Abstract
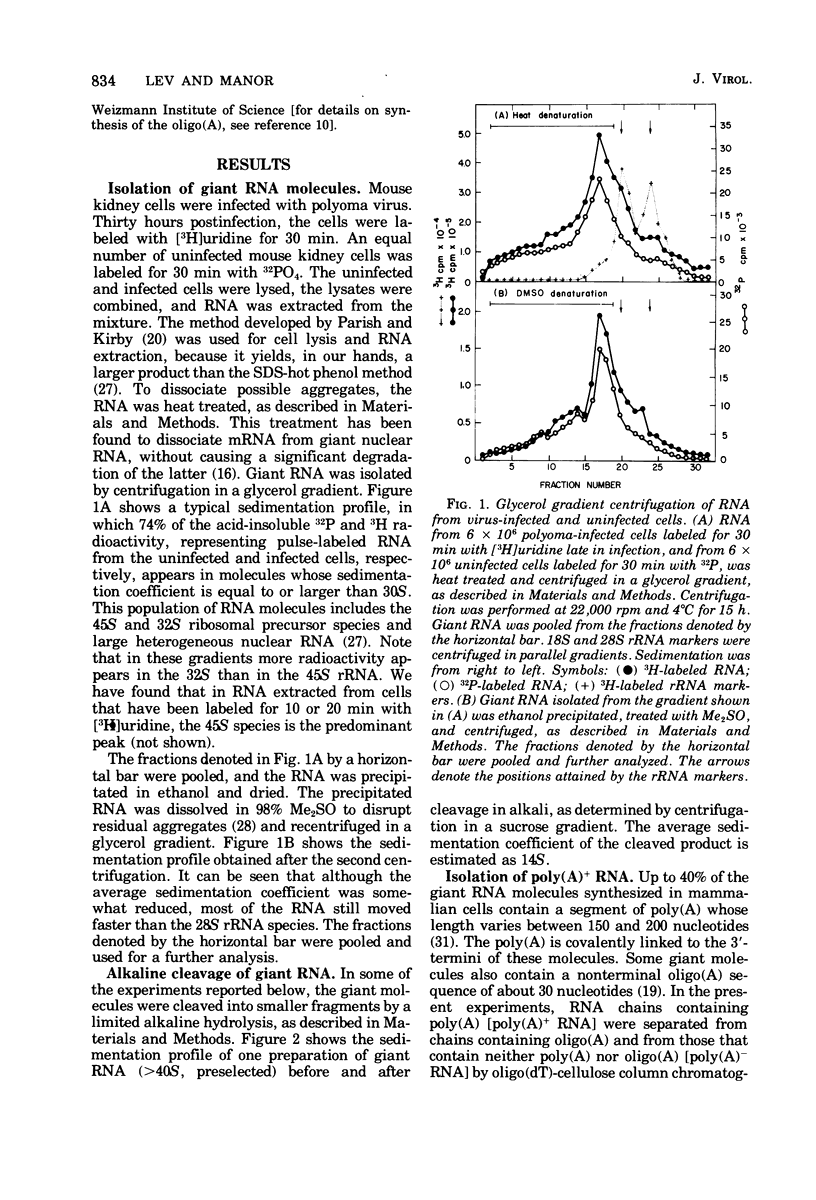
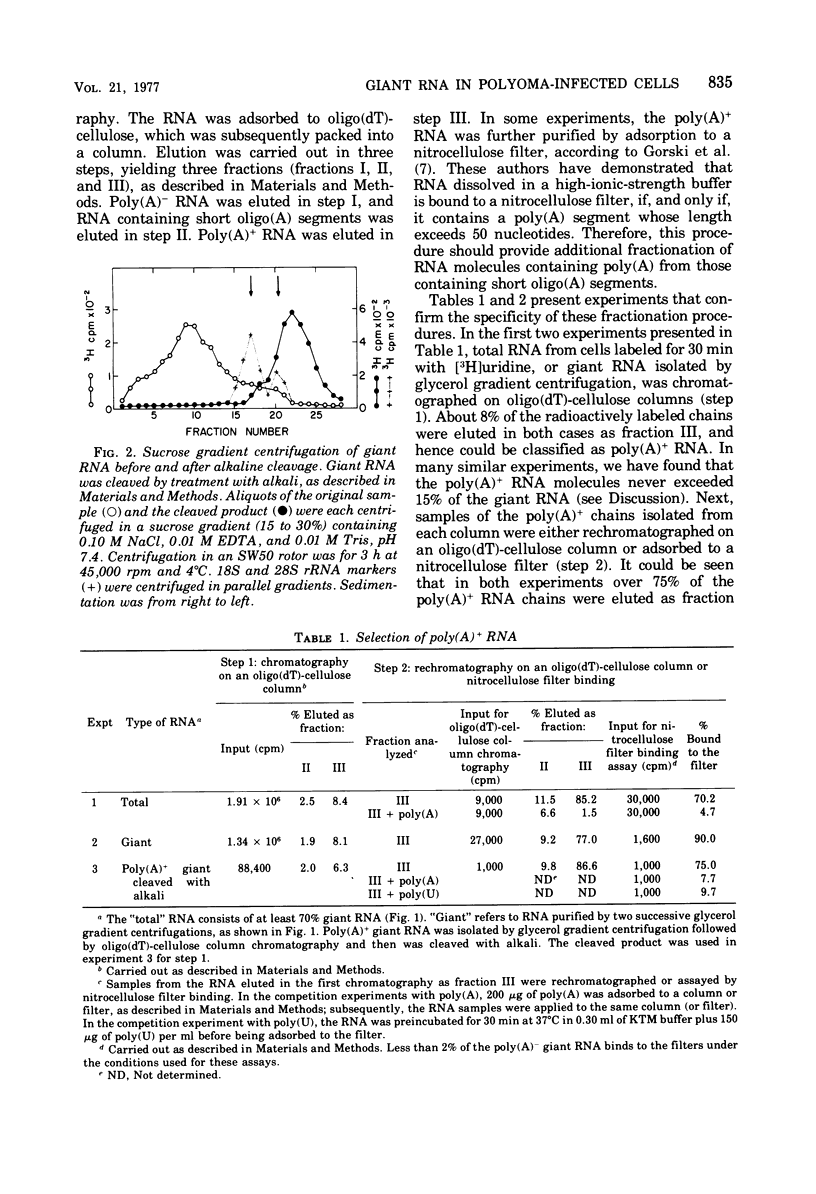
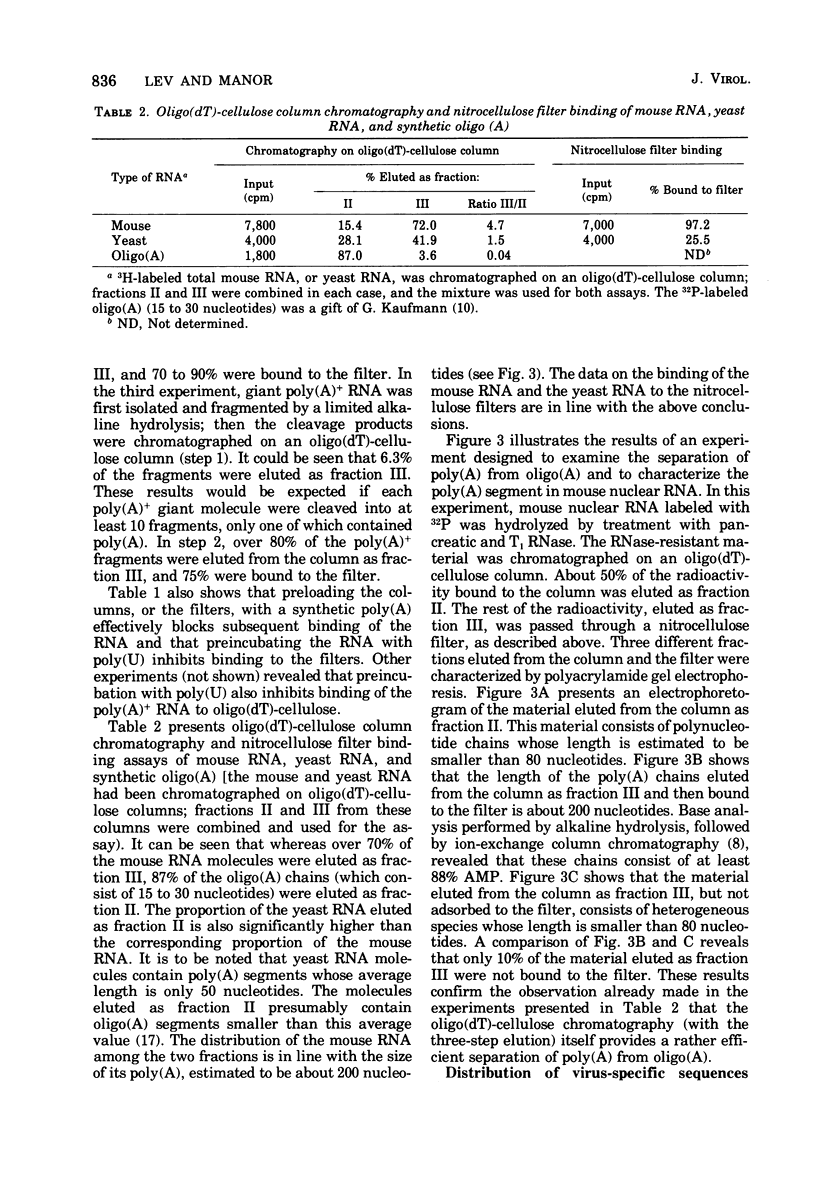
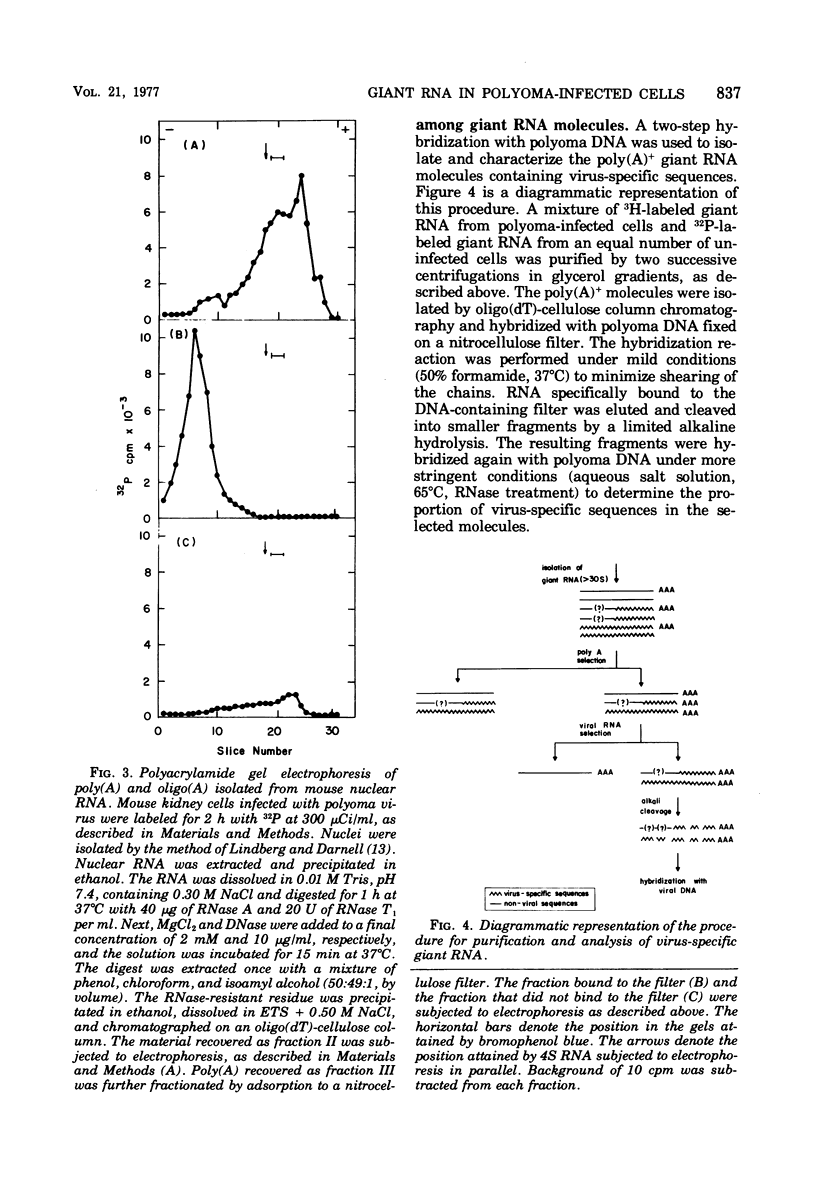
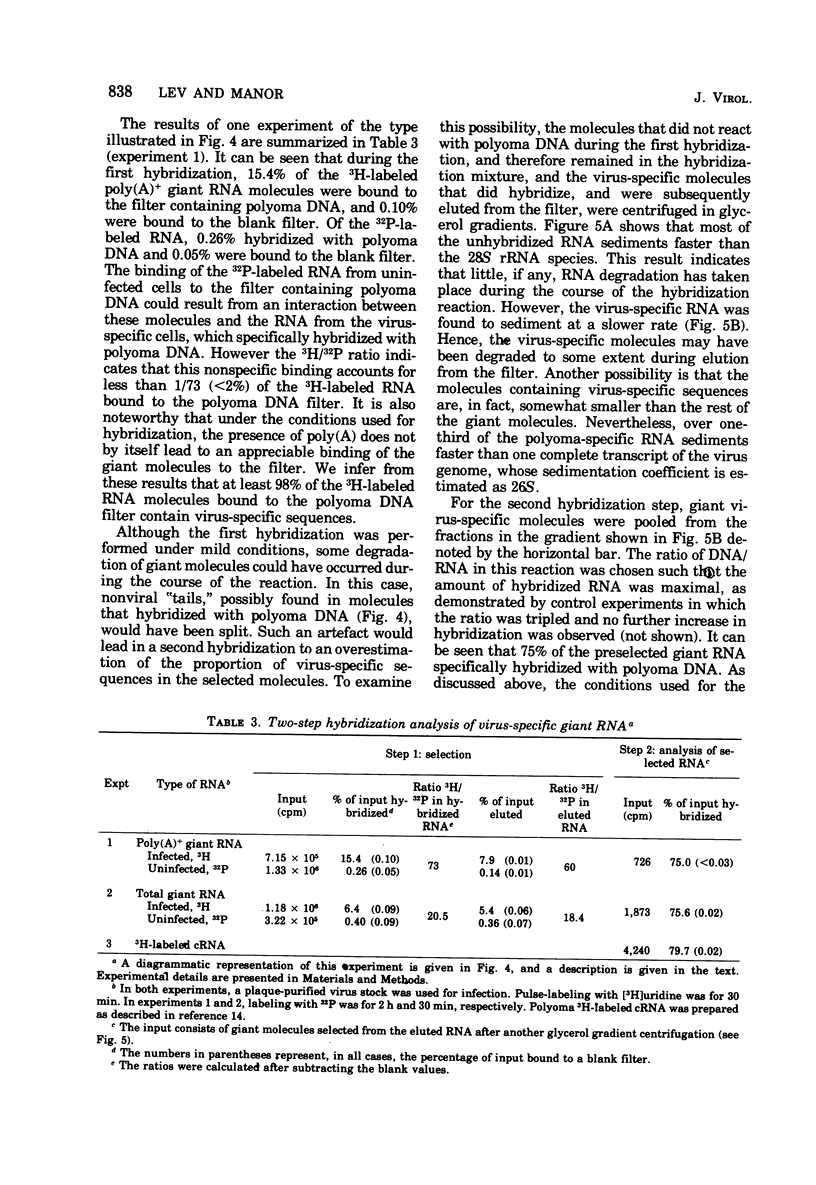
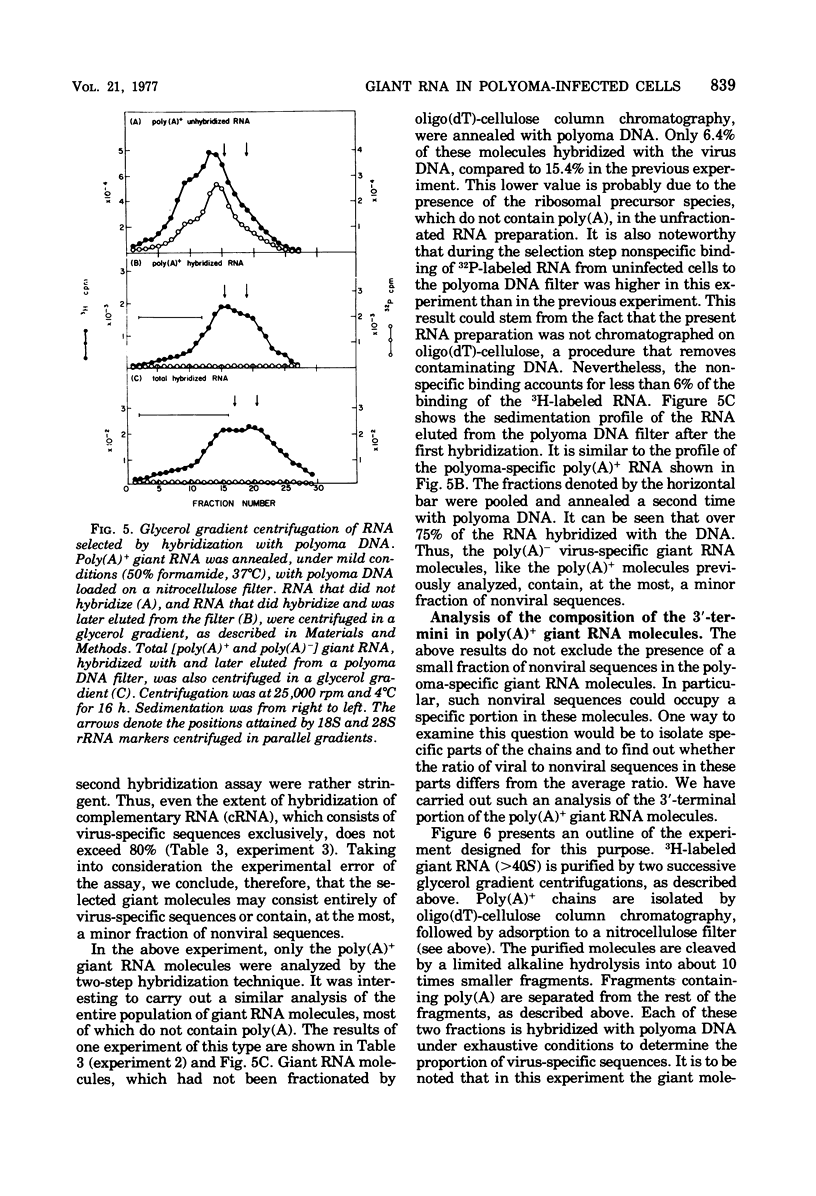
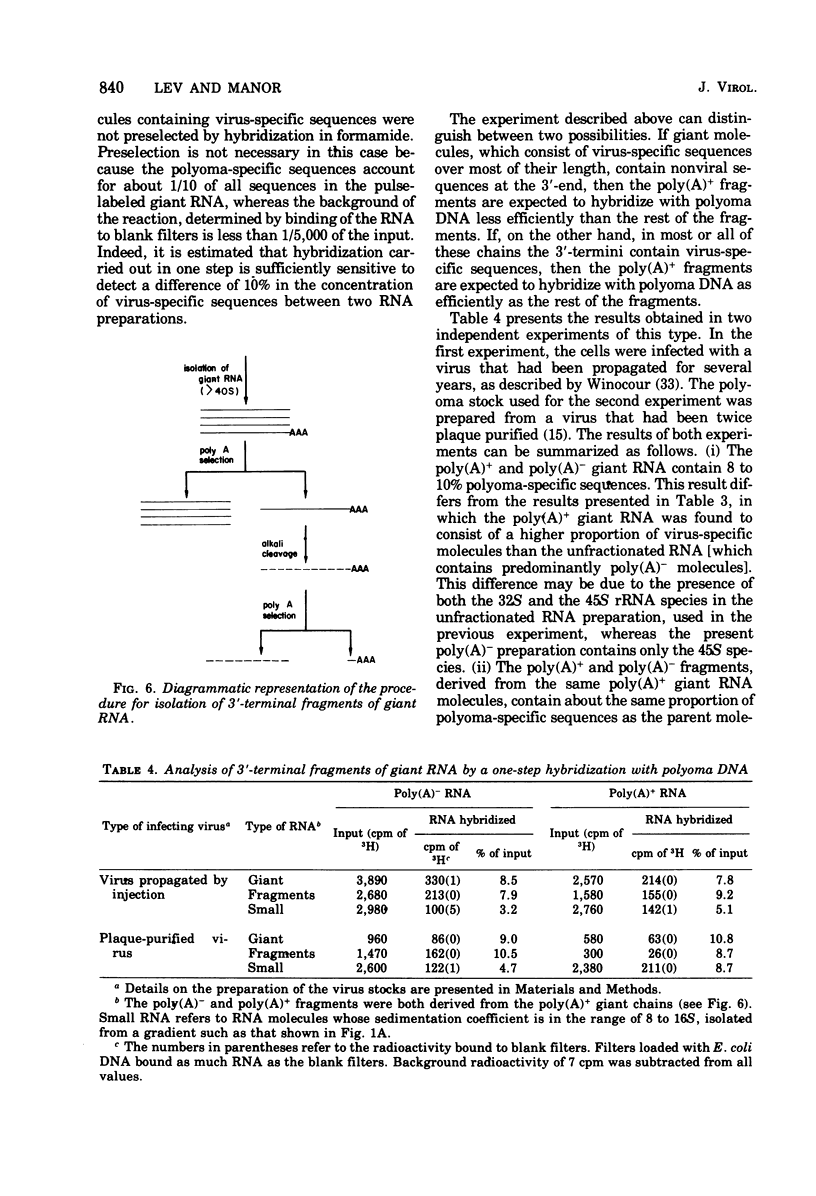
A two-step hybridization with polyoma DNA was used to study the composition of giant RNA molecules synthesized in mouse kidney cells late in productive infection by polyoma virus. Giant molecules longer than a complete transcript of the polyoma genome were purified from cells that had been pulse-labeled for 30 min with [3H]uridine and annealed, under mild conditions (50% formamide, 37 degrees C), with polyoma DNA loaded on nitrocellulose filters. Hybridized RNA (6 to 7% of the entire population of 3H-labeled molecules and up to 15% of the molecules containing polyadenylic acid [poly(A)]] was eluted and annealed a second time with polyoma DNA under more stringent conditions. In this second step, 75% of the 3H-labeled RNA formed an RNase-resistant hybrid. Under the same conditions, complementary RNA hybridized with polyoma DNA to a maximal extent of 80%. Since the difference between 75 and 80% is within the experimental error of the hybridization assay, it is inferred that the giant molecules selected by the first hybridization may consist entirely of virus-specific sequences or contain, at the most, a minor fraction of nonviral sequences. To examine the possibility that such nonviral sequences are clustered at the 3'-terminus of these molecules, poly(A)+ giant RNA, which had not been preselected by hybridization with polyoma DNA, was fragmented by a limited alkaline hydrolysis. Fragments linked to the poly(A) segment were separated from the rest of the cleavage products. A one-step hybridization with polyoma DNA revealed that both fractions contain 8 to 10% of virus-specific sequences. These results indicate that the 3'-termini of the poly(A)+ polyoma-specific giant RNA molecules consist of viral rather than nonviral sequences.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Buetti E., Scherrer K., Weil R. Transcription of the polyoma virus genome: synthesis and cleavage of giant late polyoma-specific RNA. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2231–2235. doi: 10.1073/pnas.68.9.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Winocour E., Sachs L., Torten J. Hybridization between SV40 DNA and cellular DNA's. J Mol Biol. 1969 Sep 14;44(2):333–345. doi: 10.1016/0022-2836(69)90179-x. [DOI] [PubMed] [Google Scholar]
- Buetti E. Characterization of late polyoma mRNA. J Virol. 1974 Aug;14(2):249–260. doi: 10.1128/jvi.14.2.249-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Gorski J., Morrison M. R., Merkel C. G., Lingrel J. B. Size heterogeneity of polyadenylate sequences in mouse globin messenger RNA. J Mol Biol. 1974 Jun 25;86(2):363–371. doi: 10.1016/0022-2836(74)90025-4. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., SPIEGELMAN S. The selective synthesis of informational RNA in bacteria. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1564–1580. doi: 10.1073/pnas.47.10.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Evidence for SV40 specific RNA containing virus and host specific sequences. Nat New Biol. 1972 Jan 12;235(54):46–47. doi: 10.1038/newbio235046a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Fridkin M., Zutra A., Littauer U. Z. Monofunctional substrates of polynucleotide phosphorylase. The monoaddition of 2'(3')-O-isovaleryl-nucleoside diphosphate to an initiator oligonucleotide. Eur J Biochem. 1971 Dec 22;24(1):4–11. doi: 10.1111/j.1432-1033.1971.tb19649.x. [DOI] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Accumulation of closed-circular polyoma DNA molecules containing host DNA during serial passage of the virus. Virology. 1974 Jan;57(1):296–299. doi: 10.1016/0042-6822(74)90132-9. [DOI] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Darnell J. E. SV40-specific RNA in the nucleus and polyribosomes of transformed cells. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1089–1096. doi: 10.1073/pnas.65.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor H., Fogel M., Sachs L. Integration of viral into chromosomal deoxyribonucleic acid in an inducible line of polyoma-transformed cells. Virology. 1973 May;53(1):174–185. doi: 10.1016/0042-6822(73)90476-5. [DOI] [PubMed] [Google Scholar]
- Manor H., Neer A. Effects of cycloheximide on virus RNA replication in an inducible line of polyoma-transformed rat cells. Cell. 1975 Jul;5(3):311–318. doi: 10.1016/0092-8674(75)90106-3. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Schimke R. T. Ovalbumin messenger RNA: evidence that the initial product of transcription is the same size as polysomal ovalbumin messenger. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4327–4331. doi: 10.1073/pnas.71.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Warner J. R., Edmonds M., Nakazato H., Vaughan M. H. Polyadenylic acid sequences in yeast messenger ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1466–1471. [PubMed] [Google Scholar]
- Nakazato H., Edmonds M., Kopp D. W. Differential metabolism of large and small poly(A) sequences in the heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1974 Jan;71(1):200–204. doi: 10.1073/pnas.71.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish J. H., Kirby K. S. Reagents which reduce interactions between ribosomal RNA and rapidly labelled RNA from rat liver. Biochim Biophys Acta. 1966 Dec 21;129(3):554–562. doi: 10.1016/0005-2787(66)90070-0. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Ralph R. K., Colter J. S. Evidence for the integration of polyoma virus DNA in a lytic system. Virology. 1972 Apr;48(1):49–58. doi: 10.1016/0042-6822(72)90113-4. [DOI] [PubMed] [Google Scholar]
- Rosenthal L. J., Salomon C., Weil R. Isolation and characterization of poly(A)-containing intranuclear polyoma-specific "giant" RNA'S. Nucleic Acids Res. 1976 May;3(5):1167–1183. doi: 10.1093/nar/3.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Winocour E. Covalently linked cell and SV40-specific sequences in an RNA from productively infected cells. Virology. 1972 Nov;50(2):558–566. doi: 10.1016/0042-6822(72)90407-2. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Kelly R. B., Sinsheimer R. L. Denaturation of RNA with dimethyl sulfoxide. Biopolymers. 1968 Jun;6(6):793–807. doi: 10.1002/bip.1968.360060604. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Wall R., Darnell J. E. Presence of cell and virus specific sequences in the same molecules of nuclear RNA from virus transformed cells. Nat New Biol. 1971 Jul 21;232(29):73–76. doi: 10.1038/newbio232073a0. [DOI] [PubMed] [Google Scholar]
- Weil R., Salomon E., May E., May P. A simplifying concept in tumor virology: virus-specific "pleiotropic effectors". Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):381–395. doi: 10.1101/sqb.1974.039.01.050. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Nuclear RNA metabolism. Annu Rev Biochem. 1973;42:329–354. doi: 10.1146/annurev.bi.42.070173.001553. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


