Abstract
Nonionic detergent treatments released a nucleocapsid from the enveloped bacteriphage phi6. The nucleocapsid sedimented at nearly the same rate as the whole phage in sucrose density gradients, but the buoyant density in Cs2S04 changed from 1.22 g/cm3 for the whole phage to 1.33 g/cm3 for the nucleocapsid. The detergent completely removed the lipid and 5 of the 10 proteins from the phage. Surface labeling of the phage and nucleocapsid with 125I revealed that protein P3 was on the outer surface of the whole phage and P8 was on the surface of the nucleocapsid. Both the phage and the nucleocapsid were stable between pH 6.0 and 9.5. Low concentrations of EDTA (10-4 M) dissociated the nucleocapsid but had no effect on the whole phage. The nucleocapsid contained all three double-stranded RNA segments, as well as RNA polymerase activity.
Full text
PDF


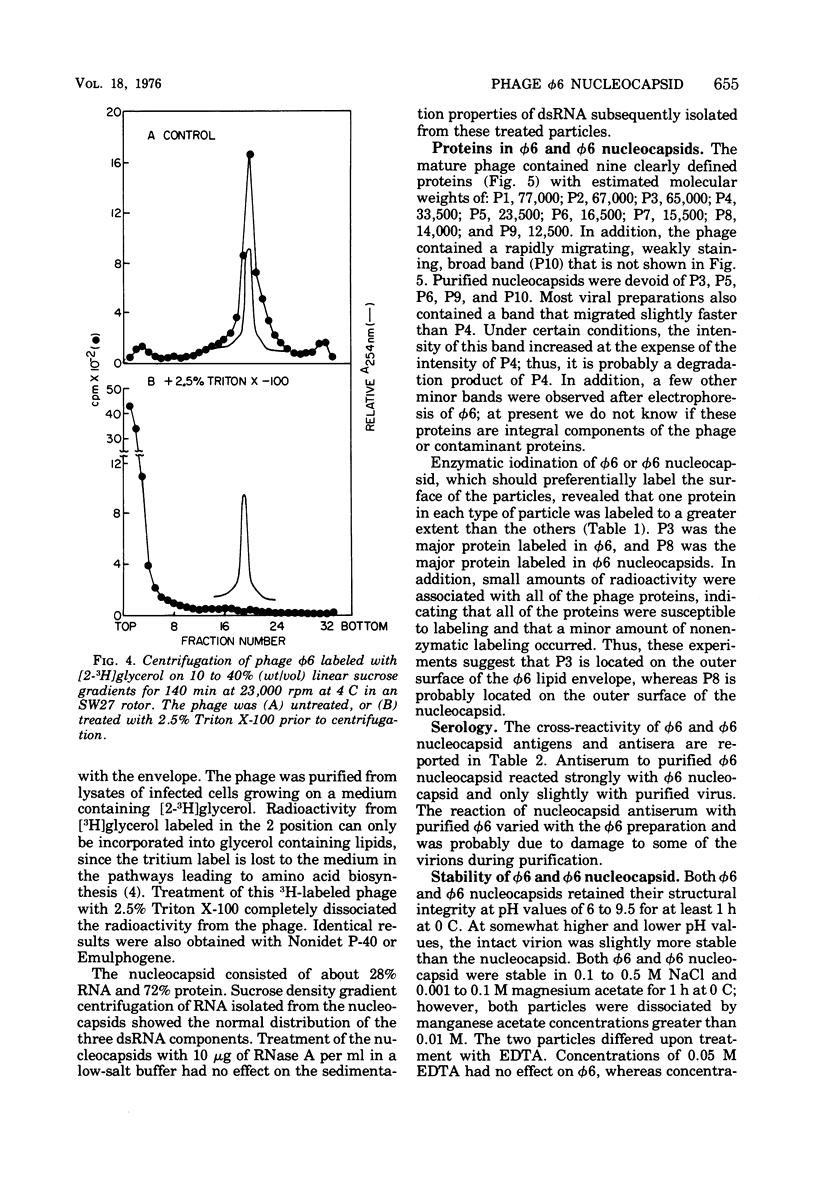



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball E. M., Brakke M. K. Analysis of antigen-antibody reactions of two plant viruses by density-gradient centrifugation and electron microscopy. Virology. 1969 Dec;39(4):746–758. doi: 10.1016/0042-6822(69)90012-9. [DOI] [PubMed] [Google Scholar]
- Brakke M. K., Van Pelt N. Linear-log sucrose gradients for estimating sedimentation coefficients of plant viruses and nucleic acids. Anal Biochem. 1970 Nov;38(1):56–64. doi: 10.1016/0003-2697(70)90155-7. [DOI] [PubMed] [Google Scholar]
- Coplin D. L., Van Etten J. L., Koski R. K., Vidaver A. K. Intermediates in the biosynthesis of double-stranded ribonucleic acids of bacteriophage phi 6. Proc Natl Acad Sci U S A. 1975 Mar;72(3):849–853. doi: 10.1073/pnas.72.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane L. C. The components of barley stripe mosaic and related viruses. Virology. 1974 Apr;58(2):323–333. doi: 10.1016/0042-6822(74)90068-3. [DOI] [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- ROCHOW W. F., BRAKKE M. K. PURIFICATION OF BARLEY YELLOW DWARF VIRUS. Virology. 1964 Nov;24:310–322. doi: 10.1016/0042-6822(64)90169-2. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Quigley J. P. Virus-induced modification of cellular membranes related to viral structure. Annu Rev Microbiol. 1974;28(0):325–351. doi: 10.1146/annurev.mi.28.100174.001545. [DOI] [PubMed] [Google Scholar]
- Sands J. A., Cupp J., Keith A., Snipes W. Temperature sensitivity of the assembly process of the enveloped bacteriophage phi6. Biochim Biophys Acta. 1974 Dec 10;373(2):277–285. doi: 10.1016/0005-2736(74)90151-5. [DOI] [PubMed] [Google Scholar]
- Sands J. A. The phospholipid composition of bacteriophage phi6. Biochem Biophys Res Commun. 1973 Nov 1;55(1):111–116. doi: 10.1016/s0006-291x(73)80066-x. [DOI] [PubMed] [Google Scholar]
- Sinclair J. F., Tzagoloff A., Levine D., Mindich L. Proteins of bacteriophage phi6. J Virol. 1975 Sep;16(3):685–695. doi: 10.1128/jvi.16.3.685-695.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Van Etten J. L., Vidaver A. K., Koski R. K., Burnett J. P. Base composition and hybridization studies of the three double-stranded RNA segments of bacteriophage phi 6. J Virol. 1974 Jun;13(6):1254–1262. doi: 10.1128/jvi.13.6.1254-1262.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Vidaver A. K., Koski R. K., Semancik J. S. RNA polymerase activity associated with bacteriophage phi 6. J Virol. 1973 Sep;12(3):464–471. doi: 10.1128/jvi.12.3.464-471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Schuster M. L. Characterization of Xanthomonas phaseoli Bacteriophages. J Virol. 1969 Sep;4(3):300–308. doi: 10.1128/jvi.4.3.300-308.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wood H. A. Viruses with double-stranded RNA genomes. J Gen Virol. 1973 Jun;20(Suppl):61–85. doi: 10.1099/0022-1317-20-Supplement-61. [DOI] [PubMed] [Google Scholar]
- Zaitlin M., Hariharasubramanian V. An improvement in a procedure for counting tritium and carbon-14 in polyacrylamide gels. Anal Biochem. 1970 May;35(1):296–297. doi: 10.1016/0003-2697(70)90038-2. [DOI] [PubMed] [Google Scholar]





