Abstract
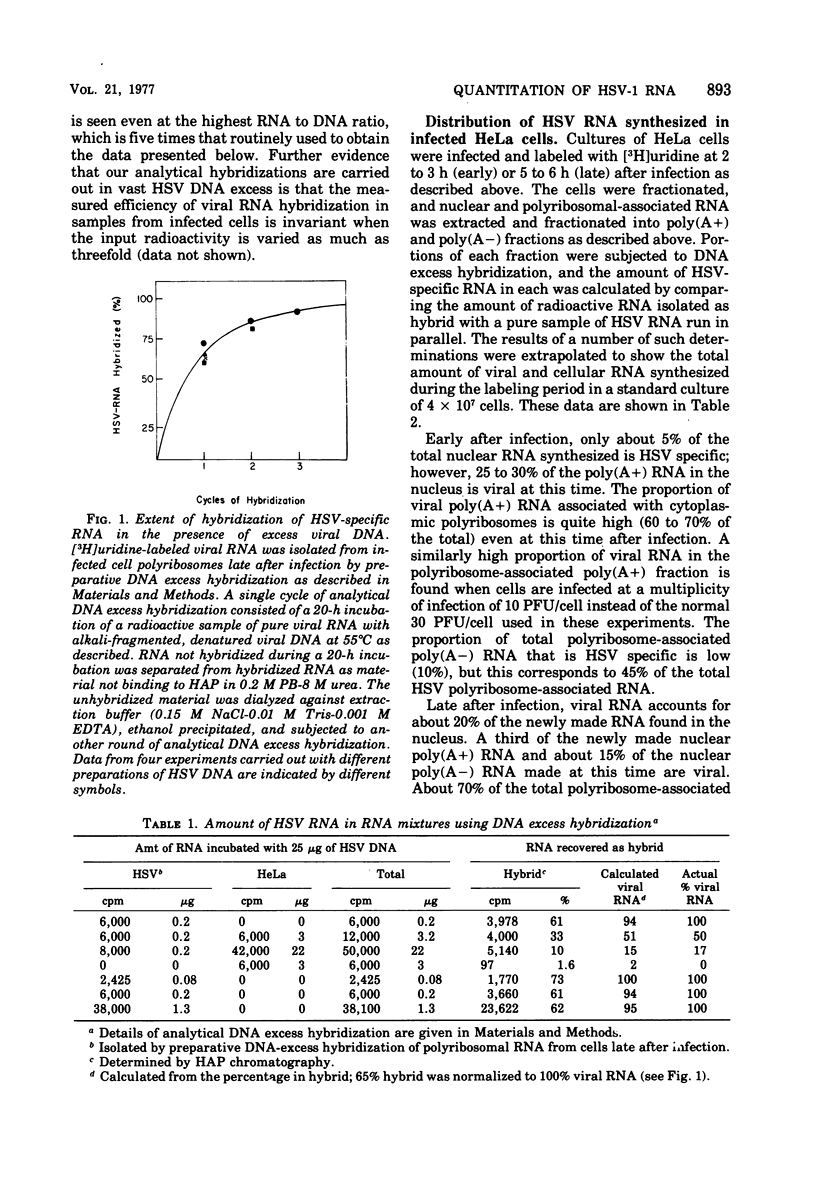
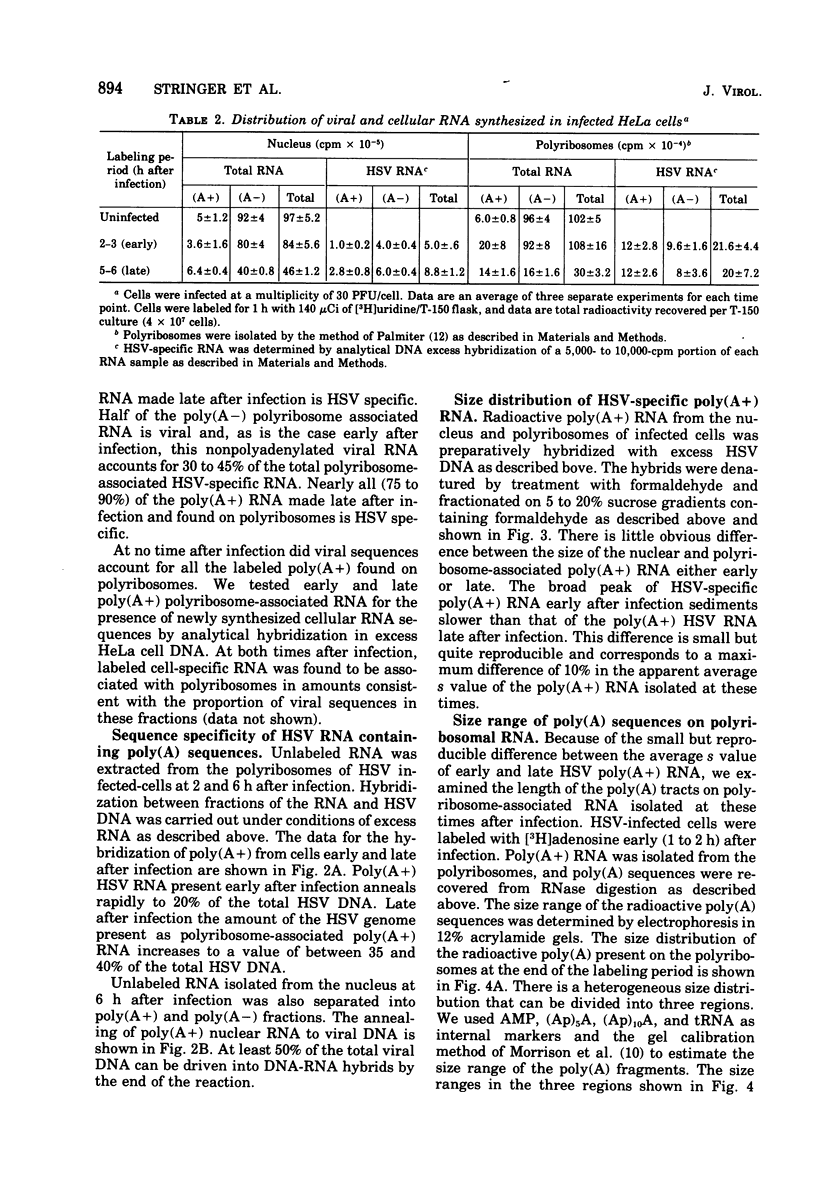
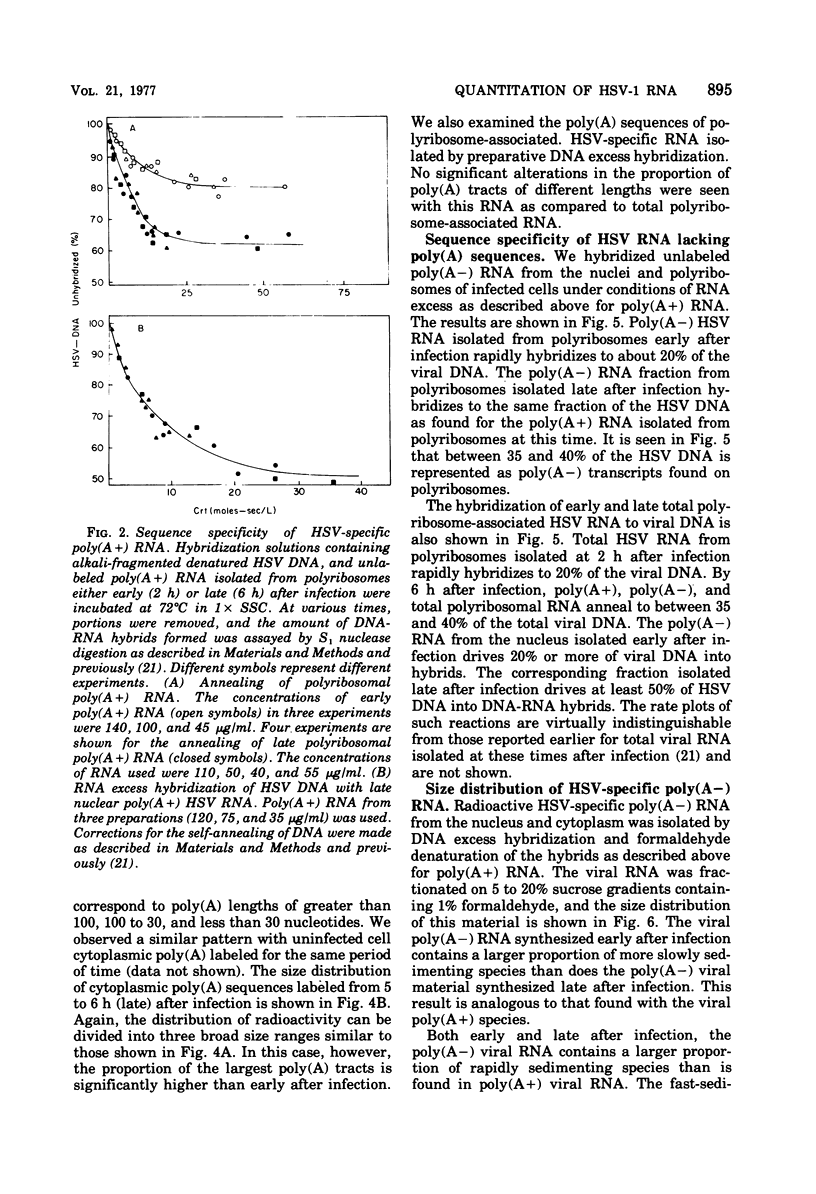
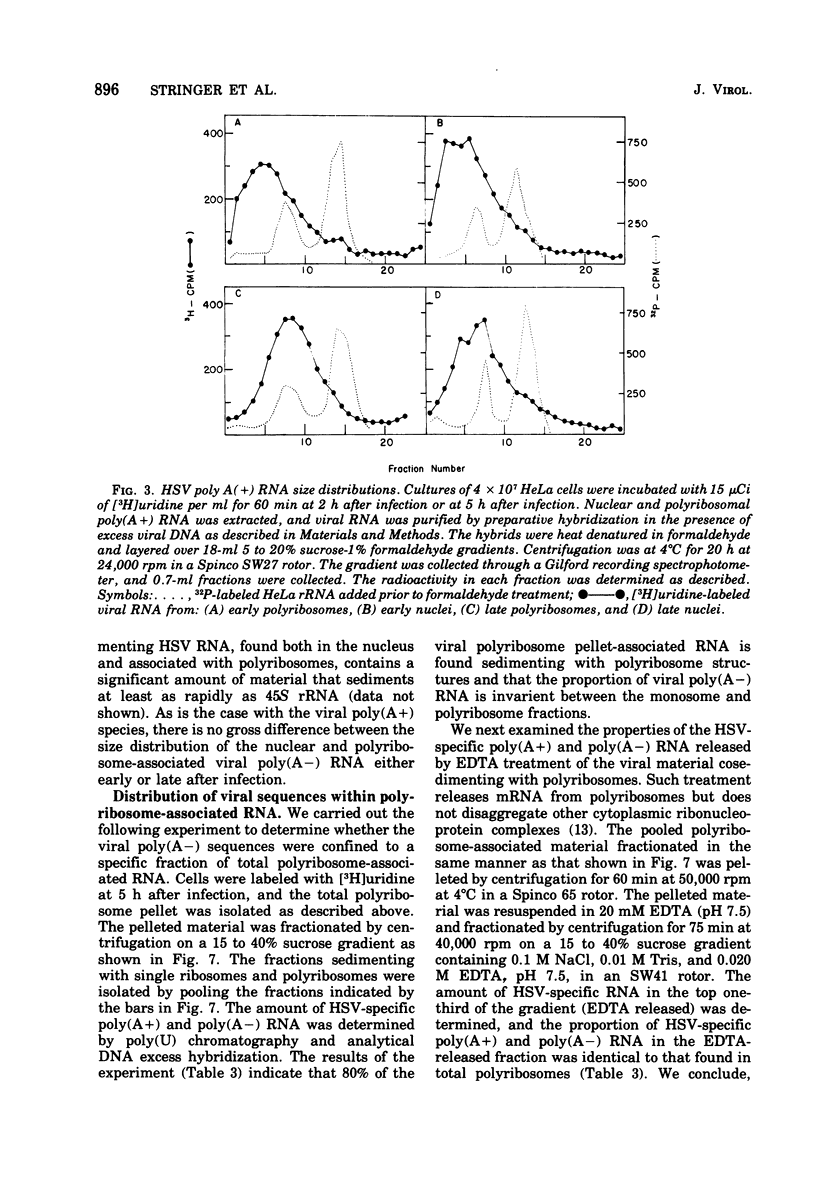
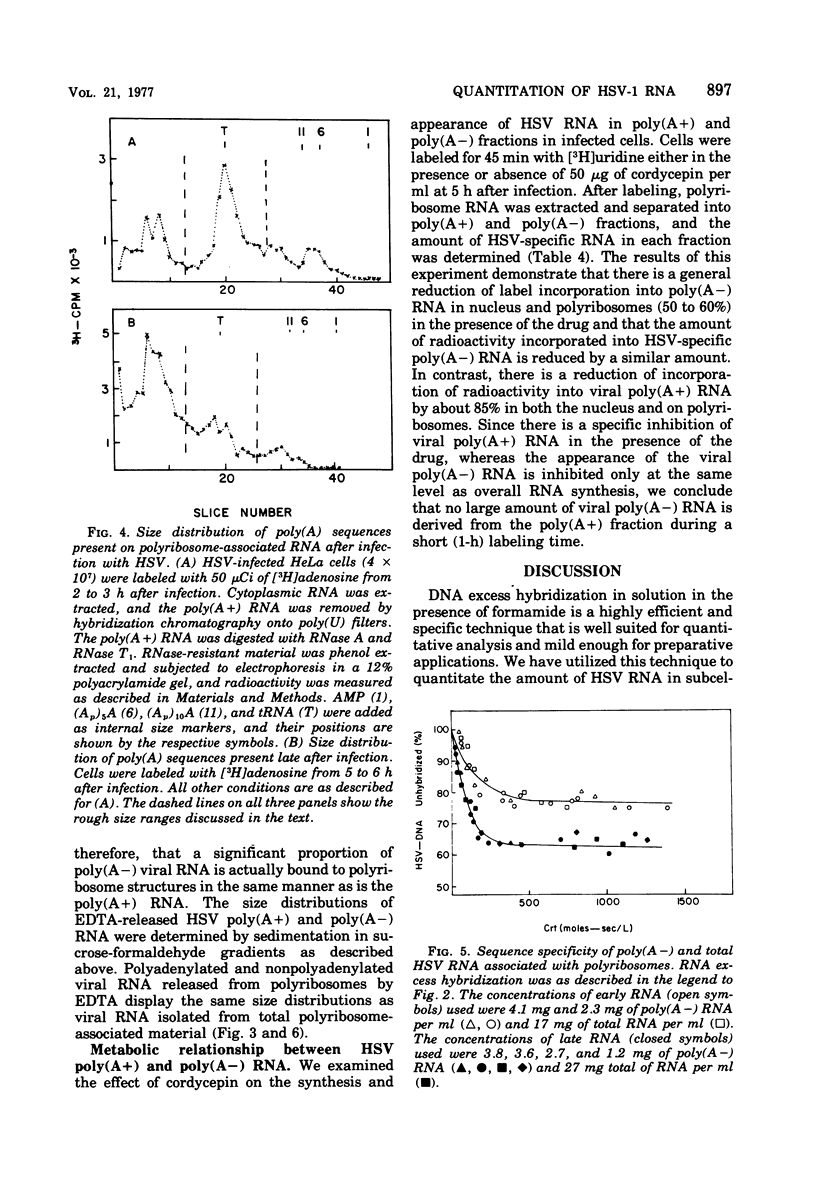
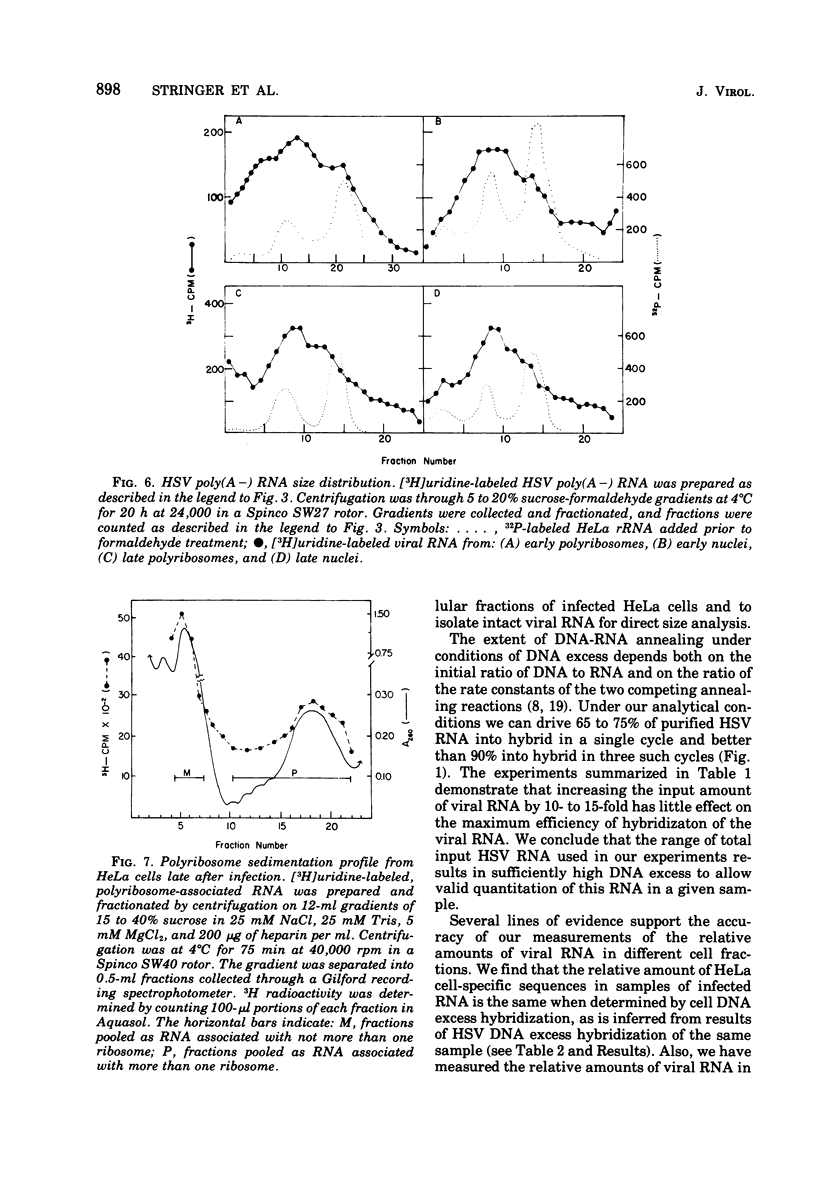
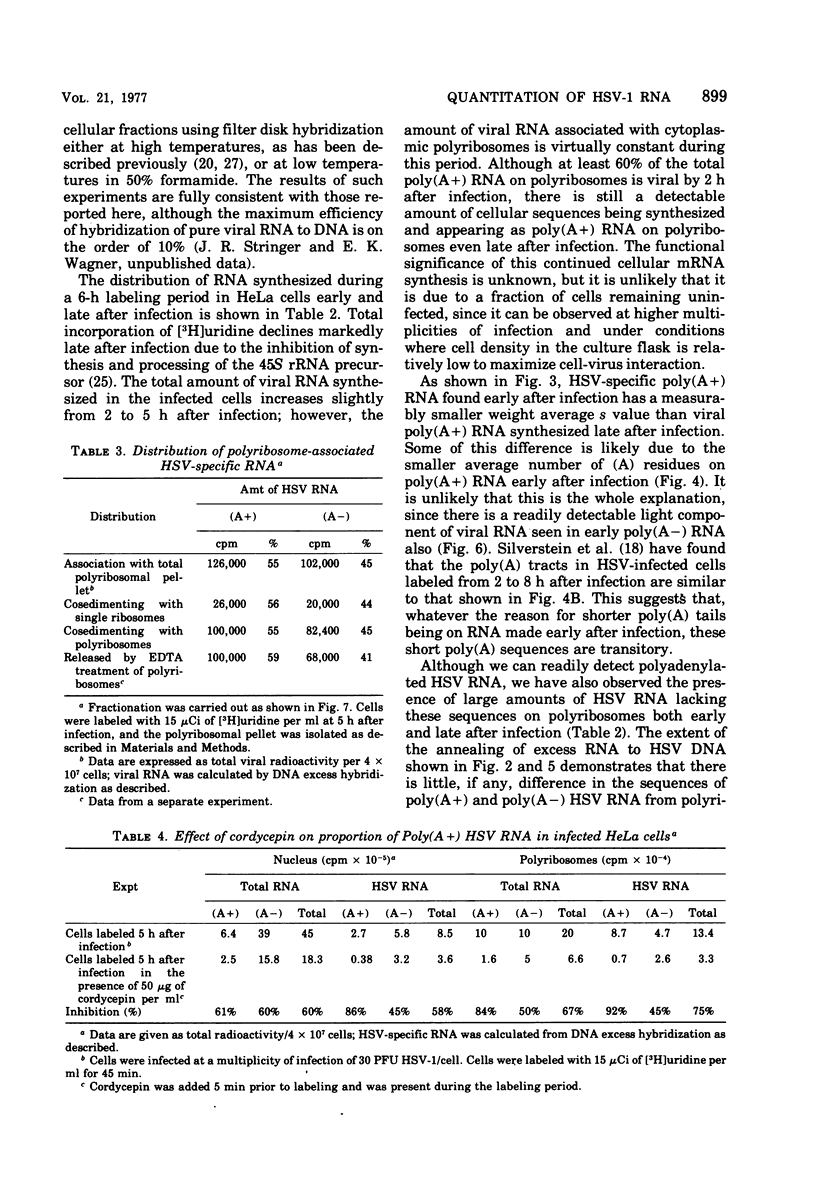
We have quantitatively analyzed the size and amount of herpes simplex virus (HSV)-specific RNA synthesized in HeLa cells using DNA and RNA excess hybridization. At 2 h after infection (early), transcripts from 20% of the total HSV DNA are present on polyribosomes as poly(A+) RNA. At this time, viral poly(A+) RNA comprises 60 to 75% of the newly synthesized poly(a+) mRNA on polyribosomes. By 6 h after infection (late), poly(A+) HSV RNA transcribed from 35 to 40% of the viral DNA is found on polyribosomes. These viral poly(A+) transcripts comprised as much as 90% of newly synthesized poly(A+) mRNA and are measurably larger than viral poly(A+) transcripts isolated early. Some but not all of this size difference is due to the fact that the poly(A) tails on early transcripts are shorter than those found on transcripts made late. Even late after infection, a small but readily measurable amount of cellular poly(A+) RNA is still being made and entering polyribosome complexes. In the nucleus, late after infection, poly(A+) HSV RNA is complementary to 50% of the total HSV DNA. Both early and late after infection, total nuclear viral transcripts are, on the average, larger than viral transcripts found on polyribosomes; however, nuclear HSV poly(A+) RNA is not measureably larger than the corresponding cytoplasmic viral poly(A+) sequences at either time. A major portion (30 to 40%) of the polyribosomal HSV RNA made either early or late after infection is not polyadenylated. This HSV poly (A-) RNA is transcribed from the same sequences as HSV poly(A+) RNA but, when labeled and isolated either early or late after infection, both nuclear and polyribosomal viral poly(A-) RNA molecules sediment faster in sucrose-formaldehyde gradients than their polyadenylated counterparts.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y. Poly A and symmetrical transcription of SV40 DNA. Nat New Biol. 1973 May 2;243(122):2–6. [PubMed] [Google Scholar]
- Bachenheimer S. L., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. VI. Polyadenylic acid sequences in viral messenger ribonucleic acid. J Virol. 1972 Oct;10(4):875–879. doi: 10.1128/jvi.10.4.875-879.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtker H. Dependence of the sedimentation coefficient on molecular weight of RNA after reaction with formaldehyde. J Mol Biol. 1968 Jul 14;35(1):61–70. doi: 10.1016/s0022-2836(68)80036-1. [DOI] [PubMed] [Google Scholar]
- Kozak M., Roizman B. Regulation of herpesvirus macromolecular synthesis: nuclear retention of nontranslated viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4322–4326. doi: 10.1073/pnas.71.11.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Persson T. Isolation of mRNA from KB-cells by affinity chromatography on polyuridylic acid covalently linked to Sepharose. Eur J Biochem. 1972 Dec 4;31(2):246–254. doi: 10.1111/j.1432-1033.1972.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Murray B. K., Benyesh-Melnick M., Biswal N. Early and late viral-specific polyribosomal RNA in herpes virus-1 and -2-infected rabbit kidney cells. Biochim Biophys Acta. 1974 Aug 29;361(2):209–220. doi: 10.1016/0005-2787(74)90348-7. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G., Davidson N. Expression of the mitochondrial genome in HeLa cells. VI. Size determination of mitochondrial ribosomal RNA by electron microscopy. J Mol Biol. 1971 Sep 28;60(3):473–484. doi: 10.1016/0022-2836(71)90182-3. [DOI] [PubMed] [Google Scholar]
- Roizman B., Kozak M., Honess R. W., Hayward G. Regulation of herpesvirus macromolecular synthesis: evidence for multilevel regulation of herpes simplex 1 RNA and protein synthesis. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):687–701. doi: 10.1101/sqb.1974.039.01.083. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S., Bachenheimer S. L., Frenkel N., Roizman B. Relationship between post-transcriptional adenylation of herpes virus RNA and messenger RNA abundance. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2101–2104. doi: 10.1073/pnas.70.7.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvertien S., Millette R., Jones P., Roizman B. RNA synthesis in cells infected with herpes simplex virus. XII. Sequence complexity and properties of RNA differing in extent of adenylation. J Virol. 1976 Jun;18(3):977–991. doi: 10.1128/jvi.18.3.977-991.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus N. A., Bonner T. I. Temperature dependence of RNA-DNA hybridization kinetics. Biochim Biophys Acta. 1972 Aug 16;277(1):87–95. doi: 10.1016/0005-2787(72)90355-3. [DOI] [PubMed] [Google Scholar]
- Swanstrom R. I., Pivo K., Wagner E. K. Restricted transcription of the herpes simplex virus genome occurring early after infection and in the presence of metabolic inhibitors. Virology. 1975 Jul;66(1):140–150. doi: 10.1016/0042-6822(75)90185-3. [DOI] [PubMed] [Google Scholar]
- Swanstrom R. I., Wagner E. K. Regulation of synthesis of herpes simplex type 1 virus mRNA during productive infection. Virology. 1974 Aug;60(2):522–533. doi: 10.1016/0042-6822(74)90346-8. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wagner E. K. Evidence for transcriptional control of the herpes simplex virus genome in infected human cells. Virology. 1972 Feb;47(2):502–506. doi: 10.1016/0042-6822(72)90289-9. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. I. Patterns of ribonucleic acid synthesis in productively infected cells. J Virol. 1969 Jul;4(1):36–46. doi: 10.1128/jvi.4.1.36-46.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Swanstrom R. I., Rice M., Howell L., Lane J. Variation in the molecular size of the DNA from closely related strains of type I herpes simplex virus. Biochim Biophys Acta. 1976 Jun 18;435(2):192–205. doi: 10.1016/0005-2787(76)90250-1. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Swanstrom R. I., Stafford M. G. Transcription of the herpes simplex virus genome in human cells. J Virol. 1972 Oct;10(4):675–682. doi: 10.1128/jvi.10.4.675-682.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Tewari K. K., Kolodner R., Warner R. C. The molecular size of the herpes simplex virus type 1 genome. Virology. 1974 Feb;57(2):436–447. doi: 10.1016/0042-6822(74)90183-4. [DOI] [PubMed] [Google Scholar]


