Abstract
A sensitive nitrocellulose filter assay that measures the retention of 125I single-stranded calf thymus DNA has been used to detect and purify DNA-binding proteins that retain a biological function from Rauscher murine leukemia virus. By consecutive purification on oligo (dT)- cellulose and DEAE-Bio-Gel columns and centrifugation in 10 to 30% glycerol gradients, RNA-dependent DNA polymerase has been separated from a second virion DNA-binding protein. The binding of this protein to DNA was strongly affected by NaCl concentration but showed little change in activity over a wide range of temperature or pH. After glycerol gradient purification, polyacrylamide gel electrophoresis of this protein showed one major band with a molecular weight of approximately 9,800. This protein binds about as well as to single-stranded Escherichia coli or calf thymus DNA or 70S type C viral RNA. The binding to 125I single-stranded calf thymus DNA is very efficiently inhibited by unlabeled single-stranded DNA from either E. coli or calf thymus and by 70S murine or feline viral RNA. Much larger amounts of double-stranded DNA are required to produce an equivalent percentage of inhibition. This protein, therefore, shows preferential binding to single-stranded DNA or viral RNA.
Full text
PDF


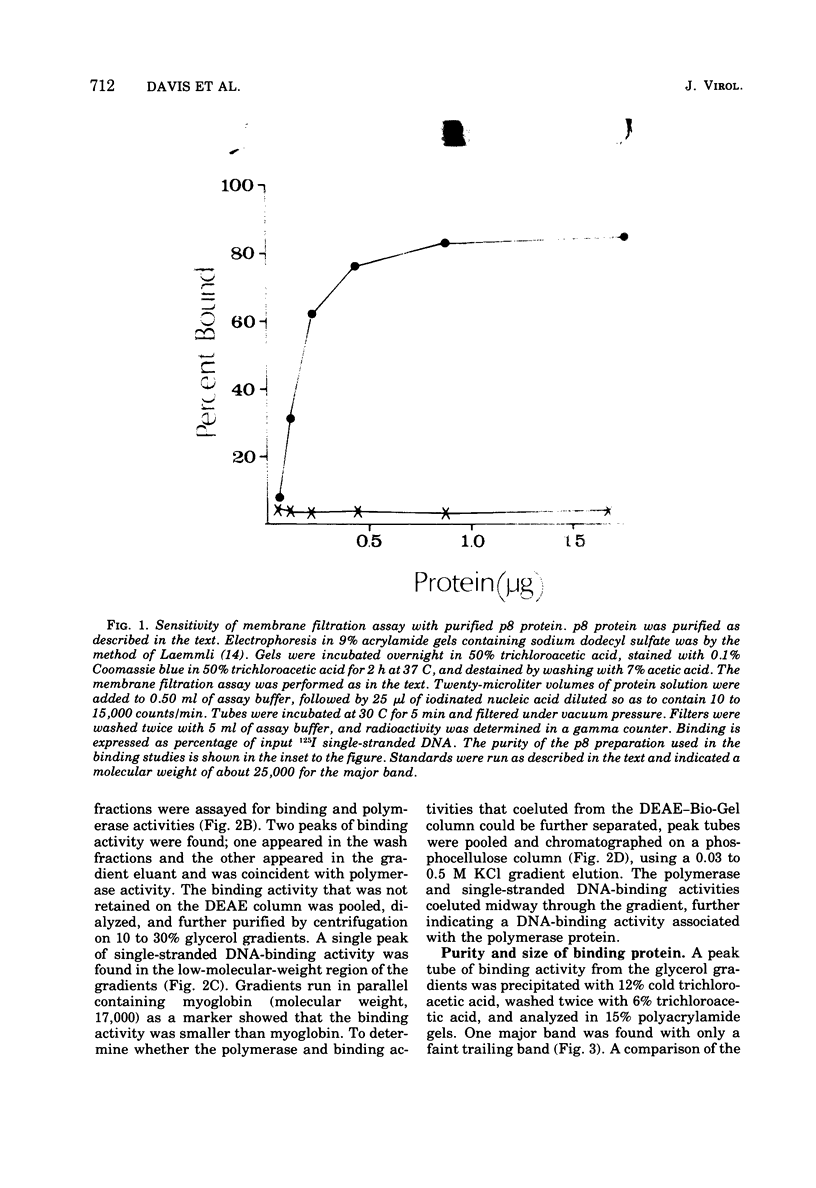

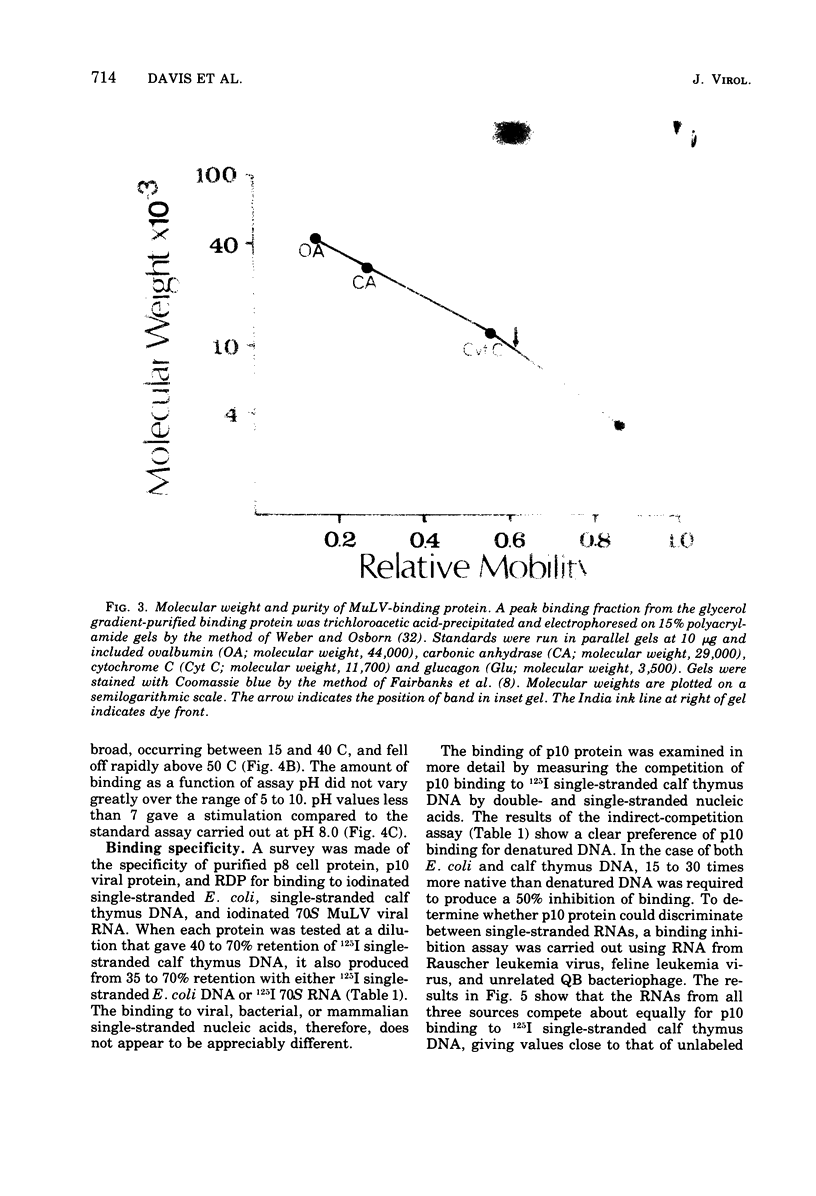

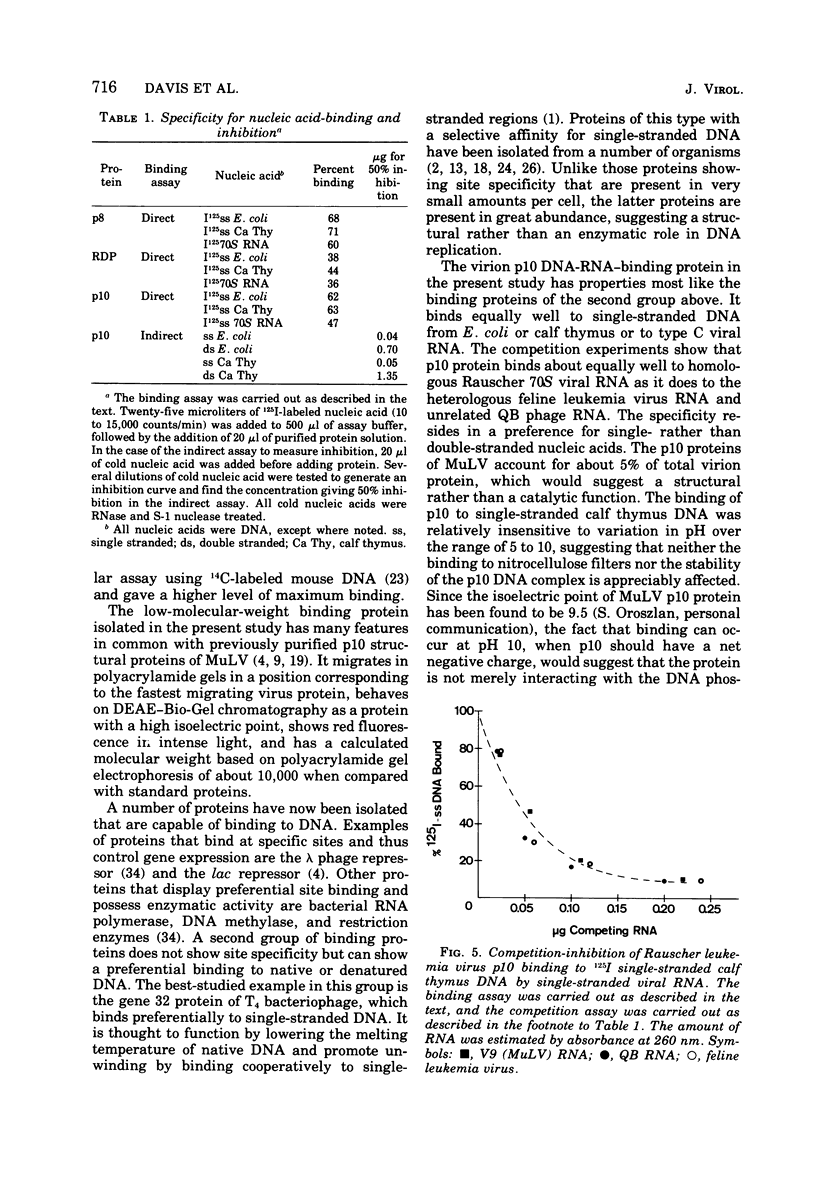


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Alberts B., Frey L., Delius H. Isolation and characterization of gene 5 protein of filamentous bacterial viruses. J Mol Biol. 1972 Jul 14;68(1):139–152. doi: 10.1016/0022-2836(72)90269-0. [DOI] [PubMed] [Google Scholar]
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Hager L., Dulbecco R. Simian virus 40 T antigen binds to DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3754–3757. doi: 10.1073/pnas.71.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Del Giudice R. A., Robillard N. F., Carski T. R. Immunofluorescence identification of Mycoplasma on agar by use of incident illumination. J Bacteriol. 1967 Apr;93(4):1205–1209. doi: 10.1128/jb.93.4.1205-1209.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fleissner E., Tress E. Isolation of a ribonucleoprotein structure from oncornaviruses. J Virol. 1973 Dec;12(6):1612–1615. doi: 10.1128/jvi.12.6.1612-1615.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. The lac operator is DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2415–2421. doi: 10.1073/pnas.58.6.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Z., Arens M. Q., Bhaduri S., Shanmugam G., Green M. Tumour antigen specificity of a DNA-binding protein from cells infected with adenovirus 2. Nature. 1975 Apr 10;254(5500):533–536. doi: 10.1038/254533a0. [DOI] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Stern H. A DNA-binding protein in meiotic cells of Lilium. Dev Biol. 1971 Sep;26(1):87–99. doi: 10.1016/0012-1606(71)90110-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long C., Sachs R., Norvell J., Huebner V., Hatanaka M., Gilden R. Specificity of antibody to the RD-114 viral polymerase. Nat New Biol. 1973 Jan 31;241(109):147–149. doi: 10.1038/newbio241147a0. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rongey R. W., Rasheed S., Sarma P. S., Huebner R. J., Hatanaka M., Oroszlan S., Gilden R. V. C-type virus released from cultured human rhabdomyosarcoma cells. Nat New Biol. 1972 Jan 5;235(53):3–6. doi: 10.1038/newbio235003a0. [DOI] [PubMed] [Google Scholar]
- Oey J. L., Knippers R. Properties of the isolated gene 5 protein of bacteriophage fd. J Mol Biol. 1972 Jul 14;68(1):125–138. doi: 10.1016/0022-2836(72)90268-9. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Summers M. R., Foreman C., Gilden R. V. Murine type-C virus group-specific antigens: interstrain immunochemical, biophysical, and amino acid sequence differences. J Virol. 1974 Dec;14(6):1559–1574. doi: 10.1128/jvi.14.6.1559-1574.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Noon M. C., Gilden R., Scolnick E. M. Serological studies with low-molecular-weight polypeptides from the Moloney strain of murine leukemia virus. J Virol. 1975 Jun;15(6):1385–1395. doi: 10.1128/jvi.15.6.1385-1395.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Specific binding of the lambda phage repressor to lambda DNA. Nature. 1967 Apr 15;214(5085):232–234. doi: 10.1038/214232a0. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Ferguson J., Davis R. W., Stark G. R. T antigen binds to simian virus 40 DNA at the origin of replication. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1605–1609. doi: 10.1073/pnas.72.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio V., Tsai W. P., Rand K., Long C. A comparison of DNA binding proteins from normal and virus-transformed mouse cells. Int J Cancer. 1973 Nov 15;12(3):545–550. doi: 10.1002/ijc.2910120302. [DOI] [PubMed] [Google Scholar]
- Salstrom J. S., Pratt D. Role of coliphage M13 gene 5 in single-stranded DNA production. J Mol Biol. 1971 Nov 14;61(3):489–501. doi: 10.1016/0022-2836(71)90061-1. [DOI] [PubMed] [Google Scholar]
- Shanmugam G., Bhaduri S., Arens M., Green M. DNA binding proteins in the cytoplasm and in a nuclear membrane complex isolated from uninfected and adenovirus 2 infected cells. Biochemistry. 1975 Jan 28;14(2):332–337. doi: 10.1021/bi00673a020. [DOI] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Dungworth D. L., Kawakami T. G., Munn R. J., Ward J. M., Harrold J. B. Experimental induction of lymphosarcoma in the cat with "C"-type virus. Cancer Res. 1970 Feb;30(2):401–408. [PubMed] [Google Scholar]
- Tronick S. R., Stephenson J. R., Aaronson S. A. Comparative immunological studies of RNA C-type viruses: radioimmunoassay for a low molecular weight polypeptide of woolly monkey leukemia virus. Virology. 1974 Feb;57(2):347–356. doi: 10.1016/0042-6822(74)90174-3. [DOI] [PubMed] [Google Scholar]
- Tsai R. L., Green H. Studies on a mammalian cell protein (P8) with affinity for DNA in vitro. J Mol Biol. 1973 Feb 19;73(3):307–316. doi: 10.1016/0022-2836(73)90344-6. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Wright B. S., O'Brien P. A., Shibley G. P., Mayyasi S. A., Lasfargues J. C. Infection of an established mouse bone marrow cell line (JLS-V9) with Rauscher and Moloney murine leukemia viruses. Cancer Res. 1967 Sep;27(9):1672–1677. [PubMed] [Google Scholar]
- Yarus M. Recognition of nucleotide sequences. Annu Rev Biochem. 1969;38:841–880. doi: 10.1146/annurev.bi.38.070169.004205. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]