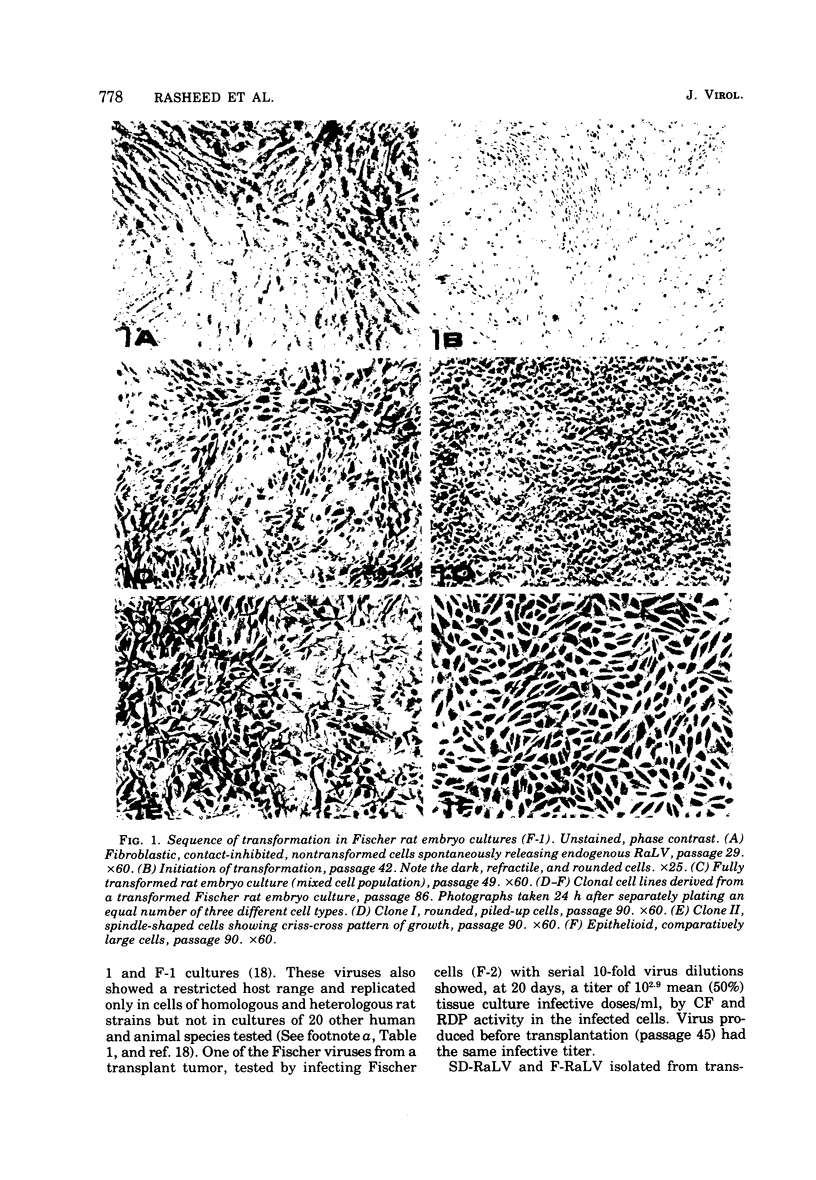
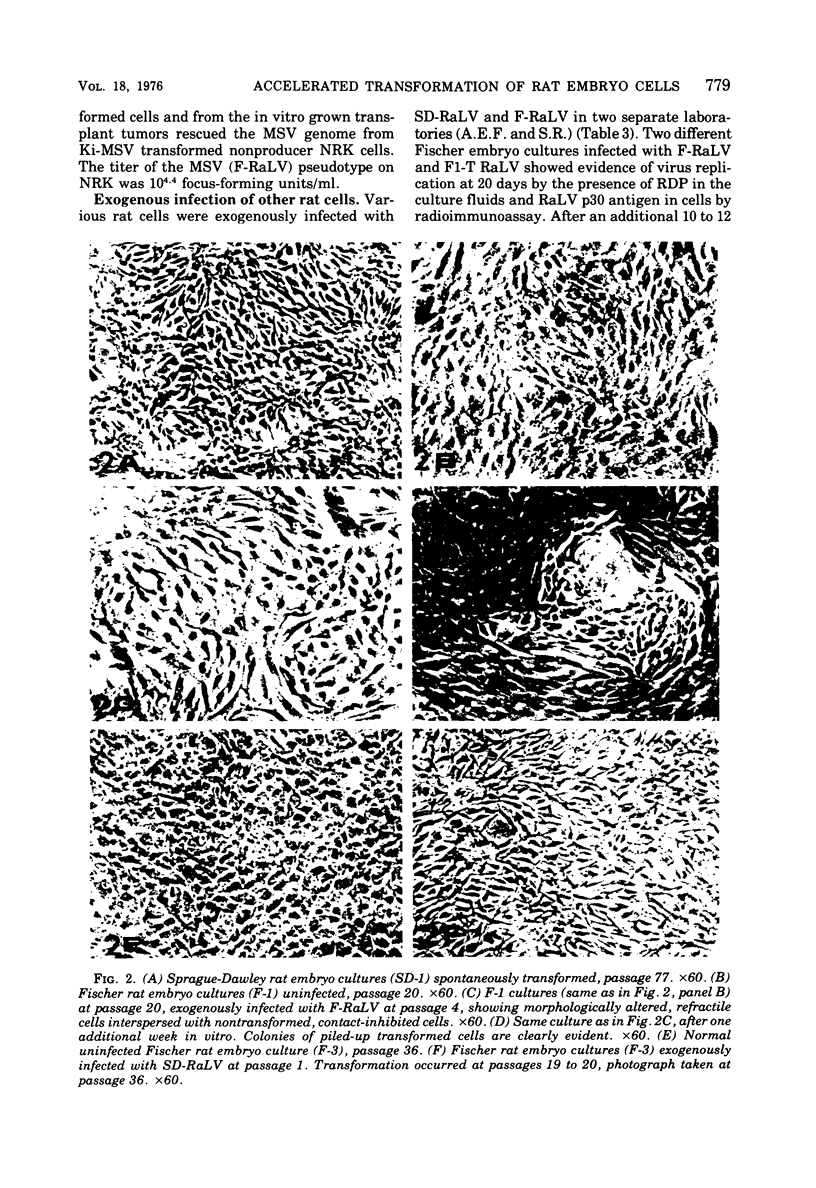
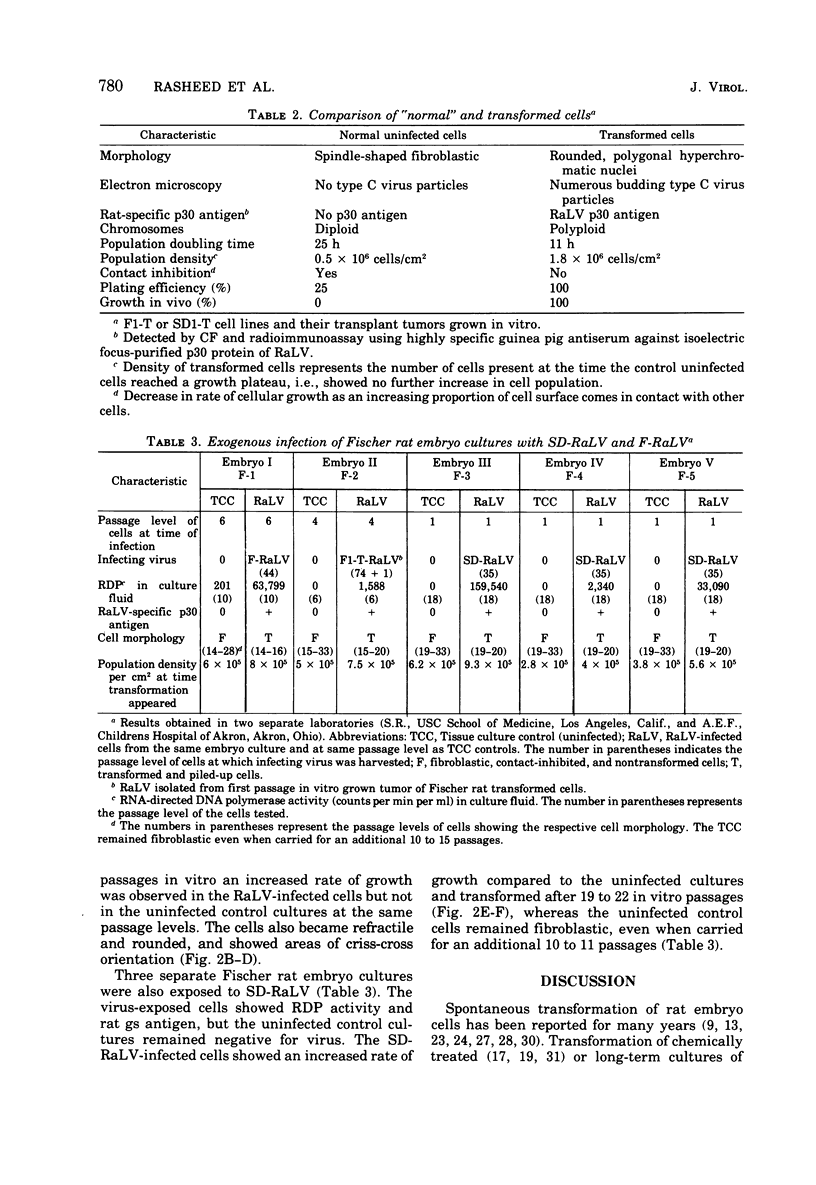
Abstract
Sprague-Dawley and Fischer rat embryo cells became spontaneously transformed about 20 passages after release of endogenous ecotropic type C virus (SD-RaLV and F-RaLV). The virus-producing transformed cells showed loss of contact inhibition, increased growth rate, and tumorigenicity in vivo. Exogenous infection of other Fischer rat embryo cultures in early passage with SD-RaLV and F-RaLV markedly accelerated their rates of transformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Korol W., Larson D., Molnar H., Schidlovsky G. Transformation of rat mammary cell cultures by R-35 virus isolated from spontaneous rat mammary adenocarcinoma. J Natl Cancer Inst. 1972 Apr;48(4):1077–1083. [PubMed] [Google Scholar]
- Cremer N. E., Taylor D. O., Oshiro L. S., Teitz Y. Transformation and virus production in normal rat thymus cells and those infected with Moloney leukemia virus. J Natl Cancer Inst. 1970 Jul;45(1):37–48. [PubMed] [Google Scholar]
- Freeman A. E., Gilden R. V., Vernon M. L., Wolford R. G., Hugunin P. E., Huebner R. J. 5-Bromo-2'-deoxyuridine potentiation of transformation of rat-embryo cells induced in vitro by 3-methylcholanthrene: induction of rat leukemia virus gs antigen in transformed cells. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2415–2419. doi: 10.1073/pnas.70.8.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. E., Igel H. J., Price P. J. I. In vitrol transformation of rat embryo cells: correlations with the known tumorigenic activities of chemicals in rodents. In Vitro. 1975 Mar-Apr;11(2):107–116. doi: 10.1007/BF02624083. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Price P. J., Igel H. J., Young J. C., Maryak J. M., Huebner R. J. Morphological transformation of rat embryo cells induced by diethylnitrosamine and murine leukemia viruses. J Natl Cancer Inst. 1970 Jan;44(1):65–78. [PubMed] [Google Scholar]
- Freeman A. E., Weisburger E. K., Weisburger J. H., Wolford R. G., Maryak J. M., Huebner R. J. Transformation of cell cultures as an indication of the carcinogenic potential of chemicals. J Natl Cancer Inst. 1973 Sep;51(3):799–808. doi: 10.1093/jnci/51.3.799. [DOI] [PubMed] [Google Scholar]
- Gazzolo L., Simkovic D., Martinberthelon M. C. The presence of C-type RNA virus particles in a rat embryo cell line spontaneously transformed in tissue culture. J Gen Virol. 1971 Sep;12(3):303–311. doi: 10.1099/0022-1317-12-3-303. [DOI] [PubMed] [Google Scholar]
- Gordon R. J., Bryan R. J., Rhim J. S., Demoise C., Wolford R. G., Freeman A. E., Huebner R. J. Transformation of rat and mouse embryo cells by a new class of carcinogenic compounds isolated from particles in city air. Int J Cancer. 1973 Jul 15;12(1):223–232. doi: 10.1002/ijc.2910120123. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioachim H. L., Berwick L. Continuous viral replication and cellular neoplastic transformation in cultures of normal rat thymus infected with gross leukemia virus. Int J Cancer. 1968 Jan 15;3(1):61–73. doi: 10.1002/ijc.2910030109. [DOI] [PubMed] [Google Scholar]
- Jackson J. L., Sanford K. K., Dunn T. B. Neoplastic conversion and chromosomal characteristics of rat embryo cells in vitro. J Natl Cancer Inst. 1970 Jul;45(1):11–23. [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Gilden R. V., Oroszlan S., Sarma P. S., Rongey R. W., Gardner M. B. Rat C-type virus induced in rat sarcoma cells by 5-bromodeoxyuridine. Nat New Biol. 1972 Aug 23;238(86):234–237. doi: 10.1038/newbio238234a0. [DOI] [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Nelson-Rees W., Gilden R. V., Oroszlan S., Rongey R. W., Gardner M. B. Spontaneous production of a C-type RNA virus in rat tissue culture lines. Int J Cancer. 1973 Nov 15;12(3):654–666. doi: 10.1002/ijc.2910120314. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Namba M., Masuji H., Sato J. Carcinogenesis in tissue culture. IX. Malignant transformation of cultured rat cells treated with 4-nitroquinoline-1-oxide. Jpn J Exp Med. 1969 Jun;39(3):253–265. [PubMed] [Google Scholar]
- Rasheed S., Bruszewski J., Rongey R., Roy-Burman P., Charman H. P., Gardner M. B. Spontaneous release of endogenous ecotropic type C virus from rat embryo cultures. J Virol. 1976 May;18(2):799–803. doi: 10.1128/jvi.18.2.799-803.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. S., Huebner R. J., Lane W. T., Vernon M. L. Neoplastic transformation of rat embryo cells induced in vitro by Rauscher leukemia virus. Proc Soc Exp Biol Med. 1970 Mar;133(3):914–920. doi: 10.3181/00379727-133-34594. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Huebner R. J. Transformation of rat embryo cells in vitro by chemical carcinogens. Cancer Res. 1973 Apr;33(4):695–700. [PubMed] [Google Scholar]
- Rhim J. S., Vass W., Cho H. Y., Huebner R. J. Malignant transformation inducedby 7,12-dimethylbenz(a)anthracene in rat embryo cells infected with Rauscher leukemia virus. Int J Cancer. 1971 Jan 15;7(1):65–74. doi: 10.1002/ijc.2910070108. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Vernon M. L., Huebner R. J., Turner H. C., Lane W. T., Gilden R. V. Spontaneous transformation of rat cells after long-term in vitro cultivation and the "switch-on" of a new complement-fixing antigen. Proc Soc Exp Biol Med. 1972 Jun;140(2):414–419. doi: 10.3181/00379727-140-36470. [DOI] [PubMed] [Google Scholar]
- STAROVEROVA N. S. Kariotypes of rat fibroblasts during "spontaneous" malignant transformation in monostratal cultures. Probl Oncol. 1961;7:1235–1241. [PubMed] [Google Scholar]
- Sanford K. K. Malignant transformation of cells in vitro. Int Rev Cytol. 1965;18:249–311. doi: 10.1016/s0074-7696(08)60556-2. [DOI] [PubMed] [Google Scholar]
- Sato J., Namba M., Usui K., Nagano D. Carcinogenesis in tissue culture. 8. Spontaneous malignant transformation of rat liver cells in long-term culture. Jpn J Exp Med. 1968 Apr;38(2):105–118. [PubMed] [Google Scholar]
- Schwartz S. A., Panem S., Stefanski E., Kirsten W. H. Endogenous type C particles from rat embryo cells treated with 5-bromodeoxyuridine. Cancer Res. 1974 Sep;34(9):2255–2259. [PubMed] [Google Scholar]
- Scolnick E. M., Maryak J. M., Parks W. P. Levels of rat cellular RNA homologous to either Kirsten sarcoma virus or rat type-C virus in cell lines derived from Osborne-Mendel rats. J Virol. 1974 Dec;14(6):1435–1444. doi: 10.1128/jvi.14.6.1435-1444.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Pollard M. Spontaneous neoplastic transformation of germ-free rat embryo cell culture. Cancer Res. 1969 Aug;29(8):1523–1526. [PubMed] [Google Scholar]
- Tsuchida N., Gilden R. V., Hatanaka M. Sarcoma-virus-related RNA sequences in normal rat cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4503–4507. doi: 10.1073/pnas.71.11.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselý P., Donner L., Kucerová M. Spontaneous malignant transformation of embryonic rat fibroblasts from an inbred Lewis strain in vitro. Folia Biol (Praha) 1968;14(5):409–410. [PubMed] [Google Scholar]
- Weinstein I. B., Gebert R., Stadler U. C., Orenstein J. M., Axel R. Type C virus from cell cultures of chemically induced rat hepatomas. Science. 1972 Dec 8;178(4065):1098–1100. doi: 10.1126/science.178.4065.1098. [DOI] [PubMed] [Google Scholar]