Summary
While JA signaling is widely accepted as mediating plant resistance to herbivores, and the importance of the roots in plant defenses is recently being recognized, the function of root-JA production or perception in aboveground plant defense remains unstudied.
To restrain JA impairment to the roots, we micrografted wild type Nicotiana attenuata shoots to the roots of transgenic plants impaired in JA signaling, and evaluated ecological relevant traits under glasshouse and field conditions.
Root-JA synthesis, conjugation, and perception are involved in regulating nicotine production in roots. Strikingly, roots regulated leaf JA and ABA levels, which in turn, explain differences in nicotine transport from the roots to the shoot via the transpiration stream. Root-JA signaling also regulates the accumulation of other shoot metabolites; these account for plant resistance against a generalist, Spodoptera littoralis, and a specialist herbivore, Manduca sexta. In N. attenuata’s native habitat, silencing root-JA synthesis increased the shoot damage inflicted by Empoasca leafhoppers, which are able to select natural jasmonate mutants. Silencing JA perception in roots also increased damage by Tupiocoris notatus.
Thus, the whole is greater than the sum of its parts: root jasmonate signaling profoundly tailors leaf defense responses to aboveground attack.
Keywords: plant defense, plant tolerance, roots, nicotine, leaf wounding, aboveground herbivores, jasmonates
Introduction
Plants have evolved refined signaling mechanisms capable of inducing responses specifically according to different stimuli. These signals can act locally, but can also travel to distal systemic parts within the plant, fine-tuning the whole-plant performance and maximizing its Darwinian fitness. An interesting example of these systemic events is the inducible responses of plants when attacked by leaf-feeders: within minutes, defense mechanisms are triggered in attacked leaves and also in unattacked leaves and roots (Wu & Baldwin, 2010). Transcriptional changes in systemic roots in response to Spodoptera littoralis leaf attack were shown to even exceed the local response of infested leaves of maize (Erb et al., 2009). Most interestingly, no overlap in induced genes was found between the local shoots and the systemic roots, suggesting that plant responses are triggered in a tissue specific manner.
Jasmonic acid (JA) and its derivatives, collectively known as jasmonates (JAs), are rapidly induced in the local tissues attacked by herbivores, and activate direct and indirect plant defenses (Kessler et al., 2004; Howe & Jander, 2008). The initial step of JA biosynthesis is the production of linolenic acid from membrane-derived polyunsaturated fatty acids (PUFAs) through a lipase enzyme activity (Howe & Schilmiller, 2002). Linolenic acid serves as substrate for two distinct branches of the lipoxigenase (LOX) pathway: 9-LOX and 13-LOX, yielding 9- and 13-hydroperoxy linolenic acid (HPOT), respectively, depending on the oxygen position in the C18 chain. 13-HPOT is converted into the intermediate 12-oxo-phytodienoic acid (OPDA) by the action of an allene oxidase synthase followed by an allene oxidase cyclase (AOC; Stenzel et al., 2003; Kallenbach et al., 2012) in chloroplasts. OPDA is then transported into the peroxisome where it is reduced and submitted to cycles of β-oxidation which finally leads to the production of JA. JA can be subsequently conjugated to a methyl group (MeJA) by the action of jasmonyl-O-methyl transferase (JMT; Seo et al., 2001; Stitz et al., 2011), or to isoleucine (JA-Ile) by jasmonate-resistant 4 (JAR4) or jasmonate-resistant 6 (JAR6; Staswick & Tiryaki, 2004; Wang et al., 2007). JA-Ile, the bioactive conjugated form of JA, interacts with coronatine-insensitive 1 (COI1) and triggers the degradation of jasmonate ZIM domain (JAZ) proteins, which releases downstream positive regulators of the JA-mediated responses (Xie et al., 1998; Chini et al., 2007; Paschold et al., 2007; Oh et al., 2012).
JA signaling is also required for the systemic defense response in the aboveground parts of a plant. Grafting experiments have dissected the local versus systemic requirements of the JA signaling components in the shoots of tomato (Li et al., 2002; Li et al., 2005). For the belowground systemic responses, JA signaling has been suggested to mediate root responses to shoot elicitation. In tobacco, leaf wounding increases JA pools in roots after a rapid increase in damaged leaves (Zhang & Baldwin, 1997). The same study also showed that 14C-labeled JA applied to wounded leaves was recovered in roots, at similar rates of endogenous root-JA, suggesting that leaf-derived JA transported to roots could alone account for the systemic increase in root-JA after leaf wounding. In a later study, Wang et al. (2008) showed that, after a treatment that mimicked herbivore attack combined to the application of 13C-labeled Ile, newly synthesized13C-labeled JA-Ile was only detected in elicited leaves, but not in roots. However, these results should be interpreted with caution, because it is possible that labeled compounds are not metabolized or transported in the same way as plant-derived compounds. Recently, Grebner et al. (2013) showed that roots when wounded can synthesize JA independently of the shoots by the action of specific JA biosynthetic enzymes, but to date it remains unexplored whether root-JA is employed in root systemic responses after shoot elicitation.
One of the best studied examples of JA-dependent systemic responses of the roots is the production of nicotine, which is synthesized in the belowground tissues of tobacco plants. Nicotine is the most abundant alkaloid found in tobacco leaves, with basal levels of 0.1 to 1% of dry mass (Baldwin, 1999). It is highly toxic to most herbivores (Glendinning, 2002), and it is effective in deterring leaf consumption of generalists, such as S. exigua, as well as specialists, like Manduca sexta (Steppuhn et al., 2004). Upon leaf damage, levels of nicotine in shoots dramatically raise (up to 10-fold) as a consequence of induced expression of the putrescine N-methyltransferase (PMT) gene in the roots (Winz & Baldwin, 2001). Hence, nicotine induction (NI) utilizes a substantial portion of a plant’s nitrogen budget: 6-8 % of a plant’s total nitrogen is in this molecule alone, without considering the nitrogen demands of nicotine biosynthesis, transport and storage (Baldwin et al., 1994). Interestingly, this investment does not seem to be recovered and reinvested in growth, even when plants are grown in nitrogen-limited conditions, meaning that inducing nicotine is a one-way investment (Baldwin & Ohnmeiss, 1994). In addition, roots can regulate the increment of nicotine in proportion to the amount of leaf damage (Baldwin & Schmelz, 1994). Hence the mechanisms underlying nicotine synthesis and accumulation after induction should be tightly regulated. Although a plant’s nicotine investment is elicited in the roots through enhanced JA levels in roots (Baldwin, 1996b), it remains unknown whether shoot-derived JA alone can account for NI.
Many components of the JAs pathway have been identified in the wild tobacco species Nicotiana attenuata, and genetically engineered N. attenuata used in field experiments have revealed the role of JA signaling in plants facing native herbivore pressure. Here we dissected the systemic signaling function of jasmonates in N. attenuata roots that regulate nicotine induction after leaf wounding with the use of micrografted plants that have impaired JA synthesis or signaling only in their roots. We show that JAs synthesis and JA-Ile perception of both shoot and roots are necessary to induce nicotine production in the roots. We also show that once nicotine is loaded into stems, root-JA synthesis and perception control its correct allocation to the leaf lamina. Strikingly, root JA signaling systematically regulates leaf levels of JA and ABA after leaf wounding. Finally, we show that root-JA synthesis and perception influence the metabolic profile of leaves, which in turn, reduces the performance of aboveground herbivores under glasshouse and field conditions.
Materials and Methods
Plant material and treatments
All lines were derived from seeds originally collected in a natural population of N. attenuata at the DI Ranch, near Santa Clara. Seed germination and plant growth are described in Kruegel et al. (2002). WT or transgenic plants harboring an empty vector (EV) construct were used as controls; all transformed and WT plants were from the same inbred generation of the same original accession. Silenced (ir) or overexpressing (ov) stably transformed plants were used to knock-down JA-Ile perception (irCOI1; Paschold et al., 2007), synthesis (irAOC; Kallenbach et al., 2012), or accumulation (irJAR4/6; Wang et al., 2008, or ovJMT; Stitz et al., 2011), and plants silenced in nicotine production (irPMT; Steppuhn et al., 2004). Seven-day-old seedlings were grafted as described in Fragoso et al. (2011), with average rate of grafting success of 77%, that did not differ significantly amongst all graft combinations (p=0.1377; 300 to 400 seedlings were approximately grafted per each graft combination).
Glasshouse plants were grown in sand or in soil with a perlite mixture as substrate, and kept at 26-28°C under 16 h of light. Five-week-old plants had 3 of their rosette leaves punctured with a pattern wheel, run 4 times on each side of leaf, parallel to the midrib (wounding treatment). After designated time points, systemic tissues (roots and pooled stalk leaves) and local leaves of control and wounded plants were sampled and stored at -80°C until analysis. In order to standardize leaf damage for global metabolic analysis, herbivore attack was mimicked by wounding, as described and applying of 20 µL per leaf of a 1:5 dilution of oral secretions with water directly to the freshly created puncture wounds. Oral secretions were collected from 3rd to 4th instar larvae of M. sexta or S. littoralis rared on WT plants or artificial diet, respectively. Three days after treatments, undamaged systemic leaves were sampled and stored at -80°C until analysis. For field experiments, seeds were imported under US Department of Agriculture Animal and Plant Health Inspection Service (APHIS) notification number 11-350-101r, and planted in a randomized manner to an experimental plot at plot at Lytle Ranch Preserve, Utah, in 2012.
RNA extraction and real time RT-PCR
Total RNA was extracted from leaf or root tissues with the Trizol reagent, from which 500 ng were used for cDNA synthesis as described in Fragoso et al. (2011). All primers were previously described (Paschold et al., 2007; Wang et al., 2007; Stitz et al., 2011; Kallenbach et al., 2012), and for NaPMT a new pair of primers was designed and tested for their ability to amplify a 93-bp-long consensus cDNA fragment of NaPMT1 and NaPMT2 genes concomitantly (NaPMT12-for 5’- TCATTGGACCAAGATCGAG-3’ and rev 5’- TGGAAATTATGATAATTACTGCAGA-3’; Winz & Baldwin, 2001). The efficiency of the primers and the estimated initial amount of template were calculated as described in Fragoso et al. (2011), and relativized to N. attenuata’s elongation factor1A (NaEF1A). All reactions used qPCR Core Kit for SYBR Green I (Eurogentec, Seraing, Belgium, http://www.eurogentec.com), and performed with at least 5 biological replicates.
Nicotine extraction and analysis by HPLC-PDA detector
Approximately 150 mg of leaf or 300 mg of root tissue was used for extraction of nicotine as described by Onkokesung et al. (2012). Plant tissues were ground with two 4 mm steel balls by Genogrinder 2000 (SPEX CertiPrep, New Jersey, USA). Samples were extracted with 1 mL of methanol : water (40:60, v/v) acidified by 0.1% (v/v) acetic acid and homogenized by vortex for 10 min. Supernatants were obtained after two rounds of centrifugation at 16,100 g at 4°C for 20 min. Aliquots of 1 µL of each sample were analyzed by an Agilent HPLC 1100 Series device (http://www.chem.agilent.com) in a Chromolith FastGradient RP-18e column (endcapped 50 x 2 mm; Merck) attached to a precolumn (Gemini NX RP-18e, 3 um, 2 x 4.6 mm; Phenomenex) as described in Oh et al. (2012). An external standard curve of an authentic and purified nicotine standard was used for quantification and peaks were identified based on retention time, spectra and samples spiked with purified compounds. All extractions were performed with at least 6 biological replicates.
Stem-feeding experiments
One day after leaf wounding, younger unwounded systemic leaves from wounded and control plants were carefully excised at the base of their petioles, weighed and transferred to vials containing water or to a 1 mM nicotine-containing solution. After one day of feeding, leaf laminas were dissected and stored at -80°C until HPLC analysis. All tubes containing solutions were weighed prior to and after the stem-feeding, so that the volume of solution transpired by the leaf could be estimated. In the nicotine treatment, the remaining solution was also analyzed for its nicotine content, so in comparing initial and final nicotine amounts in solution, nicotine absorption was calculated. Treatments were performed with at least 8 biological replicates.
Phytohormone analysis
Approximately 100 mg leaf or 200 mg root frozen tissue material was ground as described above. Phytohormones were extracted with 1 mL of ethyl acetate spiked with internal standards (200 ng of [2H2]JA and 40 ng of each JA-[13C6]-Ile, [2H4]SA, and [2H6]ABA). After vortexing for 10 min, 500 µL of the organic phase was obtained by centrifugation at 16,100 g at 4°C for 15 min. Samples were evaporated almost to dryness in a vacuum concentrator (Eppendorf) under reduced pressure at 30°C. Leaf samples were then diluted in 200 µL of methanol: water (70:30, v/v), while 100 µL was used for root samples. Analysis was performed with a Varian 1200 HPLC-MS/MS system as described in Vadassery et al. (2012), with a modification for a shortened chromatographic gradient. Sample-derived phytohormones were calculated by the ratios of their ion intensity and of their respective internal standards, and for cis-OPDA, [2H2]JA was used as an internal standard applying an experimentally determined response factor of 0.5. All extractions were performed with 6 biological replicates, and the resulting amount was then expressed per gram fresh mass plant material.
Herbivore assays
Eggs of M. sexta were obtained from Carolina Biological Supply and were derived from an in-house colony, and S. littoralis eggs were obtained from Syngenta Crop Protection AG (Stein, Switzerland). All eggs were kept in growth chambers (Snijders Scientific) at 22-26°C under 16h light and 8h dark conditions until hatching. Due to a high mortality rate during the first days of life, 4 freshly hatched neonates were placed on the rosette leaves of each grafted plant (n = 10) and after 3 days, only 2 neonates per plant were kept. Larvae performance was determined by their mass gain, measured on the 12th day of M. sexta and on the 10th day of S. littoralis feeding. Under field conditions, insect damage was measured on June 4th, 2012, in plants between the 5th and 6th week after transplanting to the plot. Insect-specific damage signature was identified according to Gaquerel et al. (2013), and quantified in standardized units of leaf area consumed relative to insect size (i.e. 5 units of mirids damage ≈ 5 units of leafhoppers damage ≈ 1 cm2 of heavily attacked leaf area).
Extraction and unbiased analysis of metabolites
Metabolites were extracted from frozen ground leaf tissue with 1 mL of 50 mM acetate buffer (40 mM acetic acid plus 44 mM ammonium acetate; 4.8 pH) in methanol (60:40, v/v). Samples were homogenized, and supernatant was recovered after two rounds of centrifugation. This procedure is optimized for extracting a wide range of metabolites in N. attenuata (Gaquerel et al., 2010), and after separation by an Agilent HPLC 1100 Series device (http://www.chem.agilent.com), eluted compounds were positively charged by Electrospray Ionization (ESI) and had their masses detected by mass spectrometry (MS), carried out with a MicroToF (Time-of-Flight, Bruker Daltonik, Bremen, Germany). Extractions used 4 to 5 biological replicates for analysis.
Raw data files were converted to netCDF format and processed by XCMS (http://fiehnlab.ucdavis.edu/staff/kind/Metabolomics/Peak_Alignment/xcms/) and CAMERA (http://bioconductor.org/packages/devel/bioc/html/CAMERA.html) R packages according to Kim et al. (2011). All peaks ranging from 40 to 450 s of ions in the mass range m/z 90 to 1400 were selected and normalized by the exact amount of plant material used. Only peaks that were found in at least 75% of the replicates with absolute intensities higher than 5 mega counts per second of the total ion count within same group were analyzed by PCA using MetaboAnalyst (http://www.metaboanalyst.ca/MetaboAnalyst/faces/Home.jsp), following a normalization by the median value and a Pareto scaling.
Statistical analysis
Data were verified for assumptions of normal distribution and homogeneity of variances, and log-transformed when adequate. Parametric or non-parametric (Kruskal-Wallis test) ANOVA, followed by Fisher LSD or Dunnett’s as post-hoc tests, were performed using StatView5 (SAS Institute, Cary, NC, USA) or SigmaPlot 12.0 (Systat Software Inc. 2008).
Results
Nicotine induction (NI) in systemic leaves in response to leaf-wounding is dependent on root JA synthesis and perception
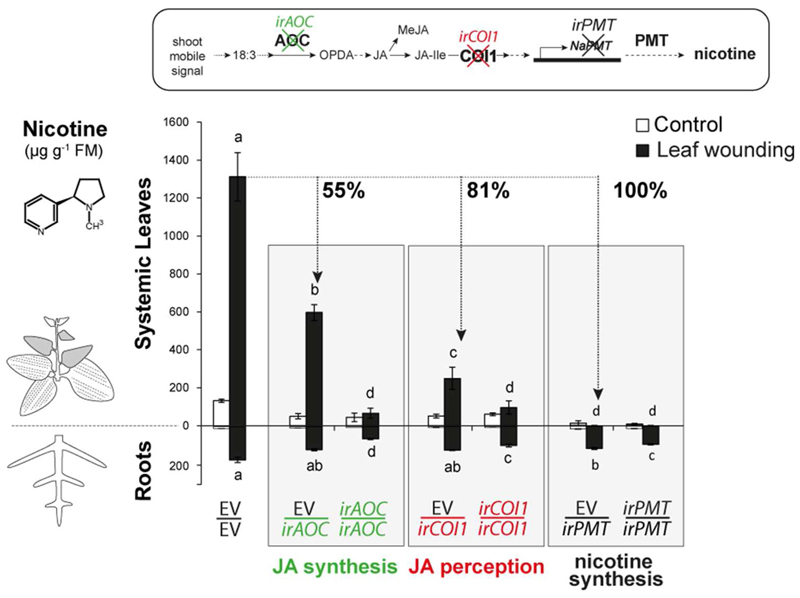
To examine the contribution of the below- and aboveground parts of N. attenuata plants to JA-dependent NI in systemic leaves, we grafted a series of transgenic lines of N. attenuata to WT shoots or roots. We used grafts of the scions and rootstocks of the same genotypes as controls. As previously described (Fragoso et al., 2011), the silencing of target genes in ir lines is only restrained to the transgenic counterpart of the grafts when those are used as rootstocks (Fig. S2,S6). However, when grafting a line ectopically expressing a transgene in a sense orientation (ov) to WT scions or rootstocks, the expression of the target gene is not affected in the WT counterpart in any of the graft combinations. We first observed that grafted empty vector plants (EV/EV) responded to leaf wounding with amounts of nicotine 10-fold higher than control EV/EV levels (Fig. 1; p <0.001). EV shoots grafted onto silenced irPMT roots (EV/irPMT) completely lacked nicotine, and did not differ from fully silenced irPMT/irPMT grafted plants (Fig. 1; p >0.94 for both control and induced nicotine levels).
Fig. 1. JA de novo synthesis and perception in roots contribute to nicotine accumulation induced in systemic leaves in response to leaf wounding.
Mean ± SE levels of nicotine accumulated in roots and systemic leaves (shaded) of control and induced grafted plants displaying roots impaired in JA synthesis (EV/irAOC), JA perception (EV/irCOI1), and nicotine synthesis (EV/irPMT), 3 days after leaf wounding. Grafts of the scions and rootstocks of the same genotypes (EV/EV, irAOC/irAOC, irCOI1/irCOI1, and irPMT/irPMT) were used as controls. Bars sharing same letters do not differ significantly (Two-way ANOVA followed by Fisher LSD test, n = 6).
When comparing NI in EV/EV plants to those of plants with impaired JA synthesis in roots (EV/irAOC), a decrease of 55% in wound NI in systemic leaves was observed (Fig. 1; p <0.001). An even more pronounced impairment in NI in systemic leaves was observed in plants lacking JA perception in roots: wounded EV/irCOI1 plants accumulated less than 20% of induced EV/EV nicotine levels (Fig. 1; p <0.001). Both graft combinations accumulated more leaf-nicotine than their respective entirely silenced graft combinations, irAOC/irAOC and irCOI1/irCOI1 (Fig. 1; p <0.001 and p =0.023, respectively). Moreover, only EV/EV, EV/irAOC and EV/irCOI1 responded to wounding with increased levels of nicotine in systemic leaves compared to control nicotine levels (p <0.001, p <0.001 and p =0.009, respectively). When analyzing NI in roots, all graft combinations accumulated similar or lower levels than those found in EV/EV roots (Fig 1).
JA conjugation in roots also influenced the induced accumulation of nicotine in response to leaf wounding (Fig. S1). Systemic leaves of plants with roots impaired in converting JA to JA-Ile (EV/irJAR4/6) accumulated 48% less nicotine than did the systemic leaves of EV/EV (Fig. S1; p <0.001). An overexpressing line (ovJMT), which depletes JA flux and standing pools through a strong sink towards the inactive MeJA (Stitz et al., 2011) was also used for grafting. Interestingly, similarly reduced levels of induced nicotine were observed in leaves of ovJMT/EV and EV/ovJMT grafted plants; they both were around 20% of nicotine levels in induced EV/EV (Fig. S1; p <0.001 in both comparisons). Interestingly, none of the systemic leaves of the grafts using ovJMT line significantly responded to this treatment; after wounding, leaf-nicotine levels of EV/ovJMT, ovJMT/EV and ovJMT/ovJMT did not differ statistically compared to their control levels (p =0.058, p =0.191 and p =0.976, respectively). On the other hand, irJAR4/6/irJAR4/6 grafts, as well as EV/irJAR4/6, showed a significant increase in leaf-nicotine levels in response to wounding (p =0.048 and p <0.001, respectively). Similar or lower levels of nicotine in response to wounding were found in roots of EV/irJAR4/6, irJAR4/6/irJAR4/6, EV/ovJMT, ovJMT/EV and ovJMT/ovJMT compared to roots induced nicotine levels of EV/EV (Fig. S1).
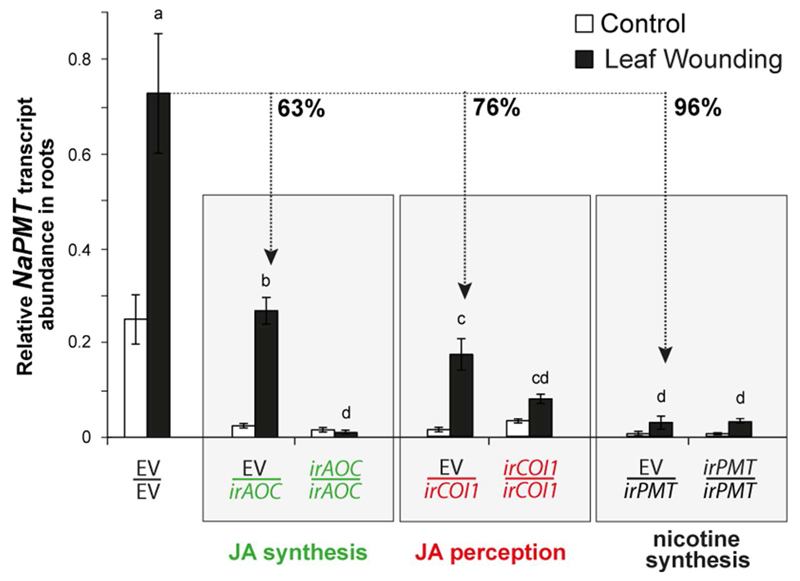
Induction of NaPMT expression in roots in response to leaf-wounding is dependent on JA synthesis and perception in roots
To test whether JA synthesis or perception in roots regulates the expression of nicotine biosynthetic genes, we analyzed the accumulation of NaPMT1 and NaPMT2 transcripts, here collectively referred as NaPMT. One day after the leaf wounding treatment, NaPMT transcript levels in roots of both EV/irPMT and irPMT/irPMT were less than 5% of the NaPMT transcript levels of EV/EV grafts (Fig. 2; p <0.001 in both comparisons). Roots of induced EV/irAOC plants accumulated 63% less NaPMT transcripts than did the roots of induced EV/EV plants (Fig. 2; p <0.001). An even more pronounced impairment in NaPMT transcript abundance was found in roots of induced EV/irCOI1 plants compared to those of EV/EV grafts (76% less, Fig. 2; p <0.001). NaPMT levels in EV/EV, EV/irAOC and EV/irCOI1 were significantly increased in response to wounding relative to their respective untreated levels (p <0.001, p <0.001 and p =0.005, respectively).
Fig. 2. JA de novo synthesis and perception in roots contribute to induced NaPMT expression in roots in response to leaf wounding.
Mean ± SE levels of relative NaPMT transcript accumulation in roots of control and induced grafted plants displaying roots impaired in JA synthesis (EV/irAOC), JA perception (EV/irCOI1), and nicotine synthesis (EV/irPMT), 1 day after leaf wounding. Grafts of the scions and rootstocks of the same genotypes (EV/EV, irAOC/irAOC, irCOI1/irCOI1, and irPMT/irPMT) were used as controls. Bars sharing same letters do not differ significantly (Two-way ANOVA followed by Fisher LSD test, n = 6).
JA conjugation in roots also influenced the induction of NaPMT transcripts in response to wounding (Fig. S3). Roots of induced EV/irJAR4/6 plants accumulated only 20% of the induced levels found in roots of EV/EV plants (p <0.001), and roots of induced EV/ovJMT and ovJMT/EV accumulated 10 and 15% of NaPMT transcripts of those in the roots of induced EV/EV (p <0.001, for both comparisons). The wounding treatment significantly increased levels of NaPMT transcripts in irJAR4/6/irJAR4/6 (Fig. S3; p =0.015).
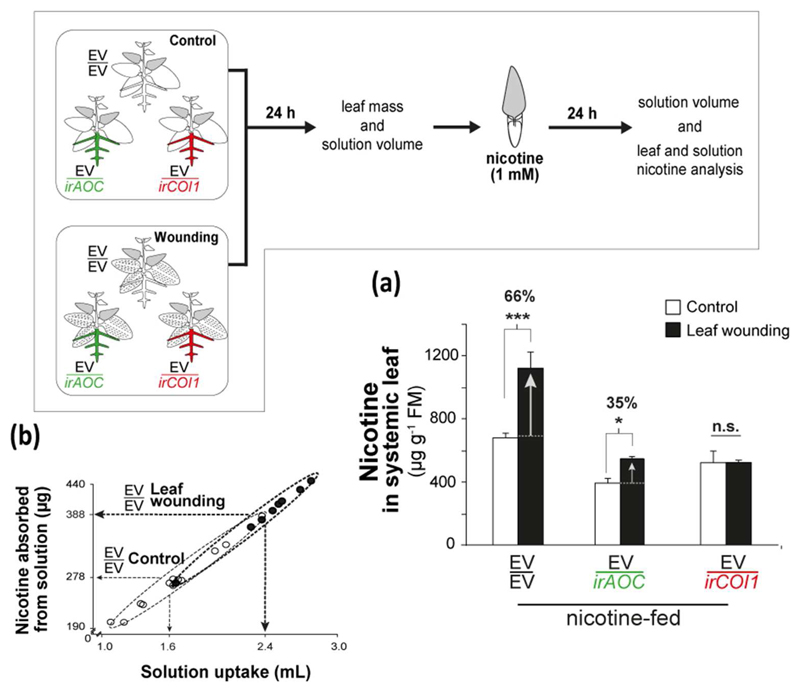
Root JA perception and synthesis control nicotine transport to leaves
To investigate whether JA synthesis or perception in roots regulates nicotine transport to leaves, a stem-feeding experiment was performed, in which excised systemic leaves of control and wounded EV/EV, EV/irAOC and EV/irCOI1 were stem-fed with a nicotine-containing solution at a physiologically relevant concentration (Fig. 3). We observed that nicotine levels in systemic leaves of wounded plants were 66% higher than those of control plants (Fig. 3a; p <0.001). This perfectly matches with endogenous wound NI found in intact leaves of non-grafted WT plants between the 24h and 48h time interval (Fig. S4).
Fig. 3. JA synthesis and perception in roots tightly control induced nicotine transport to systemic leaves after leaf wounding through higher transpiration rates.
(a) Mean ± SE levels of nicotine accumulated in leaf lamina of detached (shaded) systemic leaves of control and induced grafted plants as shown in the diagram. Asterisks refer to comparisons between control and wounding treatment within same graft kind (***, p <0.001; *, p <0.05; n.s., not significant; Two-way ANOVA followed by Fisher LSD test, n = 8). (b) Effect of wounding treatment in EV/EV samples showed in a correlation plot of solution uptake (mL) versus nicotine absorbed from solution (µg). Dashed arrows indicate average values.
Nicotine levels of systemic leaves of both EV/irAOC and EV/irCOI1 after wounding were significantly lower than those of EV/EV (Fig. 3a; p <0.001 in both comparisons). The wounding treatment only increased nicotine levels in systemic leaves of EV/irAOC plants by 35% (Fig. 3a; p =0.016), while the systemic leaves of wounded EV/irCOI1 did not take up nicotine, and remained as their respective control levels (Fig. 3a; p =0.963). In addition, when leaves were fed a nicotine-free solution, the wounding treatment failed to induce leaf-nicotine in all grafts, including EV/EV, indicating that induced nicotine levels in systemic leaves are derived from the nicotine-containing solution (Fig. S5). Interestingly, the presence of nicotine in the solution did not increase nicotine levels of control EV/EV leaves, these remained similar to those found in water-fed control EV/EV leaves (Fig. 3a,S5; p =0.654). However, systemic leaves of EV/irAOC and EV/irCOI1 in nicotine-containing solution accumulated more nicotine than did their respective leaves stem fed only water (Fig. 3a,S5; nicotine-versus water-feeding for EV/irAOC control, p =0.029 and wounding, p =0.025; for EV/irCOI1 control, p =0.013 and wounding, p =0.052).
Although the interaction of factors was statistically significant (Feeding*Treatment: p =0.047), treatment factor had a stronger effect than feeding factor in explaining how EV/EV leaves absorbed nicotine (Treatment: p <0.001; Feeding: p =0.099). In contrast, taking EV/irAOC and EV/COI1 leaves together, the feeding factor contributed more to explaining how samples varied in leaf-nicotine, and made the interaction between factors no longer significant (Feeding solution: p <0.001; Treatment: p <0.001; Feeding*Treatment: p =0.337; for both grafts analyzed individually).
The size of the leaves did not differ significantly across different grafts (p =0.104), treatments (p =0.536), and feedings (p =0.515; data not shown). As expected, nicotine uptake from the solutions was highly correlated to the volume of solution transpired by the leaf (Fig. 3b, correlation coefficient =0.99, p =7.5e-09), and wounding significantly increased the volume of solution transpired (Fig 3b; wounding versus control nicotine-fed EV/EV, p <0.001).
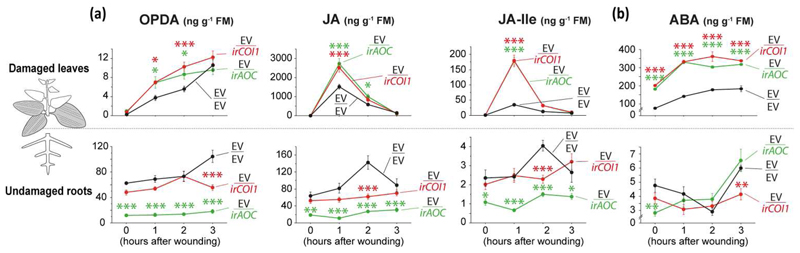
Impaired JA synthesis and perception in roots up-regulate jasmonates and ABA accumulation in local leaves
To evaluate whether the impairment in JA synthesis or perception in roots would change phytohormone accumulations in above- and belowground tissues in response to leaf wounding, we measured the levels of these compounds in EV/EV, EV/irAOC and EV/irCOI1 plants in the first hours after wounding. A burst in JA and JA-Ile accumulation was detected in local leaves of EV/EV 1 h after wounding, and this pattern was also observed in EV/EV roots 2 h after leaf treatment at much lower concentrations (Fig. 4a). In contrast, EV/irAOC and EV/irCOI1 roots completely lacked the JA and JA-Ile burst found in EV/EV roots (Fig. 4a; p <0.001, for all comparisons), while the local tissues of these grafts attained levels of phytohormones that were strikingly higher than EV/EV (Fig. 4a; p <0.001, for all comparisons). JA levels in local leaves of EV/irAOC and EV/irCOI1 1 h after wounding were 1.7-fold higher than those of EV/EV leaves, and JA-Ile levels were up to 5-fold the levels found in EV/EV leaves (Fig. 4a). Levels of leaf-OPDA were also higher in EV/irAOC and EV/irCOI1 than in EV/EV (Fig. 4a; p =0.01, for both grafts compared individually to EV/EV). In addition, ABA levels of control and treated leaves were also found to be higher in EV/irAOC and EV/irCOI1 when compared to those of EV/EV (Fig. 4b; p <0.001).
Fig. 4. Impaired JA synthesis and perception in undamaged roots up-regulates jasmonates and ABA accumulation in damaged leaves in response to wounding.
Mean ± SE levels of phytohormone accumulation in response to wounding of undamaged roots and damaged leaves (shaded) of grafted plants with roots impaired in JA synthesis and perception (***, p <0.001; **, p <0.01; *, p <0.05; Two-way ANOVA followed by Dunnett’s test, n = 6).
Interestingly, initial basal levels of JA, JA-Ile and OPDA in local tissues were similar among all grafts (p >0.8 for all comparisons against EV/EV, within “control” time-point), while EV/irAOC roots had reduced basal levels of jasmonates compared to EV/EV (JA, p =0.002; JA-Ile, p =0.011; OPDA, p <0.001).
JA synthesis and perception in roots contribute to plant resistance in glasshouse and nature
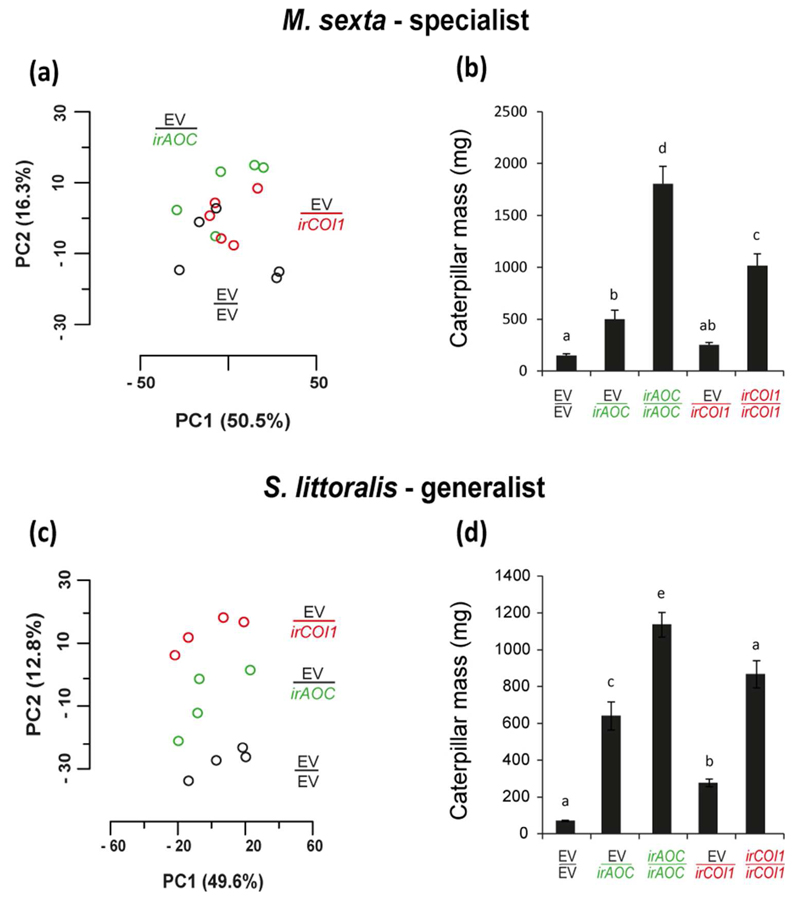
To further investigate the contribution of root-JA synthesis or perception to plant resistance to herbivore attack, we performed a series of experiments exploring plant-insect interactions, under glasshouse and field conditions. Initially, we compared the global metabolic profile of systemic leaves of EV/EV, EV/irAOC and EV/irCOI1, 3 days after simulated herbivore attack, which standardizes leaf damage across plants. Oral secretions of two herbivore species were tested: M. sexta, a specialist, and S. littoralis, a generalist. We further performed assays in which these herbivores were reared in EV/EV, EV/irAOC and EV/irCOI1 grafts and had their larval mass measured. Principal component analysis (PCA) revealed that metabolic profiles of EV/EV, EV/irAOC and EV/irCOI1 grafts were more clearly discriminated when treated with regurgitant of S. littoralis than with regurgitant of M. sexta (Fig. 5a,c). Importantly, when comparing the principal components (PC) individually across the two herbivores examined, PC1 and PC2 explained similar levels of the total variance (PC1: M. sexta, 50.5% and S. littoralis, 49.6%; PC2: M. sexta, 16.3% and S. littoralis, 12.8%). Larvae of both M. sexta and S. littoralis reared on EV/EV plants showed the smallest mass gain (Fig. 5b,d). In contrast, larvae reared on irAOC/irAOC and irCOI1/irCOI1 plants attained the largest masses; followed by those reared on EV/irAOC. Larvae of M. sexta fed on EV/irCOI1 gained as same mass as those fed on EV/EV (Fig. 5b; p =0.150). However, S. littoralis larvae fed on EV/irCOI1 plants gained significantly more mass than those on EV/EV plants (Fig. 5d; p =0.002).
Fig. 5. JA synthesis and perception in roots contribute to plant resistance against leaf-attackers, and are differentially employed depending on the level of adaptation of the herbivore species.
Untargeted principal component analysis (PCA) of metabolic profile of systemic leaves of EV/EV, EV/irAOC and EV/irCOI1 plants 3 days after simulated M. sexta (a) or S. littoralis attack (c) under glasshouse conditions. Mean ± SE levels of M. sexta (b) and S. littoralis (d) mass after 12 and 10 days, respectively, of feeding on EV/EV, EV/irAOC and EV/irCOI1 plants under glasshouse conditions. Grafts of the scions and rootstocks of the same genotypes were used as controls. Bars sharing same letters do not significantly differ (One-way ANOVA followed by Fisher LSD test, n ≈ 20).
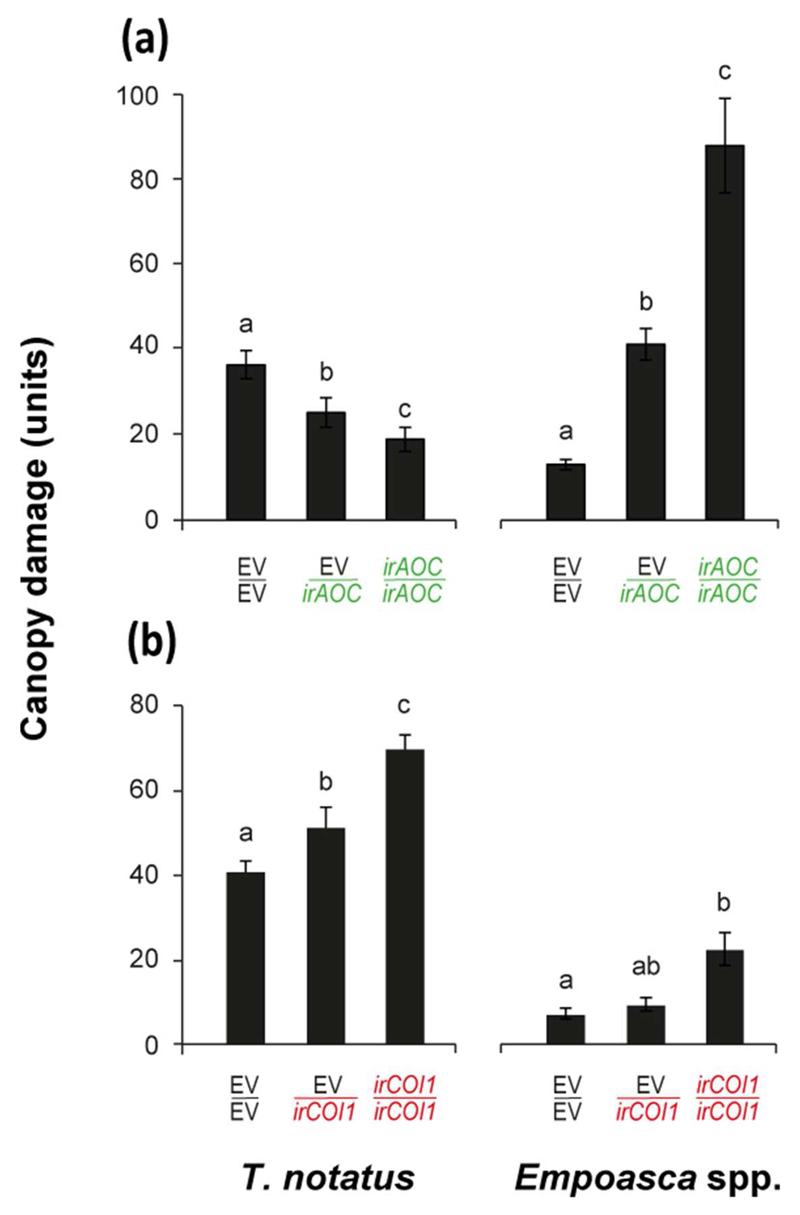
We also measured herbivore preference between EV/EV, EV/irAOC, and EV/irCO1 plants in N. attenuata’s natural habitat, the Great Basin Desert of Utah. Under field conditions, EV/irAOC plants were significantly more damaged by leafhoppers (Empoasca spp., p =0.044), and less attacked by mirids (Tupiocoris notatus, p <0.001) compared to EV/EV plants (Fig. 6a). EV/irCOI1 plants were more heavily damaged by mirids (p =0.038), but were consumed as much by leafhoppers as EV/EV plants (Fig. 6b; p =0.096). The silencing of target genes in field-grown grafts was confirmed to be restricted to the transgenic rootstocks (Fig. S6).
Fig. 6. Under field conditions, plants impaired in (a) JA synthesis (EV/irAOC) and (b) perception (EV/irCOI1) in roots are more attacked by Empoasca spp. and Tupiocoris notatus, respectively.
Mean ± SE levels of cumulative plant damage over 6 weeks after transplantation to the plot. Grafts of the scions and rootstocks of the same genotypes (EV/EV, irAOC/irAOC, and irCOI1/irCOI1) were used as controls. Bars sharing same letters do not significantly differ (One-way ANOVA followed by Fisher LSD test, n = 25).
Discussion
In this study, we investigated the JA-dependent role of roots for the resistance of aboveground plant parts. For this, we used genetically transformed plants disrupted in different components of the JA pathway and dissected the JA-dependent function of roots using micrografted plants. JA synthesis, perception, and conjugation in roots tightly controlled the accumulation of NaPMT transcript (Fig. 2,S3), nicotine accumulation (Fig. 1,S1) and its transport from roots to its fate in the shoots (Fig. 3a). Like nicotine, JA signaling in roots also regulated the accumulation of other shoot-accumulated metabolites (Fig. 5a,c), and significantly promoted plant resistance against leaf-feeders in both glasshouse and natural conditions (Fig. 5b,d,6).
Nicotine and micrografting in N. attenuata: the toolbox for the study of root-dependent JA signaling in systemic responses
Recently, Mousavi et al. (2013) showed that changes in electrical potentials of the leaf surface were triggered by larval feeding. This electrical wave spread throughout unattacked portions of the shoots, inducing jasmonate biosynthesis and defense-responsive gene expression in systemic leaves. Regardless of the identity of the systemic signal conveying the information of leaf wounding to roots, we investigated whether JA synthesis or perception in roots regulate systemic root responses after leaf wounding. We used nicotine induction as a case study to explore the function of JA signaling in roots, because nicotine is dramatically increased after leaf wounding. EV/EV plants accumulated 10-fold more nicotine in response to leaf-wounding than undamaged control grafted EV/EV plants (Fig. 1), and nicotine induction was completely absent in systemic leaves of EV/irPMT and irPMT/irPMT as well. These observations are in agreement to previous reports of wounding in Nicotiana species (Ohnmeiss et al., 1997) and confirm that nicotine biosynthetic genes are required only in roots (Winz & Baldwin, 2001). These data also highlight the value of micrografting to test root responses to leaf induction, and demonstrate that the mobile wound signal is graft-transmissible.
JA de novo synthesis and perception in roots contribute to nicotine production and tightly regulate nicotine transport to the leaf lamina
NI in response to leaf wounding seemed to be almost fully dependent on JA-Ile perception (COI1) in roots. JA de novo synthesis (AOC) and JA conjugation (JMT and JAR4/6) in roots were also required for NI in shoots (Fig. 1,S1). However, EV/irAOC, EV/irCOI1, and EV/JAR4/6 grafted plants were shown to induce nicotine levels in response to leaf wounding, suggesting a JA-independent root signaling pathway involved in NI. Alternatively, the residual NI found in leaves of these grafts might be due to minor expression levels remaining in silenced roots (Fig. S2) or a consequence of shoot-derived JAs transported to roots.
The expression levels of NaPMT transcripts in roots of all grafts were strongly correlated to the nicotine amounts found in leaves (Fig. 2), and roots did not over-accumulate nicotine (Fig. 1), suggesting that JA signaling in roots controls NI at the transcriptional level. NaPMT expression and nicotine production are known to be attenuated by ethylene emission (Kahl et al., 2000; Winz & Baldwin, 2001). However, wounding alone did not induce leaf ethylene emission in EV/EV grafts and intact wild type plants (Diezel et al., 2011); also, EV/irAOC and EV/irCOI1 produced similar or even reduced levels of ethylene emission compared to EV/EV (data not shown). It is also noteworthy that NI in Nicotiana species has allometrically-determined setpoints that control the amount of nicotine accumulated in the shoots in response to wounding. The allometric NI is proportional to the biomass of the plant, and it seems to be mainly dictated by the rate of de novo synthesized nicotine in roots, as well as by other factors regulating nicotine storage in the shoots (Baldwin, 1996a; Baldwin, 1999). Hence, a plants’ ability to store nicotine aboveground is presumably controlled by the same mechanisms as those that control nicotine synthesis in the belowground.
In a stem-feeding experiment, we mimicked the endogenous increase in the transport of nicotine via the apoplast from roots to shoot that occurs between the first and second day after leaf wounding (Fig. S4; Baldwin, 1989). As a control, water-fed leaves failed to induce nicotine in systemic leaves in respond to wounding (Fig. S5), indicating that the wound-induced NI of nicotine-fed leaves was due to the nicotine loaded into the leaf by transpiring the nicotine-containing solution. Even when nicotine was equally offered, systemic leaves of EV/irAOC and EV/irCOI1 failed to allocate nicotine correctly to the leaf lamina, suggesting that JA signaling in roots regulates physiological changes in shoots required to cope with the load of root-derived nicotine (Fig. 3a), as had been suggested by previous experiments with N. sylvestris (Baldwin & Callahan, 1993; Baldwin, 1996a). It was also observed that leaves of EV/irAOC and EV/irCOI1 have significantly fewer trichomes than EV/EV leaves on their abaxial surfaces (around 20~30% less, p =0.007), and trichomes represent one site of nicotine accumulation (Roda et al., 2003). These data add another regulatory step by which root-JA signaling fine-tunes the wound-induced nicotine response of shoots (Baldwin & Schmelz, 1994).
Wounding in stem-fed leaves increased solution uptake in systemic leaves (Fig. 3b); this is consistent with the mechanism by which wound-induced nicotine transport via xylem is facilitated through higher rates of transpiration (Baldwin, 1989; Baldwin & Schmelz, 1994). Interestingly, nicotine-fed leaves of EV/irAOC and EV/irCOI tended to absorb more solution than did their water-fed leaves, whether wounded or not (data not shown), and basal levels of nicotine in these grafts were already reduced compared to EV/EV. It is possible that nicotine per se serves as a signal to promote transpiration in shoots of these nicotine-deprived N. attenuata grafts. Taken together, these results suggest that root-JA signaling regulates directly and/or indirectly nicotine uptake by regulating transpiration rates in leaves. To explore this difference further, the levels of phytohormones, such as ABA, were analyzed.
Impaired JA signaling in roots leads to an over-accumulation of jasmonates and ABA in damaged leaves
As expected from our previous study with WT intact plants of N. attenuata (Von Dahl & Baldwin, 2004), leaf wounding did not induce either JA nor MeJA in systemic leaves of EV/EV (data not shown). Since AOC enzyme activity leads to OPDA production that serves as a substrate for JA and JA-Ile formation, basal levels of all these JAs were reduced in EV/irAOC roots (Fig. 4a). Although basal levels of root jasmonates in EV/irCOI1 plants were similar to those of EV/EV, roots of both EV/irAOC and EV/irCOI1 did not show the systemic wound-induced burst of OPDA, JA and JA-Ile observed in EV/EV roots. Surprisingly, the reduced JAs levels of EV/irAOC and EV/irCOI1 roots were associated with a hyper-responsive JA accumulation in induced leaves, suggesting the existence of a novel shoot-root-shoot loop in regulating the JA response. The impaired accumulation of systemic root-JAs possibly boosts JAs responses in the local leaf, as a compensatory effect. We ruled out the possibility that the result was an artifact (i.e. silencing of JA components in shoots) by checking gene expression in both shoot and roots of these grafted plants (Fig. S2; Fragoso et al., 2011).
Moreover, levels of ABA were found to be surprisingly higher in leaves of EV/irAOC and EV/irCOI1 when compared to those of EV/EV (Fig. 4b), suggesting a crosstalk between JA and ABA in regulating shoot-root-shoot interplay in plant defenses. This agrees with our stem-feeding data, and with the notion of facilitated nicotine transport through higher transpiration rates: higher ABA levels in EV/irAOC and EV/irCOI1 leaves would inhibit transpiration, ultimately leading to reduced nicotine contents. Furthermore, ABA-regulated water stress, rather than ABA-induced defenses, has been already suggested to be involved in leaf resistance induced by root-herbivory in maize plants (Erb et al., 2010). However, the involvement of ABA in JA-dependent responses to wounding and to herbivore attack is shown to be beyond the control of guard cells. Recently, reduced levels of ABA in leaves, and its consequent augmented transpiration rates were associated with reduced emission of defensive organic volatile compounds (VOCs) in N. attenuata plants silenced for a novel protein that suppress ABA catabolism after herbivore attack (Dinh et al., 2013).
JA synthesis and perception in roots enhance plant resistance against aboveground herbivores
The treatment using regurgitate of a generalist herbivore caused EV/irAOC and EV/irCOI1 to be metabolic more distinct from EV/EV when compared to the weak grouping found for these graft combinations when elicited with M. sexta regurgitant (Fig. 5a,c). These data suggest that other defensive metabolites in addition to nicotine are also dependent on JA signaling in roots. This result is consistent with the notion of an herbivore-induced carbon sequestration to roots as a resistance mechanism regulating defensive metabolites, rather than solely a tolerance mechanism (Schwachtje et al., 2006; Machado et al., 2013).
In the glasshouse, impaired AOC or COI1 activity in roots had a more pronounced effect on S. littoralis than on M. sexta larval performance (Fig. 5b,d). Under field conditions, COI1 activity in roots accounted for enhanced plant resistance against mirids, while it likely had no influence on the feeding choice of Empoasca leafhoppers (Fig. 6). On the other hand, the lack of AOC activity only in roots enhanced the vulnearability of plants to these leafhopper species. Empoasca spp. is able to identify in native populations natural N. attenuata JA-mutants with impaired capacity to mediate JA signaling (Kallenbach et al., 2012). Despite its hyper-response in JA accumulation after wounding (Fig. 4a), EV/irAOC plants were preferably attacked by Empoasca, suggesting that the function of AOC in roots profoundly influences JA-mediated responses of the shoots. In addition, our data support the notion that JA dependent responses are employed in an herbivore-specific way (Hettenhausen et al., 2013), and suggest a COI1-independent JA signaling in the roots.
In parallel, we observed that damage caused by mirids was negatively correlated to damage inflicted by Empoasca leafhoppers in irAOC grafts. It would be interesting to test whether the density of these herbivores is directly tailored simply by their presence/absence, or indirectly, through plants’ responses mediated by JA signaling (Kessler & Baldwin, 2004; Kallenbach et al., 2012). Mirids are specialist herbivores, and might be more adapted to N. attenuata defense metabolites than to the presence of other generalist herbivores, and this becomes apparent only in irAOC plants. In other words, mirids might prefer plants more defended against generalists. Moreover, the negative correlation between damage inflicted by mirids and leafhoppers found in irAOC grafts was not found in grafts using irCOI1. This suggests that the interaction between these herbivores is very likely plant mediated, and CO1-dependent.
The revival of the root-brain theory originally proposed by Charles and Francis Darwin (Baluska et al., 2009) has renewed attention to the function of roots as a regulatory organ of plants. How changes in roots affect shoot responses, and vice-versa, is the subject of current intense study. Here, we focused on the aboveground changes induced by leaf-attack that engage roots in a more comprehensive shoot-root-shoot loop. Based on the dramatic changes observed in how leaves respond to attack when roots are depleted of JA signaling, we conclude that roots play a central role in orchestrating aboveground processes.
Supporting information
Fig. S1. JA conjugation in roots contributes to nicotine induction in systemic leaves in response to leaf wounding.
Fig. S2. Silencing or overexpression of target genes is restricted to transgenic scions or rootstocks of grafted plants under glasshouse conditions.
Fig. S3. JA conjugation in roots contributes to induced NaPMT expression in roots in response to leaf wounding.
Fig. S4. Kinetic of endogenous nicotine (in systemic leaves) and NaPMT expression (in roots) of non-grafted WT plants in response to leaf wounding.
Fig. S5. Water-fed systemic leaves failed to induce leaf-nicotine levels in response to leaf wounding.
Fig. S6. Silencing or overexpression of target genes is restricted to transgenic scions or rootstocks of grafted plants under field conditions.
Acknowledgements
This work is supported by the European Research Council advanced grant ClockworkGreen (No. 293926) to ITB, the Global Research Lab program (2012055546) from the National Research Foundation of Korea, and the Max Planck Society. We thank the Brigham Young University for the use of Lytle Ranch Preserve; Danny Kessler, Youngjoo Oh and Felipe Yon for help with field plants; Michael Reichelt with phytohormone measurements and Youngsung Joo for help with glasshouse experiments.
References
- Baldwin IT. Mechanism of damage-induced alkaloid production in wild tobacco. Journal of Chemical Ecology. 1989;15(5):1661–1680. doi: 10.1007/BF01012392. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. Allometric limits to the induced accumulation of nicotine in native tobacco. Plant Species Biology. 1996a;11(1):107–114. [Google Scholar]
- Baldwin IT. Methyl jasmonate-induced nicotine production in Nicotiana attenuata: inducing defenses in the field without wounding. Entomologia Experimentalis Et Applicata. 1996b;80(1):213–220. [Google Scholar]
- Baldwin IT. Inducible nicotine production in native Nicotiana as an example of adaptive phenotypic plasticity. Journal of Chemical Ecology. 1999;25(1):3–30. [Google Scholar]
- Baldwin IT, Callahan P. Autotoxicity and chemical defense - nicotine accumulation and carbon gain in Solanaceous plants. Oecologia. 1993;94(4):534–541. doi: 10.1007/BF00566969. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Karb MJ, Ohnmeiss TE. Allocation of 15N from nitrate to nicotine - production and turnover of a damage-induced mobile defense. Ecology. 1994;75(6):1703–1713. [Google Scholar]
- Baldwin IT, Ohnmeiss TE. Swords into plowshares - Nicotiana sylvestris does not use nicotine as a nitrogen-source under nitrogen-limited growth. Oecologia. 1994;98(3-4):385–392. doi: 10.1007/BF00324228. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Schmelz EA. Constraints on an induced defense - the role of leaf-area. Oecologia. 1994;97(3):424–430. doi: 10.1007/BF00317335. [DOI] [PubMed] [Google Scholar]
- Baluska F, Mancuso S, Volkmann D, Barlow PW. The 'root-brain' hypothesis of Charles and Francis Darwin: Revival after more than 125 years. Plant Signaling and Behavior. 2009;4(12):1121–1127. doi: 10.4161/psb.4.12.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, Garcia-Casado G, Lopez-Vidriero I, Lozano FM, Ponce MR, Micol JL, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448(7154):666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- Diezel C, Allmann S, Baldwin IT. Mechanisms of optimal defense patterns in Nicotiana attenuata: flowering attenuates herbivory-elicited ethylene and jasmonate signaling. Journal of Integrative Plant Biology. 2011;53(12):971–983. doi: 10.1111/j.1744-7909.2011.01086.x. [DOI] [PubMed] [Google Scholar]
- Dinh ST, Baldwin IT, Galis I. The HERBIVORE ELICITOR-REGULATED1 gene enhances abscisic acid levels and defenses against herbivores in Nicotiana attenuata plants. Plant Physiology. 2013;162(4):2106–2124. doi: 10.1104/pp.113.221150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Foresti N, Turlings TCJ. A tritrophic signal that attracts parasitoids to host-damaged plants withstands disruption by non-host herbivores. BMC Plant Methods. 2010;10(247):1–11. doi: 10.1186/1471-2229-10-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Lenk C, Degenhardt J, Turlings TC. The underestimated role of roots in defense against leaf attackers. Trends in Plant Science. 2009;14(12):653–659. doi: 10.1016/j.tplants.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Fragoso V, Goddard H, Baldwin IT, Kim SG. A simple and efficient micrografting method for stably transformed Nicotiana attenuata plants to examine shoot-root signaling. BMC Plant Methods. 2011;7(34):1–8. doi: 10.1186/1746-4811-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaquerel E, Heiling S, Schoettner M, Zurek G, Baldwin IT. Development and validation of a liquid chromatography-electrospray ionization-time-of-flight mass spectrometry method for induced changes in Nicotiana attenuata leaves during simulated herbivory. Journal of Agricultural and Food Chemistry. 2010;58(17):9418–9427. doi: 10.1021/jf1017737. [DOI] [PubMed] [Google Scholar]
- Gaquerel E, Stitz M, Kallenbach M, Baldwin IT. Jasmonate signaling in the field, part II: insect-guided characterization of genetic variations in jasmonate-dependent defenses of transgenic and natural Nicotiana attenuata populations. Moethods in Molecular Biology. 2013;1011:97–109. doi: 10.1007/978-1-62703-414-2_8. [DOI] [PubMed] [Google Scholar]
- Glendinning JI. How do herbivorous insects cope with noxious secondary plant compounds in their diet? Entomologia Experimentalis Et Applicata. 2002;104(1):15–25. [Google Scholar]
- Grebner W, Stingl NE, Oenel A, Mueller MJ, Berger S. Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiology. 2013;161(4):2159–2170. doi: 10.1104/pp.113.214544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettenhausen C, Baldwin IT, Wu J. Nicotiana attenuata MPK4 suppresses a novel jasmonic acid (JA) signaling-independent defense pathway against the specialist insect Manduca sexta, but is not required for the resistance to the generalist Spodoptera littoralis. New Phytologist. 2013;199(3):787–799. doi: 10.1111/nph.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Jander G. Plant immunity to insect herbivores. Annual Review of Plant Biology. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Howe GA, Schilmiller AL. Oxylipin metabolism in response to stress. Current Opinion in Plant Biology. 2002;5(3):230–236. doi: 10.1016/s1369-5266(02)00250-9. [DOI] [PubMed] [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gabler R, Kuhnemann F, Preston CA, Baldwin IT. Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta. 2000;210(2):336–342. doi: 10.1007/PL00008142. [DOI] [PubMed] [Google Scholar]
- Kallenbach M, Bonaventure G, Gilardoni PA, Wissgott A, Baldwin IT. Empoasca leafhoppers attack wild tobacco plants in a jasmonate-dependent manner and identify jasmonate mutants in natural populations. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(24):E1548–E1557. doi: 10.1073/pnas.1200363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in the wild tobacco Nicotiana attenuata. Plant Journal. 2004;38(4):639–649. doi: 10.1111/j.1365-313X.2004.02076.x. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Baldwin IT. Silencing the jasmonate cascade: induced plant defenses and insect populations. Science. 2004;305(5684):665–668. doi: 10.1126/science.1096931. [DOI] [PubMed] [Google Scholar]
- Kim SG, Yon F, Gaquerel E, Gulati J, Baldwin IT. Tissue specific diurnal rhythms of metabolites and their regulation during herbivore attack in a native tobacco, Nicotiana attenuata. Plos One. 2011;6(10):e26214. doi: 10.1371/journal.pone.0026214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel T, Lim M, Gase K, Halitschke R, Baldwin IT. Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology. 2002;12(4):177–183. [Google Scholar]
- Li CY, Schilmiller AL, Liu GH, Lee GI, Jayanty S, Sageman C, Vrebalov J, Giovannoni JJ, Yagi K, Kobayashi Y, Howe GA. Role of beta-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell. 2005;17(3):971–986. doi: 10.1105/tpc.104.029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li C, Lee GI, Howe GA. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):6416–6421. doi: 10.1073/pnas.072072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado R, Ferrieri A, Robert C, Glauser G, Kallenbach M, Erb M, Baldwin IT. Leaf-herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytologist. 2013 doi: 10.1111/nph.12438. in press. [DOI] [PubMed] [Google Scholar]
- Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature. 2013;500(7463):422–426. doi: 10.1038/nature12478. [DOI] [PubMed] [Google Scholar]
- Oh Y, Baldwin IT, Galis I. NaJAZh regulates a subset of defense responses against herbivores and spontaneous leaf necrosis in Nicotiana attenuata plants. Plant Physiology. 2012;159(2):769–788. doi: 10.1104/pp.112.193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmeiss TE, McCloud ES, Lynds GY, Baldwin IT. Within-plant relationships among wounding, jasmonic acid, and nicotine: implications for defence in Nicotiana sylvestris. New Phytologist. 1997;137(3):441–452. doi: 10.1046/j.1469-8137.1997.00845.x. [DOI] [PubMed] [Google Scholar]
- Onkokesung N, Gaquerel E, Kotkar H, Kaur H, Baldwin IT, Galis I. MYB8 controls inducible phenolamide levels by activating three novel hydroxycinnamoyl-coenzyme A:polyamine transferases in Nicotiana attenuata. Plant Physiology. 2012;158(1):389–407. doi: 10.1104/pp.111.187229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT. Co(i)-ordinating defenses: NaCOI1 mediates herbivore-induced resistance in Nicotiana attenuata and reveals the role of herbivore movement in avoiding defenses. Plant Journal. 2007;51:79–91. doi: 10.1111/j.1365-313X.2007.03119.x. [DOI] [PubMed] [Google Scholar]
- Roda AL, Oldham NJ, Svatos A, Baldwin IT. Allometric analysis of the induced flavonols on the leaf surface of wild tobacco (Nicotiana attenuata) Phytochemistry. 2003;62(3):527–536. doi: 10.1016/s0031-9422(02)00608-8. [DOI] [PubMed] [Google Scholar]
- Schwachtje J, Minchin PE, Jahnke S, van Dongen JT, Schittko U, Baldwin IT. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(34):12935–12940. doi: 10.1073/pnas.0602316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Song JT, Cheong JJ, Lee YH, Lee YW, Hwang I, Lee JS, Choi YD. Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4788–4793. doi: 10.1073/pnas.081557298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16(8):2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel I, Hause B, Miersch O, Kurz T, Maucher H, Weichert H, Ziegler J, Feussner I, Wasternack C. Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Molecular Biology. 2003;51(6):895–911. doi: 10.1023/a:1023049319723. [DOI] [PubMed] [Google Scholar]
- Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine's defensive function in nature. PLoS Biology. 2004;2(8):1074–1080. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitz M, Baldwin IT, Gaquerel E. Diverting the flux of the JA pathway in Nicotiana attenuata compromises the plant's defense metabolism and fitness in nature and glasshouse. PLoS One. 2011;6(10):e25925. doi: 10.1371/journal.pone.0025925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadassery J, Reichelt M, Hause B, Gershenzon J, Boland W, Mithofer A. CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiology. 2012;159(3):1159–1175. doi: 10.1104/pp.112.198150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Dahl CC, Baldwin IT. Methyl jasmonate and cis-jasmone do not dispose of the herbivore-induced jasmonate burst in Nicotiana attenuata. Physiologia Plantarum. 2004;120(3):474–481. doi: 10.1111/j.0031-9317.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Allmann S, Wu J, Baldwin IT. Comparisons of LIPOXYGENASE3- and JASMONATE-RESISTANT4/6-silenced plants reveal that jasmonic acid and jasmonic acid-amino acid conjugates play different roles in herbivore resistance of Nicotiana attenuata. Plant Physiology. 2008;146(3):904–915. doi: 10.1104/pp.107.109264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Halitschke R, Kang JH, Berg A, Harnisch F, Baldwin IT. Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta. 2007;226(1):159–167. doi: 10.1007/s00425-007-0477-3. [DOI] [PubMed] [Google Scholar]
- Winz RA, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host. Nicotiana attenuata IV. Insect-induced ethylene reduces jasmonate-induced nicotine accumulation by regulating putrescine N-methyltransferase transcripts. Plant Physiology. 2001;125(4):2189–2202. doi: 10.1104/pp.125.4.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annual Review of Genetics. 2010;44:1–24. doi: 10.1146/annurev-genet-102209-163500. [DOI] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280(5366):1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- Zhang ZP, Baldwin IT. Transport of [2-14C]jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta. 1997;203(4):436–441. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.