Abstract
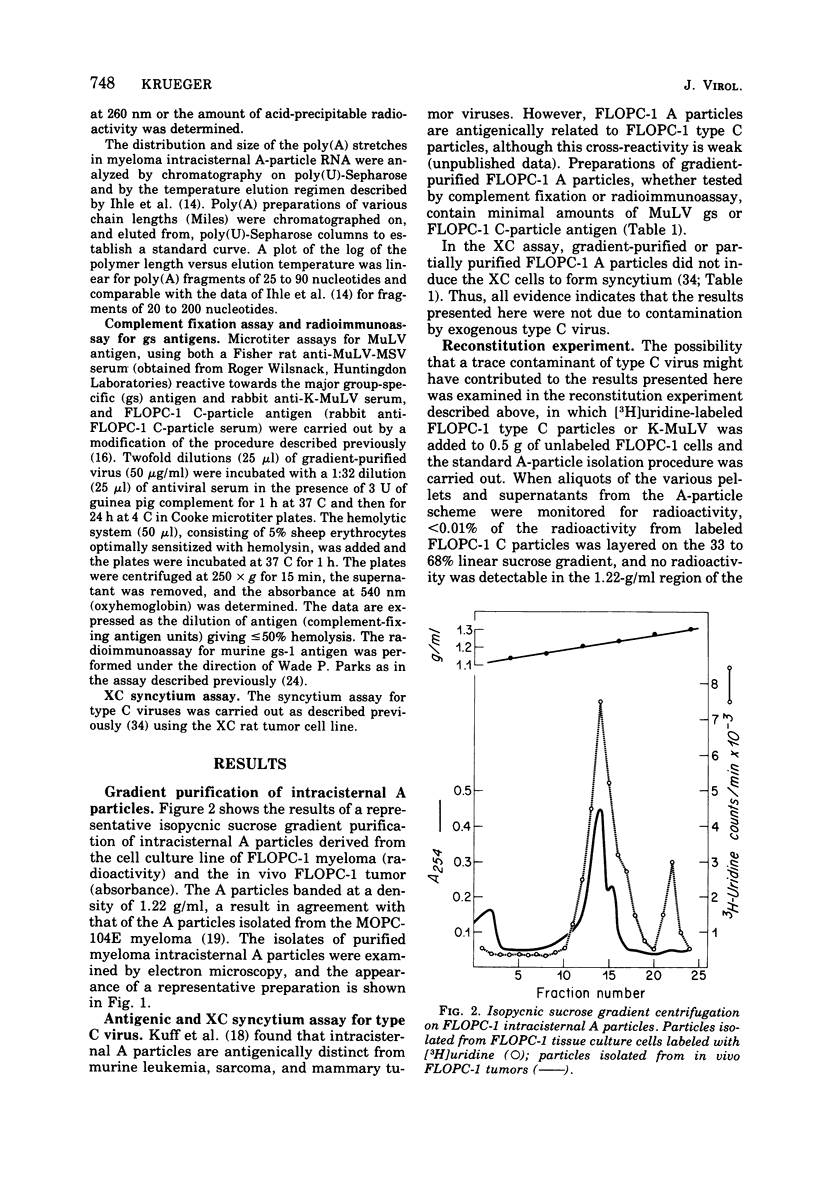
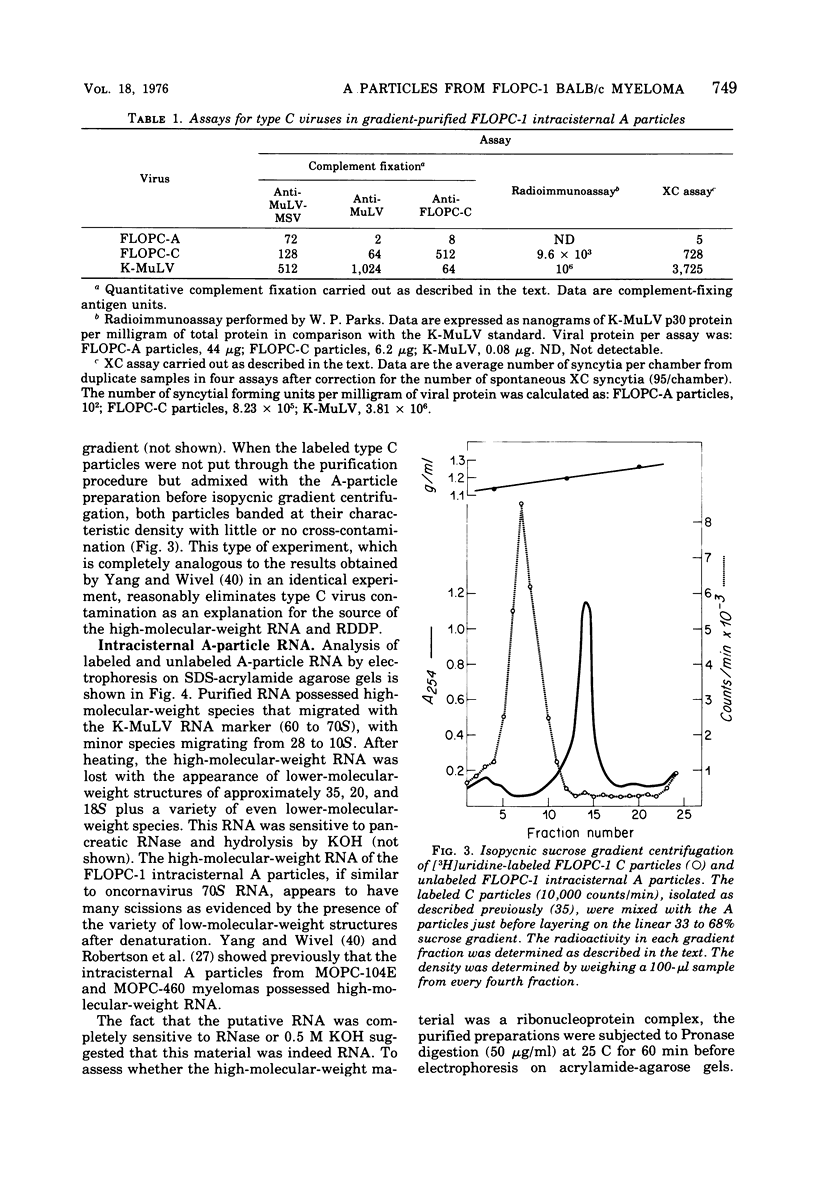
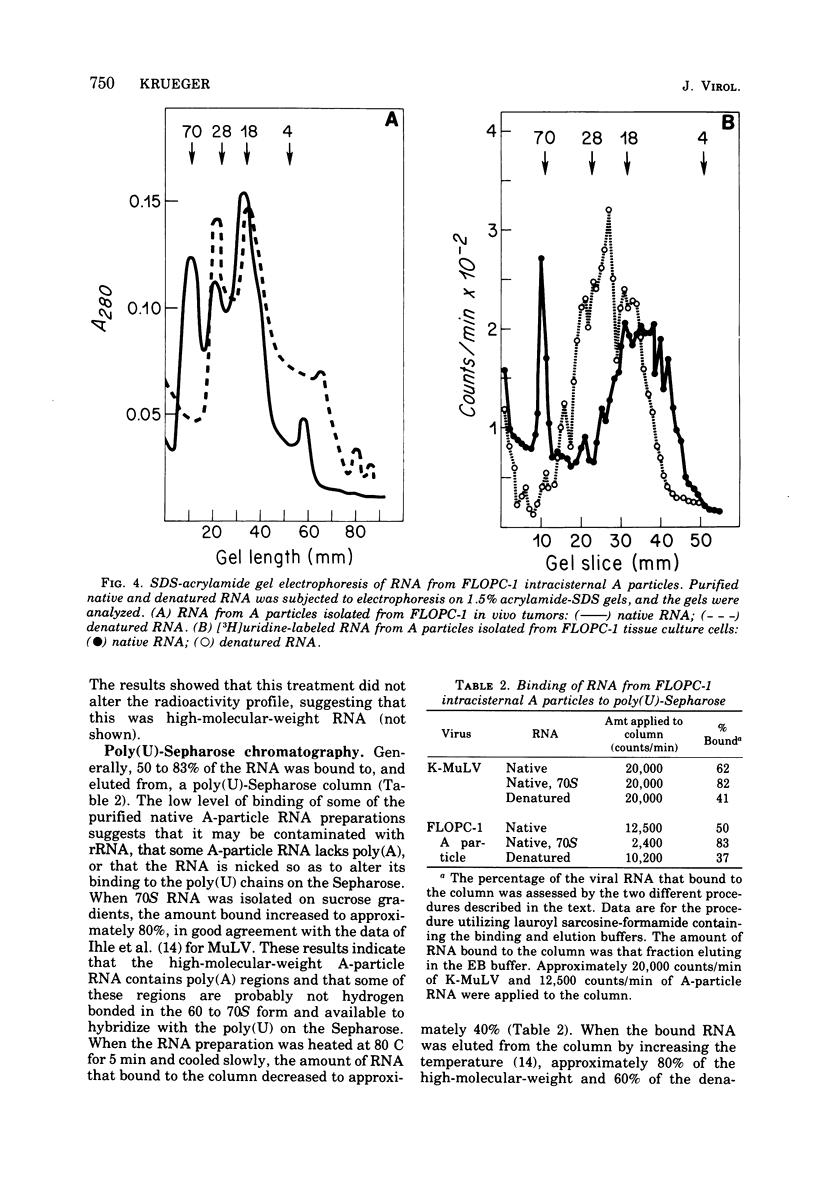
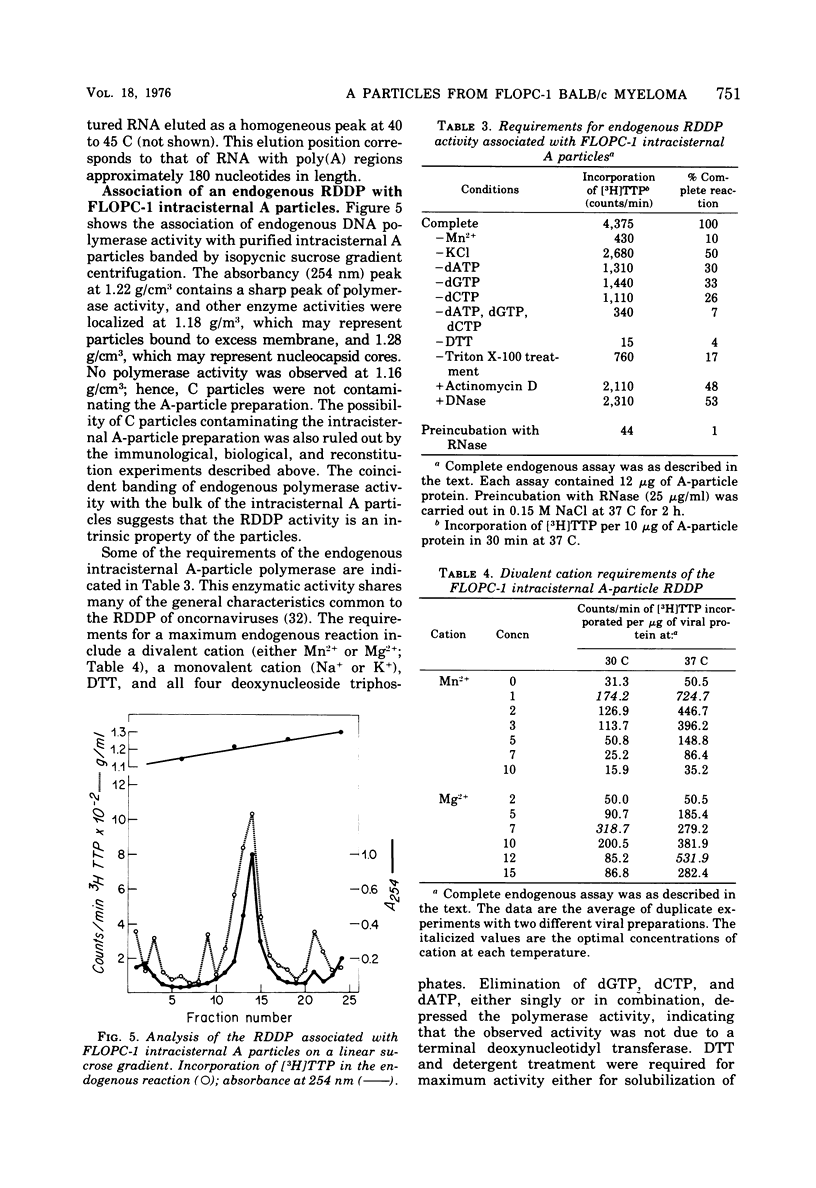
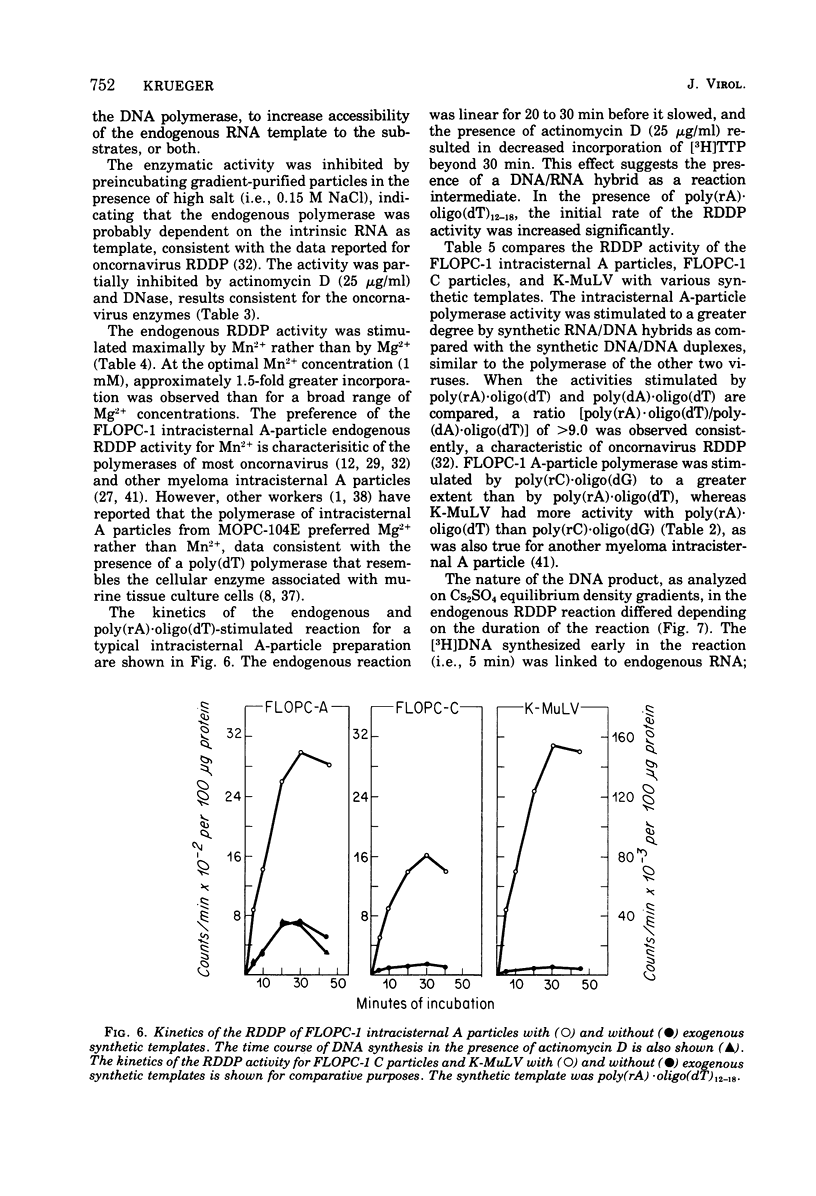
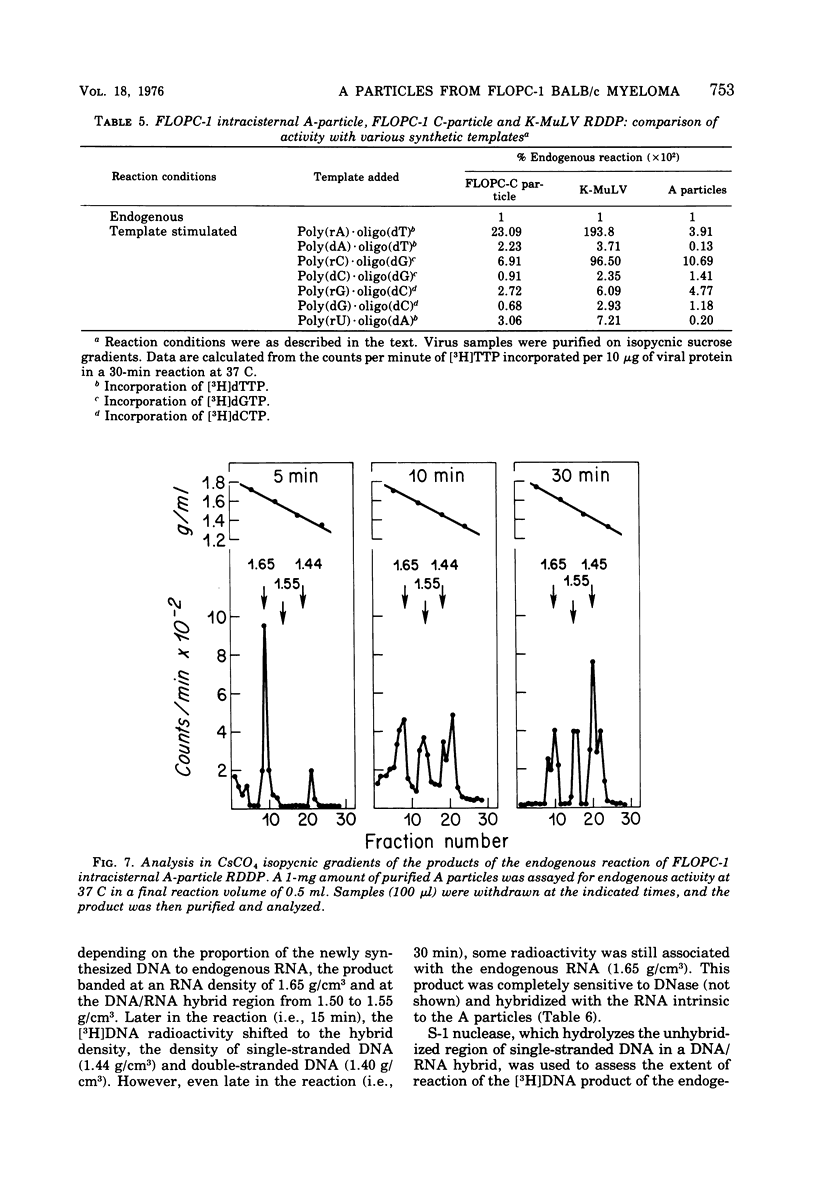
Intracisternal A particles from the FLOPC-1 line of BALB/c myeloma have been shown to contain high-molecular-weight RNA (60 to 70S) that is sensitive to RNase, alkali degradation, and heat but resistant to Pronase treatment. The intracisternal A-particle RNA contains tract of poly (A) approximately 180 nucleotides long. As shown in a reconstitution experiment, by antigenic analysis of A-particle preparation and the SC cytopathogenicity assay, the 70S RNA was not due to contamination by type C virus particles. The FLOPC-1 intracisternal A particles also possess an endogenous RNA-dependent DNA polymerase. The enzyme required Mn2+ or Mg2+, dithiothreitol, detergent, and four deoxyribonucleoside triphosphates for maximum activity. Enzymatic activity was maximally stimuated by poly (rC)-oligo (dG)12-18 and less with poly (rG)-oligo (dC)10 or poly (rA)-oligo (dT)12-18 as compare with synthetic DNA/DNA duplex templates such as poly (dA)-oligo (dT)12-18. The enzyme can utilize the A-particle endogenous RNA as template as shown by analysis of the early and late DNA products of the endogenous reaction by CsSO4 isopycnic gradient centrifuation and hybridization of purified 70S or 35S A-particle RNA with the purified complementary DNA product. Approximately 50% of the A-particle complementary DNA also hybridized with oncornavirus RNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohn E. W., Wilson S. H. Studies on the activity of the A particle-associated Dna polymerase. Cancer Res. 1974 Aug;34(8):1977–1981. [PubMed] [Google Scholar]
- DALTON A. J., FELIX M. D. The electron microscopy of normal and malignant cells. Ann N Y Acad Sci. 1956 Mar 30;63(6):1117–1140. doi: 10.1111/j.1749-6632.1956.tb32127.x. [DOI] [PubMed] [Google Scholar]
- DALTON A. J., POTTER M., MERWIN R. M. Some ultrastructural characteristics of a series of primary and transplanted plasma-cell tumors of the mouse. J Natl Cancer Inst. 1961 May;26:1221–1267. [PubMed] [Google Scholar]
- DE HARVEN E., FRIEND C. Electron microscope study of a cell-free induced leukemia of the mouse: a preliminary report. J Biophys Biochem Cytol. 1958 Mar 25;4(2):151–156. doi: 10.1083/jcb.4.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DMOCHOWSKI L., GREY C. E. Electron microscopy of tumors of known and suspected viral etiology. Tex Rep Biol Med. 1957;15(3):704–753. [PubMed] [Google Scholar]
- Dessev G. N., Grancharov K. Precipitation of RNA, DNA, and nucleoprotein particles from very dilute solutions. Anal Biochem. 1973 May;53(1):269–271. doi: 10.1016/0003-2697(73)90428-4. [DOI] [PubMed] [Google Scholar]
- FRIEDLAENDER M., MOORE D. H. Occurrence of bodies within endoplasmic reticulum of Ehrlich ascites tumor cells. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):828–831. doi: 10.3181/00379727-92-22627. [DOI] [PubMed] [Google Scholar]
- Fry M., Weissbach A. The utilization of synthetic ribonucleic acid templates by a new deoxyribonucleic acid polymerase from cultured murine cells. J Biol Chem. 1973 Apr 25;248(8):2678–2683. [PubMed] [Google Scholar]
- GRANBOULAN N., RIVIERE M. R., BERNHARD W. [Presence of particles of viral appearance in a transplantable mouse sarcoma induced by methylcholanthrene]. Bull Assoc Fr Etud Cancer. 1960 Apr-Jun;47:291–307. [PubMed] [Google Scholar]
- Gardner M. B., Officer J. E., Rongey R. W., Estes J. D., Turner H. C., Huebner R. J. C-type RNA tumour virus genome expression in wild house mice. Nature. 1971 Aug 27;232(5313):617–620. doi: 10.1038/232617a0. [DOI] [PubMed] [Google Scholar]
- Godson G. N. Action of the single-stranded DNA specific nuclease S1 on double-stranded DNA. Biochim Biophys Acta. 1973 Apr 21;308(7):59–67. doi: 10.1016/0005-2787(73)90122-6. [DOI] [PubMed] [Google Scholar]
- Green M., Rokutanda M., Fujinaga K., Ray R. K., Rokutanda H., Gurgo C. Mechanism of carcinogenesis by RNA tumor viruses. I. An RNA-dependent DNA polymerase in murine sarcoma viruses. Proc Natl Acad Sci U S A. 1970 Sep;67(1):385–393. doi: 10.1073/pnas.67.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. T., Hartley J. W., Sanford K. K. Characteristics of and relationship between C particles and intracisternal A particles in cloned cell strains. J Virol. 1968 Mar;2(3):238–247. doi: 10.1128/jvi.2.3.238-247.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Lee K. L., Kennedy F. T. Fractionation of 34 S ribonucleic acid subunits from oncornaviruses on polyuridylate-sepharose columns. J Biol Chem. 1974 Jan 10;249(1):38–42. [PubMed] [Google Scholar]
- Kakefuda T., Roberts E., Suntzeff V. Electron microscopic study of methylcholanthrene-induced epidermal carcinogenesis in mice: mitochondrial dense bodies and intracisternal A-particles. Cancer Res. 1970 Apr;30(4):1011–1019. [PubMed] [Google Scholar]
- Krueger R. G., Miller G. C. Relationship between the cellular and viral antigens of a BALB-c myeloma and murine leukemia virus. J Natl Cancer Inst. 1974 Oct;53(4):997–1004. doi: 10.1093/jnci/53.4.997. [DOI] [PubMed] [Google Scholar]
- Krueger R. G., Williams W. H., Miller G. C. Cellular and viral antigens of BALB-c myeloma. J Natl Cancer Inst. 1974 Apr;52(4):1203–1210. doi: 10.1093/jnci/52.4.1203. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Leuders K. K., Ozer H. L., Wivel N. A. Some structural and antigenic properties of intracisternal A particles occurring in mouse tumors (complement fixation-immunodiffusion-neuroblastoma-plasma-cell tumor). Proc Natl Acad Sci U S A. 1972 Jan;69(1):218–222. doi: 10.1073/pnas.69.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Wivel N. A., Lueders K. K. The extraction of intracisternal A-particles from a mouse plasma-cell tumor. Cancer Res. 1968 Oct;28(10):2137–2148. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marciani D. J., Kuff E. L. Isolation and partial characterization of the internal structural proteins from murine intracisternal A particles. Biochemistry. 1973 Dec 4;12(25):5075–5083. doi: 10.1021/bi00749a008. [DOI] [PubMed] [Google Scholar]
- Nadel E., Banfield W., Burstein S., Tousimis A. J. Virus particles associated with strain 2 guinea pig leukemia (L2C/N-B). J Natl Cancer Inst. 1967 Jun;38(6):979–981. [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Radioimmunoassay of mammalian type-C viral proteins: interspecies antigenic reactivities of the major internal polypeptide. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1766–1770. doi: 10.1073/pnas.69.7.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiol Rev. 1972 Jul;52(3):631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Baenziger N. L., Dobbertin D. C., Thach R. E. Characterization of DNA polymerase and RNA associated with A-type particles from murine myeloma cells. J Virol. 1975 Feb;15(2):407–415. doi: 10.1128/jvi.15.2.407-415.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Humphreys S., Baroni C., Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969 Sep;64(1):316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Rands E., Aaronson S. A., Todaro G. J. RNA-dependent DNA polymerase activity in five RNA viruses: divalent cation requirements. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1789–1796. doi: 10.1073/pnas.67.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. H., Andervont H. B., Dunn T. B. Attempts to detect nodule-inducing virus in strain RIII mice. J Natl Cancer Inst. 1970 Mar;44(3):657–671. [PubMed] [Google Scholar]
- Stewart S. E., Kasnic G., Jr, Draycott C., Ben T. Activation of viruses in human tumors by 5-iododeoxyuridine and dimethyl sulfoxide. Science. 1972 Jan 14;175(4018):198–199. doi: 10.1126/science.175.4018.198. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Cholon J. J. Intracisternal type A particles and properties of a continuous cell line originating from a gerbil fibroma. Proc Soc Exp Biol Med. 1971 Apr;136(4):1107–1110. doi: 10.3181/00379727-136-35439. [DOI] [PubMed] [Google Scholar]
- Volkman L. E., Krueger R. G. Characterization of C-type particles produced by a tissue culture-adapted murine myeloma. J Virol. 1973 Dec;12(6):1589–1597. doi: 10.1128/jvi.12.6.1589-1597.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L. E., Krueger R. G. XC cell cytopathogenicity as an assay for murine myeloma C-type virus. J Natl Cancer Inst. 1973 Oct;51(4):1205–1210. doi: 10.1093/jnci/51.4.1205. [DOI] [PubMed] [Google Scholar]
- Volkman L. E., Smuckler E. A., Krueger R. G. Mouse myeloma: differentiation of neoplastic cells accompanied by an increase in intracellular virus. J Natl Cancer Inst. 1971 May;46(5):953–962. [PubMed] [Google Scholar]
- Weissbach A., Schlabach A., Fridlender B., Bolden A. DNA polymerases from human cells. Nat New Biol. 1971 Jun 9;231(23):167–170. doi: 10.1038/newbio231167a0. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Bohn E. W., Matsukage A., Lueders K. K., Kuff E. L. Studies on the relationship between deoxyribonucleic acid polymerase activity and intracisternal A-type particles in mouse myeloma. Biochemistry. 1974 Mar 12;13(6):1087–1094. doi: 10.1021/bi00703a005. [DOI] [PubMed] [Google Scholar]
- Wivel N. A., Smith G. H. Distribution of intracisternal A-particles in a variety of normal and neoplastic mouse tissues. Int J Cancer. 1971 Jan 15;7(1):167–175. doi: 10.1002/ijc.2910070119. [DOI] [PubMed] [Google Scholar]
- Yang S. S., Wivel N. A. Analysis of high-molecular-weight ribonucleic acid associated with intracisternal A particles. J Virol. 1973 Feb;11(2):287–298. doi: 10.1128/jvi.11.2.287-298.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. S., Wivel N. A. Characterization of an endogenous RNA-dependent DNA polymerase associated with murine intracisternal A particles. J Virol. 1974 Mar;13(3):712–720. doi: 10.1128/jvi.13.3.712-720.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]