Abstract
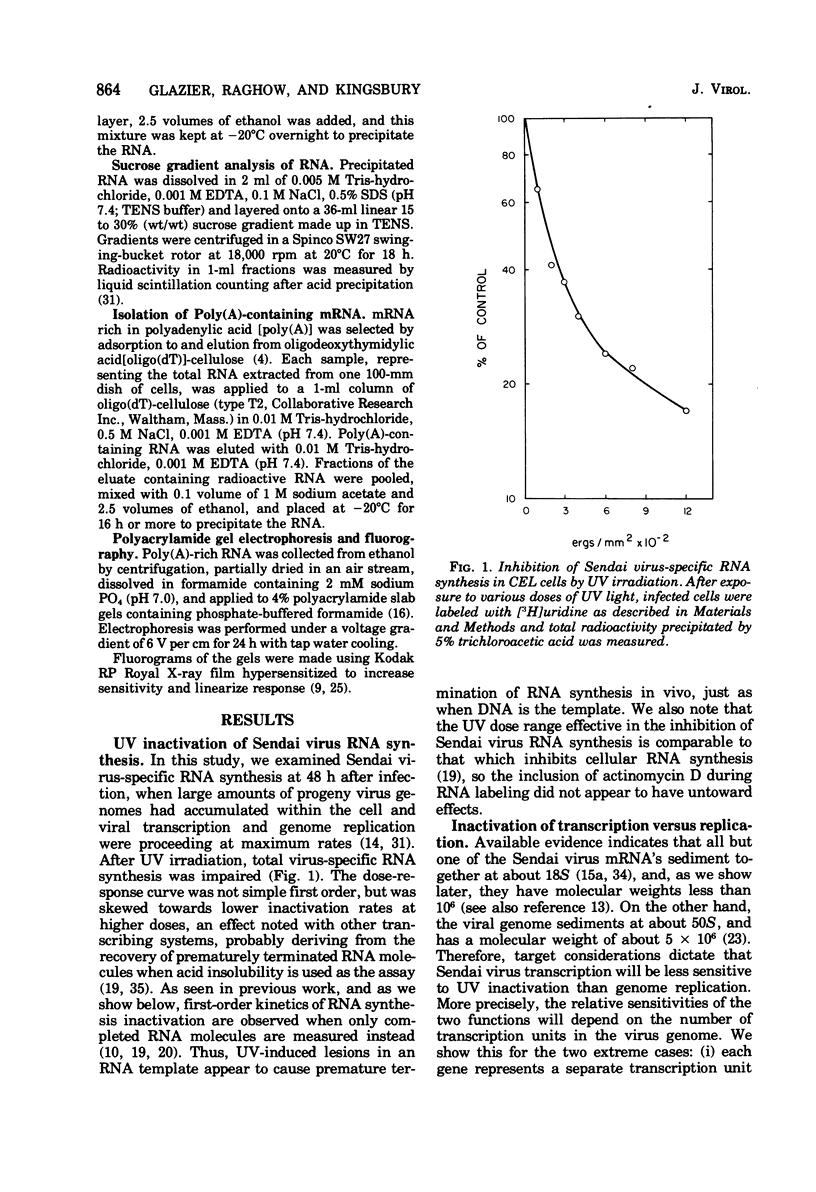
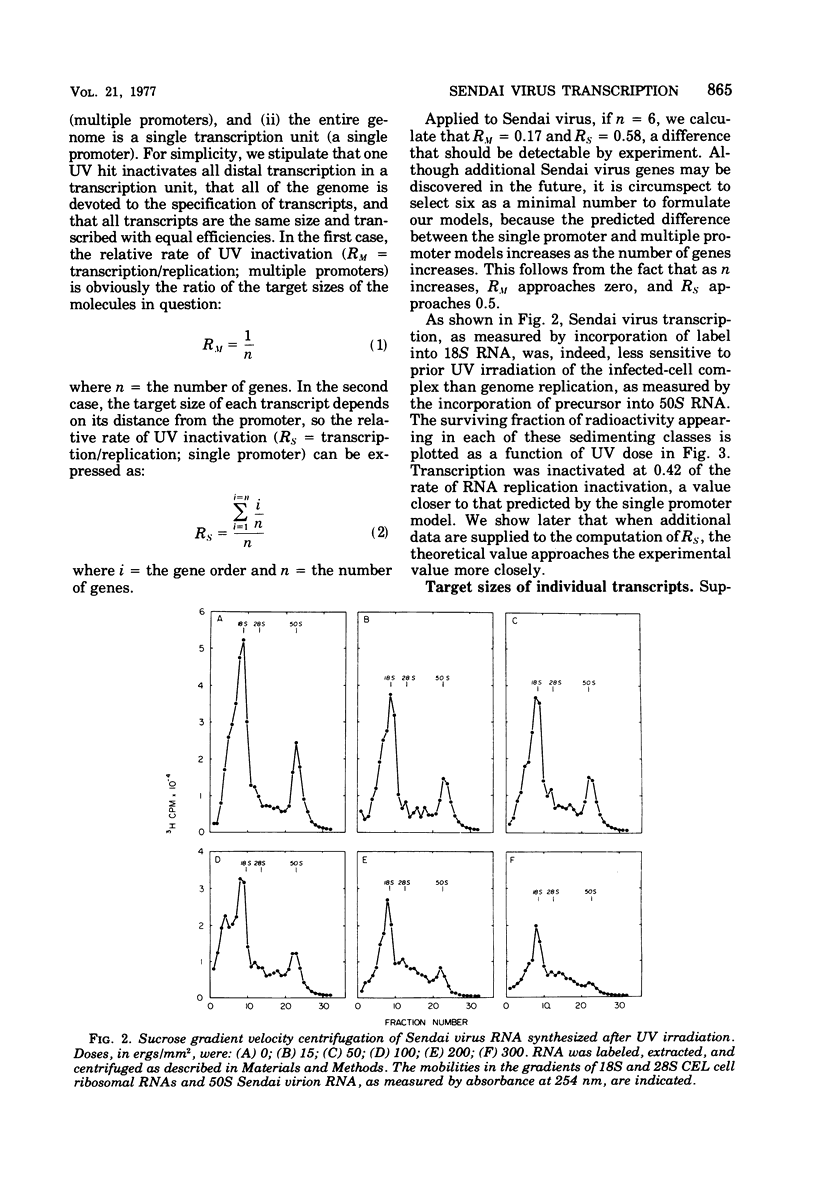
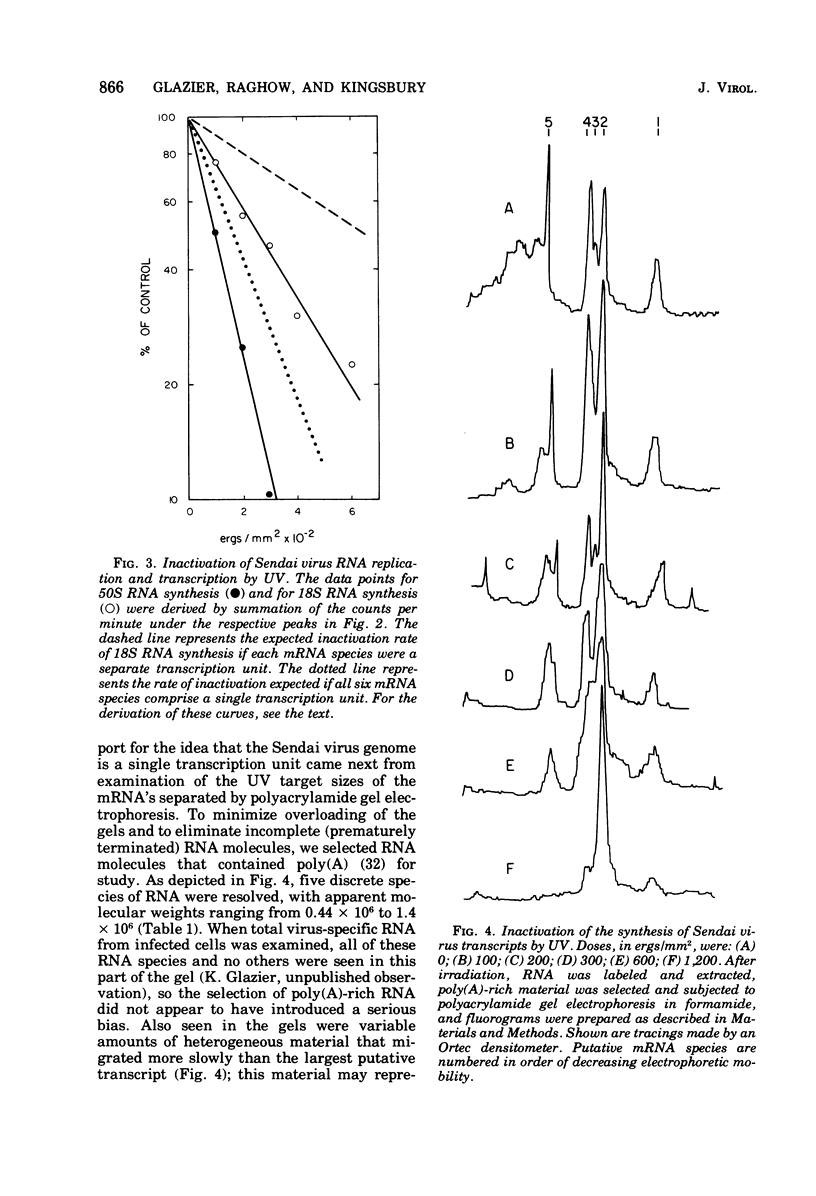
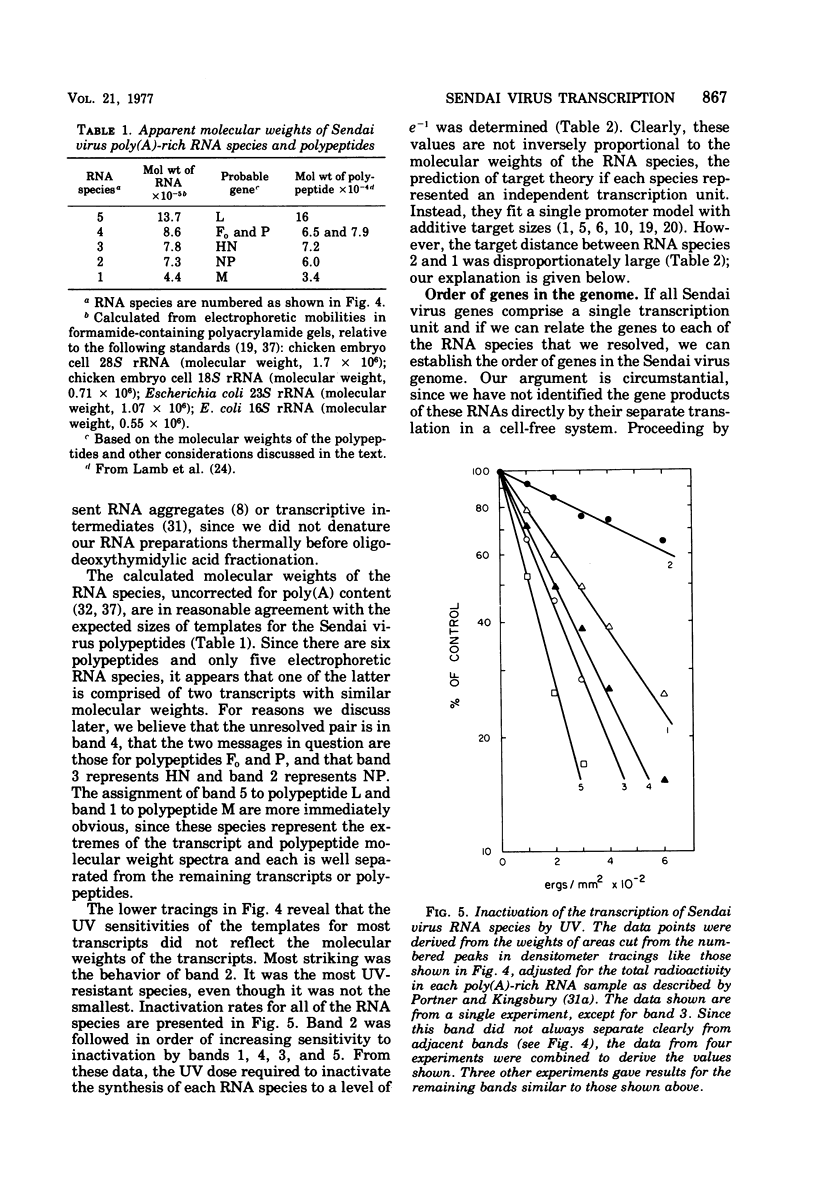
The synthesis of Sendai virus RNA species was examined after UV irradiation of cells late in infection. Compared with the inactivation of 50S genomic RNA synthesis, the synthesis of the group of mRNA species that sediments at 18S was inactivated at an average rate consistent with a process of sequential transcription from a single promoter. The rates of inactivation of the synthesis of individual mRNA's separated by polyacrylamide gel electrophoresis confirmed this and, with the aid of additional data, suggested that the order of genes in the Sendai virus genome is: 3'-NP-Fo-M-P-HM-L-5'.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham G., Rhodes D. P., Banerjee A. K. The 5' terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell. 1975 May;5(1):51–58. doi: 10.1016/0092-8674(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Adhya S., Gottesman M., De Crombrugghe B. Release of polarity in Escherichia coli by gene N of phage lambda: termination and antitermination of transcription. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2534–2538. doi: 10.1073/pnas.71.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A. Transcriptional mapping of vesicular stomatitis virus in vivo. J Virol. 1977 Jan;21(1):411–414. doi: 10.1128/jvi.21.1.411-414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971 Sep;35(3):235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Eukaryotic messenger RNA. Annu Rev Biochem. 1974;43(0):621–642. doi: 10.1146/annurev.bi.43.070174.003201. [DOI] [PubMed] [Google Scholar]
- Bräutigam A. R., Sauerbier W. Transcription unit mapping in bacteriophage T7. II. Proportionality of number of gene copies, mRNA, and gene product. J Virol. 1974 May;13(5):1110–1117. doi: 10.1128/jvi.13.5.1110-1117.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calendar R. The regulation of phage development. Annu Rev Microbiol. 1970;24:241–296. doi: 10.1146/annurev.mi.24.100170.001325. [DOI] [PubMed] [Google Scholar]
- Collins B. S., Bratt M. A. Separation of the messenger RNAs of Newcastle disease virus by gel electrophoresis. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2544–2548. doi: 10.1073/pnas.70.9.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington R. W., Portner A., Kingsbury D. W. Sendai virus replication: an ultrastructural comparison of productive and abortive infections in avian cells. J Gen Virol. 1970 Dec;9(3):169–177. doi: 10.1099/0022-1317-9-3-169. [DOI] [PubMed] [Google Scholar]
- David A. E. Control of vesicular stomatitis virus protein synthesis. Virology. 1976 May;71(1):217–229. doi: 10.1016/0042-6822(76)90107-0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Martin S. A., Paoletti E., Moss B. Modification of the 5'-terminus of mRNA by soluble guanylyl and methyl transferases from vaccinia virus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2525–2529. doi: 10.1073/pnas.72.7.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Sauerbier W. The transcriptional organization of the ribosomal RNA genes in mouse L cells. J Mol Biol. 1975 Jan 25;91(3):235–256. doi: 10.1016/0022-2836(75)90378-2. [DOI] [PubMed] [Google Scholar]
- Hercules K., Sauerbier W. Transcription units in bacteriophage T4. J Virol. 1973 Oct;12(4):872–881. doi: 10.1128/jvi.12.4.872-881.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto F., Schlessinger D. Bearing of some recent results on the mechanisms of polarity and messenger RNA stability. Mol Gen Genet. 1974;135(1):29–38. doi: 10.1007/BF00433898. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. The molecular biology of paramyxoviruses. Med Microbiol Immunol. 1974;160(2-3):73–83. doi: 10.1007/BF02121714. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Delius H. Molecular weight determination of Sendai and Newcastle disease virus RNA. J Virol. 1974 Feb;13(2):261–268. doi: 10.1128/jvi.13.2.261-268.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Reichmann M. E. The RNA of defective vesicular stomatitis virus particles in relation to viral cistrons. J Mol Biol. 1974 Jan 5;85(4):551–568. doi: 10.1016/0022-2836(74)90315-5. [DOI] [PubMed] [Google Scholar]
- Lim L. W., Kennel D. Evidence against transcription termination within the E. coli lac operon. Mol Gen Genet. 1974;133(4):367–371. doi: 10.1007/BF00332713. [DOI] [PubMed] [Google Scholar]
- Lomniczi B., Meager A., Burke D. C. Virus RNA and protein synthesis in cells infected with different strains of Newcastle disease virus. J Gen Virol. 1971 Oct;13(1):111–120. doi: 10.1099/0022-1317-13-1-111. [DOI] [PubMed] [Google Scholar]
- Perlman S. M., Huang A. S. RNA synthesis of vesicular stomatitis virus. V. Interactions between transcription and replication. J Virol. 1973 Dec;12(6):1395–1400. doi: 10.1128/jvi.12.6.1395-1400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Identification of transcriptive and replicative intermediates in Sendai virus-infected cells. Virology. 1972 Mar;47(3):711–725. doi: 10.1016/0042-6822(72)90561-2. [DOI] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Regulatory events in the synthesis of Sendai virus polypeptides and their assembly into virions. Virology. 1976 Aug;73(1):79–88. doi: 10.1016/0042-6822(76)90062-3. [DOI] [PubMed] [Google Scholar]
- Pridgen C., Kingsbury D. W. Adenylate-rich sequences in Sendai virus transcripts from infected cells. J Virol. 1972 Aug;10(2):314–317. doi: 10.1128/jvi.10.2.314-317.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S. Sendai virus RNA synthesis and nucleocapsid formation in the presence of cycloheximide. Virology. 1971 Jun;44(3):494–502. doi: 10.1016/0042-6822(71)90362-x. [DOI] [PubMed] [Google Scholar]
- Roux L., Kolakofsky D. Isolation of RNA transcripts from the entire Sendai viral genome. J Virol. 1975 Dec;16(6):1426–1434. doi: 10.1128/jvi.16.6.1426-1434.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier W., Millette R. L., Hackett P. B., Jr The effects of ultraviolet irradiation on the transcription of T4 DNA. Biochim Biophys Acta. 1970;209(2):368–386. doi: 10.1016/0005-2787(70)90735-5. [DOI] [PubMed] [Google Scholar]
- Stamminger G., Lazzarini R. A. Analysis of the RNA of defective VSV particles. Cell. 1974 Sep;3(1):85–93. doi: 10.1016/0092-8674(74)90044-0. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., Breindl M., Holland J. J. Determination of molar ratios of vesicular stomatitis virus induced RNA species in BHK21 cells. Biochemistry. 1976 Apr 20;15(8):1663–1667. doi: 10.1021/bi00653a012. [DOI] [PubMed] [Google Scholar]
- Zaides V. M., Selimova L. M., Zhirnov O. P., Bukrinskaya A. G. Protein synthesis in Sendai virus-infected cells. J Gen Virol. 1975 Jun;27(3):319–327. doi: 10.1099/0022-1317-27-3-319. [DOI] [PubMed] [Google Scholar]


