Abstract
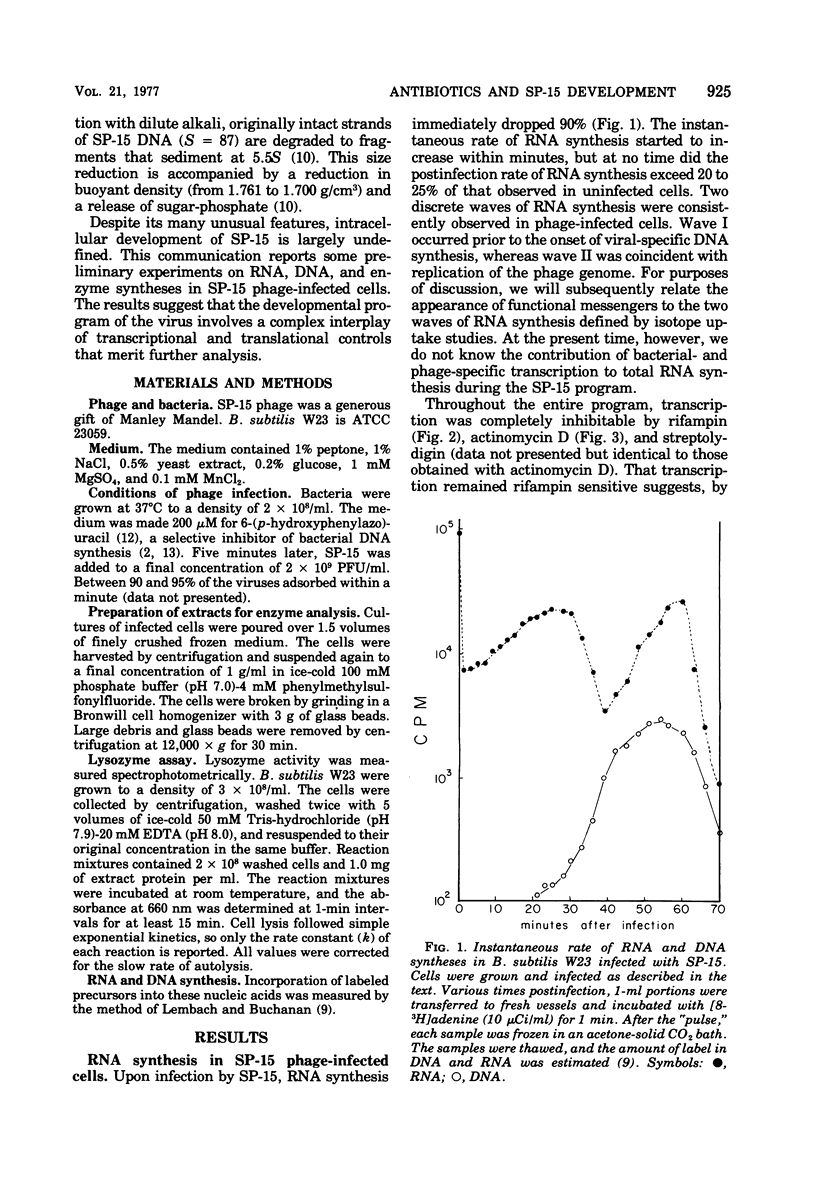
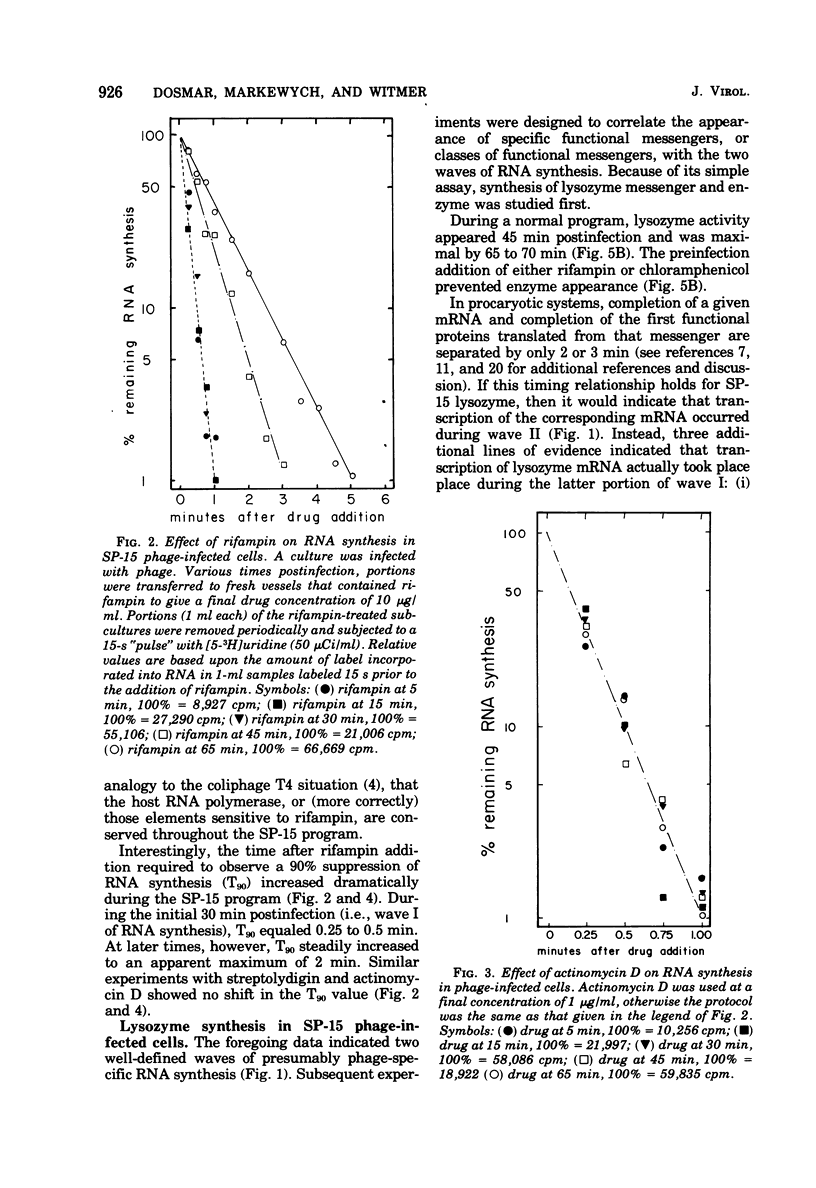
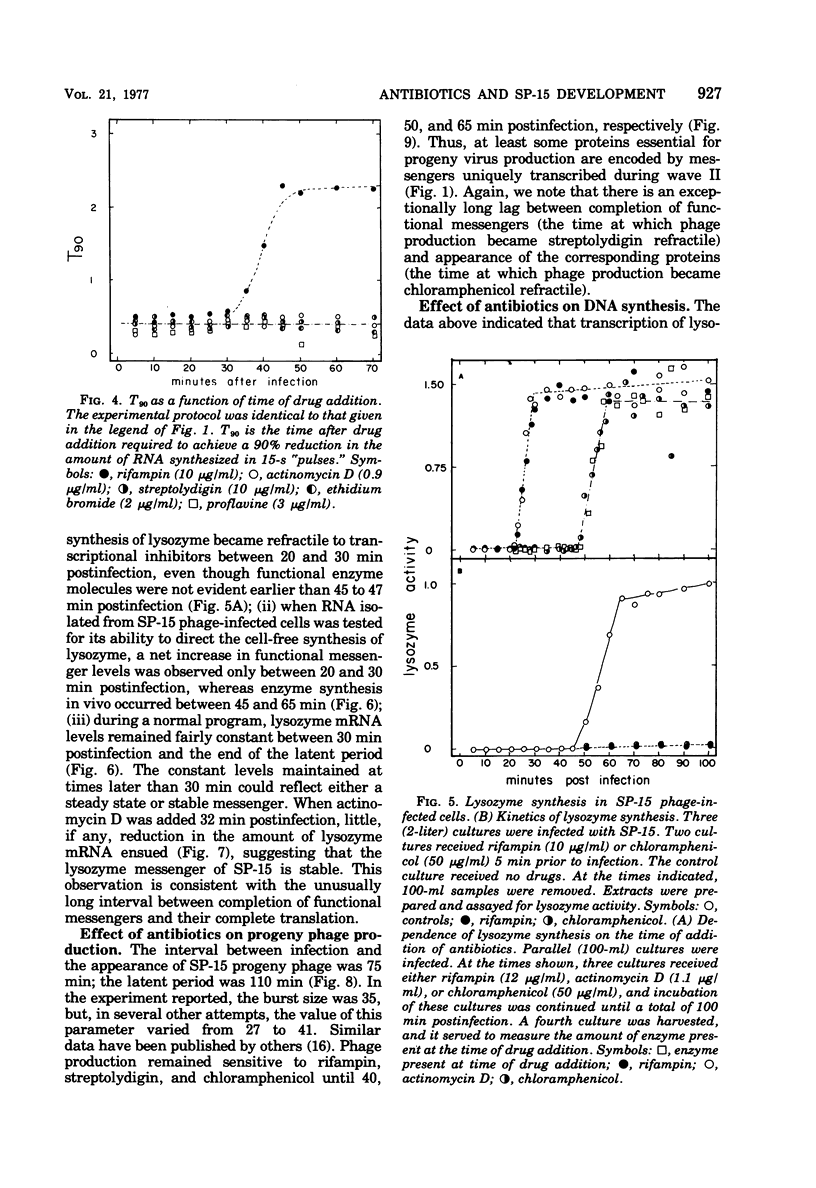
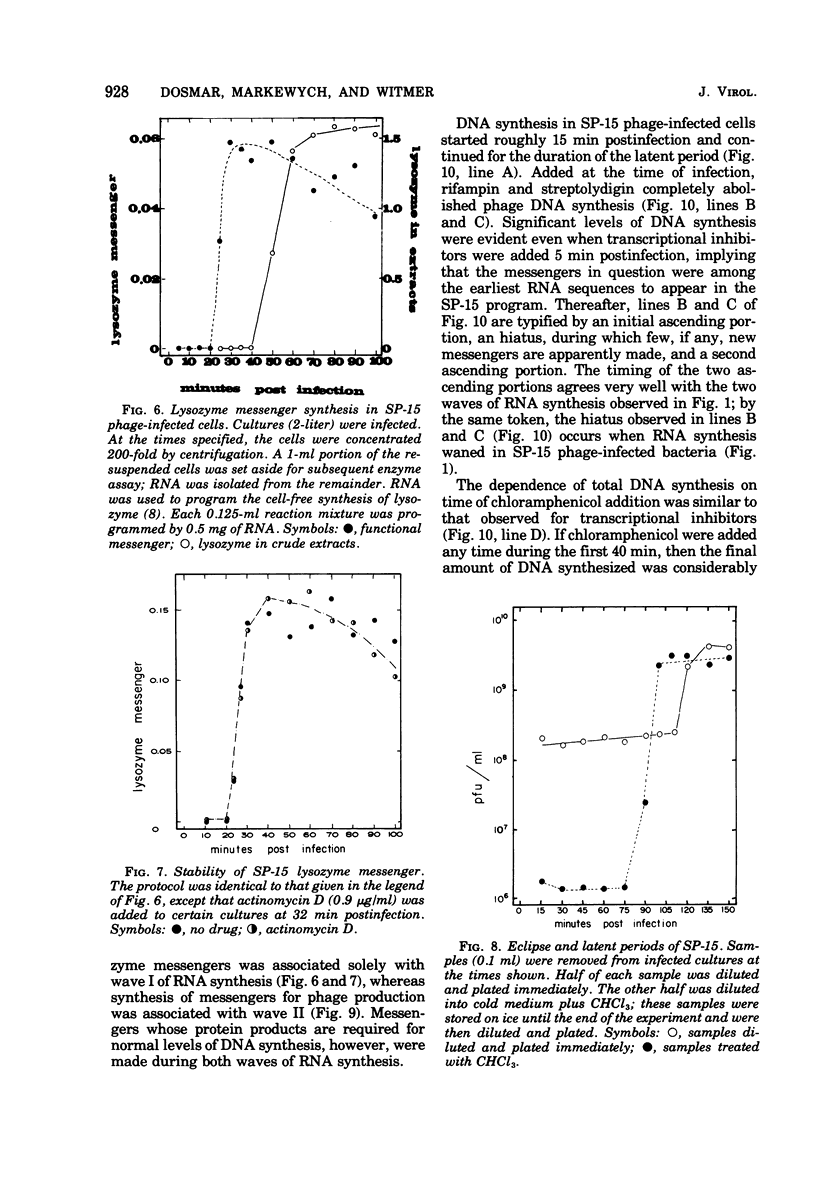
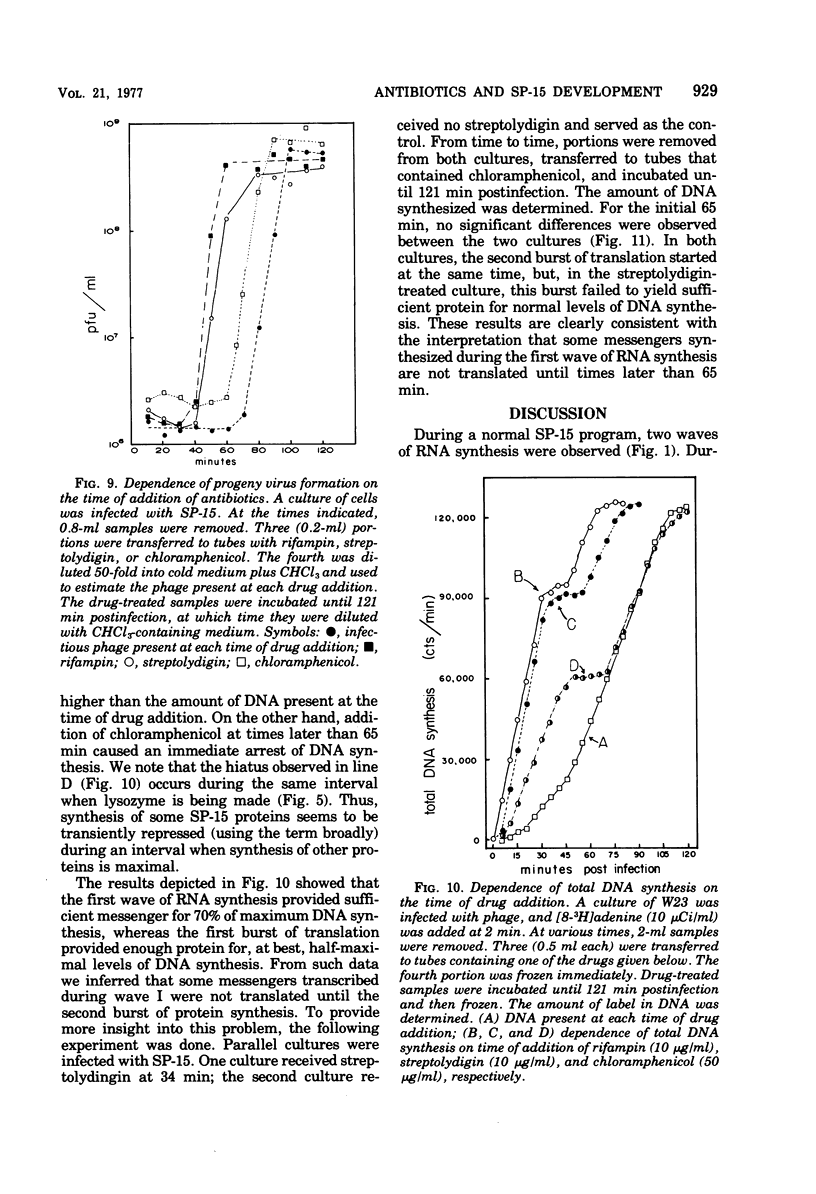
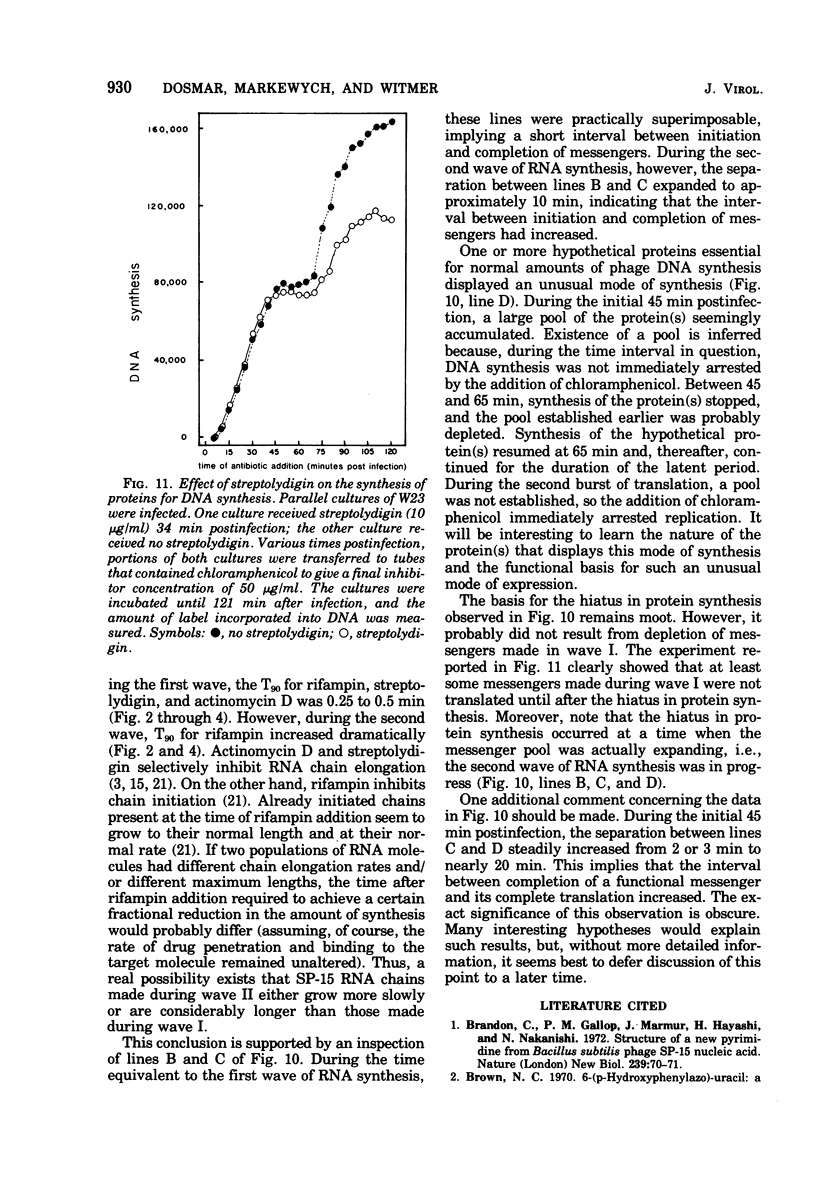
Bacillus subtilis W23 was infected with bacteriophage SP-15. Two waves of phage-specific RNA synthesis were observed. Wave I was prereplicative, and wave II was coincident with replication of the viral genome. To determine the temporal appearance of general classes of phage-coded messengers and proteins, we studied the dependence of lysozyme synthesis, phage production, and DNA synthesis on time of addition of transcriptional and translational inhibitors. Lysozyme synthesis started to become refractile to a variety of transcriptional inhibitors (rifampin, streptolydigin, and actinomycin D) between 20 and 22 min postinfection and was completely refractile by 30 min. Nevertheless, functional enzyme did not appear until 45 to 47 min postinfection; lysozyme was maximal by 65 min. Rna isolated from SP-15 phage-infected cells was used to program the cell-free synthesis of lysozyme. The messenger was synthesized exclusively between 20 and 30 min postinfection. Lysozyme messengers were stable. The data imply that lysozyme messengers were present 52 min prior to their translation. Progeny virus formation remained sensitive to transcriptional inhibitors until 40 to 50 min postinfection, and sensitivity to chloramphenicol lasted 65 min. The first progeny viruses appeared at 75 min. Again, an unusually long lag between completion of functional messengers and their translation was evident. The aforementioned data indicated that transcription of lysozyme messengers and, at least, some messengers, whose products are essential for phage production, are uniquely associated with waves I and II of RNA synthesis, respectively. However, messengers whose products are essential for normal amounts of DNA synthesis were apparently synthesized during both waves; transcription of these messengers was transiently repressed (using the term broadly) between 30 and 40 min postinfection. Judging from the dependence of DNA synthesis on time of chloramphenicol addition, proteins essential for normal amounts of DNA synthesis were also synthesized in two discrete waves, each yielding sufficient protein for half-maximal levels of DNA synthesis. An hiatus in the synthesis of the proteins in question was evident between 45 and 65 min postinfection; evidence cited in this paper indicates that this hiatus did not result from messenger depletion, which, in turn, implied some type of translational-level control. This latter conclusion is substantiated by the lysozyme synthesis that occurred during the same interval when synthesis of certain proteins for DNA replication was transiently repressed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandon C., Gallop P. M., Marmur J., Hayashi H., Nakanishi K. Structure of a new pyrimidine from Bacillus subtilis phage SP-15 nucleic acid. Nat New Biol. 1972 Sep 20;239(90):70–71. doi: 10.1038/newbio239070a0. [DOI] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P., Sklar J. Continual requirement for a host RNA polymerase component in a bacteriophage development. Nature. 1969 Mar 1;221(5183):833–836. doi: 10.1038/221833a0. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Nakanishi K., Brandon C., Marmur J. Structure and synthesis of dihydroxypentyluracil from bacteriophage SP-15 deoxyribonucleic acid. J Am Chem Soc. 1973 Dec 26;95(26):8749–8757. doi: 10.1021/ja00807a041. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Simmons C. Synthesis and decay of messenger ribonucleic acid from the lactose operon of Escherichia coli during amino-acid starvation. J Mol Biol. 1972 Oct 14;70(3):451–464. doi: 10.1016/0022-2836(72)90552-9. [DOI] [PubMed] [Google Scholar]
- Legault-Demare L., Chambliss G. H. Natural messenger ribonucleic acid-directed cell-free protein-synthesizing system of Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1300–1307. doi: 10.1128/jb.120.3.1300-1307.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembach K. J., Buchanan J. M. The relationship of protein synthesis to early transcriptive events in bacteriophage T4-infected Escherichia coli B. J Biol Chem. 1970 Apr 10;245(7):1575–1587. [PubMed] [Google Scholar]
- Marmur J., Brandon C., Neubort S., Ehrlich M., Mandel M., Konvicka J. Unique properties of nucleic acid from Bacillus subtilis phage SP-15. Nat New Biol. 1972 Sep 20;239(90):68–70. doi: 10.1038/newbio239068a0. [DOI] [PubMed] [Google Scholar]
- Natale P. J., Buchanan J. M. DNA-directed synthesis in vitro of T4 phage-specific enzymes. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2513–2517. doi: 10.1073/pnas.69.9.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubort S., Marmur J. Synthesis of the unusual DNA of Bacillus subtilis bacteriophage SP-15. J Virol. 1973 Nov;12(5):1078–1084. doi: 10.1128/jvi.12.5.1078-1084.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M. M., Brown N. C. Inhibition of a discrete bacterial DNA polymerase by 6-(p-hydroxyphenylazo)-uracil and 6-(p-hydroxyphenylazo-)-isocytosine. Nat New Biol. 1972 Nov 15;240(98):80–82. doi: 10.1038/newbio240080a0. [DOI] [PubMed] [Google Scholar]
- ROMIG W. R., BRODETSKY A. M. Isolation and preliminary characterization of bacteriophages for Bacillus subtilis. J Bacteriol. 1961 Jul;82:135–141. doi: 10.1128/jb.82.1.135-141.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhikol C., Erbstoeszer J. W., Weisblum B. Mode of action of streptolydigin. J Bacteriol. 1969 Jul;99(1):151–155. doi: 10.1128/jb.99.1.151-155.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR M. J., THORNE C. B. TRANSDUCTION OF BACILLUS LICHENIFORMIS AND BACILLUS SUBTILIS BY EACH OF TWO PHAGES. J Bacteriol. 1963 Sep;86:452–461. doi: 10.1128/jb.86.3.452-461.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Goldberg I. D. Growth and cultivation of the unusual generalized transducing Bacillus bacteriophage SP-15. Appl Microbiol. 1971 Jul;22(1):113–119. doi: 10.1128/am.22.1.113-119.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Lawton W. D., MacQuillan A. M. Sequential replication of the chromosome of Bacillus licheniformis. J Bacteriol. 1968 Jun;95(6):2062–2069. doi: 10.1128/jb.95.6.2062-2069.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Taylor M. J., Lawton W. D., Goldberg I. D. Cotransduction and cotransformation of genetic markers in Bacillus subtilis and Bacillus licheniformis. J Bacteriol. 1969 Nov;100(2):1027–1036. doi: 10.1128/jb.100.2.1027-1036.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


