 28% were below. Although Australia, Japan, Italy, Portugal, and the United Kingdom are part of the top 10 destination countries for Latin American migrants, we did not find any qualifying studies from these countries.
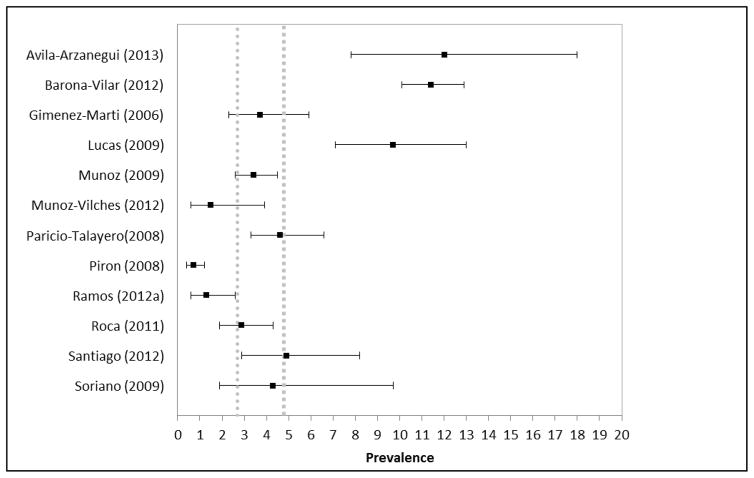
Within Spain, 7 of the 12 studies with prevalence estimates of Chagas disease in migrants were similar to the highest countrywide estimate (Del Pino and Coll, 2006; Giménez-Martí et al., 2006; Munoz-Vilches et al., 2012; Paricio-Talayero et al., 2008; Roca et al., 2011; Santiago et al., 2012; Soriano Arandes et al., 2009), two were lower(Piron et al., 2008; Ramos et al., 2012a), and three(Avila Arzanegui et al., 2013; Barona-Vilar et al., 2012; Lucas and Barba, 2009) were higher (Figure 2). One additional study was conducted in Spain, but its results are not summarized here because it was a non-representative sample of only Bolivian and Paraguayan participants (Ramos et al., 2012b). Most (85%) of the studies in Spain were hospital or clinic-based and among women who were pregnant or of childbearing age.
Figure 2.
Forest plot of prevalence estimates of Chagas disease among migrants in Spain from systematic review articles compared to estimates of countrywide prevalence (dashed grey lines indicate the range of countrywide prevalence estimates for Spain)
Studies in Canada (O’Brien et al., 2013), France (El Ghouzzi et al., 2010), and the United States (Custer et al., 2012; Edwards et al., 2013) all reported prevalence estimates among their migrant sample that were lower than PAHO estimates (Table 2) (Basile et al., 2011; Bern and Montgomery, 2009; Gascon et al., 2010). Three out of 4 of these studies were based on samples of blood donors (Custer et al., 2012; El Ghouzzi et al., 2010; O’Brien et al., 2013) and one was a sample of pregnant women from Latin America (Edwards et al., 2013). One other study in Canada (Steele et al., 2007) used a sample of community clinics and had a prevalence similar to the PAHO based estimate. One study in France (Lescure et al., 2008) based on study volunteers from South American communities was above PAHO estimates. Two studies of clinics in Geneva, Switzerland found prevalence rates among migrants higher than that of the countrywide estimates(Jackson et al., 2009) and one found rates that were similar (Martinez de Tejada et al., 2009).
Table 2.
Prevalence estimates of Chagas disease among migrants from systematic review articles compared to estimates of countrywide prevalence, by country of origin (endemic) or country of destination (non-endemic)
| Country | Study | N | Migrant Study Prevalence | Calculated Migrant Study 95% C.I. | Countrywide Prevalence | Migrant vs. Countrywide Prevalencea |
|---|---|---|---|---|---|---|
| Endemic countries | ||||||
| Argentina | Barona-Vilar (2012a) | 74 | 5.3 | 2.1 – 12.9 | 4.13b | ≃ |
| Munoz-Vilches (2012) | 56 | 1.8 | 0.3 – 9.4 | ≃ | ||
| Gimenez-Marti (2006) | 8 | 12.5 | 2.2 – 47.1 | ≃ | ||
| Piron (2008) | 298 | 0.7 | 0.2–2.4 | Below | ||
| Bolivia | See figure 3 | See figure 3 | See figure 3 | See figure 3 | 6.75 b | See figure 3 |
| Brazil | Luitgards-Moura (2005) | 1,239 | 1.6 | 1.0 – 2.5 | 1.02b | ≃ |
| Avila-Arzanegui (2013) | 7 | 14.3 | 2.6–51.3 | Above | ||
| Chile | Lucas (2009) | 8 | 12.5 | 2.2 – 47.1 | 0.99b | Above |
| Colombia | Lucas (2009) | 63 | 4.8 | 1.6 – 13.1 | 0.96b | Above |
| Patricio-Talayero (2008) | 131 | 1.5 | 0.4 – 5.4 | ≃ | ||
| Gimenez-Marti (2006) | 185 | 0.5 | 0.1 – 3.0 | ≃ | ||
| Ecuador | Barona-Vilar (2012a) | 571 | 0.2 | 0 – 1.0 | 1.74b | Below |
| Lucas (2009) | 118 | 1.7 | 0.5 – 6.0 | ≃ | ||
| Paricio-Talayero (2008) | 195 | 1.5 | 0.5 – 4.4 | ≃ | ||
| Gimenez-Marti (2006) | 185 | 0.5 | 0.1 – 3.0 | ≃ | ||
| Piron (2008) | 223 | 0.5 | 0.1–2.5 | ≃ | ||
| El Salvador | El Ghouzzi (2010) | 14 | 14.3 | 4.0 – 39.9 | 3.37b | Above |
| Honduras | Barona-Vilar (2012a) | 55 | 3.6 | 1.0 – 12.3 | 3.05b | ≃ |
| Mexico | Custer (2012) | 26,541 | 0.12 | 0.1 – 0.2 | 1.03b | Below |
| Nicaragua | Lucas (2009) | 5 | 20.0 | 3.6 – 62.4 | 1.14b | Above |
| Paraguay | See figure 4 | See figure 4 | See figure 4 | See figure 4 | 2.54b | See figure 4 |
| Lucas (2009) | 6 | 16.7 | 3.0 – 56.4 | Above | ||
| Ramos (2012b) | 128 | 4.6 | 2.2 – 9.8 | ≃ | ||
| Ramons (2012a) | 46 | 6.5 | 2.2 – 17.5 | ≃ | ||
| Piron (2008) | 15 | 6.7 | 1.2 – 29.8 | ≃ | ||
| Avila-Arzanegui (2013) | 21 | 9.5 | 2.7–28.9 | Above | ||
| Peru | Lucas (2009) | 10 | 10.0 | 1.8 – 40.4 | 0.69b | Above |
| Munoz-Vilches (2012) | 44 | 2.3 | 0.4 – 11.8 | ≃ | ||
| Non-endemic countries | ||||||
| Australia | n/a | n/a | n/a | n/a | 1.7c | n/a |
| Canada | O’Brien (2013) | 3,675 | 0.3 | 0.2 – 0.5 | 1.8c | Below |
| Steele (2007) | 102 | 1.0 | 0.2 – 5.4 | ≃ | ||
| France | El Ghouzzi (2010) | 972 | 0.31 | 0.1 – 0.9 | 1.3 – 1.7d | Below |
| Lescure (2008) | 254 | 20.1 | 15.6 – 25.4 | Above | ||
| Japan | n/a | n/a | n/a | 0.97c | n/a | |
| Italy | n/a | n/a | n/a | 1.7 – 3.1d | n/a | |
| Portugal | n/a | n/a | n/a | 1.0d | n/a | |
| Spain | See figure 2 | See figure 2 | See figure 2 | See figure 2 | 2.7 – 4.8d | See figure 2 |
| Switzerland | Jackson (2010) | 1,012 | 12.8 | 10.9 – 15.1 | 2 – 4.8d | Above |
| Jackson (2009) | 72 | 9.7 | 4.8 – 18.7 | ≃/Above | ||
| Martinez de Tejada (2009) | 305 | 2.0 | 1.0 – 4.2 | ≃ | ||
| United Kingdom | n/a | n/a | n/a | n/a | 1.3 – 2.4d | n/a |
| United States | Custer (2012) | 31,615 | 0.17 | 0.1 – 0.2 | 1.3 – 1.4c,e | Below |
| Edwards (2013) | 3,090 | 0.32 | 0.2 – 0.6 | Below | ||
Migrant prevalence was classified as the same as countrywide prevalence if it fell within the countrywide confidence intervals
Estimates come from Pan American Health Organization, 2006
Estimates come from Gascon, 2010
Estimates come from Basile, 2011
Estimates come from Bern, 2009
n/a= not available
3.3. Prevalence of Chagas Disease: Migrant vs. Endemic Countrywide Prevalence Estimates
We compared reviewed literature on prevalence rates of Chagas disease among Latin American migrants stratified by birth country to countrywide prevalence estimates in these endemic countries. Overall, there were a total of 43 prevalence rates of Chagas disease by endemic country; 44% were above endemic countrywide prevalence estimates and 49% were similar.
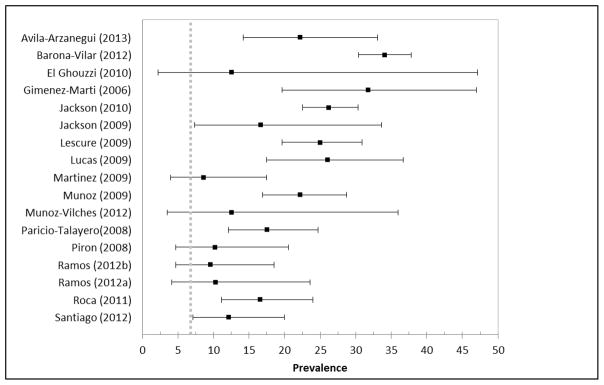
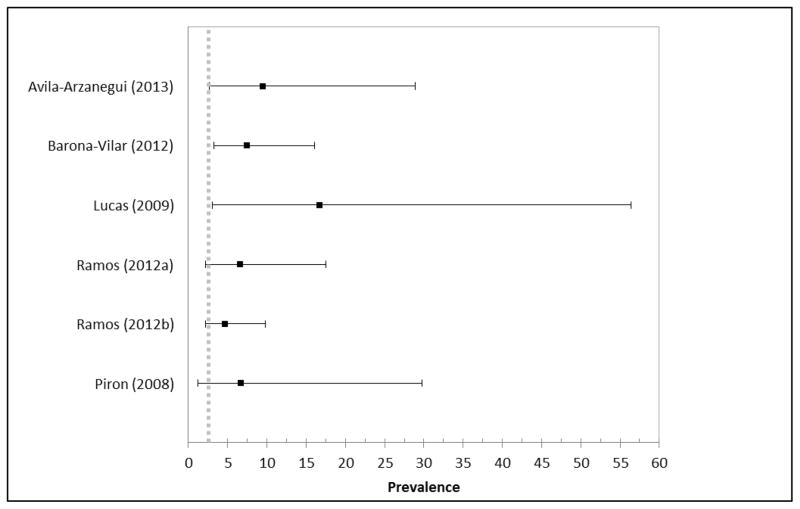
Seventeen of the studies had prevalence estimates specifically among Bolivian migrants (12 from Spain, 3 from Switzerland, and 2 from France) (Figure 3). Of these studies, 11 reported higher prevalence estimates as compared to PAHO (Barona-Vilar et al., 2012; Jackson et al., 2010; Jackson et al., 2009; Lucas and Barba, 2009; Paricio-Talayero et al., 2008; Roca et al., 2011) and 6 had similar estimates (El Ghouzzi et al., 2010; Ramos et al., 2012a; Ramos et al., 2012b). For Paraguayan migrants, 3 studies had similar prevalence estimates to countrywide estimates (Ramos et al., 2012a; Ramos et al., 2012b), while 3 were higher (Figure 4) (Avila Arzanegui et al., 2013; Barona-Vilar et al., 2012; Lucas and Barba, 2009). However, it should be noted that one sample that was higher was only based on 6 individuals, and thus was very imprecise (95% CI: 3.0–56.4) (Lucas and Barba, 2009). Prevalence estimates were also availabile for Argentina, Brazil, Chile, Colombia, Ecuador, El Salvador, Honduras, Mexico, Nicaragua, and Peru (Table 2).
Figure 3.
Forest plot of prevalence estimates of Chagas disease among migrants from Bolivia from systematic review articles compared to estimates of countrywide prevalence (dashed grey lines indicate the countrywide prevalence estimate for Bolivia)
Figure 4.
Forest plot of prevalence estimates of Chagas disease among migrants from Paraguay from systematic review articles compared to estimates of countrywide prevalence (dashed grey lines indicate the countrywide prevalence estimate for Paraguay)
3.4. Intraregional Migration Prevalence
We only found one study among migrants within Latin America. It compared the seroprevalence of Chagas disease in migrants to locals in a primarily enzoonotic area of Brazil. The prevalence among agricultural settlers born in the state was 0.9, compared to 1.6 in migrants (Luitgards-Moura et al., 2005). This migrant prevalence was not significantly different from the PAHO countrywide estimates of 1.02 (Pan American Health Organization, 2006).
3.5. Risk Factors for Chagas Disease Among Migrants
Of the 23 studies, 8 conducted significance testing for risk factors of T. cruzi among migrants. Older age was significantly (p<0.05) associated with Chagas disease in two studies (Jackson et al., 2010; Ramos et al., 2012b). Contrary to most Chagas literature, younger age was associated with Chagas disease in 1 study(Lucas and Barba, 2009) and had no association in another (Roca et al., 2011). Other variables found to be significantly associated with Chagas disease included: living in a rural area (Custer et al., 2012; Muñoz et al., 2009; Roca et al., 2011), living in a mud/adobe house (Avila Arzanegui et al., 2013; Custer et al., 2012; Muñoz et al., 2009; Roca et al., 2011), hearing about Chagas disease (Roca et al., 2011), knowing someone with Chagas disease (Roca et al., 2011), having lived in a place with a thatched roof (Custer et al., 2012), having a mother or grandmother born in an endemic country (Custer et al., 2012), having a mother or family member who has had Chagas disease (Jackson et al., 2010; Lescure et al., 2009; Lucas and Barba, 2009; Ramos et al., 2012b), being bitten by a triatomine (Custer et al., 2012), or knowledge of the vector (Avila Arzanegui et al., 2013).
Having had a previous transfusion was only found to have a positive association with Chagas disease in one Swiss study (Jackson et al., 2010). No studies found an association between disease status and gender.
Descriptively, three other studies found higher percentages of infected migrants than non-infected migrants living in mud or adobe houses or a rural area, but they did no formal significance testing (Piron et al., 2007; Ramos et al., 2012b; Roca et al., 2011). A sample of migrants in Switzerland found that only 8% of Latin American migrants had ever previously been tested for Chagas disease.(Jackson et al., 2010) Finally, within a sample of migrants from Paraguay and Bolivia living in Spain, 40% lacked knowledge about Chagas disease (Ramos et al., 2012b).
4. Discussion
An estimated 94–96% of migrants living with Chagas disease in non-endemic areas are unaware of their disease (Basile et al., 2011). Underestimating the burden of disease among Latin American migrants likely means a continued lack of priority for a neglected disease and migrant health generally.
We found a trend that the prevalence of Chagas disease in migrant groups, when stratified by birth country, was generally higher than endemic countrywide estimates of prevalence. In non-endemic countries, the trend was that prevalence estimates based on blood donors were lower than PAHO estimates of Chagas disease and those based on hospital or clinic samples were the same or higher.
Because the prevalence of Chagas disease in non-endemic countries is inherently dependent on the prevalence in migrant groups from endemic countries, we would have expected similar patterns in trends. One explanation for the discrepancy is that blood donation centers are not representative of the population as a whole. A study of characteristics of US blood donors found that Hispanics (vs. non-Hispanic whites) and individuals born outside the United States (vs. native-born) were less likely to donate blood (Gillum et al., 2008). Additionally, undocumented migrants or migrants who have Chagas disease and are aware of their status or risk may self-select not to donate blood. Thus the reliance on blood donor data in generating non-endemic countrywide estimates is likely underrepresenting the burden of disease among migrants (Sedyaningsih-Mamahit et al., 2004). This is in line with findings of a recent review of Chagas disease in Europe (Requena-Mendez et al., 2015).
Studies of disease prevalence in interregional migrants were sparse, with only one study uncovered. While we found two additional studies conducted in non-endemic areas of Bolivia and Northern Mexico, they did not capture the migration history of the participants and therefore were excluded (Brutus et al., 2008; Galavíz-Silva et al., 2009). Given the potential risk of transmission from rural to peri-urban or urban areas, this dearth of data is concerning (Bayer et al., 2009; Pinazo and Gascon, 2015).
Risk factors for Chagas disease were similar to those published in studies of non-migrants and included living in mud or adobe housing, living in a rural area, having a family member with Chagas disease, or knowing of either the vector or disease. It is hypothesized that the burden of Chagas disease is higher among migrants than the general population in endemic countries, however we did not identify any studies directly testing for risk factors of Chagas disease between migrants and non-migrants.
Past qualitative research suggested migrants would have less access to healthcare and lower health knowledge than non-migrants and descriptive statistics in studies from this literature review supported that finding. However, the difference between access to care and knowledge among migrants and non-migrants was not formally tested in any study. Additionally, none of the studies quantitatively reported on migrant occupation or socioeconomic status, however one study did anecdotally note that most of their sample travel from endemic zones to non-endemic zones for seasonal work (Brutus et al., 2008). Given that Latin American migrants are a highly heterogeneous group, well designed epidemiologic studies controlling for age, location and other confounding factors are needed to determine if migrants are at increased risk of contracting the disease.
A major limitation of this review is that there was high heterogeneity in prevalence estimates by the type of study. Most of the studies utilized convenience samples and therefore the proportions of migrants in the studies were likely not representative of the country as a whole. Thus this review was limited to a more descriptive summary of the literature.
This literature review was also subject to publication bias, which might favor studies that show an association between migration and Chagas disease. Finally, although we restricted our sample to exclude studies that recruited among tropical infectious disease or cardiac patients, we did allow for participants recruited from general hospitals or clinics. This may have overestimated the prevalence of Chagas disease if symptoms prompted patients to come in to their provider. However, because chronic Chagas disease is often asymptomatic for long periods and because the primary group sampled was pregnant women, this bias was likely minimal.
Despite the limitations, the results of this review raise some recommendations for future studies. First, there is a clear need for more accurate epidemiologic data on migrants. In receiving communities, the more complete capture of place of origin (i.e., state or region) for Latin American migrants is suggested. This would serve the dual purpose of strengthening estimates of disease in the receiving country as well as informing control efforts in regions of the sending country. In many studies, even country of origin was not captured and individuals were only identified as being from “Latin America”. Establishment of better measures for tracking migrant health are informative to other conditions beyond Chagas disease and reflect a prioritization of understanding and caring for migrant communities (Cabieses et al., 2013).
Secondly, we believe the global response to Chagas disease is not proportional to the burden of disease, especially in non-endemic countries. Studies in non-endemic countries have found that knowledge of Chagas disease is limited in both providers (Stimpert and Montgomery, 2010; Verani et al., 2010) and patients (Minneman et al., 2012; Pérez de Ayala Balzola et al., 2009; Sanchez et al., 2014). Supplying more accurate estimates of the burden of disease among migrants will help countries appropriately scale up their response to the disease. A recent economic evaluation of Chagas disease screening programs in Spain found that a no-screening strategy was the most costly course of action (Imaz-Iglesia et al., 2015). Tension between the belief that this disease has been controlled through the vector spraying campaigns and the reality of individuals who are asymptomatic and chronically infected mean it may not get the attention it deserves in non-endemic countries. Without improved education on Chagas disease, especially in the fields of obstetrics-gynecology and cardiology, patients will remain unaware of their status.
Lastly, the ascertainment of accurate estimates of Chagas disease in migrants is especially important in areas with the potential for vector-borne transmission. Differences in prevalence of Chagas disease in non-endemic countries may be due to differences in country of origin, regional differences within countries, as well as factors related to migration (e.g., poor housing conditions, outdoor occupations). Determining where these differences lie have implications for establishing the true burden of disease in receiving communities, as well as establishing if interventions targeted towards helping migrants within an endemic country are necessary. Ideally, region-specific surveillance data are needed in addition to studies comparing prevalence of migrants to that of individuals remaining in sending communities. Given that the disease cannot likely be eradicated, improved surveillance and reporting is vital to continuing control efforts in areas with the potential for transmission.
5. Conclusions
Our findings from this literature review, along with recent work by others to establish more accurate prevalence estimates in Europe, are that Chagas disease estimates among migrants are generally poor (Requena-Mendez et al., 2015). No longer only a disease of Latin America, control of Chagas disease now requires the joint collaboration of endemic and non-endemic countries.
Despite challenges, the benefit of better estimates and surveillance are manifold and include improved distribution of scarce public health resources to combat Chagas disease, finding cases at earlier stages in order to prevent long-term sequelae or secondary transmission, and providing data vital to the control of this disease. Improved estimates of Chagas disease among migrants would likely serve to highlight the real need for better screening, diagnostics, and treatment of individuals living with the disease.
Highlights.
Human migration has transformed Chagas disease into a global problem
We led a systematic review of prevalence estimates of Chagas disease among migrants
Improved surveillance and epidemiologic studies among migrants are needed
Acknowledgments
Erin Conners was supported by a UC MEXUS dissertation research grant titled “The Potential Role of Migration in Chagas Disease Expansion” and the University of California, San Diego, Center for AIDS Research (CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcon de Noya B, Diaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Zavala-Jaspe R, Suarez JA, Abate T, Naranjo L, Paiva M, Rivas L, Castro J, Marques J, Mendoza I, Acquatella H, Torres J, Noya O. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. The Journal of infectious diseases. 2010;201:1308–1315. doi: 10.1086/651608. [DOI] [PubMed] [Google Scholar]
- Ault SK. Pan American Health Organization’s Regional Strategic Framework for addressing neglected diseases in neglected populations in Latin America and the Caribbean. Mem Inst Oswaldo Cruz. 2007;102:99–107. doi: 10.1590/s0074-02762007005000094. [DOI] [PubMed] [Google Scholar]
- Avila Arzanegui O, Liendo Arenaza P, Martinez Indart L, Martinez Astorkiza T, Pocheville Guruceta MI, Egurbide Arberas MV. Prevalence of Trypanosoma cruzi infection and vertical transmission in Latin-American pregnant women in a health area of Biscay. Enfermedades Infecciosas y Microbiologia Clinica. 2013;31:210–216. doi: 10.1016/j.eimc.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Barona-Vilar C, Giménez-Martí MJ, Fraile T, González-Steinbauer C, Parada C, Gil-Brusola A, Bravo D, Gómez MD, Navarro D, Perez-Tamarit A, Fernandez-Silveira L, Fullana-Montoro A, Borrás R. Prevalence of Trypanosoma cruzi infection in pregnant Latin American women and congenital transmission rate in a non-endemic area: The experience of the Valencian Health Programme (Spain) Epidemiology and Infection. 2012;140:1896–1903. doi: 10.1017/S0950268811002482. [DOI] [PubMed] [Google Scholar]
- Basile L, Jansa JM, Carlier Y, Salamanca DD, Angheben A, Bartoloni A, Seixas J, Van Gool T, Canavate C, Flores-Chavez M, Jackson Y, Chiodini PL, Albajar-Vinas P. Chagas disease in European countries: the challenge of a surveillance system. Euro Surveill. 2011:16. [PubMed] [Google Scholar]
- Bayer AM, Hunter GC, Gilman RH, Del Carpio JGC, Naquira C, Bern C, Levy MZ. Chagas disease, migration and community settlement patterns in Arequipa, Peru. PLoS Neglected Tropical Diseases. 2009:3. doi: 10.1371/journal.pntd.0000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C, Montgomery SP. An estimate of the burden of chagas disease in the United States. Clinical Infectious Diseases. 2009;49:e52–e54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- Briceno-Leon R, Galvan JM. The social determinants of Chagas disease and the transformations of Latin America. Memorias Do Instituto Oswaldo Cruz. 2007;102:109–112. doi: 10.1590/s0074-02762007005000095. [DOI] [PubMed] [Google Scholar]
- Brown LD, Cai TT, DasGupta A, Agresti A, Coull BA, Casella G, Corcoran C, Mehta C, Ghosh M, Santner TJ, Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion - Comment - Rejoinder. Stat Sci. 2001;16:101–133. [Google Scholar]
- Brutus L, Schneider D, Postigo J, Romero M, Santalla J, Chippaux JP. Congenital Chagas disease: Diagnostic and clinical aspects in an area without vectorial transmission, Bermejo, Bolivia. Acta Tropica. 2008;106:195–199. doi: 10.1016/j.actatropica.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Cabieses B, Tunstall H, Pickett KE, Gideon J. Changing patterns of migration in Latin America: how can research develop intelligence for public health? Rev Panam Salud Publica. 2013;34:68–74. [PubMed] [Google Scholar]
- Carballo M, Nerukar A. Migration, refugees, and health risks. Emerg Infect Dis. 2001;7:556–560. doi: 10.3201/eid0707.017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier Y, Sosa-Estani S, Luquetti AO, Buekens P. Congenital Chagas disease: an update. Mem Inst Oswaldo Cruz. 2015;110:363–368. doi: 10.1590/0074-02760140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura JR. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions--a comprehensive review. Mem Inst Oswaldo Cruz. 2015;110:277–282. doi: 10.1590/0074-0276140362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura JR, Dias JCP. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem Inst Oswaldo Cruz. 104:31–40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- Custer B, Agapova M, Bruhn R, Cusick R, Kamel H, Tomasulo P, Biswas H, Tobler L, Lee TH, Caglioti S, Busch M. Epidemiologic and laboratory findings from 3 years of testing United States blood donors for Trypanosoma cruzi. Transfusion. 2012;52:1901–1911. doi: 10.1111/j.1537-2995.2012.03569.x. [DOI] [PubMed] [Google Scholar]
- Del Pino M, Coll O. Chagas’ disease, foetal maternal transmision and experience in our hospital. Enfermedades Emergentes. 2006;8:37–39. [Google Scholar]
- Dias J, Schofield C. The evolution of Chagas disease (American trypanosomiasis) control after 90 years since Carlos Chagas discovery. Mem Inst Oswaldo Cruz. 1999;94(Suppl 1):103–121. doi: 10.1590/S0074-02761999000700011. [DOI] [PubMed] [Google Scholar]
- Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- Edwards MS, Rench MA, Todd CW, Czaicki N, Steurer FJ, Bern C, Montgomery SP. Perinatal Screening for Chagas Disease in Southern Texas. Journal of the Pediatric Infectious Diseases Society. 2013 doi: 10.1093/jpids/pit056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ghouzzi MH, Boiret E, Wind F, Brochard C, Fittere S, Paris L, Mazier D, Sansonetti N, Bierling P. Testing blood donors for Chagas disease in the Paris area, France: First results after 18 months of screening. Transfusion. 2010;50:575–583. doi: 10.1111/j.1537-2995.2009.02476.x. [DOI] [PubMed] [Google Scholar]
- Enger KS, Ordoñez R, Wilson ML, Ramsey JM. Evaluation of risk factors for rural infestation by Triatoma pallidipennis (Hemiptera: Triatominae), a Mexican vector of Chagas disease. J Med Entomol. 2004;41:760–767. doi: 10.1603/0022-2585-41.4.760. [DOI] [PubMed] [Google Scholar]
- Galavíz-Silva L, Molina-Garza DP, González-Santos MA, Mercado-Hernández R, González-Galavíz JR, Rosales-Encina JL, Molina-Garza ZJ. Update on seroprevalence of anti-Trypanosoma cruzi antibodies among blood donors in northeast Mexico. American Journal of Tropical Medicine and Hygiene. 2009;81:404–406. [PubMed] [Google Scholar]
- Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010;115:22–27. doi: 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Gillum F, Eder AF, McLaurin-Jones TL. Hispanic ethnicity, race and blood donation in the United States. Transfus Med. 2008;18:366–370. doi: 10.1111/j.1365-3148.2008.00894.x. [DOI] [PubMed] [Google Scholar]
- Giménez-Martí MJ, Gil-Brusola A, Gómez MD, Pemán J, Gobernado M. Prevalence of Trypanosoma cruzi antibodies in immigrants living in Valencia (Spain) Enfermedades Emergentes. 2006;8:189–193. [Google Scholar]
- Gürtler RE. Sustainability of vector control strategies in the Gran Chaco Region: Current challenges and possible approaches. Memorias do Instituto Oswaldo Cruz. 2009;104:52–59. doi: 10.1590/s0074-02762009000900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS neglected tropical diseases. 2008;2:e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaz-Iglesia I, Garcia-San Miguel L, Ayala-Morillas LE, Garcia-Perez L, Gonzalez-Enriquez J, Blasco-Hernandez T, Martin-Agueda MB, Sarria-Santamera A. Economic evaluation of Chagas disease screening in Spain. Acta Tropica. 2015;148:77–88. doi: 10.1016/j.actatropica.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Jackson Y, Castillo S, Hammond P, Besson M, Brawand-Bron A, Urzola D, Gaspoz JM, Chappuis F. Metabolic, mental health, behavioural and socioeconomic characteristics of migrants with Chagas disease in a non-endemic country. Tropical Medicine and International Health. 2012;17:595–603. doi: 10.1111/j.1365-3156.2012.02965.x. [DOI] [PubMed] [Google Scholar]
- Jackson Y, Gétaz L, Wolff H, Holst M, Mauris A, Tardin A, Sztajzel J, Besse V, Loutan L, Gaspoz JM, Jannin J, Vinas PA, Luquetti A, Chappuis F. Prevalence, clinical staging and risk for blood-borne transmission of chagas disease among latin American migrants in Geneva, Switzerland. PLoS Neglected Tropical Diseases. 2010:4. doi: 10.1371/journal.pntd.0000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Y, Myers C, Diana A, Marti HP, Wolff H, Chappuis F, Loutan L, Gervaix A. Congenital transmission of chagas disease in Latin American immigrants in Switzerland. Emerging Infectious Diseases. 2009;15:601–603. doi: 10.3201/eid1504.080438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. Global economic burden of Chagas disease: a computational simulation model. The Lancet Infectious diseases. 2013;13:342–348. doi: 10.1016/S1473-3099(13)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure FX, Canestri A, Melliez H, Jaureguiberry S, Develoux M, Dorent R, Guiard-Schmid JB, Bonnard P, Ajana F, Rolla V, Carlier Y, Gay F, Elghouzzi MH, Danis M, Pialoux G. Chagas disease, France. Emerg Infect Dis. 2008;14:644–646. doi: 10.3201/eid1404.070489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure FX, Paris L, Elghouzzi MH, Le Loup G, Develoux M, Touafek F, Mazier D, Pialoux G. Experience of targeted screening of Chagas disease in Île-de-France. Bull Soc Pathol Exot. 2009;102:295–299. [PubMed] [Google Scholar]
- Levy MZ, Bowman NM, Kawai V, Waller LA, Del Carpio JGC, Benzaquen EC, Gilman RH, Bern C. Periurban Trypanosoma cruzi-infected Triatoma infestans, Arequipa, Peru. Emerging infectious diseases. 2006;12:1345–1352. doi: 10.3201/eid1209.051662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RMO, Barba MCP. Prevalence of American tripanosomiasis in pregnant women from a health area of Valencia, Spain. 2005–2007. Revista Espanola de Salud Publica. 2009;83:543–555. [PubMed] [Google Scholar]
- Luitgards-Moura JF, Borges-Pereira J, Costa J, Zauza PL, Rosa-Freitas MG. On the possibility of autochthonous chagas disease in Roraima, Amazon Region, Brazil, 2000–2001. Revista do Instituto de Medicina Tropical de Sao Paulo. 2005;47:45–54. doi: 10.1590/s0036-46652005000100008. [DOI] [PubMed] [Google Scholar]
- Mackey TK, Liang BA. Threats from emerging and re-emerging neglected tropical diseases (NTDs) Infection ecology & epidemiology. 2012:2. doi: 10.3402/iee.v2i0.18667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey TK, Liang BA, Cuomo R, Hafen R, Brouwer KC, Lee DE. Emerging and reemerging neglected tropical diseases: a review of key characteristics, risk factors, and the policy and innovation environment. Clinical microbiology reviews. 2014;27:949–979. doi: 10.1128/CMR.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez de Tejada B, Jackson Y, Paccolat C, Irion O. Congenital Chagas disease in Geneva: diagnostic and clinical aspects. Revue medicale suisse. 2009;5:2091–2092. 2094–2096. [PubMed] [Google Scholar]
- Medrano-Mercado N, Ugarte-Fernandez R, Butrón V, Uber-Busek S, Guerra HL, De Araújo-Jorge TC, Correa-Oliveira R. Urban transmission of Chagas disease in Cochabamba, Bolivia. Memorias do Instituto Oswaldo Cruz. 2008;103:423–430. doi: 10.1590/s0074-02762008000500003. [DOI] [PubMed] [Google Scholar]
- Minneman RM, Hennink MM, Nicholls A, Salek SS, Palomeque FS, Khawja A, Albor LC, Pennock CC, Leon JS. Barriers to Testing and Treatment for Chagas Disease among Latino Immigrants in Georgia. J Parasitol Res. 2012;2012:295034. doi: 10.1155/2012/295034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Garza ZJ, Rosales-Encina JL, Mercado-Hernandez R, Molina-Garza DP, Gomez-Flores R, Galaviz-Silva L. Association of Trypanosoma cruzi infection with risk factors and electrocardiographic abnormalities in northeast Mexico. BMC infectious diseases. 2014;14:117. doi: 10.1186/1471-2334-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Vilches MJ, Salas J, Cabezas T, Metz D, Vazquez J, Soriano MJ. Chagas screening in pregnant Latin-American women. Experience in Poniente Almeriense (Almeria, Spain) Enferm Infecc Microbiol Clin. 2012;30:380–382. doi: 10.1016/j.eimc.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Muñoz J, Coll O, Juncosa T, Vergés M, Pino MD, Fumado V, Bosch J, Posada EJ, Hernandez S, Fisa R, Boguña JM, Gállego M, Sanz S, Portús M, Gascón J. Prevalence and vertical transmission of Trypanosoma cruzi infection among pregnant latin american women attending 2 maternity clinics in barcelona, spain. Clinical Infectious Diseases. 2009;48:1736–1740. doi: 10.1086/599223. [DOI] [PubMed] [Google Scholar]
- O’Brien SF, Scalia V, Goldman M, Fan W, Yi QL, Dines IR, Huang M, Ndao M, Fearon MA. Selective testing for Trypanosoma cruzi: The first year after implementation at Canadian Blood Services. Transfusion. 2013;53:1706–1713. doi: 10.1111/j.1537-2995.2012.03950.x. [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization. Quantitative estimation of Chagas Disease in the Americas, Montevideo, Uruguay. 2006. [Google Scholar]
- Pan American Health Organization. Chagas Disease. 2014. [Google Scholar]
- Paricio-Talayero JM, Benlloch-Muncharaz MJ, Collar-del-Castillo JI, Rubio-Soriano A, Serrat-Pérez C, Magraner-Egea J, Landa-Rivera L, Sánchez-Palomares M, Beseler-Soto B, Santos-Serrano L, Ferriol-Camacho M, Mut-Buigues J, Tomás-Vila M, Alonso-Jiménez MDC, Domínguez-Márquez V, Igual-Adell R. Epidemiological surveillance of vertically-transmitted Chagas disease at three maternity hospitals in the Valencian Community. Enfermedades Infecciosas y Microbiologia Clinica. 2008;26:609–613. doi: 10.1016/s0213-005x(08)75276-5. [DOI] [PubMed] [Google Scholar]
- Pérez de Ayala Balzola A, Pérez-Molina J, Navarro Beltrá M, López-Vélez R. Enfermedad de Chagas en personas procedentes de latinoamérica residentes en España [Chagas disease in persons coming from Latin America living in Spain] Ministerio de Sanidad y Politicaca Social; Madrid: 2009. [Google Scholar]
- Pinazo MJ, Gascon J. The importance of the multidisciplinary approach to deal with the new epidemiological scenario of Chagas disease (global health) Acta Trop. 2015;151:16–20. doi: 10.1016/j.actatropica.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Piron M, Maymó RM, Hernández JM, Vergés M, Muñoz J, Portús M, Casamitjana N, Puig L, Gascón J, Sauleda S. Round table: European blood banks and Latin American immigration - Preliminary results of the blood bank study, Catalonia. Enfermedades Emergentes. 2007;9:36–38. [Google Scholar]
- Piron M, Verges M, Munoz J, Casamitjana N, Sanz S, Maymo RM, Hernandez JM, Puig L, Portus M, Gascon J, Sauleda S. Seroprevalence of Trypanosoma cruzi infection in at-risk blood donors in Catalonia (Spain) Transfusion. 2008;48:1862–1868. doi: 10.1111/j.1537-2995.2008.01789.x. [DOI] [PubMed] [Google Scholar]
- Ramos JM, Milla A, Rodríguez JC, López-Chejade P, Flóres M, Rodríguez JM, Gutiérrez F. Chagas disease in Latin American pregnant immigrants: Experience in a non-endemic country. Archives of Gynecology and Obstetrics. 2012a;285:919–923. doi: 10.1007/s00404-011-2081-9. [DOI] [PubMed] [Google Scholar]
- Ramos JM, Ponce Y, Gallegos I, Flores-Chavez M, Canavate C, Gutierrez F. Trypanosoma cruzi infection in Elche (Spain): comparison of the seroprevalence in immigrants from Paraguay and Bolivia. Pathog Glob Health. 2012b;106:102–106. doi: 10.1179/2047773212Y.0000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JM, Alvear AL, Ordoñez R, Muñoz G, Garcia A, Lopez R, Leyva R. Risk factors associated with house infestation by the Chagas disease vector Triatoma pallidipennis in Cuernavaca metropolitan area, Mexico. Medical and veterinary entomology. 2005;19:219–228. doi: 10.1111/j.0269-283X.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. The Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Requena-Mendez A, Aldasoro E, de Lazzari E, Sicuri E, Brown M, Moore DAJ, Gascon J, Munoz J. Prevalence of Chagas Disease in Latin-American Migrants Living in Europe: A Systematic Review and Meta-analysis. PLoS Neglected Tropical Diseases. 2015:9. doi: 10.1371/journal.pntd.0003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca C, Pinazo MJ, Lopez-Chejade P, Bayo J, Posada E, Lopez-Solana J, Gallego M, Portus M, Gascon J. Chagas disease among the Latin American adult population attending in a primary care center in Barcelona, Spain. PLoS Negl Trop Dis. 2011;5:e1135. doi: 10.1371/journal.pntd.0001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez DR, Traina MI, Hernandez S, Smer AM, Khamag H, Meymandi SK. Chagas disease awareness among Latin American immigrants living in Los Angeles, California. The American journal of tropical medicine and hygiene. 2014;91:915–919. doi: 10.4269/ajtmh.14-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago B, Blázquez D, López G, Sainz T, Muñoz M, Alonso T, Moro M. Serological profile of immigrant pregnant women against HIV, HBV, HCV, rubella, Toxoplasma gondii, Treponema pallidum, and Trypanosoma cruzi. Enfermedades Infecciosas y Microbiologia Clinica. 2012;30:64–69. doi: 10.1016/j.eimc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Schmunis GA. Epidemiology of Chagas disease in non-endemic countries: The role of international migration. Memorias do Instituto Oswaldo Cruz. 2007;102:75–85. doi: 10.1590/s0074-02762007005000093. [DOI] [PubMed] [Google Scholar]
- Sedyaningsih-Mamahit E, Schinaia N, Lazzari S, Walker N, Vercauteren G. The use of blood donor data for HIV surveillance purposes. AIDS. 2004;18:1849–1851. doi: 10.1097/00002030-200409030-00016. [DOI] [PubMed] [Google Scholar]
- Soriano Arandes A, Munoz Gutierrez J, Verges Navarro M, Castells Domenech C, Portus Vinyeta M, Gascon Brustenga J. Prevalence of Chagas disease in the Latin American immigrant population in a primary health centre in Barcelona (Spain) Acta Trop. 2009;112:228–230. doi: 10.1016/j.actatropica.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Steele LS, MacPherson DW, Kim J, Keystone JS, Gushulak BD. The sero-prevalence of antibodies to trypanosoma cruzi in Latin American refugees and immigrants to Canada. Journal of immigrant and minority health/Center for Minority Public Health. 2007;9:43–47. doi: 10.1007/s10903-006-9014-x. [DOI] [PubMed] [Google Scholar]
- Stimpert KK, Montgomery SP. Physician awareness of Chagas disease, USA. Emerg Infect Dis. 2010;16:871–872. doi: 10.3201/eid1605.091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affairs. International migrant stock by destination and origin. 2013a. [Google Scholar]
- United Nations Department of Economic and Social Affairs. International migration report. 2013b. [Google Scholar]
- Ventura-Garcia L, Roura M, Pell C, Posada E, Gascon J, Aldasoro E, Munoz J, Pool R. Socio-cultural aspects of Chagas disease: a systematic review of qualitative research. PLoS Negl Trop Dis. 2013;7:e2410. doi: 10.1371/journal.pntd.0002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verani JR, Montgomery SP, Schulkin J, Anderson B, Jones JL. Survey of obstetrician-gynecologists in the United States about Chagas disease. The American journal of tropical medicine and hygiene. 2010;83:891–895. doi: 10.4269/ajtmh.2010.09-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR. Population: An Introduction to Concepts and Issues. 12. Cengage Learning; Boston, MA: 2015. [Google Scholar]
- Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. [Google Scholar]
- World Health Organization. Control of Chagas Disease: second report of the WHO expert committee WHO Technical Report Series. Geneva: 2002. [Google Scholar]
- World Health Organization. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. Geneva: 2010. [Google Scholar]
- World Health Organization. DALY estimates, WHO regions, 2000–2012. 2012. [Google Scholar]
- World Health Organization. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second WHO Report on Neglected Tropical Diseases. 2013. [Google Scholar]