Abstract
Context
Marine cyanobacteria offer a robust resource for natural products drug discovery due to the secondary metabolites they produce.
Objective
To identify novel cyanobacterial compounds that exhibit CNS psychoactive effects.
Materials and methods
Cyanobacteria were collected from Las Perlas Archipelago, Panama and subjected to dichloromethane/methanol extraction and fractionation by column chromatography before being screened for affinity against a panel of CNS targets. A 50:50 ethyl acetate:methanol fraction of one cyanobacterial extract (2064H) was subjected to HPLC and the major peak was isolated (2064H3). At a dose of 20 μg per animal, 2064H and 2064H3 were tested in mice using behavioral assays that included the forced swim, open field, and formalin tests.
Results
2064H was shown to bind to the serotonin 2C (5-HT2C) receptor, a known target for depression and pain treatment. 2064H showed 59.6% inhibition of binding of [3H]-mesulergine with an IC50 of 179 ng/mL and did not show inhibition of binding greater than 45% with any other receptors tested. Both 2064H and 2064H3 decreased immobility time in the first min of the tail suspension test. 2064H increased time, distance and number of entries in the center region in the first half of the open field test. 2064H increased overall nocifensive behaviors in the formalin test.
Discussion and Conclusion
Overall, manipulating the 5-HT2C receptor with these receptor-specific ligands derived from cyanobacteria altered pain, depression and anxiety-like behaviors, illustrating the importance of this receptor in affective behaviors. These results demonstrate the potential of cyanobacteria as a source for CNS active compounds.
Keywords: pharmacognosy, depression, anxiety, pain, GPCR
Introduction
Cyanobacteria are a diverse group of organisms that are ubiquitous in aquatic and terrestrial environments around the world. These organisms are photosynthetic prokaryotes that often form multicellular, filamentous colonies. Cyanobacteria are known to produce a wide variety of chemically complex secondary metabolites, some of which are thought to act as a means of protection by exuding antiherbivory effects on other organisms (Nagle & Paul 1999). For many years, marine pharmacognosy researchers have explored the pharmacological potential of some of these marine cyanobacteria metabolites (Dixit & Suseela 2013). These compounds have been shown to have diverse bioactivities, including anticancer, antibacterial, and antiparasitic activity (Gutierrez et al. 2010; Tripathi et al. 2011; Tripathi et al. 2012; Costa et al. 2014). Interestingly, many of the metabolites produced by marine cyanobacteria are cyclic peptides, short linear peptides, and mixed polyketide/peptide sections that resemble endogenous mammalian ligands for G-protein coupled receptors (GPCRs) (Tan 2007). Several species from the genus Moorea (formerly known as Lyngbya), for example, produce compounds that were serendipitously shown to have activity on the GPCR subfamily of cannabinoid receptors (Sitachitta & Gerwick 1998; Han et al. 2003; Gutierrez et al. 2011; Montaser et al. 2012). The goal of our work is to screen marine cyanobacteria extracts against a large panel of CNS-active targets in order to find novel ligands with a focused approach against CNS targets. Cyanobacterial extracts were screened for activity against a panel of 48 known CNS GPCRs, ion channels, and transporters for affinity to receptors involved in depression, anxiety, and pain.
Serotonin, one of the main monoamine neurotransmitters in the nervous system, acts as a ligand for the family of seven serotonin receptors (5-HT1-7), and plays a role in many physiological processes including learning, memory, mood, behavior, pain, sleep, and appetite (Pytliak et al. 2011). Many mood disorders are associated with dysfunction involving the 5-HT receptors. Of particular interest, the serotonin receptor subtype 2C (5-HT2C) has been implicated as a target for depression and anxiety treatment (Artigas 2013). Pharmacological manipulation of this receptor can modulate depression and anxiety-like behaviors in animal models. In both mice and rats, antagonists for 5-HT2C tend to decrease depression-like behaviors (Dekeyne et al. 2008; Nahata et al. 2013). Changes in anxiety-like behaviors tend to occur with activation of 5-HT2C receptors in the basolateral amygdala (BLA), dorsal periaqueductal gray (PAG) or ventral hippocampus (Campbell & Merchant 2003; Alves et al. 2004; Overstreet et al. 2006; Gomes & Nunes-De-Souza 2009; Yamashita et al. 2011; Pockros-Burgess et al. 2014; Vicente & Zangrossi 2014). Similarly, ligands for 5-HT2C can also alter pain-like behavior. Spinal or peripheral activation of 5-HT2C decreases pain-like behavior while brain activation of 5-HT2C increases pain-like behavior in animals (Jeong et al. 2004; Obata et al. 2004; Nakajima et al. 2009; Nakai et al. 2010; Baptista et al. 2012; Gregoire & Neugebauer 2013).
In the clinical setting, the 5-HT2C receptor antagonist agomelatine was approved for the treatment of major depressive disorder (MDD) by the European Medicines Agency in 2009 (Gahr 2014) and was found to be significantly more effective than placebo in reversing depression symptoms as measured by the Hamilton Rating Scale for Depression (Taylor et al. 2014). In humans, this drug was also found to work as effectively as other antidepressant agents such as the selective serotonin reuptake inhibitors (SSRIs) fluoxetine, paroxetine, sertraline, and escitalopram as well as the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine (Guaiana et al. 2013). The 5-HT2C receptor antagonist Agomelatine works to inhibit both anxiety- and depression-like behaviors, as seen in coat state degradation and home cage activity in mice with chronic corticosterone treatment as well as responses in the open field test, novelty suppressed feeding, splash test, and forced swim test (Rainer et al. 2012). Given the similarity of cyanobacterial secondary metabolites to endogenous ligands of GPCRs and the integral role of the GPCR 5HT2C in pain, depression, and anxiety, we sought to find novel 5-HT2C receptor-binding ligands derived from marine cyanobacteria that could induce similar in vivo effects in a mouse model and that could also serve as novel scaffolds for the design of more effective compounds for treating psychiatric disturbances in humans.
In our study, extracts of cyanobacteria collected in the Las Perlas Archipelago, Panama, were used for screening. Crude extracts from the cyanobacteria were fractionated using silica gel chromatography and screened by the Psychoactive Drug Screening Program (PDSP) at the University of North Carolina Chapel Hill (Besnard et al. 2012). The PDSP screens researcher-supplied fractions for affinity to an array of GPCRs, ion channels, and transporters, including the 5-HT2C receptor. Fractions are screened through competitive inhibition assays to determine binding affinity at specific targets. Fractions with selective affinity for the 5-HT2C receptor were injected into the lateral ventricle of mice via intracerebroventricular (ICV) injections. Following compound administration, mice were tested in a series of depression, anxiety and pain behavioral paradigms, including the tail suspension, open field, and formalin tests. We hypothesized that fractions demonstrating selective affinity to the 5-HT2C receptor would induce pain-like behavior in animals when applied through ICV injection. We further hypothesized that these fractions would similarly impact anxiety-like and depression-like behavior in these animals.
Materials and methods
Field Sampling
A green cyanobacterial mat was collected in September 2011 (collection code PAL-22Sep11-4) by hand using SCUBA in 8–10 ft of water off of sandy bottom substrate near Mogo Mogo in the Las Perlas Archipelago, Panama (GPS coordinates: N8 34.837 W79 01.177; Figure 1). The cyanobacterial mat had large green filaments and grew in dispersed clumps in the sand. A voucher sample was taken for storage at the Smithsonian Tropical Research Institute in Panama. Upon collection, the cyanobacteria were excreting a reddish pigment into the water, which was saved in seawater and combined with cyanobacterial biomass for extraction purposes. The total volume collected was 4 L.
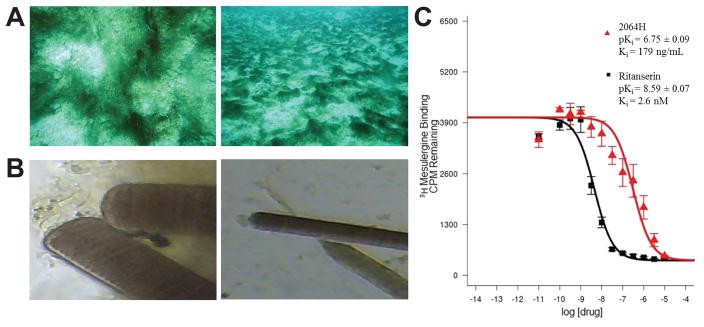
Figure 1. Picture of cyanobacteria 2064 and apparent Ki curve for fraction 2064H.
(A) Photo of the cyanobacterium in the field; (B) microscope image of the cyanobacterium shown at 40X (left) and 10X magnification; (C) Apparent Ki curve for fraction 2064H showing the standard 5-HT2c ligand ritanserin for comparison.
Compound Extraction
Collected cyanobacterial biomass was transported in sea-water and stored at −20°C until extraction. A small sample was preserved in an RNA preserving solution (RNALater, Qiagen), but no viable genetic material could be obtained, making phylogenetic determination impossible. Cyanobacterial biomass and water were extracted repeatedly with a 2:1 mixture of dichloromethane:methanol (5 × 500 mL for each extraction) and filtered through cheese cloth. Organic layers obtained from each extraction were combined and concentrated by rotary evaporation at 25°C to obtain 4.2 g of the crude extract. This crude extract was then subjected to flash silica gel column chromatography and eluted with hexane:ethyl acetate (100:0, 80:20, 60:40, 40:60, 20:80 and 0:100) and ethyl acetate:methanol (75:25, 50:50 and 0:100) to obtain nine fractions (2064A to 2064I). Fractions and crude extracts were then subjected to bioassays.
Leishmania bioassay
Axenically grown (cell free) amastigotes of Leishmania donovani (LD-1S/MHOM/SD/00-strain 1S), the species responsible for the visceral and lethal forms of leishmaniasis, were used to assess parasite growth and survival. Samples were tested in duplicate at 10 μg/mL. The results were expressed as a percentage of growth inhibition (IG) compared to controls. Samples that showed above 70% IG were considered active and were then assayed at four different concentrations (0.08, 0.4, 2, and 10 μg/mL) to determine IC50 values. Amphotericin B was used as a positive control with the typical IC50 response of 0.08–0.13 μM (Williams et al. 2003).
Chagas’ disease bioassays
Trypanosoma cruzi bioassays were performed using a colorimetric method, and the inhibition of parasite growth was assessed by the expression of the reporter gene for β-galactosidase (β-Gal) in the recombinant Tulahuen clone C4 of T. cruzi. Assays were performed in duplicate on the amastigote, the intracellular form of the parasite infecting African green monkey kidney (Vero) cells, exposed during 120 h to different concentrations (10, 2 and 0.4 μg/mL) of the test compounds at 37°C under an atmosphere of 5% CO2/95% air. The resulting color from the cleavage of chlorophenol red-β-D-galactoside (CPRG) by β-Gal expressed by the parasite was measured at 570 nm. The concentration that inhibited 50% expression of β-Gal (IC50) was calculated by log regression of the obtained optical density values, and compared with the untreated controls. Nifurtimox was used as a positive control (IC50 10–16 μM) (Buckner et al. 1996).
Malaria Bioassays
Antiplasmodial activity was evaluated using a fluorometric method based on the detection of parasite DNA with the fluorochrome PicoGreen using a chloroquine-resistant strain (Indochina W2) of Plasmodium falciparum. The sample was considered active if it inhibited >70% of the growth of the parasites as compared to their untreated controls at 10 μg/mL. The IC50 was calculated by normal regression of the resulting inhibition percentages at 0.08, 0.4, 2, and 10 μg/mL. The parasites were maintained in vitro by a modification of the method of Trager and Jensen (Trager & Jensen 1976). Chloroquine was used as a positive control (IC50 80–100 nM) (Corbett et al. 2004).
Cytotoxicity Assay
H-460 cells were added to 96-well plates at 3.33x104 cells/mL of Roswell Park Memorial Institute (RPMI) 1640 medium with fetal bovine serum (FBS) and 1% penicillin/streptomycin. The cells, in a volume of 180 μL per well, were incubated overnight (37°C, 5% CO2) to allow recovery before treatment with test compounds. Compounds were dissolved in DMSO to a stock concentration of 10 mg/mL. Working solutions of the compounds were made in RPMI 1640 medium without FBS, with a volume of 20 μL added to each well to give a final compound concentration of either 30 or 3 μg/mL. An equal volume of RPMI 1640 medium with FBS was added to wells designated as negative controls for each plate. Plates were incubated for approximately 48 h before staining with dimethylthiazolyl-diphenyltetrazolium bromide MTT. Using a ThermoElectron Multiskan Ascent plate reader, plates were read at 570 and 630 nm (Mevers et al. 2011).
Psychoactive Drug Screening Program (PDSP) CNS-activity screening
All binding assays for 2064 (including crude fraction and fractions 2064A to 2064I) were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP) using a radioligand competition-binding assay. Experimental details are available online at http://pdsp.med.unc.edu/. Fractions that showed greater than 50% inhibition of binding of [3H]-mesulergine at 4 μg/mL were further analyzed to determine IC50 at the specific target of interest. [3H]-mesulergine binds with high affinity to the serotonin 2 receptor (Closse 1983).
HPLC
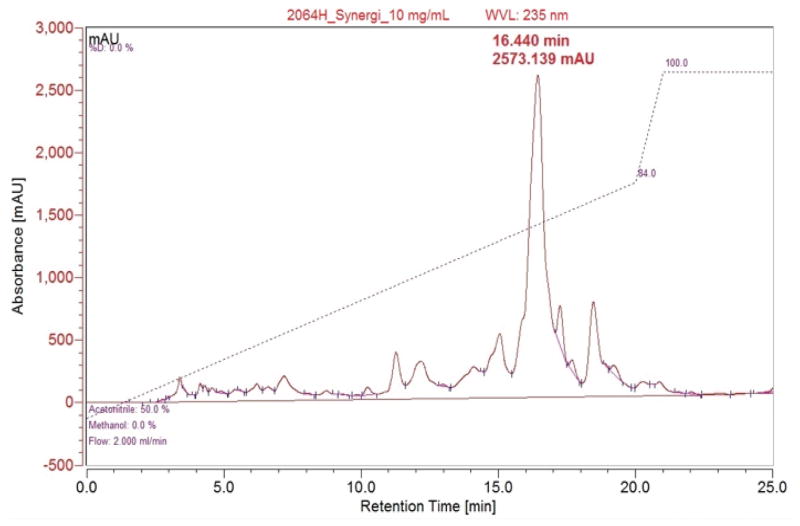
HPLC separation was carried out on a Dionex UltiMate 3000 equipped with a diode array detector. A Synergi 4u Fusion-RP-80A (150 × 10 mm, 4 micron) column was used for the separation. The reverse phase separation started at a constant flow rate of 2 mL/min with a linear gradient from 50% acetonitrile (ACN) in water to 84% ACN over 20 min followed by an increase to 100% ACN and holding at 100% ACN for 10 min. For each run, 100 μL of each sample was injected at a concentration of 10 mg/mL. Fractions were collected based on UV absorption at 235 nm and 2064H3 was collected at a retention time of 16.4 min.
Animals
All animal procedures were reviewed and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Animals and the Institutional Animal Care and Use Committee at Duquesne University. All experiments were carried out on male C57Bl/6J mice that were 8–10 weeks old. Animals were individually housed and maintained on a 12 h light/dark cycle (lights on between 7:00 AM and 7:00 PM) with ad libitum access to food and water. All procedures were carried out during the light cycle. All behaviors performed were recorded and analyzed by a male experimenter blinded to experimental treatment.
Surgical Procedures
Animals were anesthetized with 3% isoflurane/0.6% O2 during all surgical procedures. Intracerebroventricular (ICV) cannulation surgeries were performed as described previously (Glascock et al. 2011). Briefly, mice were placed in a stereotaxic frame and an 8.00 mm steel cannula was placed into the right lateral ventricle at the following anatomical coordinates: 0.5 mm anterior to bregma, 1.0 mm lateral to midline and 2.0 mm ventral to the skull. A dental cement skullcap secured with two bone screws was used to hold the cannula in place. Mice recovered on heating pads and were given one week of recovery prior to the beginning of behavioral testing. Following all behavioral tests, cannula placement was verified with necropsy.
Compound Preparation and Administration
Cyanobacterial extracts from collection 2064, fraction H (2064H) and HPLC peak 3 (2064H3) were dissolved in a mixture of 50% DMSO and 50% artificial cerebrospinal fluid (aCSF) containing the following (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2.0 CaCl2, 1.0 MgCl2 and 25 D-glucose, bubbled with 95% O2/5% CO2 for 20 min prior to use. Microinjections were performed using a 32-gauge injector that extended 0.5 mm beyond the tip of the ICV cannula. The injector was attached to flexible tubing and a 1.0 μL syringe (Hamilton) that was used to deliver a total volume of 0.5 μL over a 2 min period. The injector was kept in place for an additional 1 min to allow complete compound infusion. For all injections, fraction 2064H and 2064H3 were administered at a 20 μg dose. 50%/50% aCSF/DMSO was used as a vehicle control.
Behavioral Testing
Mice were habituated for 1 h with 60 dB of white noise to block any ambient noise. All animals received fraction/compound (2064H/2064H3) or vehicle 5 min prior to the beginning of testing. Depression-like behaviors were measured using the tail suspension test as described previously (Can et al. 2012). Briefly, mice were suspended by the distal ends of their tail in a Plexiglas enclosure (35 × 25 × 25 cm) with white sides. The animals’ movements were recorded for scoring offline in 1-min bins for a total of 6 min. Immobility was defined as when the mouse was not exhibiting any outward movement of its limbs or body. Anxiety-like behaviors were measured with the open field test as described previously (Seibenhener & Wooten 2015). In this assay, mice were placed in a brightly lit Plexiglas box with high walls (25 × 25 × 35 cm) and tracked with an over-head camera and ANY-maze software (Stoelting Co., version 4.98). Time spent in the center and outside regions of the box were recorded for the 10 min long test. Finally, spontaneous pain-like behaviors were measured using the formalin test as described previously (Bhave et al. 2001). Mice were habituated in clear, ventilated Plexiglas boxes (12 × 12 × 20 cm) for 2 h. Five min after compound or vehicle injection, the animal received a 10 μL intradermal injection of 4% formalin into the right paw and was videotaped for 1 h followed by offline scoring. The mice were analyzed for nocifensive behaviors (lifting, biting, lifting and flinching of the injected paw) in 5 min bins.
Results
Cyanobacteria Sample, Screening and Pharmacology Reveals a 5-HT2C Compound
A green cyanobacterial mat was collected by hand using SCUBA from a sandy bottom in ~10 feet of water off of Mogo Mogo, an island in the Las Perlas Archipelago, Panama (Figure 1A). The cyanobacterium was excreting a reddish pigment upon being removed from the sandy substrate, so both seawater and the cyanobacterial mass were saved and used for extraction. Based on morphology, the collection was given the field identification of Oscillatoria sp. but could also be from the Moorea or Okeania genera (Figure 1A and B). Phylogenetic determination was not possible since no viable genetic material could be obtained. A total of 4 L of cyanobacterial biomass and pigmented water were brought back to the laboratory in Panama City for extraction.
Following extraction and fractionation, fractions of extract 2064 were screened in antiparasitic (L. donovanii, P. falciparum, T. cruzi) and cytotoxicity assays, as well as screened by the PDSP for CNS activity. For the PDSP screening, the fractions were screened against a panel of 48 targets (receptors, transporters and ion channels) with importance in the CNS, ranging from serotonergic and opioid receptors to dopaminergic among others. The PDSP first screens compounds with a competitive radioligand-binding assay. Anything showing greater than 50% inhibition of binding is further screened to determine a Ki. Typically, a Ki value would be reported as a molar concentration but because our extracts are mixtures of compounds rather than a pure compound of known molecular weight, we refer to these secondary values from PDSP as “apparent Ki” or IC50 and report them in units of ng/mL. The apparent Ki is very important for our prioritization of “hits” in that it allows us to determine if inhibition of binding appears to be because of a truly potent compound (i.e. low apparent Ki) or simply a large concentration of a weakly binding compound (i.e. high apparent Ki).
No fractions showed any activity in the antiparasitic or cytotoxicity assays (data not shown), but there were a number of hits from the PDSP screen (Table 1). Fractions that showed greater than 50% inhibition of binding, but an apparent Ki > 10 uM, were considered non-specific hits. Fractions 2064H and 2064I showed greater than 50% inhibition of binding (Table 1) and also a low apparent Ki (ng/mL) (Table 2) for the 5-HT2c receptor, meaning that these fractions possessed true affinity for the receptor. Figure 1C shows the apparent Ki curve for 2064H and also gives a comparison curve for ritanserin, a potent serotonin receptor antagonist (Boess & Martin 1994). The curve shows a clear affinity dose response profile for 2064H and gives an apparent Ki value in the ng/mL range. 2064H was chosen for further HPLC purification due to the fact that it showed higher affinity than 2064I (179 ng/mL versus 512 ng/mL, respectively). 2064H also showed higher specificity given that 2064I also showed some non-specific binding to 5-HT3, delta opioid and kappa opioid receptors (Tables 1 and 2).
Table 1. Selected bioassay results of 2064H.
Hits are defined as fractions with greater than 50% inhibition of binding/growth in the initial screen and are shown as grey boxes.
| Chagasa | Malariaa | Cancera | Leisha | 5-HT2Ab | 5-HT2Bb | 5-HT2Cb | 5-HT3b | DORb | KORb | MORb | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | 2.18 | 3.5 | 6.67 | 0.4 | −7.1 | 93.3 | 13.6 | 8.8 | 3.7 | 73.4 | 26.2 |
| A | 0.46 | 7.1 | 5.32 | 4.0 | −16.6 | 91.2 | 12.3 | −15 | 9.8 | 26.3 | −1.4 |
| B | 0.67 | 19.4 | 5.48 | 8.3 | 1.2 | 96.2 | 13.4 | 8.8 | 9.8 | 20.4 | 15.9 |
| C | 2.12 | 10.1 | 4.26 | 9.2 | −2.5 | 96.4 | 25 | 3.5 | 11.2 | 42.1 | 18.9 |
| D | 3.57 | 8.2 | 8.71 | 9.6 | 18.4 | 95 | 13.3 | −5.1 | 14.4 | 18.3 | 19.6 |
| E | 1.57 | 4.4 | 8.65 | 13.2 | 7 | 94.4 | 12.4 | −4.8 | 19.5 | 26.8 | 18.3 |
| F | 4.29 | 17.1 | 4.97 | 4.1 | 7.8 | 10.9 | 4.9 | −5.1 | 23.4 | 15.2 | 55.8 |
| G | 2.60 | 0.0 | 3.99 | 0.0 | 9.7 | 3.2 | 15.8 | 2.1 | 26 | 31.1 | 51.5 |
| H | 1.30 | 10.9 | 3.40 | 0.0 | −1.9 | 7.9 | 59.6 | 20.1 | 40 | 36.1 | 43.5 |
| I | 0.72 | 3.5 | 0.00 | 0.0 | −0.8 | 33.5 | 50 | 56.3 | 66.8 | 50 | 39.6 |
Assays performed by the Spadafora lab as described in Experimental Section. Data represent percent inhibition of growth (N = 2).
Assays performed by PDSP as described in Experimental Section. Data represent average percent inhibition of binding of a standard radiolabeled ligand (N = 4). No fractions showed any affinity for other receptors screened against so they have been omitted.
DOR = delta opioid receptor, KOR = kappa opioid receptor, MOR = mu opioid receptor.
Table 2. Apparent Ki for PDSP hits.
Data are presented as apparent Ki in ng/mL. Ki values are calculated from best-fit IC50 values using the Cheng-Prusoff equation. ND = not determined (fraction was not a hit in the initial screen).
| 5-HT2B | 5-HT2C | 5-HT3 | DOR | KOR | MOR | |
|---|---|---|---|---|---|---|
| Crude | >10,000 | ND | ND | ND | >10,000 | ND |
| A | >10,000 | ND | ND | ND | ND | ND |
| B | >10,000 | ND | ND | ND | ND | ND |
| C | >10,000 | ND | ND | ND | ND | ND |
| D | 8270 | ND | ND | ND | ND | ND |
| E | 6868 | ND | ND | ND | ND | ND |
| F | ND | ND | ND | ND | ND | >10,000 |
| G | ND | ND | ND | ND | ND | >10,000 |
| H | ND | 179 | ND | ND | ND | ND |
| I | ND | 512 | >10,000 | >10,000 | >10,000 | ND |
Chromatography
The initial HPLC showed a single major peak with a number of smaller peaks (Figure 2). HPLC conditions were determined to separate the major peak with a short run time. Final HPLC conditions used were the following: a gradient from 50% acetonitrile(ACN):water to 84% ACN:water over 20 min followed by increasing to 100% ACN and holding for 10 min before re-equilibration. The major peak had a retention time of 16.440 min, with some small satellite peaks nearby. The major peak (2064H3) was isolated for additional analysis.
Figure 2. HPLC chromatogram of 2064H.
Reverse Phase HPLC (100 μL injection; 10 mg/mL); Major peak at 16.440 min and 2573 mAU was isolated as pure compound 2064H3.
Alteration of Depression, Anxiety and Pain-like Behaviors in vivo with 2064H
After determining that 2064H showed affinity for the 5-HT2C receptor, we sought to test its potential effects using an in vivo mouse model. Since the structure and exact nature of the active compound in this fraction were unknown, intracerebroventricular (ICV) administration was used to directly administer the compounds into the cerebrospinal fluid of the lateral ventricle of the brain (Glascock et al. 2011), circumventing the need for a compound structure capable of crossing the blood-brain barrier. Through this administration route, compounds disperse throughout the CNS. This is important because it is unknown where the active component of 2064H acts in the brain or if it has the ability to cross the blood-brain barrier.
We first sought to test if there might be any effects induced by the vehicle used to dissolve both 2064H and 2064H3 (50% artificial cerebrospinal fluid (aCSF)/ 50% DMSO). We found no statistically significant differences between the 100% aCSF, 50% DMSO/50% aCSF and 100% DMSO vehicles (administered ICV 0.5 μL) in the open field test (mean time in center of open field (sec) ± SEM – 100% aCSF: 44.28 ± 7.75, 50% DMSO/50% aCSF: 38.30 ± 2.63, 100% DMSO: 47.72 ± 7.94, one-way ANOVA p=0.602; mean distance traveled in open field (m) +/− SEM – 100% aCSF: 34.14 ± 2.91, 50% DMSO/50% aCSF: 38.06 ± 3.72, 100% DMSO: 32.43 ± 3.79, one-way ANOVA p=0.521). These data confirm that vehicle administration alone did not influence basal measures of rodent activity indicating that it was a suitable vehicle to proceed with further behavioral assays.
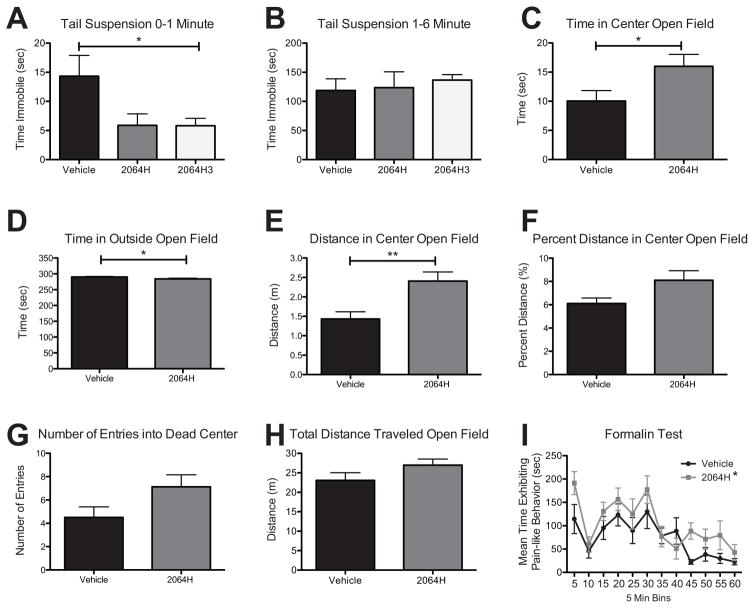
The first set of behavioral experiments were conducted to test both the fraction 2064H and the purified HPLC peak 2064H3 via the tail suspension test. This assay measures the amount of time an animal spends immobile when it is suspended upside down by the distal end of its tail. A longer duration of immobility is a proxy for greater depression-like behavior and a shorter duration of immobility indicates a compound demonstrates antidepressant-like effects. We verified the efficacy of ICV antidepressant delivery with a pilot study using the well-characterized SSRI, fluoxetine. Following ICV pretreatment with fluoxetine or vehicle (aCSF), fluoxetine-treated mice spent statistically significantly lower amount of time immobile compared to control mice (fluoxetine n = 4 128.8 ± 14.8 sec; control n = 4 177.8 ± 4.9 sec; t-test p = 0.02). Following a 5 min pretreatment with 2064H3 or vehicle via ICV injection, mice spent a statistically significantly lower amount of time immobile in the first min of the test compared to vehicle-treated mice (Figure 3A, unpaired t-test, *p = 0.048). Similarly, mice pretreated with 2064H showed a trend towards lower immobility compared to vehicle-injected mice also in the first min of the test (Figure 3A, unpaired t-test, p = 0.067). This effect was not seen in the latter half of the test during the one-to-six min time period [Figure 3B, unpaired t-test, p = 0.893 (2064H), p = 0.439 (2064H3)]. These decreases in immobility time suggest a modest antidepressant-like effect of the active compound contained in 2064H and 2064H3 vs vehicle.
Figure 3. Behavioral effects of fraction 2064H and pure compound 2064H3 in vivo.
(A) Mice treated with 2064H3 (n = 6) significantly decrease time immobile compared to vehicle (n = 6) in the first min of the tail suspension test; mice treated with 2064H (n = 6) show a similar trend. (B) These same animals show no difference in immobility time in the latter half of the test. (C) Mice treated with 2064H (n = 8) spend more time in the center of the open field box compared to vehicle treated mice (n = 8). (D) 2064H-treated mice also show a decrease in the time spent in the outside of the open field apparatus. (E) Mice treated with 2064H travel more in the center of the open field box compared to vehicle. (F) Mice treated with 2064H show a trend of having a greater percent distance traveled in the center of the open field box compared to vehicle (p = 0.051). (G) 2064H-treated mice also show a trend in entering the dead center of the open field box more than vehicle-treated mice (p = 0.075). (H) 2064H-treated mice show no difference in total distance traveled compared to vehicle-treated mice. (I) Mice treated with 2064H (n = 8) show an overall main effect of treatment (2-way ANOVA) of increasing pain-like behaviors compared to vehicle-treated mice (n = 8) in the formalin test. A,C,D,F - * p<0.05, **p<0.01, unpaired t-test; I - * p<0.01, 2-Way ANOVA main effect of treatment. Data shown as mean ± SEM.
In addition to effects in depression assays, anxiety and locomotor behaviors were measured using the open field test. Due to the limited amount of pure compound available, only fraction 2064H, which includes the 2064H3 compound, was tested in this assay. Here, 5 min pretreatment of 2064H significantly increased the amount of time that animals spent in the center region of the open field apparatus compared to vehicle-injected animals during the first half of the test (Figure 3C, unpaired t-test, *p = 0.047) suggesting antianxiety effects of the compound. Similarly, 2064H-treated animals spent less time in the outside region of the open field box compared to vehicle-treated animals during this same portion of the test (Figure 3D, unpaired t-test, *p = 0.047). Together, these measurements indicate an antianxiety-like effect of 2064H. The open field test also allows for the measurement of locomotion by tracking how far an animal travels in the open field apparatus. Mice treated with 2064H traveled more in the center of the open field box during the first half of the test (Figure 3E, unpaired t-test, p = **0.006) further indicating an anxiolytic-like effect. Moreover, 2064H caused mice to trend towards entering the dead center of the open field apparatus a greater number of times as compared to vehicle-treated mice in the first half of the test (Figure 3G, unpaired t-test, p = 0.075). Finally, 2064H did not alter overall locomotive behavior. Mice treated with 2064H did not travel farther in the apparatus compared to vehicle-treated mice (Figure 3H, unpaired t-test, p = 0.1408). This suggests that the effects of 2064H are likely anxiolytic and not hyperactive or hypoactive. Nonetheless, to account for any overall changes in locomotion, the percent distance traveled in the center zone was calculated (center distance/total distance × 100). Animals treated with 2064H had a small, but statistically insignificant increase in percent distance traveled in the center of the open field box compared to vehicle-injected animals in the first part of the test (Figure 3F, unpaired t-test, p = 0.051). These data also suggest an antianxiety effect of 2064H that was not likely confounded by hyper or hypolocomotion. No differences in any of these parameters of the open field test were seen in the latter half of the test (data not shown). Finally, 2064H was tested in an inflammatory pain model, the formalin test. In this assay, 2064H had an overall effect of increasing pain-like behaviors compared to vehicle-injected mice (Figure 3I, 2-way ANOVA, overall effect of treatment, *p = 0.0011).
Discussion
Overall, our results show that fraction 2064H and HPLC peak 2064H3 induce antidepressant, anxiolytic, and nocifensive behavioral effects in an in vivo mouse model. These behaviors correspond to those obtained when manipulating the 5-HT2C receptor with previously described ligands. In tests of depression, antidepression-like behaviors were seen with administration of 5-HT2C antagonists (Dekeyne et al. 2008; Nahata et al. 2013), data suggesting that blocking the 5-HT2C receptor induces these antidepression-like behaviors. In terms of anxiety-like behaviors, more anatomical specific data is known. Activation of 5-HT2C in the lateral/basolateral amygdala (BLA) (Campbell & Merchant 2003; Pockros-Burgess et al. 2014; Vicente & Zangrossi 2014) or ventral hippocampus induced anxiety-like effects (Alves et al. 2004). Administration of a 5-HT2C inverse agonist reversed anxiety-like behavior in rats (Overstreet et al. 2006). In dorsal PAG administration, agonists of the 5-HT2C receptor induced anxiolytic-like effects (Gomes & Nunes-De-Souza 2009), while others have found activation of 5-HT2C receptors increased anxiety-like behaviors (Yamashita et al. 2011). Taken together, these known effects on depression and anxiety-like behaviors suggest that the active compound in 2064H may be an antagonist of the 5-HT2C receptor. Since activation of 5-HT2C in the amygdala and hippocampus increases anxiety-like behavior (Campbell & Merchant 2003; Alves et al. 2004; Gomes & Nunes-De-Souza 2009; Pockros-Burgess et al. 2014; Vicente & Zangrossi 2014), it is possible that the antagonist in 2064H/2064H3 is acting in these regions to cause the anxiolytic-like and antidepressant-like effects demonstrated in this study. The amygdala and hippocampus are also located close to the site of ICV injection, making this a distinct possibility. Future studies will be necessary to address the anatomical specificity of 2064H behavioral effects.
Ligands of the 5-HT2C receptor have also been shown to alter pain-like behaviors, with anatomical-dependent effects. Local paw administration of 5-HT2C receptor antagonists reduced the total number of flinches in phase 2 of the formalin test (Nakajima et al. 2009). Activation of 5-HT2C in the spinal cord reduced pain in the formalin test (Jeong et al. 2004) and prevented the development of allodynia in rats with infraorbital nerve ligation (Nakai et al. 2010) or spinal nerve ligation (Obata et al. 2004). While there are limited data on the effects of the 5-HT2C receptor in specific brain regions, it is possible that the compound in 2064H could be acting to increase pain-like behavior by binding with these receptors in the PAG. Activation of 5-HT2C receptors in this region is analgesic (Baptista et al. 2012), so it is possible that antagonism causes an increase in nociceptive responses. The PAG also surrounds the cerebral aqueduct, which contains the cerebrospinal fluid where 2064H is dispersed in ICV injections, further supporting this hypothesis.
The behavioral results seen in this study and their effects seen in depression, anxiety, and pain assays were expected based on the known comorbidity that exists between depression, anxiety, and pain. Patients that suffer from a chronic pain condition often report accompanying feelings of depression – 15 to 100% of patients that suffer from depression are described as also having some type of chronic pain, with percentages varying with the type of pain studied (Bair et al. 2003). Correspondingly, rates of depression among chronic pain patients are also high, ranging from 13 to 82% (Bair et al. 2003). Comorbidity of chronic pain and depression in turn appears to amplify each individual condition, with patients often reporting more intense symptoms when the diseases occur together (Von Korff et al. 1992). There is also a synchronicity between anxiety and pain over time, with patients who have anxiety reporting more severe pain and higher levels of pain even after remission compared to healthy controls (Gerrits et al. 2015). This overlap implies that a common mechanism may link psychiatric conditions and pain and that a single treatment used for one disorder may prevent the development of the other. Most early drug screenings look only at affective assays (i.e. depression and anxiety) or pain assays, not both. Novel compounds that have activity at receptors such as the 5-HT2C should be screened for effects in affective and pain conditions. Finding one compound that could help alleviate the effects of both conditions would lead to a better understanding of how these comorbid conditions arise. In the present study, our 5-HT2C compound had opposing therapeutic effects on depression and anxiety compared to pain. However, it should be appreciated that the lack of anatomical targeting in the present study may contribute to an incomplete picture of the potential for 5HT2C in treating comorbid pain and depression. Direct administration of the 5-HT2C receptor antagonist SB242084 to the BLA along with intraperitoneal administration of the SSRI fluvoxamine inhibited pain-like vocalizations and anxiety-like behaviors in rats using an arthritis pain model (Gregoire & Neugebauer 2013), suggesting that specific antagonism of 5-HT2C receptors in this brain region may be a key to understanding comorbid pain and depression.
Our results also highlight that differences exist between the screening of a fraction of raw compounds (2064H) and an isolated HPLC peak obtained from that fraction (2064H3). These differences are illustrated in the tail suspension data (Figure 3A and 3B), the only assay in which we were able to test both 2064H and 2064H3 (we were not able to test 2064H3 in any other behavioral assays due to the low amount obtained following purification). During the first min of the tail suspension test, the pure compound 2064H3 induced a significantly lower immobility time in these mice compared to vehicle-treated mice. In contrast, when the fraction 2064H was administered, a similar, but not statistically significant trend was seen. These data imply that the behavioral trends seen with 2064H were due to the presence of 2064H3 in that fraction. In both cases, 20 μg of 2064H or 2064H3 was injected. When the pure compound is administered in isolation at a full 20 μg dose, the effect becomes significant. The presence of other inactive compounds in fraction 2064H (such as chlorophylls and other inactive metabolites) means that less than 20 μg of the active 2064H3 in this mixture is being administered.
Conclusions
Marine cyanobacterial natural products is an area of active investigation in the search for new pharmacological agents. Due to the similarity of marine cyanobacterial secondary metabolites to endogenous GPCR ligands (Tan 2007), these organisms are ideal candidates for screening at CNS targets for ligand discovery. In contrast to previous screenings that looked only at cannabinoid receptor ligands from cyanobacteria (Sitachitta & Gerwick 1998; Han et al. 2003; Gutierrez et al. 2011; Montaser et al. 2012), our study is the first to broadly screen cyanobacteria for GPCR ligands and other CNS targets. We were able to show that one fraction, 2064H with affinity for the 5-HT2C receptor and a purified HPLC peak isolated from this fraction, 2064H3, induced a series of behavioral effects in mice including antidepressant-like effects in the tail suspension test, anxiolytic-like effects in the open field test, and an increase in pain-like behaviors in the formalin test. Our results highlight the potential of cyanobacterial-derived compounds in CNS drug discovery.
Acknowledgments
We acknowledge Dr. Marcy Balunas for help in cyanobacterial collection, Ms. Jamie Moy for initial extraction, and Dr. Maria Elena Morales for scientific editing. Project was funded through (1) a grant provided by the American Pain Society and the Sharon S. Keller Fund for Chronic Pain Management Research (BK and KT), (2) a grant from the NIH (NCCIH R15AT008060; BK and KT), (3) support for this research was provided by a Fogarty International Center (FIC) International Cooperative Biodiversity Group (ICBG) grant based in Panama (2U01TW006634-06) (KT), (4) a grant from Duquesne University Faculty Development Fund (KT and BK), and (5) a Beta Beta Beta Research Award (CI). Ki determinations and receptor binding profiles were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2008-025C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- Alves SH, Pinheiro G, Motta V, Landeira-Fernandez J, Cruz AP. Anxiogenic effects in the rat elevated plus-maze of 5-HT(2C) agonists into ventral but not dorsal hippocampus. Behav Pharmacol. 2004;15:37–43. doi: 10.1097/00008877-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Artigas F. Serotonin receptors involved in antidepressant effects. Pharmacology & Therapeutics. 2013;137:119–131. doi: 10.1016/j.pharmthera.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: A literature review. Archives of Internal Medicine. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Baptista D, Nunes-de-Souza RL, Canto-de-Souza A. Activation of 5-HT2C receptors in the dorsal periaqueductal gray increases antinociception in mice exposed to the elevated plus-maze. Behavioural Brain Research. 2012;235:42–47. doi: 10.1016/j.bbr.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, et al. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–20. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Carlton SM, Gereau RW, Iv, Karim F. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nature Neuroscience. 2001;4:417. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Buckner FS, Verlinde CL, La Flamme AC, Van Voorhis WC. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob Agents Chemother. 1996;40:2592–7. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BM, Merchant KM. Serotonin 2C receptors within the basolateral amygdala induce acute fear-like responses in an open-field environment. Brain Res. 2003;993:1–9. doi: 10.1016/s0006-8993(03)03384-5. [DOI] [PubMed] [Google Scholar]
- Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD. The tail suspension test. J Vis Exp. 2012:e3769. doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closse A. [3H]Mesulergine, a selective ligand for serotonin-2 receptors. Life Sci. 1983;32:2485–95. doi: 10.1016/0024-3205(83)90375-2. [DOI] [PubMed] [Google Scholar]
- Corbett Y, Herrera L, Gonzalez J, Cubilla L, Capson TL, Coley PD, Kursar TA, Romero LI, Ortega-Barria E. A novel DNA-based microfluorimetric method to evaluate antimalarial drug activity. Am J Trop Med Hyg. 2004;70:119–24. [PubMed] [Google Scholar]
- Costa M, Garcia M, Costa-Rodrigues J, Costa MS, Ribeiro MJ, Fernandes MH, Barros P, Barreiro A, Vasconcelos V, Martins R. Exploring bioactive properties of marine cyanobacteria isolated from the Portuguese coast: high potential as a source of anticancer compounds. Mar Drugs. 2014;12:98–114. doi: 10.3390/md12010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekeyne A, Mannoury la Cour C, Gobert A, Brocco M, Lejeune F, Serres F, Sharp T, Daszuta A, Soumier A, Papp M, et al. S32006, a novel 5-HT2C receptor antagonist displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology (Berl) 2008;199:549–68. doi: 10.1007/s00213-008-1177-9. [DOI] [PubMed] [Google Scholar]
- Dixit R, Suseela MR. Cyanobacteria: potential candidates for drug discovery. Antonie van Leeuwenhoek. 2013;103:947–961. doi: 10.1007/s10482-013-9898-0. [DOI] [PubMed] [Google Scholar]
- Gahr M. Agomelatine in the treatment of major depressive disorder: an assessment of benefits and risks. Curr Neuropharmacol. 2014;12:287–398. doi: 10.2174/1570159X12999140619122914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MM, van Marwijk HW, van Oppen P, van der Horst H, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78:64–70. doi: 10.1016/j.jpsychores.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Glascock JJ, Osman EY, Coady TH, Rose FF, Shababi M, Lorson CL. Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. J Vis Exp. 2011 doi: 10.3791/2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes KS, Nunes-De-Souza RL. Implication of the 5-HT2A and 5-HT2C (but not 5HT1A) receptors located within the periaqueductal gray in the elevated plus-maze test–retest paradigm in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:1261–1269. doi: 10.1016/j.pnpbp.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Gregoire S, Neugebauer V. 5-HT2CR blockade in the amygdala conveys analgesic efficacy to SSRIs in a rat model of arthritis pain. Mol Pain. 2013;9:41. doi: 10.1186/1744-8069-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaiana G, Gupta S, Chiodo D, Davies SJ, Haederle K, Koesters M. Agomelatine versus other antidepressive agents for major depression. Cochrane Database Syst Rev. 2013;12:CD008851. doi: 10.1002/14651858.CD008851.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M, Pereira AR, Debonsi HM, Ligresti A, Di Marzo V, Gerwick WH. Cannabinomimetic lipid from a marine cyanobacterium. J Nat Prod. 2011;74:2313–7. doi: 10.1021/np200610t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez M, Tidgewell K, Capson TL, Engene N, Almanza A, Schemies J, Jung M, Gerwick WH. Malyngolide dimer, a bioactive symmetric cyclodepside from the panamanian marine cyanobacterium Lyngbya majuscula. J Nat Prod. 2010;73:709–11. doi: 10.1021/np9005184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, McPhail KL, Ligresti A, Di Marzo V, Gerwick WH. Semiplenamides A-G, fatty acid amides from a Papua New Guinea collection of the marine cyanobacterium Lyngbya semiplena. J Nat Prod. 2003;66:1364–8. doi: 10.1021/np030242n. [DOI] [PubMed] [Google Scholar]
- Jeong CY, Choi JI, Yoon MH. Roles of serotonin receptor subtypes for the antinociception of 5-HT in the spinal cord of rats. European Journal of Pharmacology. 2004;502:205–211. doi: 10.1016/j.ejphar.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Mevers E, Liu WT, Engene N, Mohimani H, Byrum T, Pevzner PA, Dorrestein PC, Spadafora C, Gerwick WH. Cytotoxic veraguamides, alkynyl bromide-containing cyclic depsipeptides from the marine cyanobacterium cf. Oscillatoria margaritifera. J Nat Prod. 2011;74:928–36. doi: 10.1021/np200077f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaser R, Paul VJ, Luesch H. Marine cyanobacterial fatty acid amides acting on cannabinoid receptors. Chembiochem. 2012;13:2676–81. doi: 10.1002/cbic.201200502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle DG, Paul VJ. Production of secondary metabolites by filamentous tropical marine cyanobacteria: eclogical functions of the compounds. Journal of Phycology. 1999;35:1412–1421. [Google Scholar]
- Nahata M, Muto S, Nakagawa K, Ohnishi S, Sadakane C, Saegusa Y, Iizuka S, Hattori T, Asaka M, Takeda H. Serotonin 2C receptor antagonism ameliorates novelty-induced hypophagia in aged mice. Psychoneuroendocrinology. 2013;38:2051–64. doi: 10.1016/j.psyneuen.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Nakai K, Nakae A, Oba S, Mashimo T, Ueda K. 5-HT2C receptor agonists attenuate pain-related behaviour in a rat model of trigeminal neuropathic pain. European Journal of Pain. 2010;14:999–1006. doi: 10.1016/j.ejpain.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Obata H, Ito N, Goto F, Saito S. The nociceptive mechanism of 5-hydroxytryptamine released into the peripheral tissue in acute inflammatory pain in rats. Eur J Pain. 2009;13:441–7. doi: 10.1016/j.ejpain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Obata H, Saito S, Sakurazawa S, Sasaki M, Usui T, Goto F. Antiallodynic effects of intrathecally administered 5-HT(2C) receptor agonists in rats with nerve injury. Pain. 2004;108:163–9. doi: 10.1016/j.pain.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Angel RA, Navarro M, Breese GR. Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT1A and 5-HT2C ligands. Psychopharmacology (Berl) 2006;187:1–12. doi: 10.1007/s00213-006-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros-Burgess LA, Pentkowski NS, Der-Ghazarian T, Neisewander JL. Effects of the 5-HT2C receptor agonist CP809101 in the amygdala on reinstatement of cocaine-seeking behavior and anxiety-like behavior. Int J Neuropsychopharmacol. 2014;17:1751–62. doi: 10.1017/S1461145714000856. [DOI] [PubMed] [Google Scholar]
- Pytliak M, Vargova V, Mechirova V, Felsoci M. Serotonin receptors - from molecular biology to clinical applications. Physiol Res. 2011;60:15–25. doi: 10.33549/physiolres.931903. [DOI] [PubMed] [Google Scholar]
- Rainer Q, Xia L, Guilloux JP, Gabriel C, Mocaer E, Hen R, Enhamre E, Gardier AM, David DJ. Beneficial behavioural and neurogenic effects of agomelatine in a model of depression/anxiety. Int J Neuropsychopharmacol. 2012;15:321–35. doi: 10.1017/S1461145711000356. [DOI] [PubMed] [Google Scholar]
- Seibenhener ML, Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015:e52434. doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitachitta N, Gerwick WH. Grenadadiene and grenadamide, cyclopropyl-containing fatty acid metabolites from the marine cyanobacterium Lyngbya majuscula. J Nat Prod. 1998;61:681–4. doi: 10.1021/np970576a. [DOI] [PubMed] [Google Scholar]
- Tan LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007;68:954–79. doi: 10.1016/j.phytochem.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Taylor D, Sparshatt A, Varma S, Olofinjana O. Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. BMJ. 2014;348:g1888. doi: 10.1136/bmj.g1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Fang W, Leong DT, Tan LT. Biochemical studies of the lagunamides, potent cytotoxic cyclic depsipeptides from the marine cyanobacterium Lyngbya majuscula. Mar Drugs. 2012;10:1126–37. doi: 10.3390/md10051126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Puddick J, Prinsep MR, Rottmann M, Chan KP, Chen DY, Tan LT. Lagunamide C, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Lyngbya majuscula. Phytochemistry. 2011;72:2369–75. doi: 10.1016/j.phytochem.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Vicente MA, Zangrossi H., Jr Involvement of 5-HT2C and 5-HT1A receptors of the basolateral nucleus of the amygdala in the anxiolytic effect of chronic antidepressant treatment. Neuropharmacology. 2014;79:127–35. doi: 10.1016/j.neuropharm.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Ormel J, Katon W, Lin EH. Disability and depression among high utilizers of health care. A longitudinal analysis. Arch Gen Psychiatry. 1992;49:91–100. doi: 10.1001/archpsyc.1992.01820020011002. [DOI] [PubMed] [Google Scholar]
- Williams C, Espinosa OA, Montenegro H, Cubilla L, Capson TL, Ortega-Barria E, Romero LI. Hydrosoluble formazan XTT: its application to natural products drug discovery for Leishmania. J Microbiol Methods. 2003;55:813–6. doi: 10.1016/j.mimet.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Yamashita PS, de Bortoli VC, Zangrossi H., Jr 5-HT2C receptor regulation of defensive responses in the rat dorsal periaqueductal gray. Neuropharmacology. 2011;60:216–22. doi: 10.1016/j.neuropharm.2010.09.001. [DOI] [PubMed] [Google Scholar]