Abstract
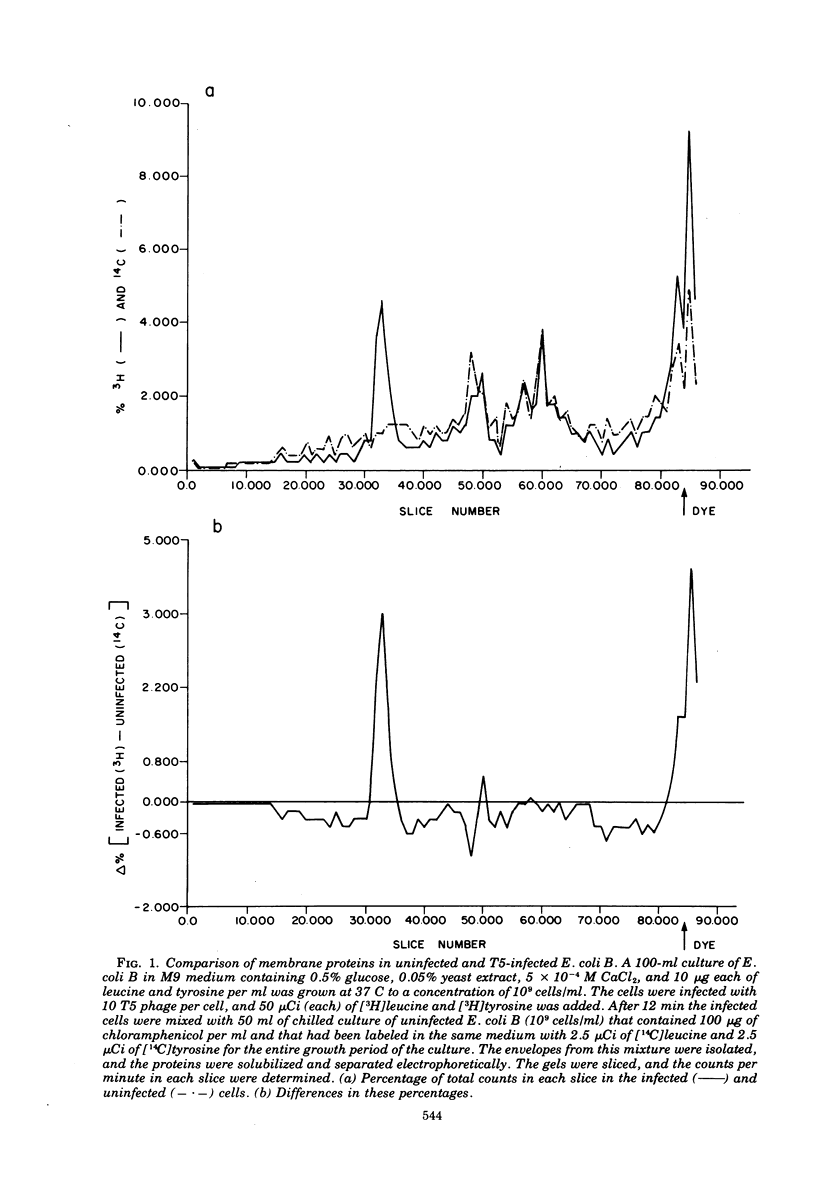
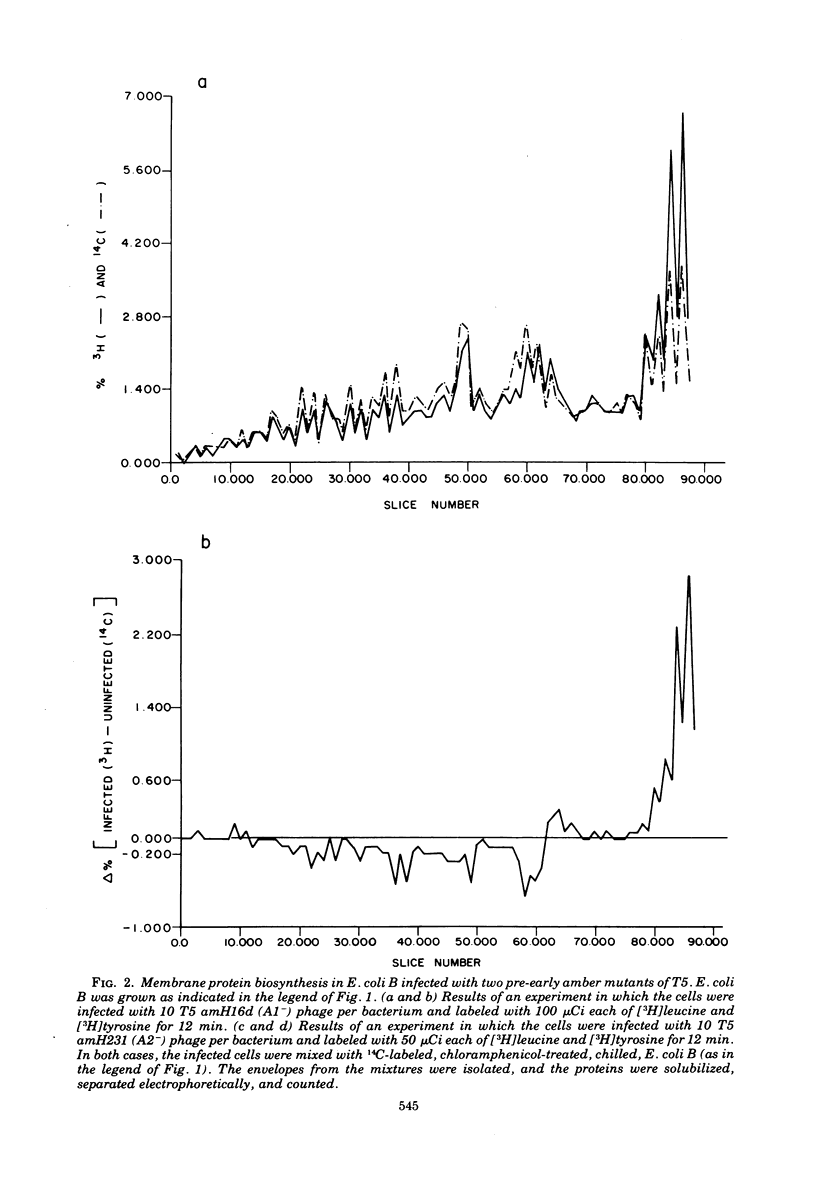
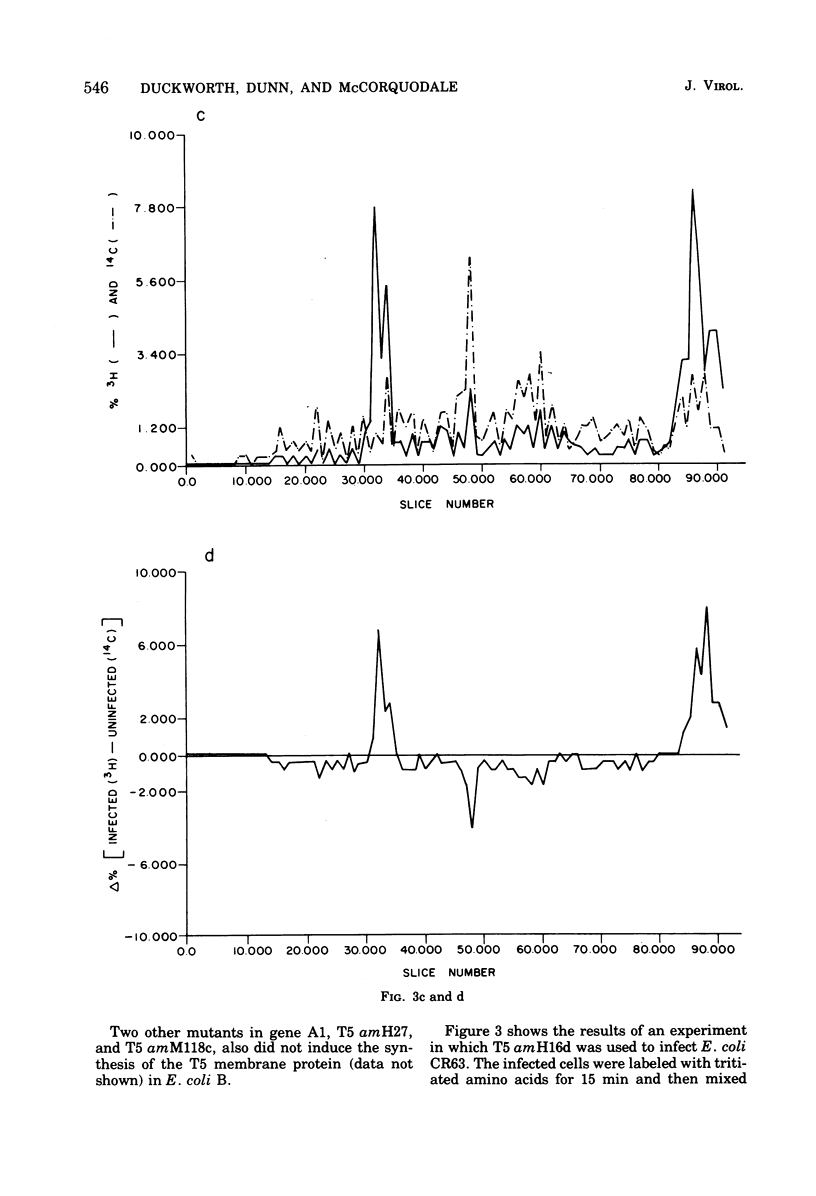
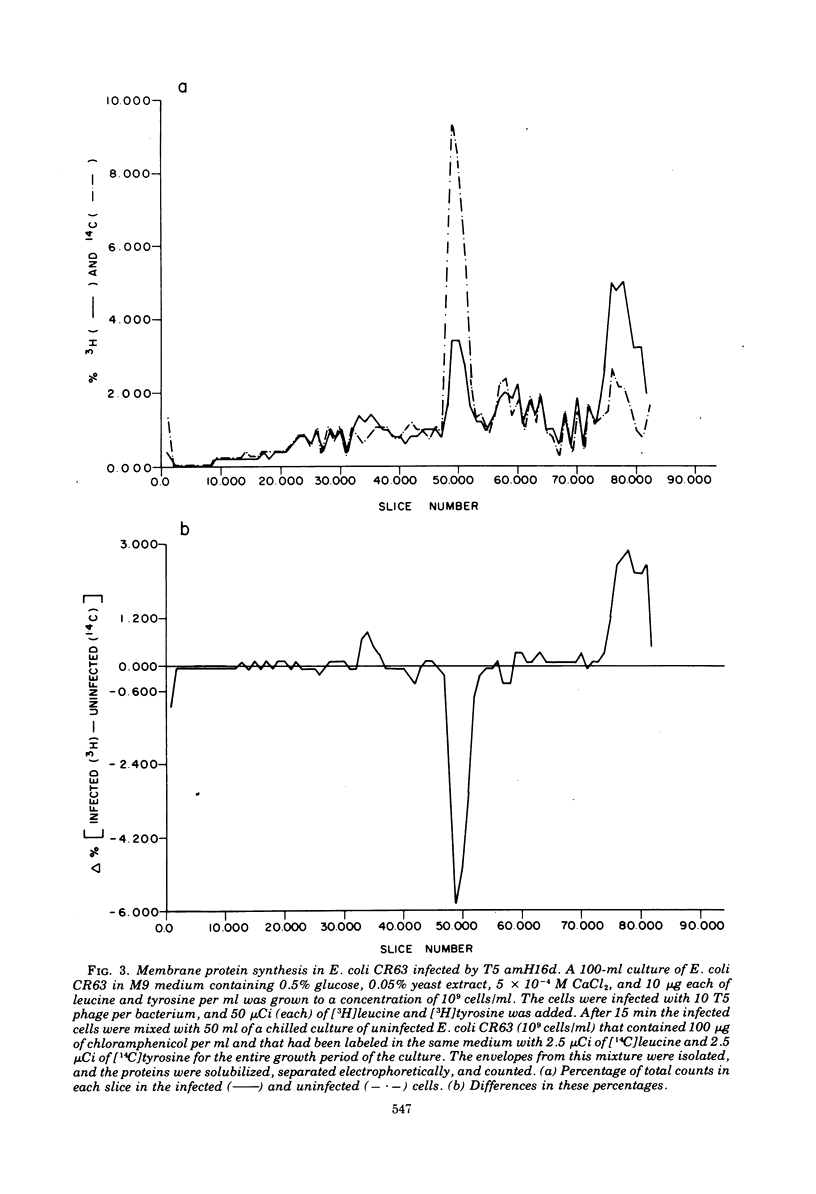
After infection of Escherichia coli B by bacteriophage T5, a major new protein species, as indicated by polyacrylamide gel electrophoresis, appears in the cells' membranes. Phage mutants with amber mutations in the first-step-transfer portion of their DNA have been tested for their ability to induce membrane protein synthesis after they infect E. coli B. We have found that phage with mutations in the Al gene of T5 do not induce the synthesis of the T5-specific major membrane protein, whereas phage that are mutant in the A2 gene do induce its synthesis. We conclude that gene Al must function normally for T5-specific membrane protein biosynthesis to occur and that only the first 8% (first-step-transfer piece) of the DNA need be present in the cell for synthesis to occur.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman L. D., Anderson G. C., McCorquodale D. J. Arrangement on the chromosome of the known pre-early genes of bacteriophages T5 and BF23. J Virol. 1973 Nov;12(5):1191–1194. doi: 10.1128/jvi.12.5.1191-1194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman L. D., Hoffman M. S., McCorquodale D. J. Pre-early proteins of bacteriophage T5: structure and function. J Mol Biol. 1971 Dec 28;62(3):551–564. doi: 10.1016/0022-2836(71)90155-0. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Dunn G. B. Membrane protein biosynthesis in T5 bacteriophage-infected Escherichia coli. Arch Biochem Biophys. 1976 Feb;172(2):319–328. doi: 10.1016/0003-9861(76)90083-7. [DOI] [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. Preparation, purification, and properties of E. coli virus T2. J Gen Physiol. 1952 May;36(1):17–28. doi: 10.1085/jgp.36.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson H. E., McCorquodale D. J. Genetic and physiological studies of bacteriophage t5 I. An expanded genetic map of t5. J Virol. 1971 May;7(5):612–618. doi: 10.1128/jvi.7.5.612-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. M. Membrane-associated proteins of T4-infected Escherichia coli. Virology. 1975 Aug;66(2):508–521. doi: 10.1016/0042-6822(75)90223-8. [DOI] [PubMed] [Google Scholar]
- Jacquemin-Sablon A., Lanni Y. T. Lambda-repressed mutants of bacteriophage T5. I. Isolation and genetical characterization. Virology. 1973 Nov;56(1):230–237. doi: 10.1016/0042-6822(73)90302-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanni Y. T. First-step-transfer deoxyribonucleic acid of bacteriophage T5. Bacteriol Rev. 1968 Sep;32(3):227–242. doi: 10.1128/br.32.3.227-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- McCorquodale D. J., Buchanan J. M. Patterns of protein synthesis in T5-infected Escherichia coli. J Biol Chem. 1968 May 25;243(10):2550–2559. [PubMed] [Google Scholar]
- McCorquodale D. J., Lanni Y. T. Patterns of protein synthesis in Escherichia coli infected by amber mutants in the first-step-transfer DNA of T5. J Mol Biol. 1970 Feb 28;48(1):133–143. doi: 10.1016/0022-2836(70)90224-x. [DOI] [PubMed] [Google Scholar]
- Mizobuchi K., McCorquodale D. J. Abortive infection by bacteriophage BF23 due to the colicin Ib factor. II. Involvement of pre-early proteins. J Mol Biol. 1974 May 5;85(1):67–74. doi: 10.1016/0022-2836(74)90129-6. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Fu A. S., Szabo C. Regulation of bacteriophage T5 development by ColI factors. J Virol. 1972 May;9(5):804–812. doi: 10.1128/jvi.9.5.804-812.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. F., Kievitt K. D., Ennis H. L. Membrane protein synthesis after infection of Escherichia coli B with phage T4: the rIIB protein. Virology. 1972 Nov;50(2):520–527. doi: 10.1016/0042-6822(72)90403-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Weintraub S. B., Frankel F. R. Identification of the T4rIIB gene product as a membrane protein. J Mol Biol. 1972 Oct 14;70(3):589–615. doi: 10.1016/0022-2836(72)90561-x. [DOI] [PubMed] [Google Scholar]


