Abstract
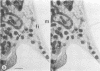
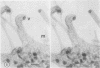
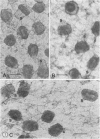
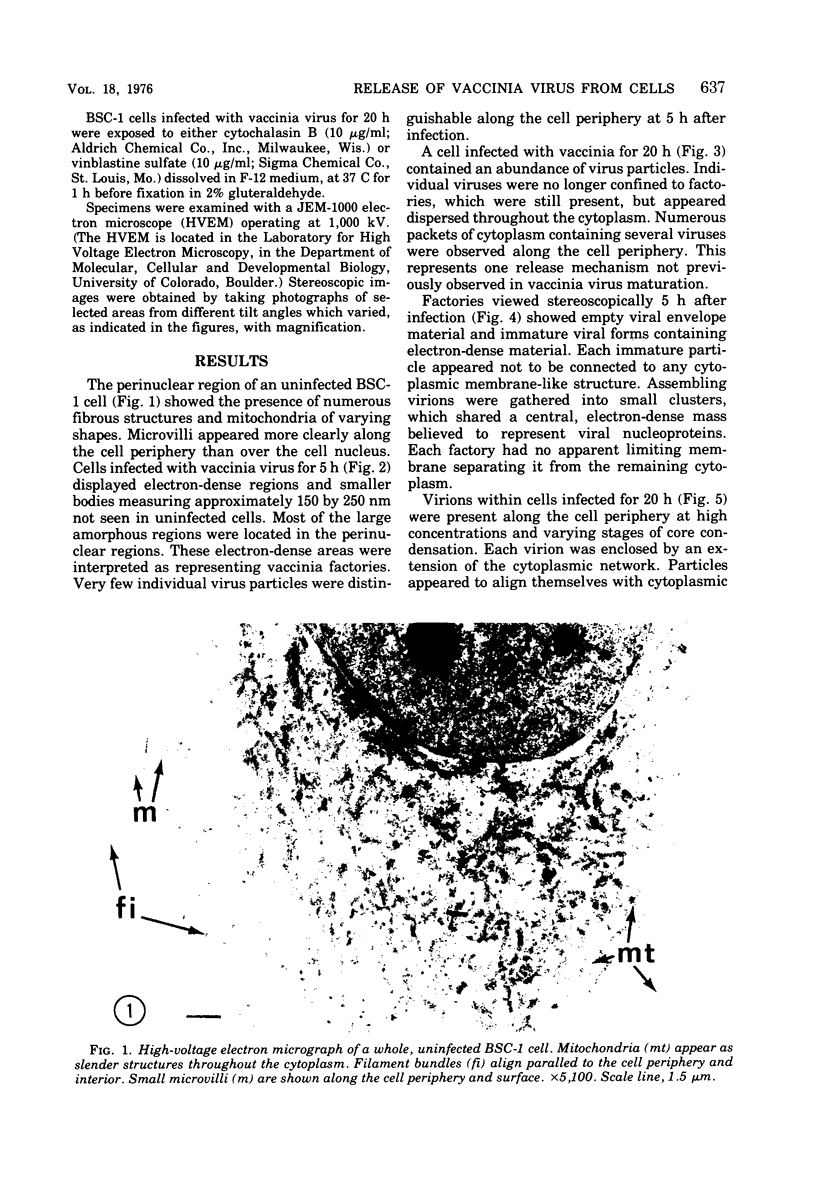
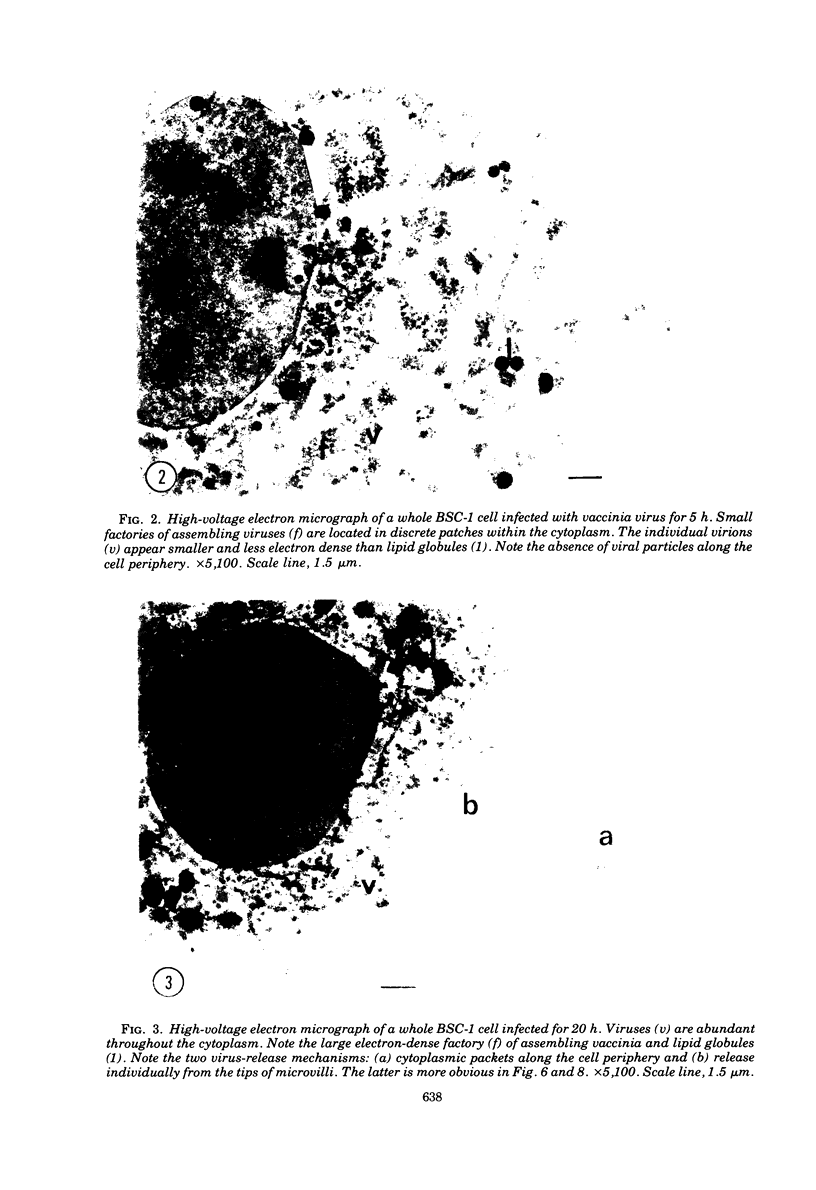
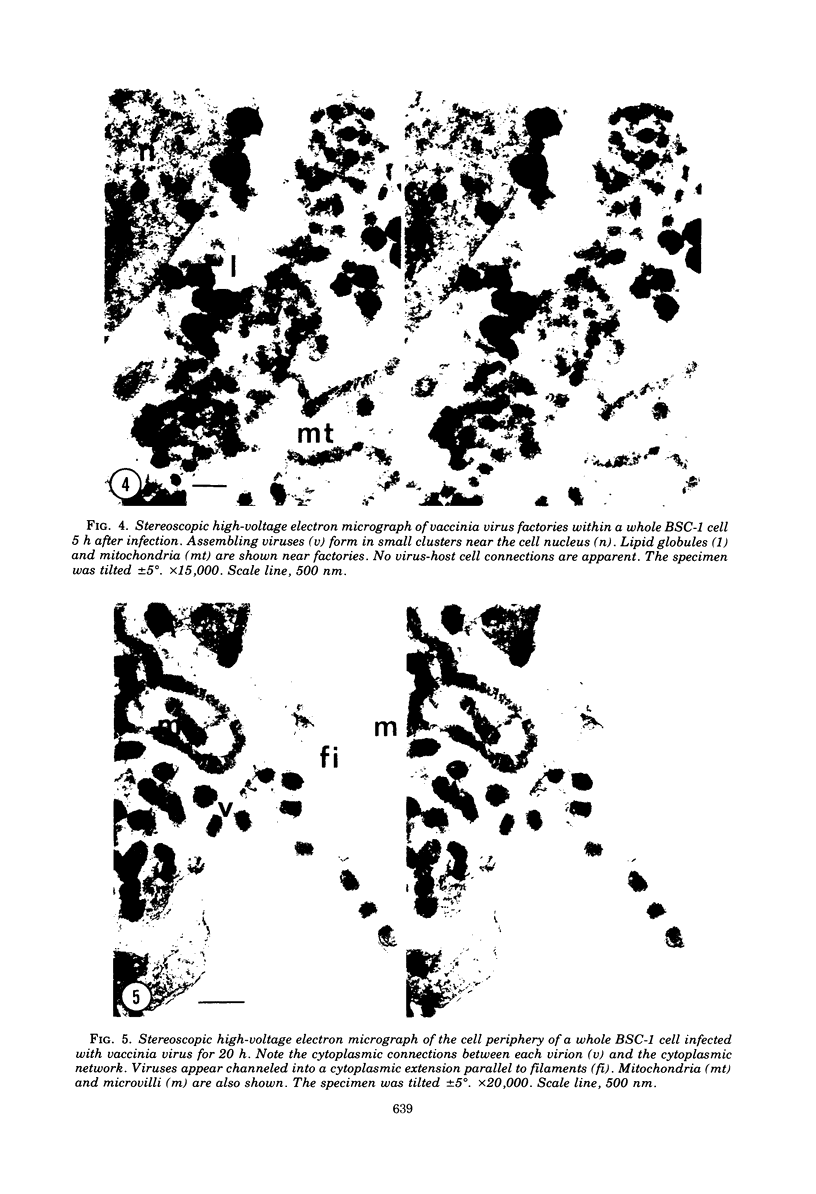
High-voltage (1,000-kV) electron microscope examination of whole BSC-1 cells infected with vaccinia virus at different times after infection revealed the presence of increasing numbers of virions no longer confined to factories but situated along the cell periphery of monolayer cells. Stereoscopic images showed each virus enclosed within a membrane-like component of the host cell cytoplasm. Viruses within factories appeared to lack similar enclosures. Cytochalasin B, but not vinblastine, caused the enclosures to disrupt. Vaccinia viruses were observed to escape the host cell individually from the tips of microvillie and within packets of cytoplasm. Observations suggest that the intracellular movement and release of vaccinia virus utilize a host cell cytoplasmic network that involves microfilaments for stability.
Full text
PDF
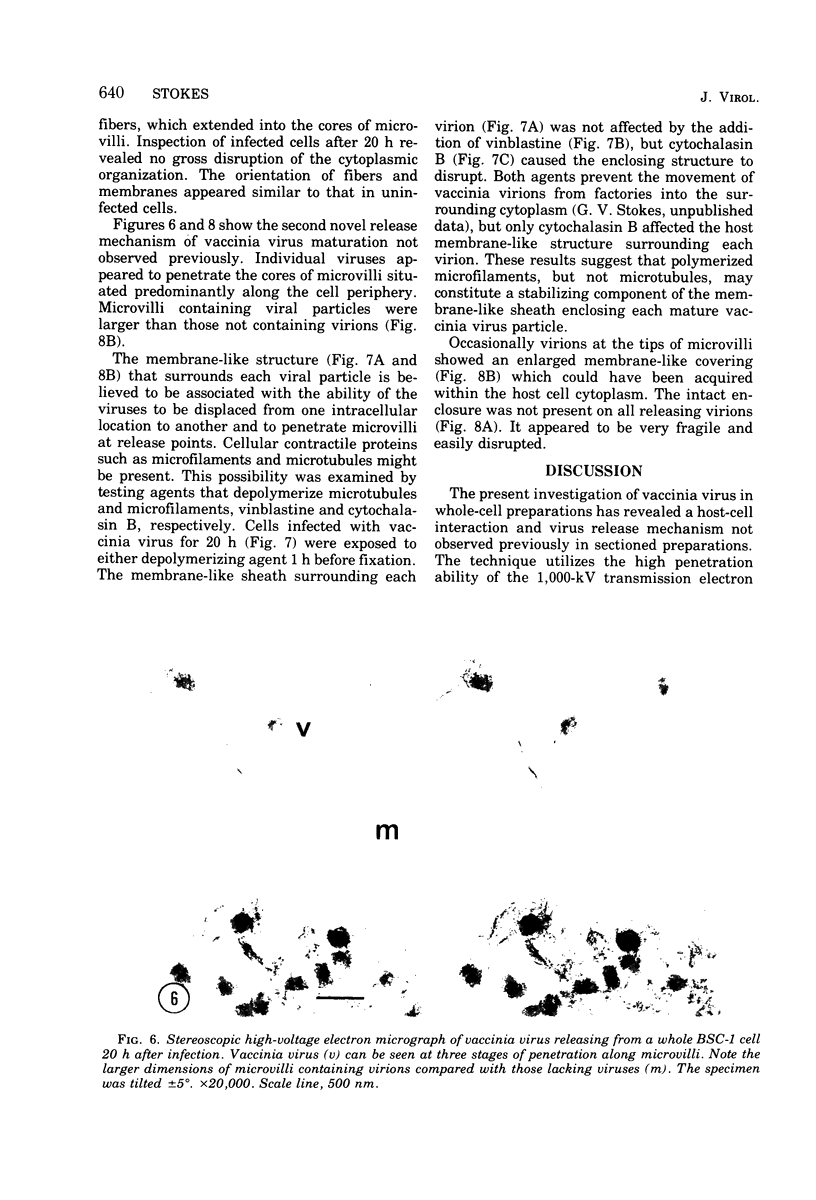
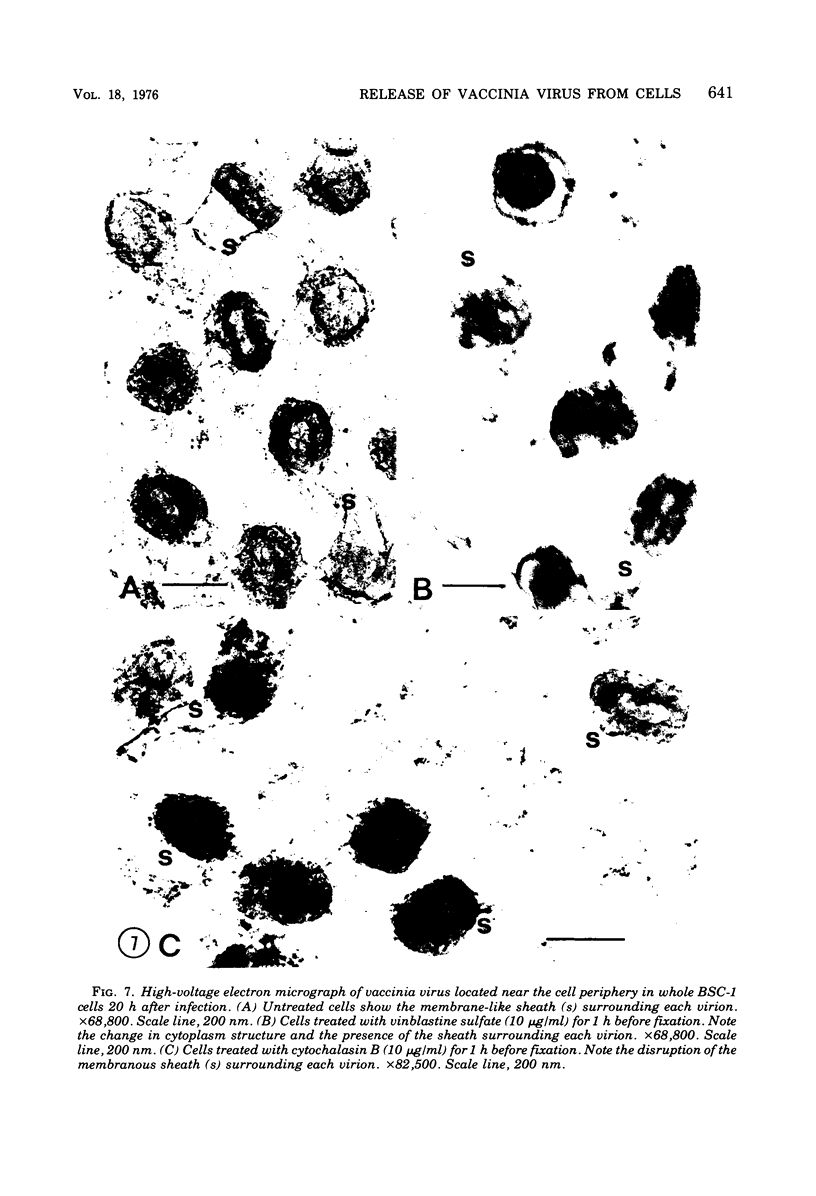


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley I. K. Three dimensional fine structure of cultured cells: possible implications for subcellular motility. Tissue Cell. 1975;7(1):51–72. doi: 10.1016/s0040-8166(75)80007-3. [DOI] [PubMed] [Google Scholar]
- Dales S., Chardonnet Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology. 1973 Dec;56(2):465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- Dales S. Pentration of animal viruses into cells. Prog Med Virol. 1965;7:1–43. [PubMed] [Google Scholar]
- Easterbrook K. B. Crystalline aggregates observed in the vicinity of freeze-etched poxvirus inclusions. Can J Microbiol. 1972 Apr;18(4):403–406. doi: 10.1139/m72-064. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Weihing R. R. Adenovirus binds to rat brain microtubules in vitro. J Virol. 1975 Sep;16(3):696–706. doi: 10.1128/jvi.16.3.696-706.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew D. E., Carrol T. W. Barley stripe mosaic virions assoicated with spindle microtubules. Science. 1974 Sep 13;185(4155):957–958. doi: 10.1126/science.185.4155.957. [DOI] [PubMed] [Google Scholar]
- NAGINGTON J., HORNE R. W. Morphological studies of orf and vaccinia viruses. Virology. 1962 Mar;16:248–260. doi: 10.1016/0042-6822(62)90245-3. [DOI] [PubMed] [Google Scholar]
- Weintraub S., Dales S. Biogenesis of poxviruses: genetically controlled modifications of structural and functional components of the plasma membrane. Virology. 1974 Jul;60(1):96–127. doi: 10.1016/0042-6822(74)90369-9. [DOI] [PubMed] [Google Scholar]