Abstract
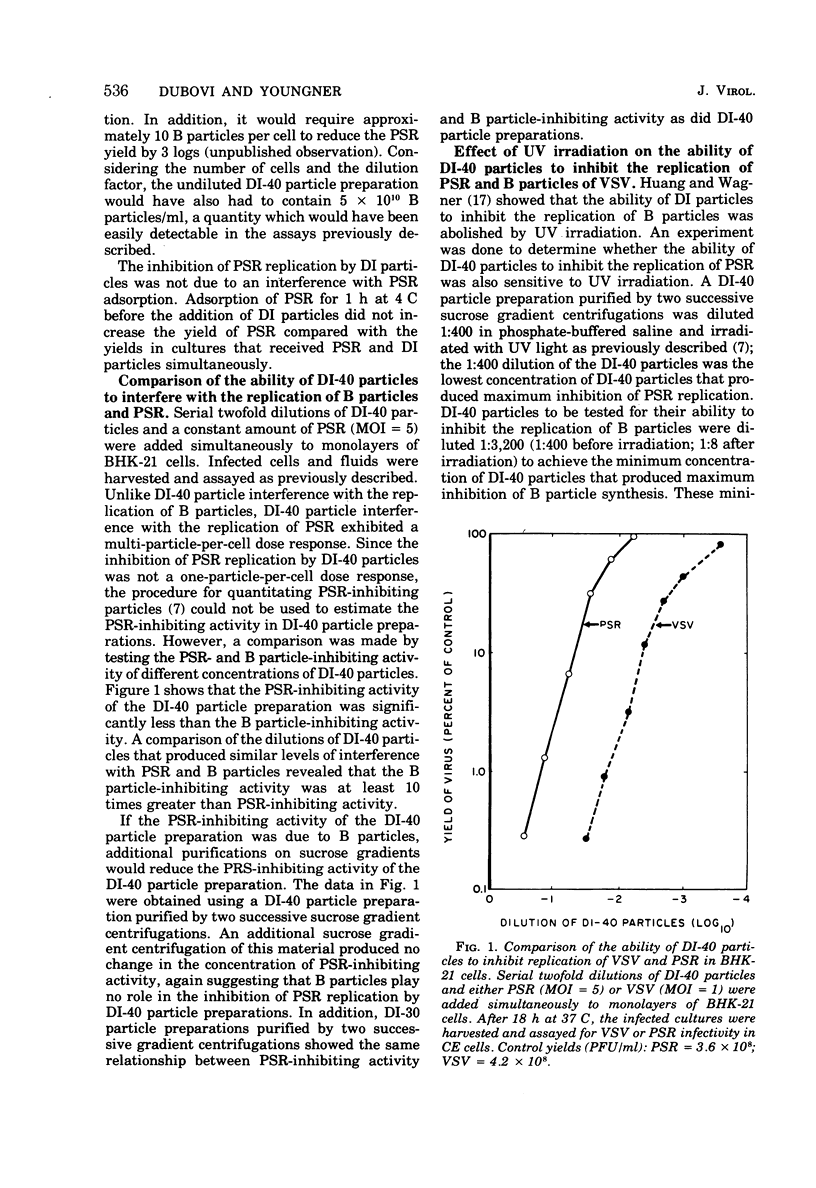
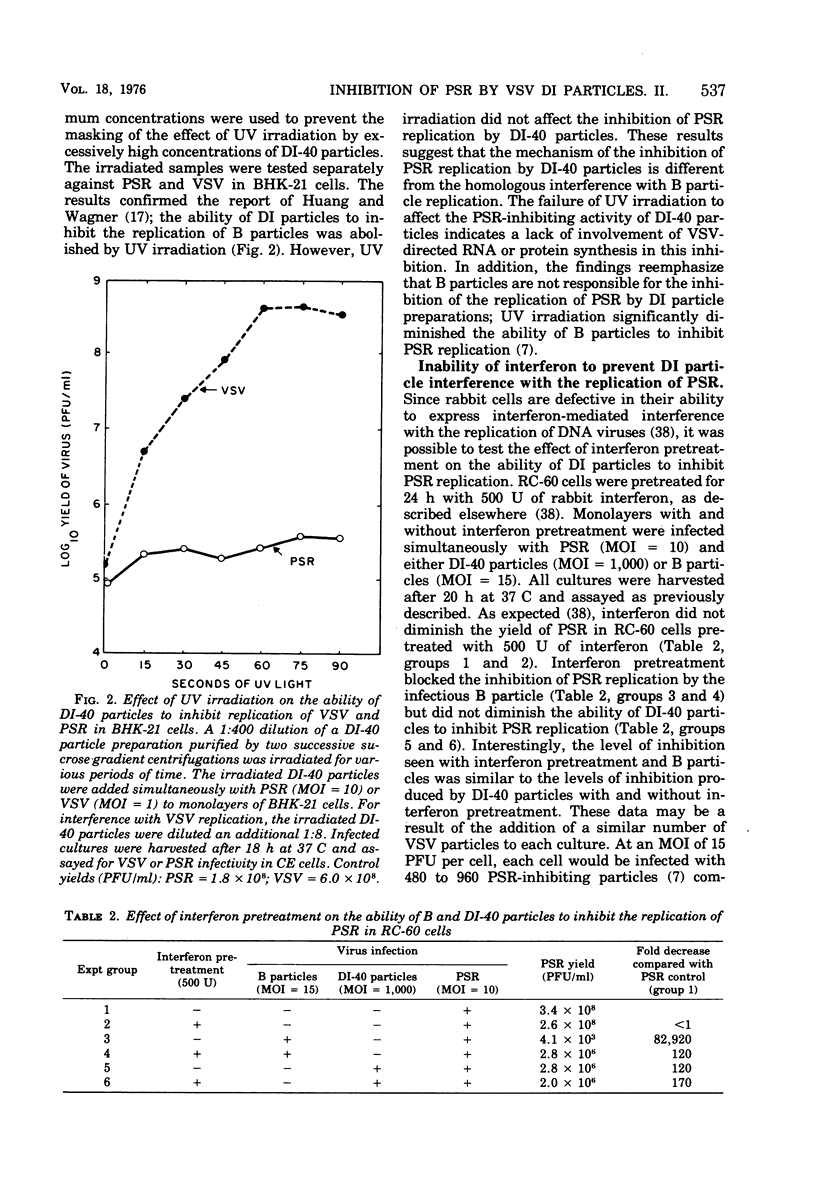
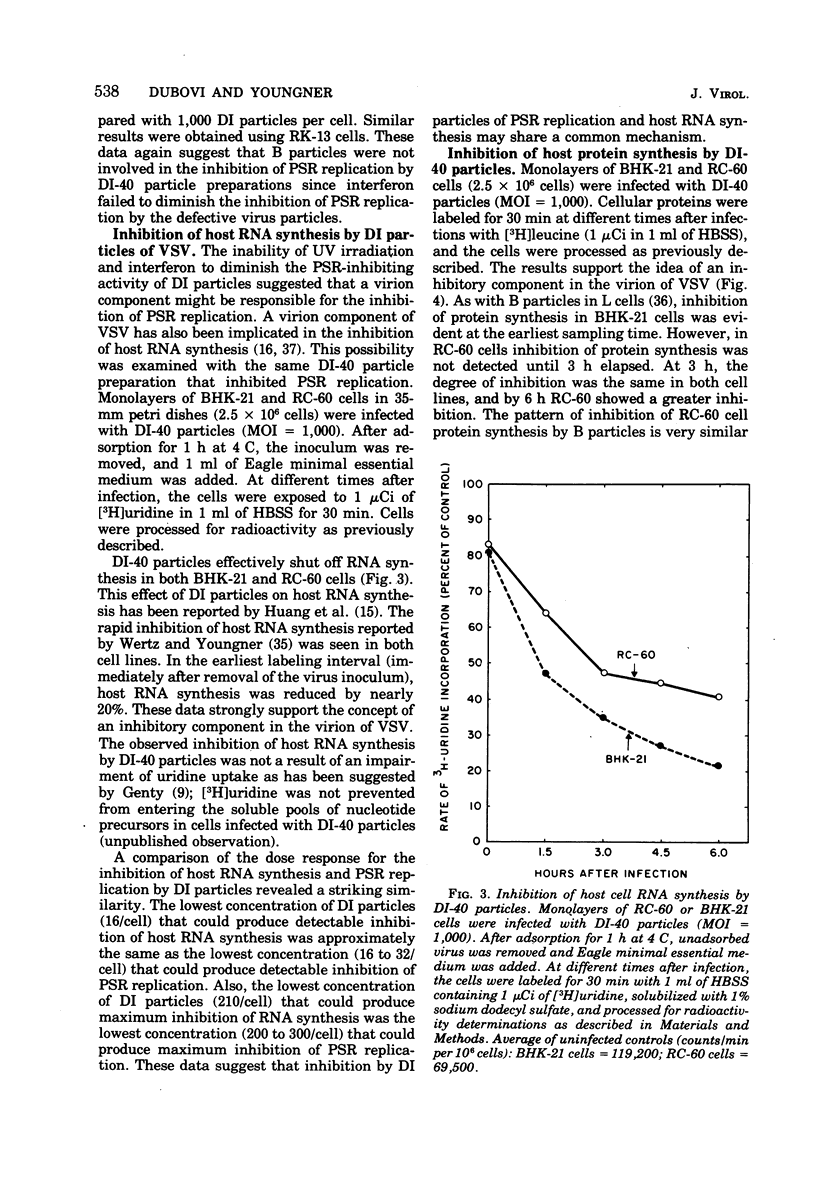
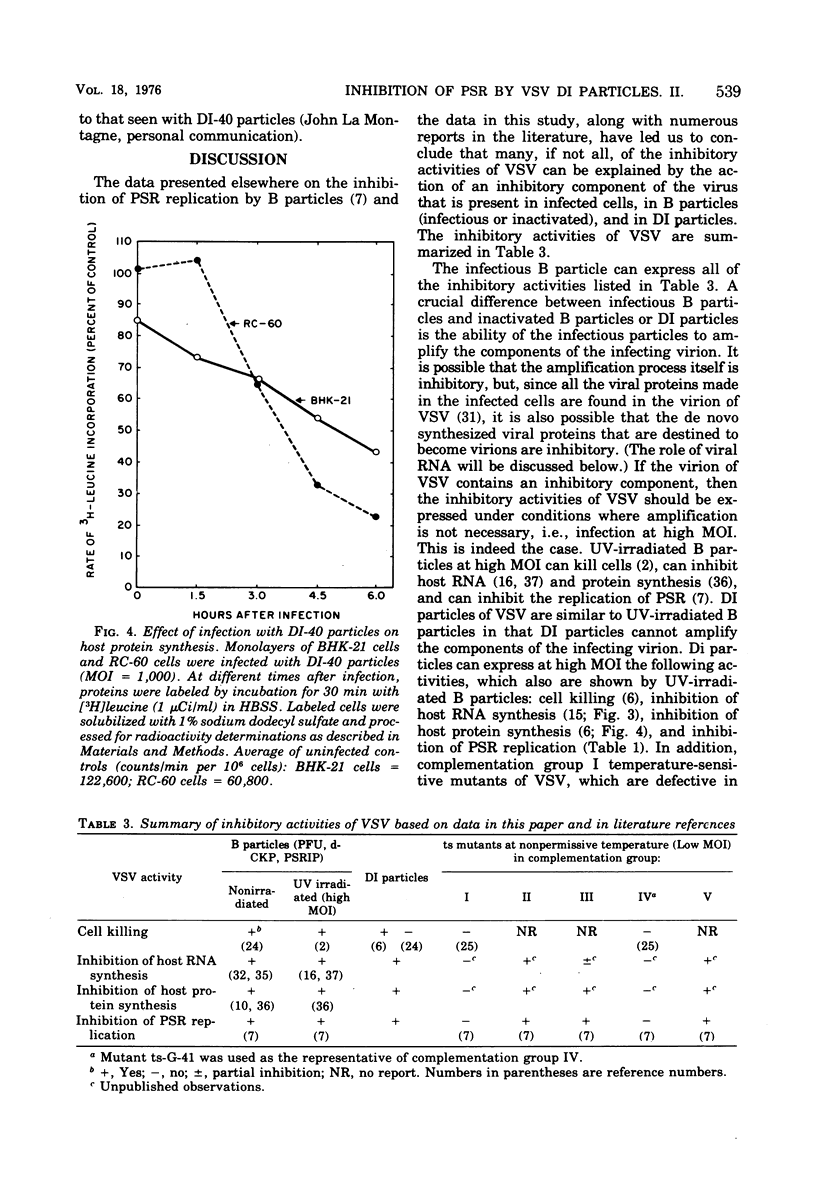
Purified defective interfering (DI) particles of vesicular stomatitis virus (VSV) inhibit the replication of a heterologous virus, pseudorabies virus (PSR), in hamster (BHK-21) and rabbit (RC-60) cell lines. In contrast to infectious B particles of VSV, UV irradiation of DI particles does not reduce their ability to inhibit PSR replication. However, UV irradiation progressively reduces the ability of DI particles to cause homologous interference with B particle replication. Pretreatment with interferon does not affect the ability of DI particles to inhibit PSR replication in a rabbit cell line (RC-60) in which RNA, but not DNA, viruses are sensitive to the action of interferon. Under similar conditions of interferon pretreatment, the inhibition of PSR by B particles is blocked. These data suggest that de novo VSV RNA or protein synthesis is not required for the inhibition of PSR replication by DI particles. DI particles that inhibit PSR replication also inhibit host RNA and protein synthesis in BHK-21 and RC-60 cells. Based on the results described and data in the literature, it is proposed that the same component of VSV B and DI particles is responsible for most, if not all, of the inhibitory activities of VSV, except homologous interference.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELLETT A. J., COOPER P. D. Some properties of the transmissible interfering component of vesicular stomatitis virus preparations. J Gen Microbiol. 1959 Dec;21:498–509. doi: 10.1099/00221287-21-3-498. [DOI] [PubMed] [Google Scholar]
- CANTELL K., SKURSKA Z., PAUCKER K., HENLE W. Quantitative studies on viral interference in suspended L cells. II. Factors affecting interference by UV-irradiated Newcastle disease virus against vesicular stomatitis virus. Virology. 1962 Jun;17:312–323. doi: 10.1016/0042-6822(62)90122-8. [DOI] [PubMed] [Google Scholar]
- Collins F. D., Roberts W. K. Mechanism of Mengo virus-induced cell injury in L cells: use of inhibitors of protein synthesis to dissociate virus-specific events. J Virol. 1972 Nov;10(5):969–978. doi: 10.1128/jvi.10.5.969-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell-Stewart B., Taylor M. W. Effect of viral double-stranded RNA on protein synthesis in intact cells. J Virol. 1973 Feb;11(2):232–237. doi: 10.1128/jvi.11.2.232-237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick J., Brown F. Interference as a measure of cross-relationships in the vesicular stomatitis group of rhabdo viruses. J Gen Virol. 1973 Jan;18(1):79–82. doi: 10.1099/0022-1317-18-1-79. [DOI] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Prophylaxis and immunization in mice by use of virus-free defective T particles to protect against intracerebral infection by vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2105–2108. doi: 10.1073/pnas.70.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Youngner J. S. Inhibition of pseudorabies virus replication by vesicular stomicles virus I. Activity of infectious and inactivated B particles. J Virol. 1976 May;18(2):526–533. doi: 10.1128/jvi.18.2.526-533.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty N. Analysis of uridine incorporation in chicken embryo cells infected by vesicular stomatitis virus and its temperature-sensitive mutants: uridine transport. J Virol. 1975 Jan;15(1):8–15. doi: 10.1128/jvi.15.1.8-15.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty N., Berreur P. Métabolisme des acides ribonucléiques et des protéines de cellules d'embryon de poulet infectées par le virus de la stomatite vésiculaire: étude des effets de mutants thermosensibles. Ann Microbiol (Paris) 1973 Jan;124(1):133–145. [PubMed] [Google Scholar]
- Heine J. W., Schnaitman C. A. Entry of vesicular stomatitis virus into L cells. J Virol. 1971 Nov;8(5):786–795. doi: 10.1128/jvi.8.5.786-795.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M., Breitbart H., Atlan H. Interactions between membrane functions and protein synthesis in reticulocytes. Effects of valinomycin and dicyclohexyl-18-crown-6. Eur J Biochem. 1974 Jun 1;45(1):161–170. doi: 10.1111/j.1432-1033.1974.tb03540.x. [DOI] [PubMed] [Google Scholar]
- Holloway A. F., Wong P. K., Cormack D. V. Isolation and characterization of temperature-sensitive mutants of vesicular stomatitis virus. Virology. 1970 Dec;42(4):917–926. doi: 10.1016/0042-6822(70)90340-5. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Inhibition of cellular RNA synthesis by nonreplicating vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1579–1584. doi: 10.1073/pnas.54.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Inhibition of cellular protein synthesis by double-stranded RNA: inactivation of an initiation factor. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1222–1226. doi: 10.1073/pnas.70.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay F. Envelope proteins of vesicular stomatitis virus: effect of temperature-sensitive mutations in complementation groups III and V. J Virol. 1974 Nov;14(5):1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Encephalomyocarditis virus infection of mouse plasmacytoma cells. I. Inhibition of cellular protein synthesis. J Virol. 1974 Sep;14(3):598–610. doi: 10.1128/jvi.14.3.598-610.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamnson R. N., Reichmann M. E. The RNA of defective vesicular stomatitis virus particles in relation to viral cistrons. J Mol Biol. 1974 Jan 5;85(4):551–568. doi: 10.1016/0022-2836(74)90315-5. [DOI] [PubMed] [Google Scholar]
- Manders E. K., Tilles J. G., Huang A. S. Interferon-mediated inhibition of virion-directed transcription. Virology. 1972 Aug;49(2):573–581. doi: 10.1016/0042-6822(72)90508-9. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. I. Comparison of cell-killing, plaque-forming, and defective-interfering particles of vesicular stomatitis virus. Virology. 1974 Feb;57(2):321–338. doi: 10.1016/0042-6822(74)90172-x. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. II. Cell killing by vesicular stomatitis virus: a requirement for virion-derived transcription. Virology. 1975 Jan;63(1):176–190. doi: 10.1016/0042-6822(75)90383-9. [DOI] [PubMed] [Google Scholar]
- McSharry J., Benzinger R. Concentration and purification of vesicular stomatitis virus by polyethylene glycol "precipitation". Virology. 1970 Mar;40(3):745–746. doi: 10.1016/0042-6822(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Pong S. S., Nuss D. L., Koch G. Inhibition of initiation of protein synthesis in mammalian tissue culture cells by L-1-tosylamido-2-phenylethyl chloromethyl ketone. J Biol Chem. 1975 Jan 10;250(1):240–245. [PubMed] [Google Scholar]
- Pringle C. R. Conditional lethal mutants of vesicular stomatitis virus. Curr Top Microbiol Immunol. 1975;69:85–116. doi: 10.1007/978-3-642-50112-8_2. [DOI] [PubMed] [Google Scholar]
- Soria M., Little S. P., Huang A. S. Characterization of vesicular stomatitis virus nucleocapsids. I. Complementary 40 S RNA molecules in nucleocapsids. Virology. 1974 Sep;61(1):270–280. doi: 10.1016/0042-6822(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Unger J. T., Reichmann M. E. RNA synthesis in temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1973 Sep;12(3):570–578. doi: 10.1128/jvi.12.3.570-578.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Huang A. S. Inhibition of RNA and interferon synthesis in Krebs-2 cells infected with vesicular stomatitis virus. Virology. 1966 Jan;28(1):1–10. doi: 10.1016/0042-6822(66)90300-x. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J Virol. 1972 Jan;9(1):85–89. doi: 10.1128/jvi.9.1.85-89.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Interferon production and inhibition of host synthesis in cells infected with vesicular stomatitis virus. J Virol. 1970 Oct;6(4):476–484. doi: 10.1128/jvi.6.4.476-484.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoi Y., Mitsui H., Amano M. Effect of U.v.-irradiated vesicular stomatitis virus on nucleic acid synthesis in chick embryo cells. J Gen Virol. 1970 Sep;8(3):165–172. doi: 10.1099/0022-1317-8-3-165. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Thacore H. R., Kelly M. E. Sensitivity of ribonucleic acid and deoxyribonucleic acid viruses to different species of interferon in cell cultures. J Virol. 1972 Aug;10(2):171–178. doi: 10.1128/jvi.10.2.171-178.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



