Abstract
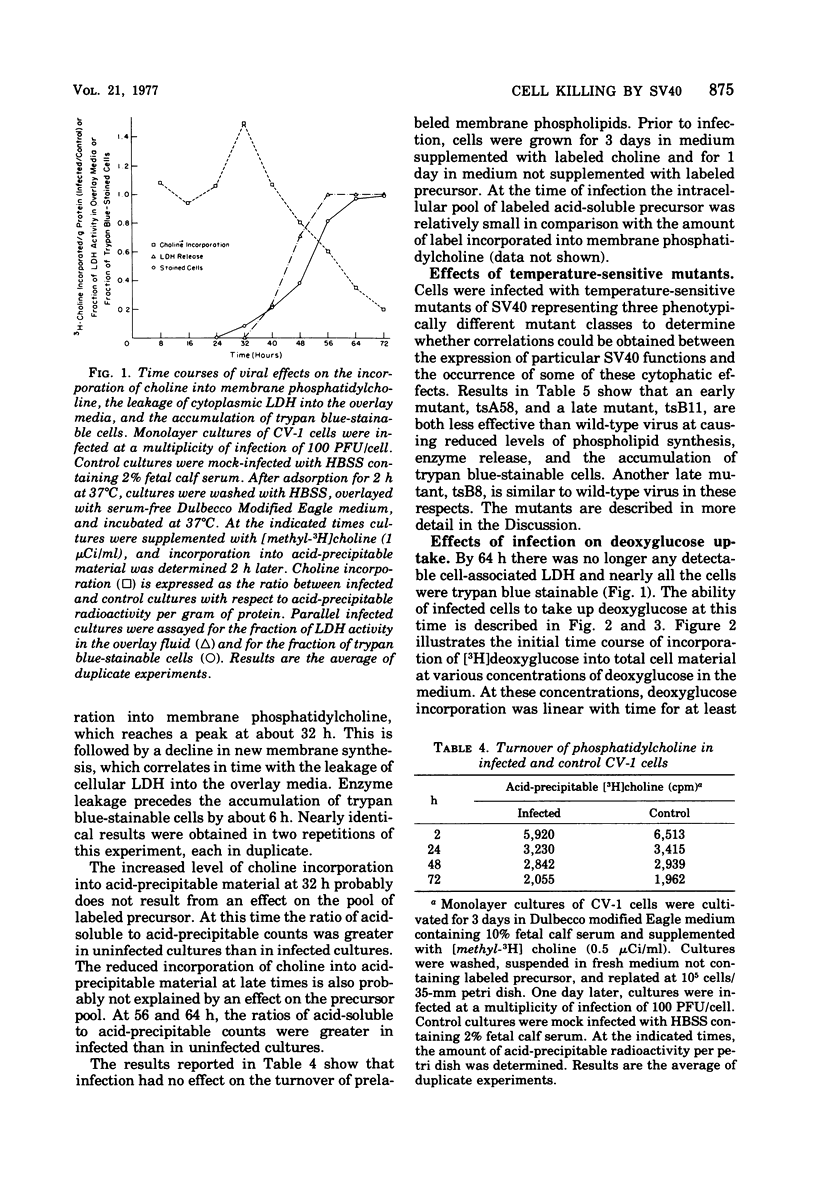
Simian virus 40 infection of the CV-1 line of green monkey kidney cells results in the release of mitochondrial malic dehydrogenase as early as 24 h. Released malic dehydrogenase is detected in the cytoplasm prior to its appearance in the overlay medium. Infected cells lose the ability to consume oxygen between 48 and 56 h, and damage to the elctron transport system is indicated. Nevertheless, cellular ATP levels remain high as late as 72 h. Infection leads to a stimulation of membrane phospholipid synthesis, which reaches a peak at about 32 h. This is followed by a severe decline in new membrane synthesis, which correlates in time with the release of cytoplasmic lactic dehydrogenase into the overlay media. Lactic dehydrogenase release precedes the accumulation of trypan blue-stainable cells by about 6 h. Infection had no effect on the turnover of prelabeled membrane phospholipids. An early simian virus 40 mutant, tsA58, and a late mutant, tsB11, are both less effective than wild-type virus at causing reduced levels of phospholipid synthesis, enzyme release, and the accumulation of trypan blue-stainable cells. Another late mutant, tsB8, is similar to wild-type virus in these respects. At 64 h, there is no detectable cell-associated lactic dehydrogenase and nearly all the cells are trypan blue stainable. Nevertheless, at concentrations of deoxyglucose in the medium below the transport Km, deoxyglucose uptake was similar in infected and control cultures. With higher concentrations of deoxyglucose in the medium, uptake by the infected cultures exceeded that by the control cultures.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., SANDELIN K. Activation of lysosomal enzymes in virus-infected cells and its possible relationship to cytopathic effects. J Exp Med. 1963 Jun 1;117:879–887. doi: 10.1084/jem.117.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. D., Brennan P. J. Effect of Sendai virus infection on lipid metabolism in chick embryo fibroblasts. J Virol. 1972 May;9(5):813–822. doi: 10.1128/jvi.9.5.813-822.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. D., Roberts W. K. Mechanism of Mengo virus-induced cell injury in L cells: use of inhibitors of protein synthesis to dissociate virus-specific events. J Virol. 1972 Nov;10(5):969–978. doi: 10.1128/jvi.10.5.969-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUSH C. R., YOUNGNER J. S. Lipids of virus infected cells. II. Lipid analysis of HeLa cells infected with vaccinia virus. Proc Soc Exp Biol Med. 1963 Apr;112:1082–1085. doi: 10.3181/00379727-112-28257. [DOI] [PubMed] [Google Scholar]
- GILBERT V. E. ENZYME RELEASE FROM TISSUE CULTURES AS AN INDICATOR OF CELLULAR INJURY BY VIRUSES. Virology. 1963 Dec;21:609–616. doi: 10.1016/0042-6822(63)90234-4. [DOI] [PubMed] [Google Scholar]
- Guskey L. E., Smith P. C., Wolff D. A. Patterns of cytopathology and lysosomal enzyme release in poliovirus-infected HEp-2 cells treated with either 2-(alpha-hydroxybenzyl)-benzimidazole or guanidine HCl. J Gen Virol. 1970 Jan;6(1):151–161. doi: 10.1099/0022-1317-6-1-151. [DOI] [PubMed] [Google Scholar]
- Katzman J., Wilson D. E. Newcastle disease virus-induced plasma membrane damage. J Gen Virol. 1974 Jul;24(1):101–113. doi: 10.1099/0022-1317-24-1-101. [DOI] [PubMed] [Google Scholar]
- Kiehn E. D. Protein metabolism in SV40-infected cells. Virology. 1973 Nov;56(1):313–333. doi: 10.1016/0042-6822(73)90309-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McIntosh K., Payne S., Russell W. C. Studies on lipid metabolism in cells infected with adenovirus. J Gen Virol. 1971 Mar;10(3):251–265. doi: 10.1099/0022-1317-10-3-251. [DOI] [PubMed] [Google Scholar]
- Norkin L. C., Ouellette J. Cell killing by simian virus 40: variation in the pattern of lysosomal enzyme release, cellular enzyme release, and cell death during productive infection of normal and simian virus 40-transformed simian cell lines. J Virol. 1976 Apr;18(1):48–57. doi: 10.1128/jvi.18.1.48-57.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENMAN S. STIMULATION OF THE INCORPORATION OF CHOLINE IN POLIOVIRUS-INFECTED CELLS. Virology. 1965 Jan;25:149–152. doi: 10.1016/0042-6822(65)90263-1. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. THE SOURCE OF THE RIBONUCLEIC ACID AND PHOSPHOLIPID OF SINDBIS VIRUS. Virology. 1963 Jul;20:446–456. doi: 10.1016/0042-6822(63)90093-x. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Choline metabolism and membrane formation in rat hepatoma cells grown in suspension culture. I. Incorporation of choline into phosphatidylcholine of mitochondria and other membranous structures and effect of metabolic inhibitors. Arch Biochem Biophys. 1968 Oct;128(1):70–87. doi: 10.1016/0003-9861(68)90009-x. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Cleveland P. H., Shea M. A. Effect of mengovirus replication on choline metabolism and membrane formation in novikoff hepatoma cells. J Virol. 1970 Dec;6(6):800–812. doi: 10.1128/jvi.6.6.800-812.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Richey D. P. Transport of nucleosides, nucleic acid bases, choline and glucose by animal cells in culture. Biochim Biophys Acta. 1974 Dec 16;344(3-4):263–305. doi: 10.1016/0304-4157(74)90010-0. [DOI] [PubMed] [Google Scholar]
- Poste G. Virus-induced polykaryocytosis and the mechanism of cell fusion. Adv Virus Res. 1970;16:303–356. doi: 10.1016/s0065-3527(08)60026-3. [DOI] [PubMed] [Google Scholar]
- Renner E. D., Plagemann P. G., Bernlohr R. W. Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem. 1972 Sep 25;247(18):5765–5776. [PubMed] [Google Scholar]
- Robb J. A., Tegtmeyer P., Ishikawa A., Ozer H. L. Antigenic phenotypes and complementation groups of temperature-sensitive mutants of simian virus 40. J Virol. 1974 Mar;13(3):662–665. doi: 10.1128/jvi.13.3.662-665.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELOKOV A., VOGEL J. E., CHI L. Hemadsorption (adsorption-hemagglutination) test for viral agents in tissue culture with special reference to influenza. Proc Soc Exp Biol Med. 1958 Apr;97(4):802–809. doi: 10.3181/00379727-97-23884. [DOI] [PubMed] [Google Scholar]
- Studzinski G. P., Gierthy J. F., Cholon J. J. An autoradiographic screening test for mycoplasmal contamination of mammalian cell cultures. In Vitro. 1973 May-Jun;8(6):466–472. doi: 10.1007/BF02615948. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Ozer H. L. Temperature-sensitive mutants of simian virus 40: infection of permissive cells. J Virol. 1971 Oct;8(4):516–524. doi: 10.1128/jvi.8.4.516-524.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Robb J. A., Widmer C., Ozer H. L. Altered protein metabolism in infection by the late tsB11 mutant of simian virus 40. J Virol. 1974 Oct;14(4):997–1007. doi: 10.1128/jvi.14.4.997-1007.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


