Abstract
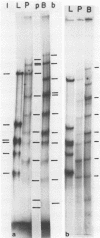
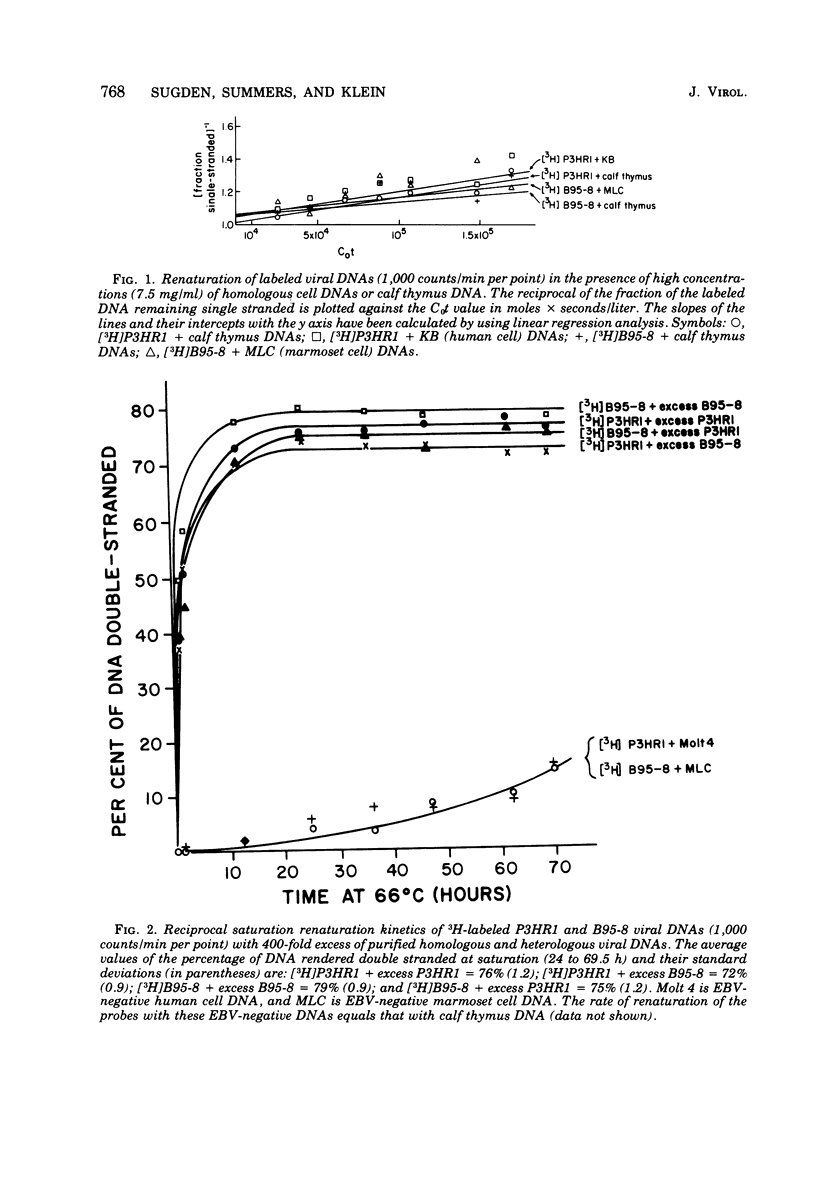
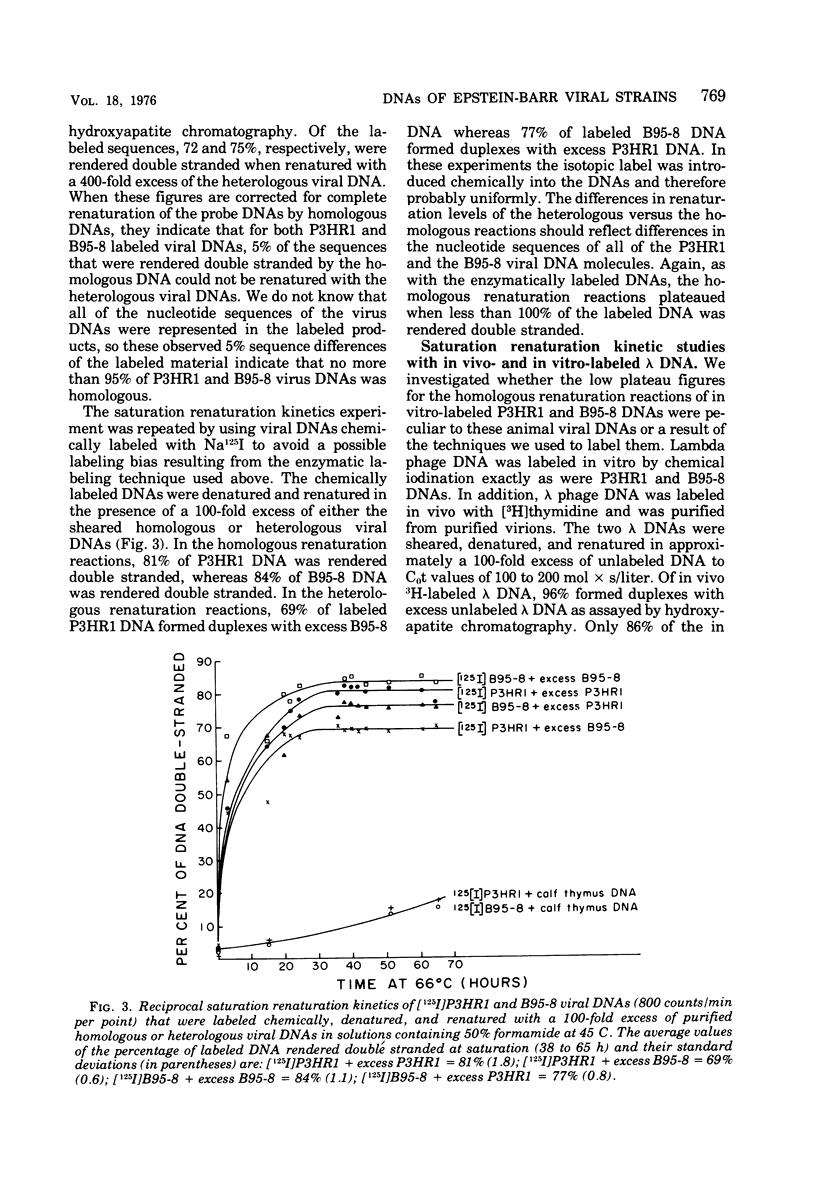
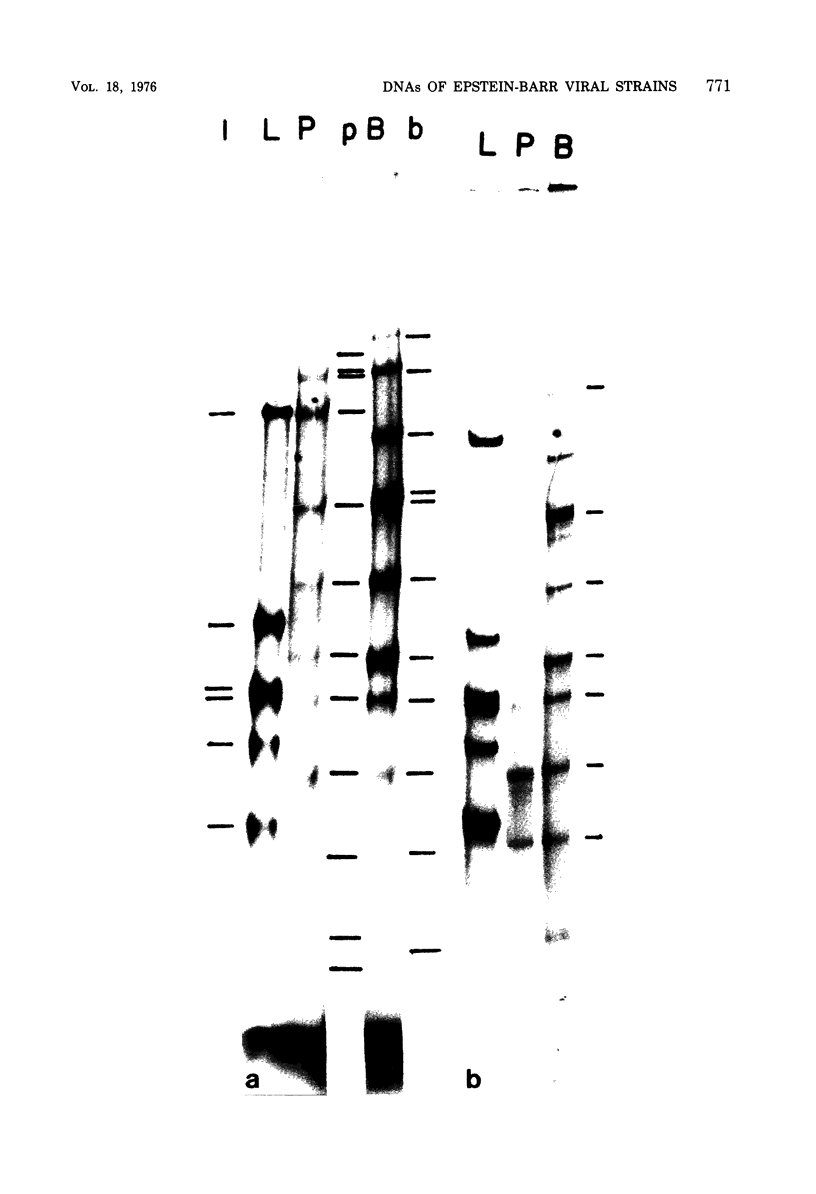
Viral DNA molecules were purified from a nontransforming and a transforming strain of Epstein-Barr virus. Each viral DNA was labeled in vitro and renatured in the presence of an excess of either one or the other unlabeled viral DNA. Both viral DNAs were also digested with the Eco R1 restriction endonuclease and subsequently labeled by using avian myeloblastosis virus DNA polymerase to repair either the EcoR1 nuclease-generated single-stranded ends of the DNAs or their single-stranded ends produced by a second digestion with exonuclease III after the first EcoR1 nuclease digestion. The results of these experiments support three general conclusions: (i) the DNAs of these two strains of Epstein-Barr virus share approximately 90% of their nucleotide sequences; (ii) both viral DNA populations are reasonably homogenous; and (iii) both DNAs contain repetitions or inverted repetitions of some of their nucleotide sequences.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. Concentration of Epstein-Barr virus from cell culture fluids with polyethylene glycol. J Gen Virol. 1973 Sep;20(3):391–394. doi: 10.1099/0022-1317-20-3-391. [DOI] [PubMed] [Google Scholar]
- Adams A., Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkower I., Leis J., Hurwitz J. Isolation and characterization of an endonuclease from Escherichia coli specific for ribonucleic acid in ribonucleic acid-deoxyribonucleic acid hybrid structures. J Biol Chem. 1973 Sep 10;248(17):5914–5921. [PubMed] [Google Scholar]
- Botchan M., McKenna G., Sharp P. A. Cleavage of mouse DNA by a restriction enzyme as a clue to the arrangement of genes. Cold Spring Harb Symp Quant Biol. 1974;38:383–395. doi: 10.1101/sqb.1974.038.01.041. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Grafstrom R. H., Alwine J. C., Steinhart W. L., Hill C. W. Terminal repetitions in herpes simplex virus type 1 DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):679–681. doi: 10.1101/sqb.1974.039.01.081. [DOI] [PubMed] [Google Scholar]
- Hedgpeth J., Goodman H. M., Boyer H. W. DNA nucleotide sequence restricted by the RI endonuclease. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3448–3452. doi: 10.1073/pnas.69.11.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. H., Sugden B. Inhibition by -amanitin of simian virus 40-specific ribonucleic acid synthesis in nuclei of infected monkey cells. J Virol. 1972 Nov;10(5):1086–1089. doi: 10.1128/jvi.10.5.1086-1089.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- Klein G., Sugden B., Leibold W., Menezes J. Infection of EBV-genome-negative and -positive human lymphoblastoid cell lines with biologically different preparations of EBV. Intervirology. 1974;3(4):232–244. doi: 10.1159/000149760. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L. Immortalizing and nonimmortalizing laboratory strains of Epstein-Barr Virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):773–781. doi: 10.1101/sqb.1974.039.01.089. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- Orosz J. M., Wetmur J. G. In vitro iodination of DNA. Maximizing iodination while minimizing degradation; use of buoyant density shifts for DNA-DNA hybrid isolation. Biochemistry. 1974 Dec 31;13(27):5467–5473. doi: 10.1021/bi00724a003. [DOI] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J., Arrand J. R., Keller W. The length of the terminal repetition in adenovirus-2 DNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3829–3833. doi: 10.1073/pnas.71.10.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberg N. H., Refetoff S. The preparation of carrier-free iodine isotope-substituted cytosine nucleotides. Biochim Biophys Acta. 1974 Apr 10;340(4):446–451. doi: 10.1016/0005-2787(74)90065-3. [DOI] [PubMed] [Google Scholar]
- Scherberg N. H., Refetoff S. The radioiodination of ribopolymers for use in hybridizational and molecular analyses. J Biol Chem. 1974 Apr 10;249(7):2143–2150. [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Skare J., Summers W. P., Summers W. C. Structure and function of herpesvirus genomes. I. comparison of five HSV-1 and two HSV-2 strains by cleavage their DNA with eco R I restriction endonuclease. J Virol. 1975 Apr;15(4):726–732. doi: 10.1128/jvi.15.4.726-732.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Tibbetts C., Johansson K., Philipson L. Hydroxyapatite chromatography and formamide denaturation of adenovirus DNA. J Virol. 1973 Aug;12(2):218–225. doi: 10.1128/jvi.12.2.218-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Skare J., Summers W. C. Analysis of DNA of defective herpes simplex virus type 1 by restriction endonuclease cleavage and nucleic acid hybridization. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):683–686. doi: 10.1101/sqb.1974.039.01.082. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Hydroxyapatite chromatography of short double-helical DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):333–340. doi: 10.1016/0005-2787(73)90019-1. [DOI] [PubMed] [Google Scholar]