Abstract
Cell signalling via inositol phosphates, eg the second messenger myo-inositol 1,4,5-trisphosphate, and phosphoinositides comprises a huge field of biology. Of nine 1,2,3,4,5,6-cyclohexanehexol isomers, myo-inositol is pre-eminent, with “other” inositols (cis-, epi-, allo-, muco-, neo-, l-chiro-, d-chiro- and scyllo-) and derivatives rarer or thought not to exist in nature. However, recently, neo- and d-chiro-inositol hexakisphosphates were revealed in both terrestrial and aquatic ecosystems, highlighting the paucity of knowledge of the origins and potential biological functions of such stereoisomers, a prevalent group of environmental organic phosphates, and their parent inositols. Some “other” inositols are medically relevant, e.g. scyllo-inositol (neurodegenerative diseases), and d-chiro-inositol (diabetes). It is timely to consider exploration of roles and applications of “other” isomers and their derivatives, likely by exploiting techniques now well developed for the myo-series.
Keywords: Cyclitols, Isomers, Inositol, Phosphate, Synthesis
1. Introduction
myo-Inositol (1) (cis-1,2,3,5-trans-4,6-cyclohexanehexol) and its derivatives, particularly its phosphates, are common in biology. These compounds have a multitude of functions across the various taxa[1] including roles in regulating ion channel permeability,[2] phosphate levels,[3] metabolic flux,[4] transcription, mRNA export and translation,[5] insulin signalling, embryonic development[6] and the stress response.[7] myo-Inositol is also a component of membrane-incorporated phosphatidylinositols.[8] Reviews of the various roles of myo-inositol and its derivatives continue to be published.[9]
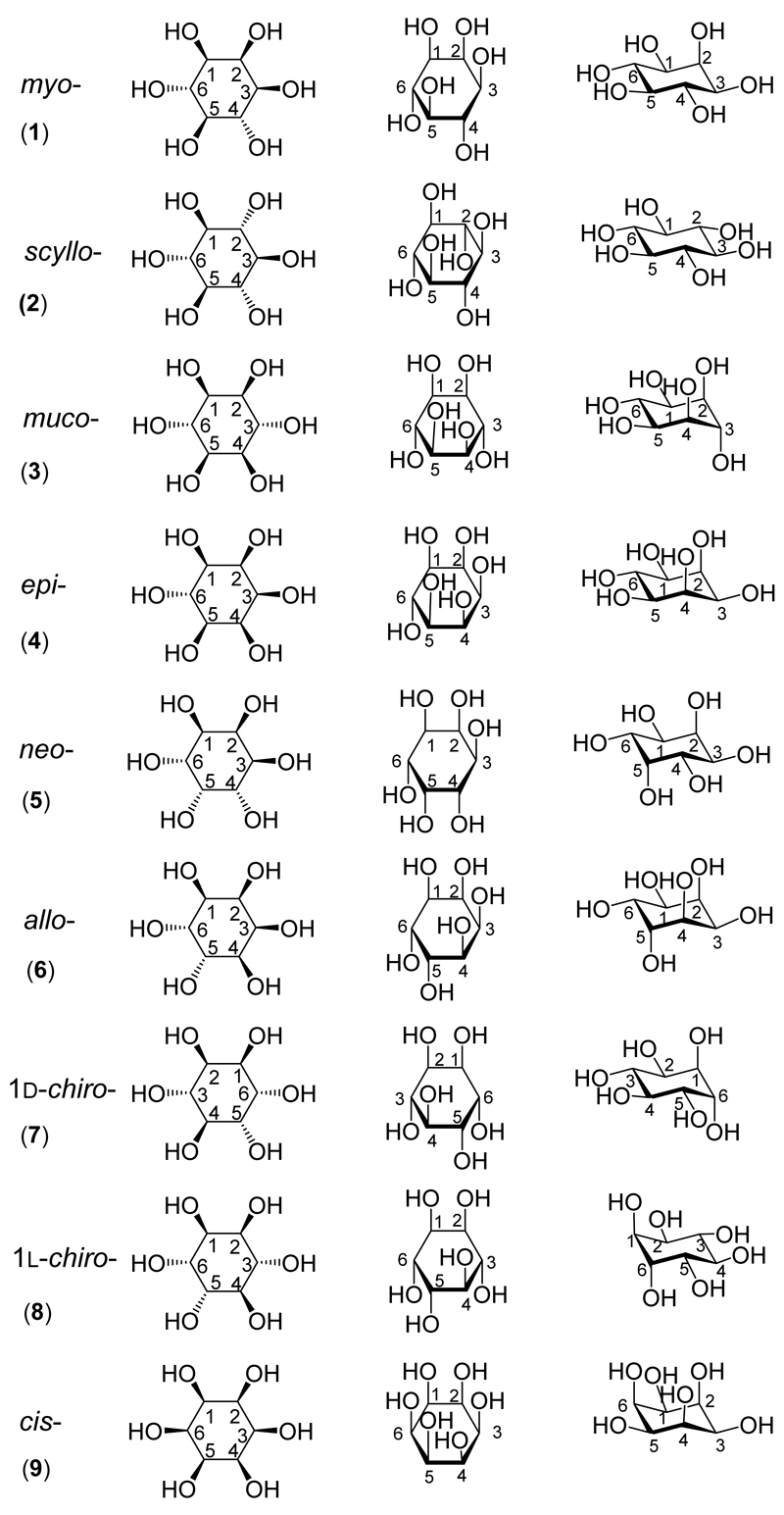
However, myo-inositol is only one of nine possible structural isomers of inositol (1,2,3,4,5,6-cyclohexanehexol; Figure 1). As illustrated on the frontispiece central superimposition, a commonly used structural mnemonic to aid biochemists in particular is “Agranoff’s turtle”*, as explained in a recent review.[10] The “other” inositols include seven that are naturally occurring (scyllo-(2), muco-(3), epi-(4), neo-(5), allo-(6) and both the d-(7) and l-chiro-inositols (8)), and one that is not known to occur naturally, cis-inositol (9). It is these compounds that are the focus of this review which looks at the synthesis and the biological and medicinal roles of each of them in turn. myo-Inositol and its derivatives have been much studied – a search of PubMed using ‘myo-inositol’ as the search term returns more than 40,000 references. In comparison searching for each of the other inositols in turn returns a total of fewer than 400 references.
Figure 1. The structures of the inositol isomers.
Three projections of each of the inositols are shown. The first column is a Mills projection. The second column is a Haworth projection. The third column shows a more realistic three-dimensional structure (not necessarily the most stable structure) for each of the inositols. The numbering of the carbons in the ring is shown.
A stimulus to compile this review was provided by results from our recent report where we used 31P NMR spectroscopy to reveal the presence of neo- and d-chiro-inositol hexakisphosphates in both terrestrial and aquatic ecosystems.[11] This report and a related commentary highlighted the paucity of our knowledge on the origins and biological functions of the inositol hexakisphosphate stereoisomers, despite the fact that they constitute one of the most prevalent groups of organic phosphates in the environment.[12] By implication also, this lack of knowledge extends to the parent inositol stereoisomers and any polyphosphate or other derivative thereof.
In the past few years the inositols and their derivatives have been the subject of several books,[e.g. 13] though these concentrate on myo-inositol and its derivatives. An older text mentions what little was known about the other inositols and their phosphates but, again, is predominantly about myo-inositol.[14] We are unaware of any recent publication that tries to summarise concisely the accumulated knowledge on the “other” inositols and this paper is an attempt to fill the gap in the literature. After brief general sections covering the synthesis and biology of the inositols and their phosphates each inositol is considered in more detail, looking at the chemical synthesis, and roles in biology and medicine. Advances in myo-inositol chemistry since 2010 are then discussed to illustrate novel chemical approaches that may be applied to the chemistry of the “other” inositols. A concluding section highlights possible directions for further study. Space constraints preclude a discussion of the nomenclature of the inositols and their phosphates, but this is discussed in detail in the accompanying Supporting Information (SI_1). For the same reason the discussion in this paper of the biology and medicine is largely limited to that pertaining to mammals: the roles of the “other” inositols in non-mammalian species (and in the environment) are also detailed in the accompanying Supporting Information (SI_2).
2. General Synthetic Routes to the “Other” Inositols
The following subjects have been previously reviewed: the regioselective protection and deprotection of inositol hydroxyl groups;[15] the chemical and chemoenzymatic synthesis of deoxyfluoro inositols;[16] the chemoenzymatic synthesis of inositols and their analogues;[17] and a general overview of recent advances in inositol chemistry.[18]
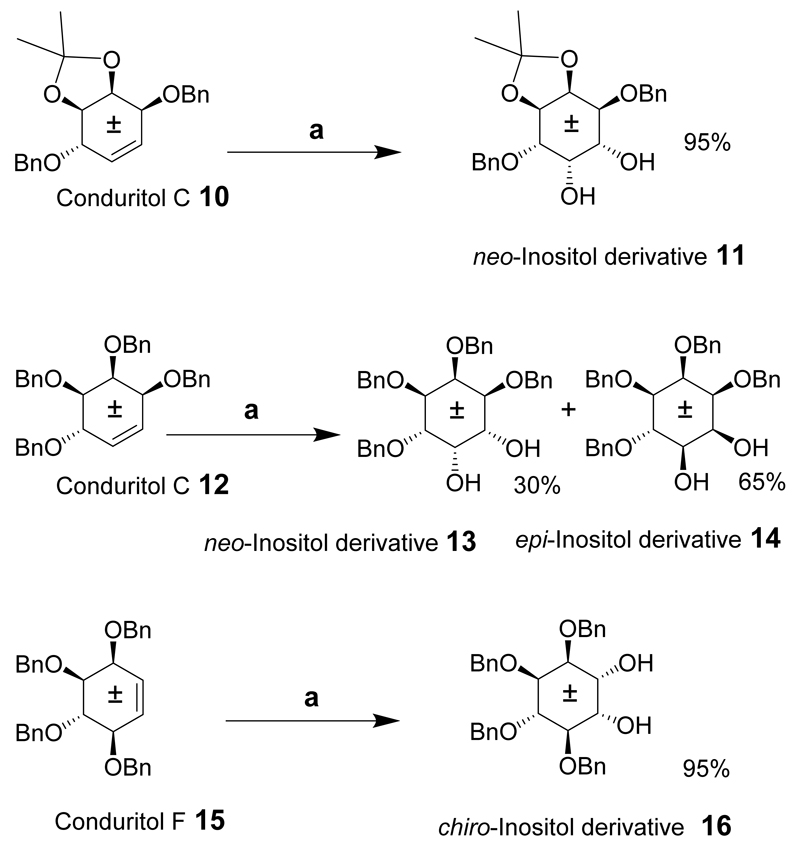
Synthetic routes to each of the inositols are described in this section. First, some general approaches are discussed to illustrate the versatility of a given route for the synthesis of a number of inositols from several intermediates. In principle, all the isomers may be derived from myo-inositol by inversion of the configuration at either one or two of the carbons. A chemical synthesis for six of the meso-inositol isomers, (neo-, epi-, scyllo-, allo-, myo- and muco-) and a synthesis of racemic chiro-inositol derivatives from myo-inositol via conduritol intermediates has been described.[19] The route makes this a quick and attractive pathway for accessing inositol derivatives on a multigram scale from intermediates derived from a one-pot reaction. A cis-inositol derivative was also synthesized via a different route but using simple readily available starting materials. Conduritol C, F and B-derivatives (Schemes 1 and 2), 10, 12, 15 and 17 were prepared from known racemic benzoylated inositol derivatives. Initially, the benzoylated conduritol precursors (not shown) were used to effect the transformation into inositol derivatives. However, the resulting products were insoluble in many cases, and benzyl groups replaced the intermediate benzoyl protecting groups. The conduritol C and F derivatives were then transformed into benzylated inositol compounds that could be hydrogenated to give the corresponding inositols. Conduritol C derivative (10) was dihydroxylated to give a cis-diol and the neo-inositol derivative (11) in 95% yield. The epi-inositol derivative 14 together with a neo-inositol derivative 13 was provided by dihydroxylation of the conduritol C derivative 12 under the same conditions. In a similar fashion, the dihydroxylation of conduritol F derivative 15 gave the racemic tetrabenzylated chiro-inositol derivate 16 in good yield.
Scheme 1.
Reaction conditions: (a) OsO4, NMO, acetone (aq).
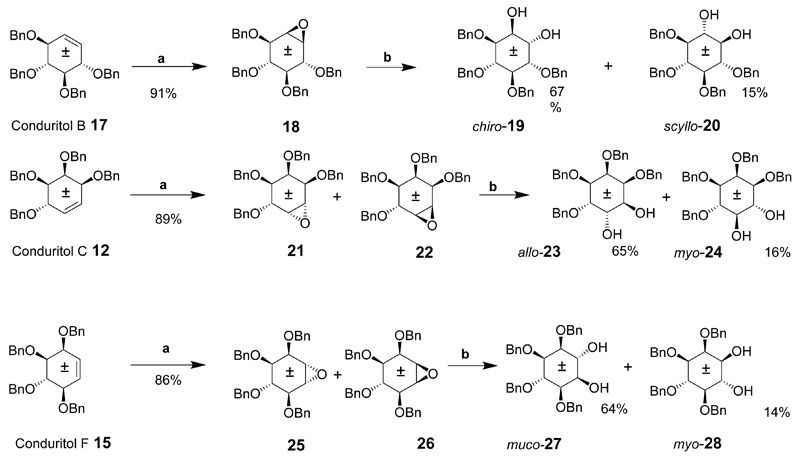
Scheme 2.
Reaction conditions: (a) I2, Ag2O, dioxane (aq), 90°C, (b) CF3COOH(aq), THF, 50°C.
The benzylated conduritol B, C and F derivatives 17, 12 and 15 (Scheme 2) were epoxidized in the presence of iodine and silver (I) oxide. Conduritol B derivative 17 gave epoxide 18 in high yield which was opened in the presence of acid to give racemic 1,4,5,6-tetra-O-benzyl chiro-inositol (19) and racemic 1,2,3,4-tetra-O-benzyl-scyllo-inositol (20). However, epoxidation of C and F derivatives 12 and 15 gave a mixture of epoxides 21 and 22 from 12 and 25 and 26 from 15. The epoxide derivatives 21 and 22 were subjected to acidic hydrolysis to provide racemic 1,2,3,6-tetra-O-benzyl-allo-inositol (23) and racemic 1,2,3,4-tetra-O-benzyl-myo-inositol (24). Ring opening of epoxides 25and 26 under acidic conditions gave racemic 1,2,5,6-tetra-O-benzyl-muco-inositol (27) and racemic 2,3,4,5-tetra-O-benzyl-myo-inositol (28). Further deprotection of these derivatives was not discussed however simple hydrogenation in the presence of a palladium on carbon catalyst will give the respective inositols in high yield.
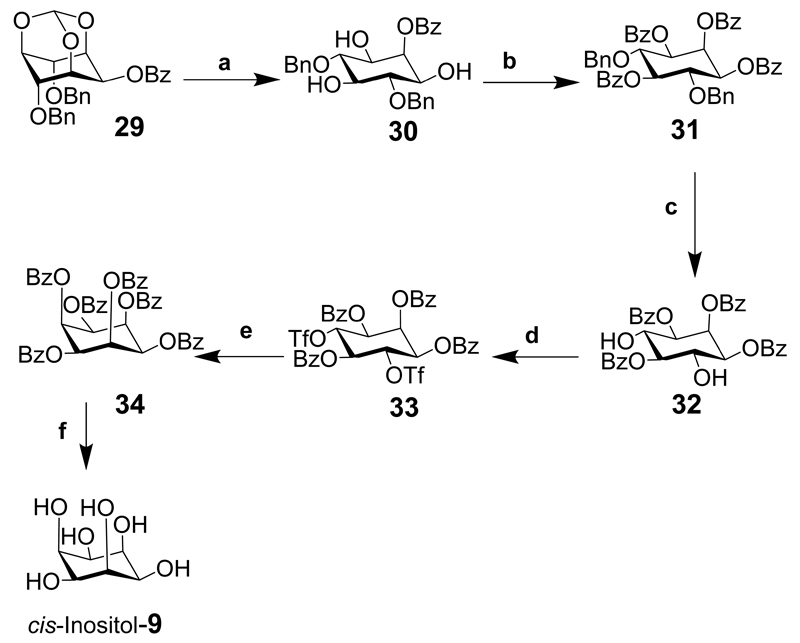
cis-Inositol hexabenzoate (34), was prepared in five steps from 2-O-benzoyl-4,6-di-O-benzyl-myo-inositol orthoformate (29) and was not accessible via the conduritol route (Scheme 3: note that some axial bonds are exaggerated to lessen the clashing with other substituents). The orthoester was hydrolyzed under acidic conditions to give 2-O-benzoyl-4,6-di-O-benzyl-myo-inositol (30) which was further benzoylated to give 1,2,3,5-tetra-O-benzoyl-4,6-di-O-benzyl myo-inositol (31). Hydrogenolysis of the benzyl groups to expose the hydroxyl groups gave compound 32 and was triflated to provide 33 that was then heated with potassium benzoate in DMSO to give the cis-inositol derivative 34. This derivative can then be deblocked with base to give cis-inositol.[19]
Scheme 3.
Reaction conditions: (a) PTSA, MeOH, reflux; (b) BzCl, pyr., 91%; (c) Pd(OH)2/C, MeOH, (50 psi) H2, 96%; (d) Tf2O, CH2Cl2, pyr., –42°C→ rt, 89%; (e) KOBz, DMSO, 100°C 32%; (f) base (step not shown in original literature).
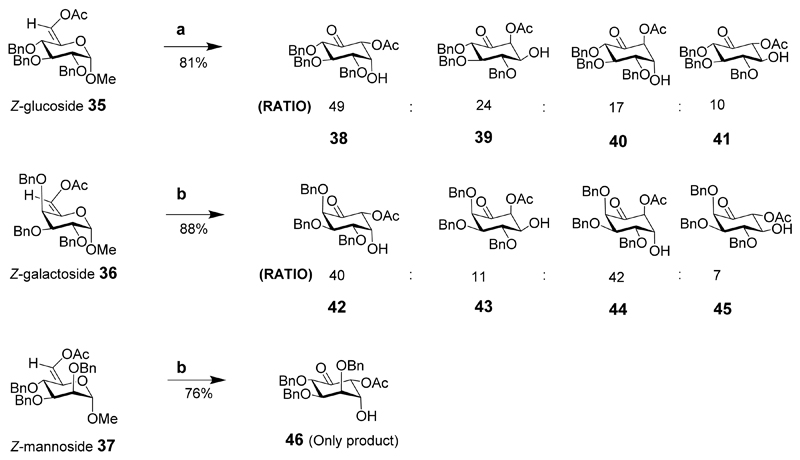
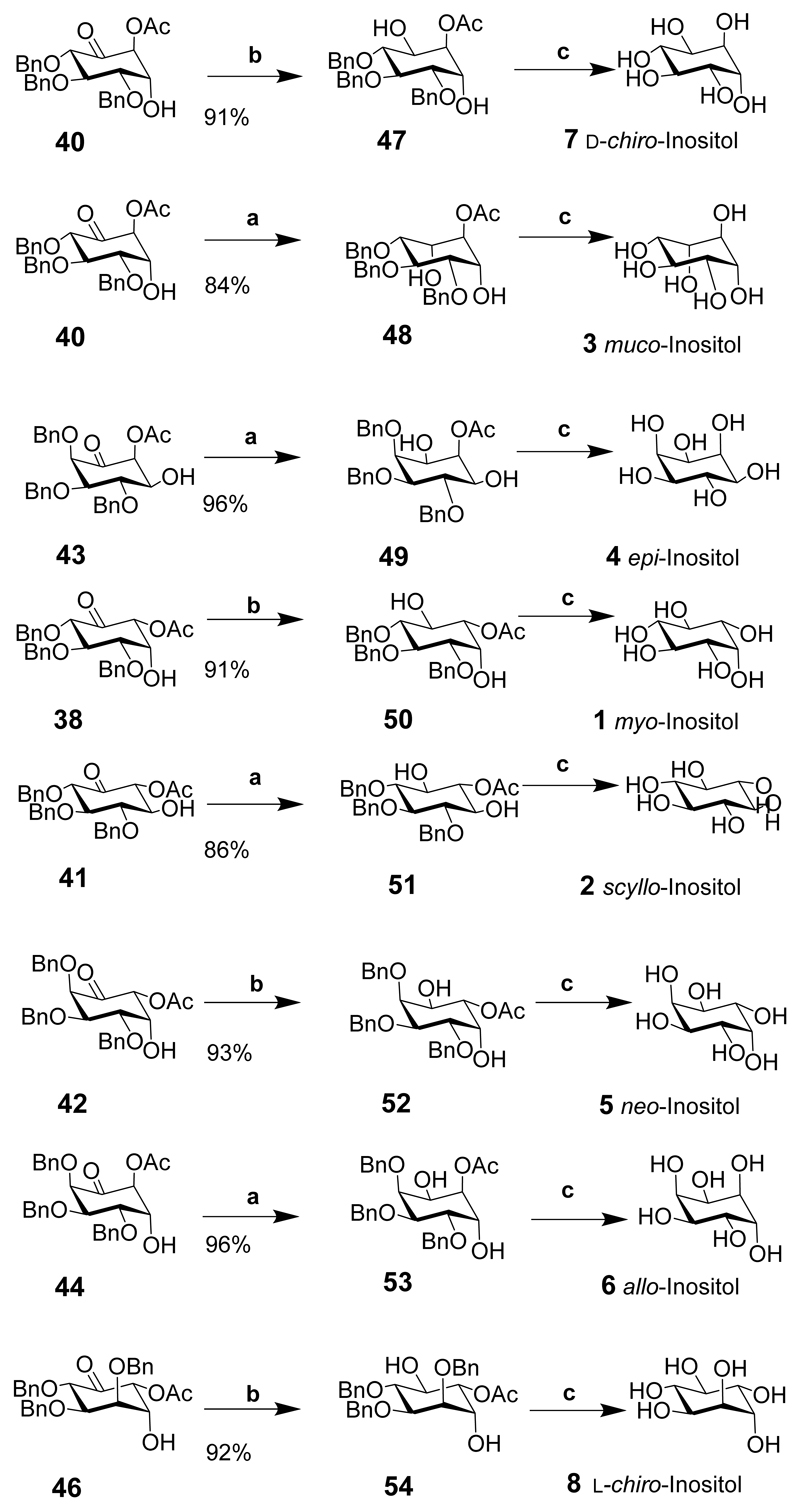
All nine inositol diastereoisomers were synthesized using three different pyranosides (glucose, galactose and mannose) as the starting materials (Schemes 4 and 5).[20] The cost of the raw materials and the inherent chirality of the starting materials make the synthesis of inositol derivatives particularly attractive, especially for d- and l-chiro-inositols. Functional group manipulation of the intermediates should give suitably protected compounds and provide any inositol or its phosphorylated derivative. These sugars were used to prepare the 6-O-acetyl-5-enopyranoside intermediates 35, 36, 37 which were transformed into chiral-substituted cyclohexanones, in a Ferrier carbocyclisation mediated by palladium dichloride (PdCl2). For the glucoside derivative, the Z-isomer 35 was treated with PdCl2 in aqueous dioxane to effect the transformation to the cyclohexanones in 81% yield and a ratio of (49:24:17:10 for 38, 39, 40 and 41). The E-isomer did not give any product under these conditions. Similarly, the galactoside Z-isomer under similar conditions as described for the glucoside (dioxane-water 2:1) gave a mixture of cyclohexanone derivatives in a ratio of (40:11:42:7, for 42, 43, 44 and 45) in 88% overall yield. The E-isomer (not shown) of the galactoside also gave a similar ratio but in poor yield. The mannoside Z-isomer provided a single product 46 in 76% yield. The E-isomer mannoside (not shown) also gave the single product 46, but in lower yield.
Scheme 4.
Reaction conditions: (a) 5% mol PdCl2, Dioxane-H2O, 4:1, 81%; (b) 5% mol PdCl2, dioxane-H2O, 2:1.
Scheme 5.
Reaction conditions: (a) NaBH4, MeOH, 0°C, 30 min; up to 99:1 of desired product; (b) Me4NBH(OAc)3, 5.0 eq, MeCN, AcOH, 0°C, 3 h, up to 99:1 of desired product; (c) NaOH, MeOH, 0°C, then Pd(OH)2, H2, MeOH.
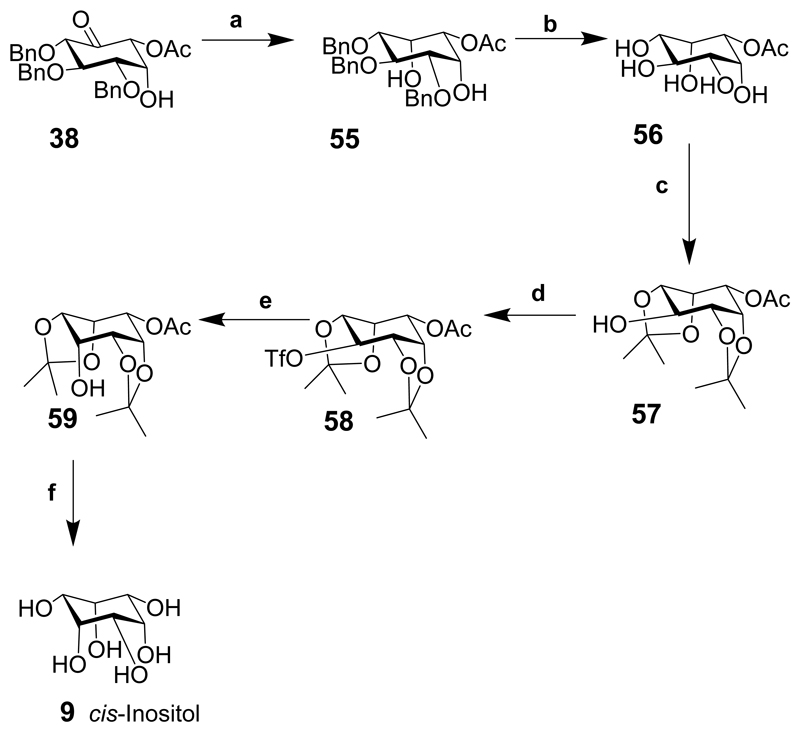
Eight of the nine inositols were prepared by the stereoselective reduction of the carbonyl to either an axial or equatorial alcohol followed by alkaline hydrolysis and hydrogenation of the benzyl groups. cis-Inositol (9) could not be synthesized directly in three steps from any of the intermediates, so was synthesized in six steps from intermediate 38.
Intermediate 38 was reduced using sodium borohydride in methanol to give the axial alcohol and the epi-inositol derivative 55 (Scheme 6; note that some of the bonds are exaggerated in cis-inositol derivatives to lessen the clashes with other functional groups). Hydrogenolysis as described for other intermediates (Scheme 5) provided the acetate 56, which was protected with isopropylidene groups to give intermediate 57. Triflation of the free hydroxyl gave 58 followed by inversion from an equatorial position to give an axial derivative then hydrolysis of the trifluoroacetate provided the cis-inositol derivative 59 that was hydrolysed under acidic conditions to give cis-inositol (9).
Scheme 6.
Reaction conditions: (a) NaBH4, MeOH, 0°C, 30 min., 97%; (b) Pd(OH)2 on carbon, H2, MeOH, 12 h, quantitative yield; (c) H2SO4 (conc.), Me2C=O, 0°C, 1 h 83%; (d) Tf2O, pyridine, CH2Cl2, rt, 1 h, 89%; (e) CF3CO2Cs, 18-crown-6, toluene, DMF, 80°C, 1.5 h, then saturated NaHCO3, rt, 1 h, 78% from 57; (f) TFA, MeOH, 60°C, 3 h.
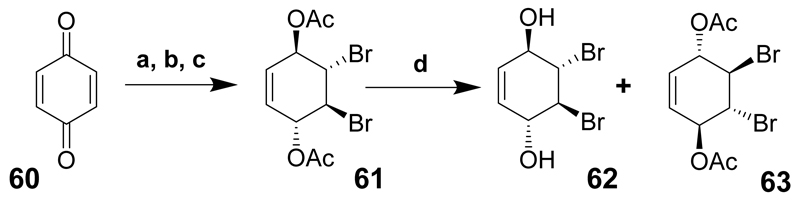
The transformation of p-benzoquinone (60) into seven of the nine inositols (cis-inositol, 9, and muco-inositol, 3, were not made) was described more than ten years ago.[21] Although this paper reports that the meso-inositols can be synthesised from racemic precursors (that are crystalline and easier to handle), the intermediates were resolved then eventually deblocked to give meso-compounds. This may appear a waste of chiral precursor to synthesise meso-inositols, but the ultimate goal was to produce chiral inositol phosphates for biological evaluation from the same precursor, and it was easier to use the same common chiral intermediate. The chiral derivatives were also used to prepare the only two chiral inositols d- and l-chiro-inositol (7 and 8, respectively).
Benzoquinone (60) was brominated and the two carbonyls of the quinone were reduced to the alcohols then acetylated to give diacetate 61 that was resolved in the presence of porcine pancreatic lipase (PPL) suspended in phosphate buffer to provide the fully deacetylated product 62 and the chiral diacetate 63 (Scheme 7).
Scheme 7.
Reaction conditions: (a) Br2, CHCl3, 0°C, (98%); (b) NaBH4, Et2O, –20°C to rt (88%); (c) pyridine, acetic anhydride, overnight, (68%); (d) PPL phosphate buffer (pH 7), 4 days (38% of each).
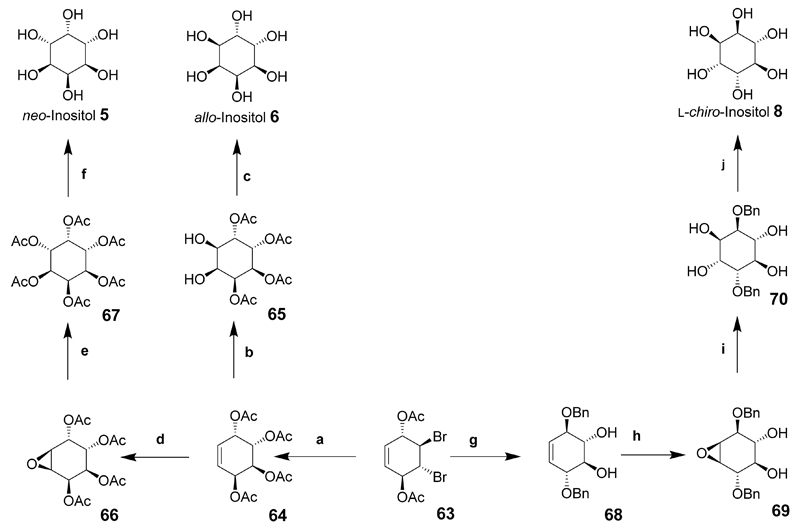
Compound 63 was central to the synthesis of further inositol derivatives including neo-inositol, d- and l-chiro-inositol and allo-inositol. For the synthesis of allo-inositol (6), compound 63 was heated with sodium acetate in acetic acid for 10 days to give conduritol-E derivative 64 then dihydroxylated to give 65, and catalytic deacetylation using sodium methoxide led to allo-inositol (6) on a multigram scale (Scheme 8).
Scheme 8.
Reaction conditions (a) NaOAc, AcOH (95%), 10 days, 125°C; Ac2O, CH2Cl2, DMAP; (b) RuCl3, NaIO4, MeCN; (c) NaOMe, MeOH; (d) (CF3CO)2O, H2O2, CH2Cl2, NaHCO3; (e) Ac2O, pyridine; (f) NaOMe, MeOH, then water/NaOH; (g) NaOBn, BnOH/THF; (h) (CF3CO)2O, H2O2, CH2Cl2, Na2CO3; (i) H2SO4, dioxane, H2O; (j) Pd/C, H2, ethanol/water.
neo-Inositol was prepared from conduritol-E tetra-acetate (64) that was epoxidized to give 66 and ring-opening gave 2,3,4,5-tetra-O-acetyl-neo-inositol that was further acetylated to give neo-inositol hexa-acetate (67). Catalytic deacetylation then provided neo-inositol (5) in near quantitative yield (Scheme 8).
l-chiro-Inositol was prepared using intermediate 63 by the formation of epoxides that were ring-opened at the allylic position when reacted with sodium benzylate below 0°C then warmed to room temperature giving the chiral 1,4-di-O-benzyl-conduritol B derivative 68 in good yield. Epoxidation of 68 and ring opening under acidic conditions gave 2,5-di-O-benzyl-l-chiro-inositol (70) in good yield. Hydrogenolysis in the presence of palladium on carbon gave l-chiro-inositol (8) in near quantitative yield (Scheme 8). d-chiro-Inositol was synthesized using the enantiomer of compound 63 and the same chemical transformations, (not shown).
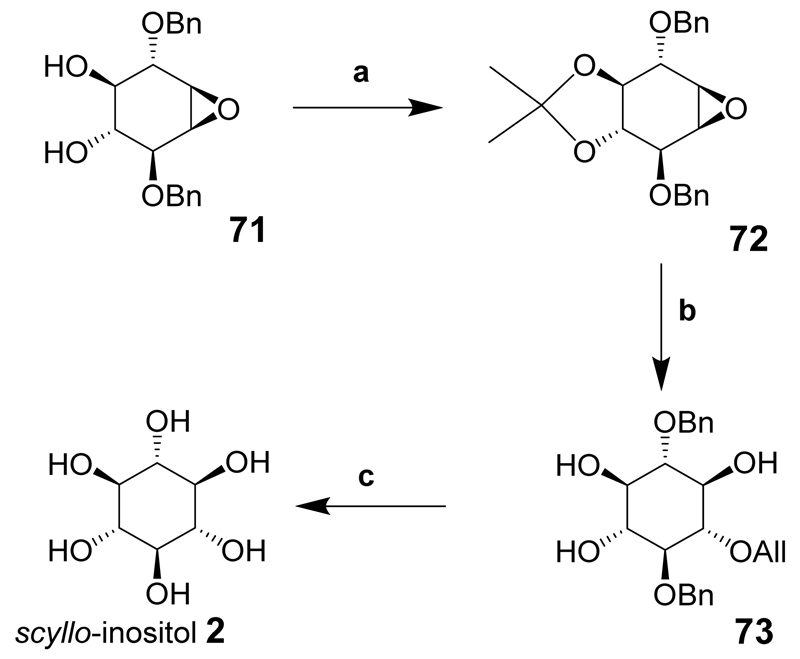
scyllo-Inositol was synthesized when the diol 71 was protected with an isopropylidene acetal to give fully blocked epoxide 72 that was opened with the sodium salt of allyl alcohol to give the scyllo-inositol derivative 73 in good yield. The allyl group was then isomerized and the resulting enol ether cleaved under acidic conditions. Hydrogenolysis of the remaining benzyl groups using palladium on carbon as catalyst provided scyllo-inositol (2) (Scheme 9).
Scheme 9.
Reaction conditions: (a) 2,2-dimethoxypropane, acetone, PPTS, (b) 1. NaOAll, 90°C, 2. HCl; (c) 1. Pd/C, MeOH; 2. HCl, 3. Pd/C, H2.
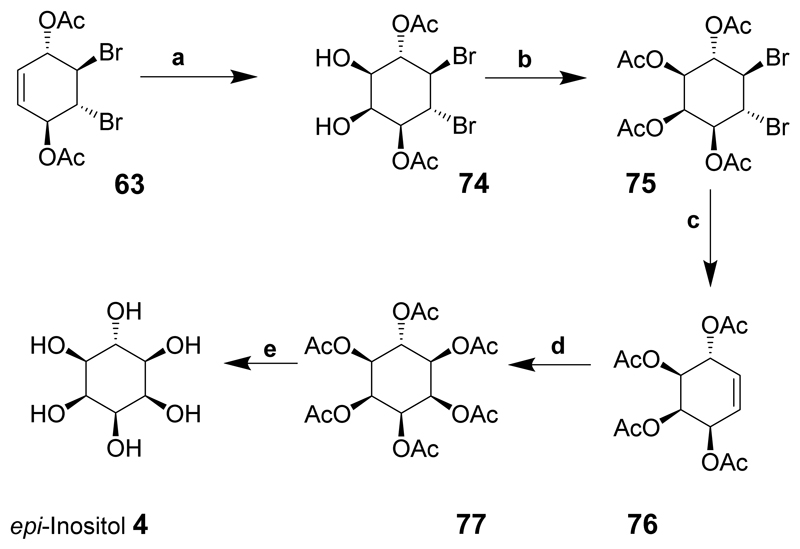
epi-Inositol was derived from the important intermediate 63 which was cis-dihydroxylated in the presence of ruthenium trichloride and sodium periodate to give diol 74 and acetylated to provide compound 75. The reductive removal of bromine using zinc produced the conduritol-C tetra-acetate derivative 76. cis-Dihydroxylation and further acetylation gave 77 and treatment with sodium methoxide followed by sodium hydroxide solution and neutralisation led to complete deacetylation to give epi-inositol (4) in near quantitative yield (Scheme 10).
Scheme 10.
Reaction conditions: (a) RuCl3, NaIO4, acetonitrile; (b) Ac2O, pyridine; (c) Zn, Et2O, AcOH; (d) 1. RuCl3, NaIO4, acetonitrile; 2 Ac2O, pyridine; (e) NaOMe, MeOH.
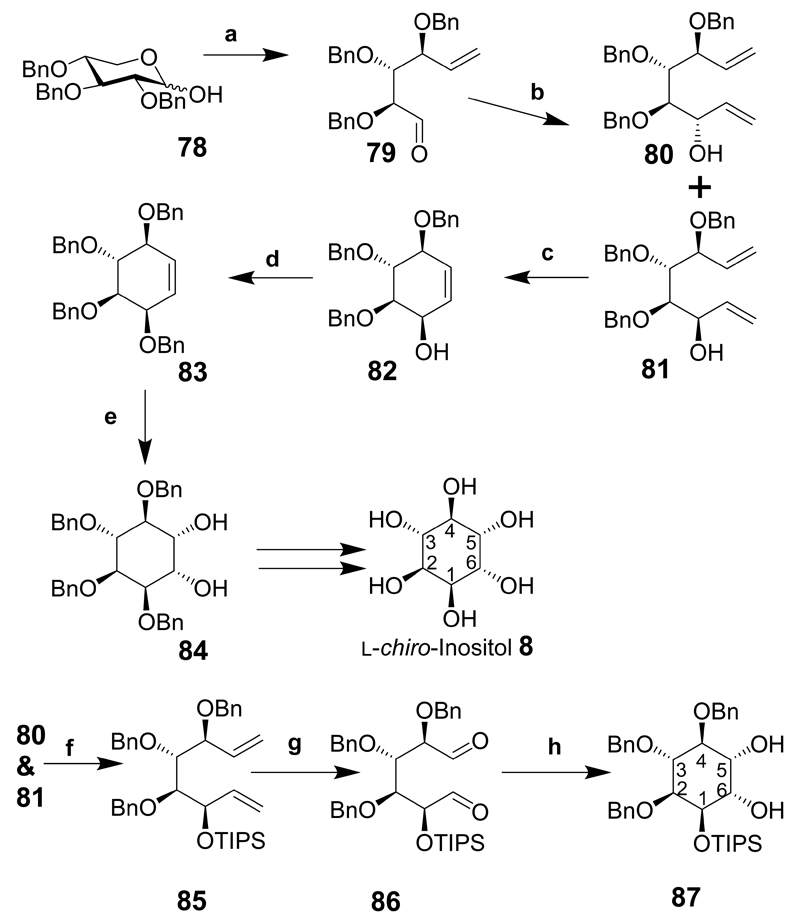
l-chiro-Inositol and d-myo-inositol derivatives were prepared from 2,3,4-tri-O-benzyl-d-xylose (78 in Scheme 11) that was transformed into myo- and l-chiro-inositol intermediates.[22–24] Compound 78 was transformed into 79 via a Wittig reaction followed by oxidation of the primary alcohol to an aldehyde.[25] In the presence of vinyl magnesium bromide the anti-alcohol is mainly formed (80 and 81, ratio 1:8) to give two inseparable dienes. Ring closure of the mixture of compounds (80 and 81) using Grubbs’ catalyst provided conduritols B (not shown) and F (82 major product). Benzylation of 82 provided 83 and dihydroxylation gave 1,2,3,4-tetra-O-benzyl-l-chiro-inositol 84.[23] Alternatively, when the dienes (80 and 81) are silylated the required compound (85) can be separated from the mixture by chromatography. Subsequent ozonolysis gave the dialdehyde (86) that was used immediately and subjected to a pinacol-coupling reaction in the presence of samarium iodide to provide l-chiro-inositol derivative, 87.[24] If 87 is subjected to desilylation and hydrogenolysis l-chiro-inositol should be produced in good yield. Both enantiomers of 78 are available and the l-xylose derivative should provide a route to d-chiro-inositol intermediates, making it suitable for the synthesis of several inositol compounds.
Scheme 11.
Reaction conditions: (a) CH2=PPh3, THF, 45°C, 10 h; then COCl2, Me2SO, CH2Cl2, –78°C, 20 min, Et3N, –78°C to rt; (b) vinyl magnesium bromide, MgBr2·OEt2, –78°C, CH2Cl2, 3 h; (c) (CyP)2RuCl2(CHPh), 10 mol %, CH2Cl2, 15 min, 99%; (d) BnBr, DMF, NaH, 94%; (e) OsO4, NMO, Me2C=O/H2O, 93%; (f) TIPS-Cl, DMF, AgNO3, Separate compounds, (yield not given for this step, but 54% over 3 preceding steps); (g) O3, CH2Cl2, pyridine, then Me2S, (h) SmI2, tert-BuOH, THF, –78°C, 3 h, then 20°C, O/N.
2.1. Summary
A number of general synthetic methods are described above for the synthesis of several or all of the inositols. One procedure [19] describes earlier work where all the compounds in the crude mixture were isolated and used.[26] These compounds include three main benzoylated isopropylidene and di-O-isopropylidene protected compounds that were used to provide an inexpensive route to racemic conduritol B, C and F-derivatives, making it a good method to synthesise all the non-chiral inositols. The benzoyl groups were changed to benzyl groups due to insoluble benzoylated intermediates. From an economic standpoint the synthesis made use of the organic-soluble benzoylated derivatives, which usually go to waste solvent. The highly insoluble 3,6-di-O-benzoyl-1,2:4,5-di-O-isopropylidene-myo-inositol derivative was a precursor to conduritol C. cis-Inositol cannot be obtained from conduritol derivatives and needed a separate starting material to be transformed into this compound. This is an economical method to synthesise some of the meso-inositols from the benzoylated isopropylidene intermediates once the derivatives are isolated from the crude mixture.
All nine inositol isomers can be synthesised in relatively few steps using the Ferrier II carbocyclization of a pyranoside enol acetate derived from glucose, galactose and mannose.[20] The synthesis of cis-inositol requires six extra chemical transformations after the carbocyclisation. This method is the most complete for synthesising any inositol and uses palladium (II) chloride for ring closure, making the reaction less toxic than the original mercury (II) chloride catalyst. However, seven of the nine inositols are achiral, and the synthesis uses chiral material to produce achiral inositols. The overall purpose of the synthesis is to produce chiral inositol phosphate derivatives that do not need resolving via diastereoisomeric derivatives. If temporary protecting groups (for example p-methoxybenzyl) can be used to provide intermediates that give a specific protection pattern, and that are then removed without affecting the remaining benzyl groups, the remaining hydroxyl groups could be phosphorylated and global deprotection will lead to the desired phosphorylated inositol derivative. On balance, the synthesis of inositols from carbohydrate precursors appears to be the most complete general method to give all the inositols and is worth considering if chemists and biologists require some of the rare inositols.
Another general method for the synthesis of inositols has been described.[21] The important intermediate was the enantiomerically pure 63 that was used to prepare all the inositol derivatives apart from cis- and muco-inositols. p-Benzoquinone is inexpensive and the only starting material needed to prepare seven of the nine inositols in multigram quantities making this a diverse synthesis from one starting material. The intermediates were resolved using the racemic derivative of 63 (via enzymatic resolution) providing a pathway to give both d- and l-enantiomers of other inositol derivatives. However, some of the reaction conditions, such as the conversion of 63 to 64, were rather sluggish taking up to 10 days to complete.The time course could potentially be shortened using a different salt-form such as cesium acetate. The overall purpose of the synthesis was to prepare chiral inositol phosphates of myo- and epi- configuration from simple starting materials as well as the synthesis of chiro-, neo-, scyllo-, and allo-inositol hexakisphosphates in reasonable quantities required for biological investigation. Furthermore, the synthesis of each of the inositols has been achieved in fewer than ten steps (including resolution) using p-benzoquinone as the starting material.
2,3,4-Tri-O-benzyl-d-xylose is the starting material used to make d-myo-inositol and l-chiro-inositol derivatives, but the synthetic approach used could be applicable to the synthesis of further inositol derivatives.[23, 24] It is a noteworthy synthesis since the diene derived from xylose intermediate 81 can be ring-closed using a Grubbs’ catalyst to give 82 and provides the chiral derivative 83 after benzylation. Introduction of hydroxyl groups from the upper face of the cyclohexene ring would provide an epi-inositol derivative. Similarly, a different method of ring closure such as a samarium iodide pinacol-type reaction produces a single l-chiro-inositol derivative (87).[24] If 2,3,5-tri-O-benzyl-l-xylose is used the corresponding d-chiro-inositol derivative could be made giving access to more inositols. Further inositol derivatives could be made using dialdehydes 130 (see section 7.1) and 147 (see section 10.1) to give epi- and allo-inositol derivatives, respectfully. The Ferrier II carbocyclisation provides the best method for preparing all inositol derivatives and those prepared from benzoquinone come a close second since only seven of the nine inositols were synthesised. The most economical route to the meso-inositols is derived from the benzoylated isopropylidene derivatives and the use of Grubbs’ catalyst or a samarium iodide ring closure requires a greater knowledge of chemistry.
3. Biology Overview
As stated in the introduction cis-inositol is not known to occur naturally but the other eight inositols, or derivatives of them, have been observed in nature. myo-Inositol is both widespread and much studied but the others are rarer and relatively little studied. This chapter provides a very brief overview of the biology of the “other” inositols and is unreferenced: for details and references see the chapters on the individual inositol isomers in both this paper and the Supplementary Information (SI_2).
Occurrence. Apart from cis-inositol all the inositol isomers, or derivatives of them, have been found in plants. scyllo-Inositol has been observed, also, in mammals, non-mammalian animals and bacteria. d-chiro-Inositol has been detected in mammals, protozoa and bacteria, and neo-inositol in insects and protozoa. Phosphatidylinositol containing scyllo-inositol has been found in plants and protozoa, but not in mammals. neo-Inositol phosphates have been detected in protozoa. Pinitol, the 3-O-methyl derivative of d-chiro-inositol has been detected in insects.
Metabolism. Not much is known about the metabolism of the inositol isomers. An enzyme in a calf brain extract that converts d-glucose 6-phosphate into d-myo-inositol 3-phosphate has also been shown to convert d-mannose 6-phosphate into l-neo-inositol 1-phosphate. Similarly, mammalian l-myo-inositol-1-phosphate synthase is able to catalyse the conversion of galactose 6-phosphate to muco-inositol 1-phosphate. Epimerases have been shown to convert myo-inositol to scyllo-, d-chiro- and neo-inositols. scyllo- And d-chiro-inositols enter mammalian cells through both active and passive transport processes. The hexakisphosphates of both scyllo- and d-chiro-inositols are degraded by soil bacteria.
Medicine. Both scyllo- and d-chiro-inositols have potential roles in medicine. scyllo-Inositol has been found to interact with the amyloid-β peptide: it helps prevent the formation of insoluble amyloid fibres that are a feature of Alzheimer’s disease, thereby alleviating memory deficits, decreasing disease symptoms and improving cognitive function. Proton magnetic resonance spectroscopy has been used to detect scyllo-inositol in the brain and in cancers. d-chiro-Inositol can act as an insulin mimetic by restoring insulin sensitivity and reducing hyperglycaemia. It also aids recovery of normal ovulation in those suffering from polycystic ovary syndrome.
4. scyllo-Inositol
4.1. scyllo-Inositol Synthesis
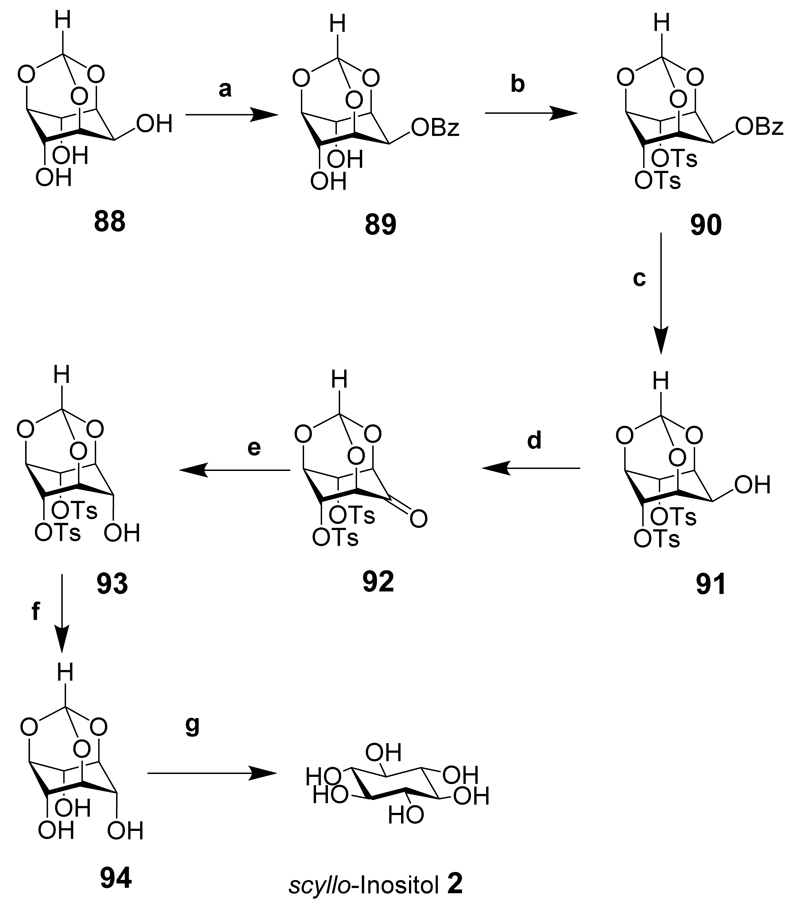
scyllo-Inositol (2) has been isolated from a mixture of inositol isomers formed during the hydrogenation of benzenehexol under high pressure and using Raney nickel as a catalyst.[27] The synthesis of scyllo-inositol from myo-inositol via the orthoformate has been described (Scheme 12).[28] This synthesis illustrated that a large amount of scyllo-inositol could be produced from inexpensive starting materials. myo-Inositol orthoformate (88) was prepared without any chromatographic purification then selectively benzoylated at the 2-hydroxyl to give 89. Tosylation of the 4- and 6-hydroxyl groups gave 90 and selective debenzoylation gave the 2-hydroxy derivative 91. Swern oxidation gave the ketone 92 that was reduced with sodium borohydride to provide the scyllo-inositol derivative 93 in excellent yield. Further deprotection of the tosyl groups under basic conditions led to 94 then hydrolysis under acidic conditions gave scyllo-inositol (2).
Scheme 12.
Reaction conditions: (a) NaH, BzCl, DMF, rt; (b) Tosyl chloride, Pyr. 80-100°C; (c) iso-butylamine, MeOH, reflux; (d) (COCl)2, DMSO, CH2Cl2, –78°C, then Et3N, rt; (e) NaBH4, MeOH-THF, rt; (f) NaOMe, MeOH, reflux; (g) TFA-water (4:1).
4.2. Overview of scyllo-Inositol Derivatives
A range of fluorinated, methylated and deoxy-scyllo-inositols have been prepared and evaluated for their ability to inhibit amyloid-β aggregation.[29] The synthesis and purification of all twelve possible regioisomers of scyllo-inositol bis-, tris- and tetrakis-phosphates in meso or racemic forms has been described.[30] scyllo-Inositol was generated from myo-inositol by stereoinversion of the vicinal cis-diol under Mitsunobu conditions. The phosphorylated products were obtained from scyllo-inositol benzoate intermediates. The same research group went on to synthesize and purify all three enantiomeric pairs of scyllo-inositol phosphates (scyllo-inositol 1,2-bisphosphate, scyllo-inositol 1,2,4-trisphosphate and scyllo-inositol 1,2,3,4-tetrakisphosphate) from enzymatically resolved conduritol B derivatives.[31] The syntheses of myo-inositol 1,3,4,5,6-pentakisphosphate and its C2 epimer scyllo-inositol pentakisphosphate starting from myo-inositol orthoformate have been described.[32]
4.3. Biology and Medicine
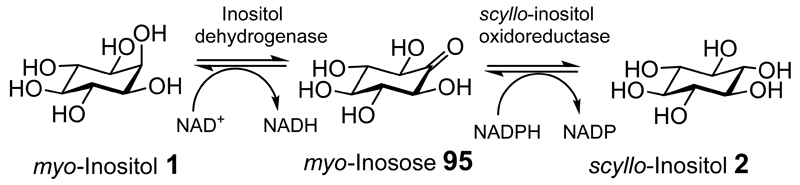
The non-mammalian biology and the role of scyllo-inositol in the environment are discussed in the Supporting Information (SI_2). The detection of scyllo-inositol in mammals was first reported in the 1950s when it was found in urine.[33] The finding of scyllo-inositol in whole rat homogenates, and in rat tissues, was accompanied by evidence that it can be generated from myo-inositol via a myo-inosose-2 (95) intermediate (Figure 2).[34] After oral ingestion by women of reproductive age myo-inositol rapidly entered the bloodstream with a small amount being converted to scyllo-inositol.[35]
Figure 2. The conversion of myo-inositol to scyllo-inositol via myo-inosose.
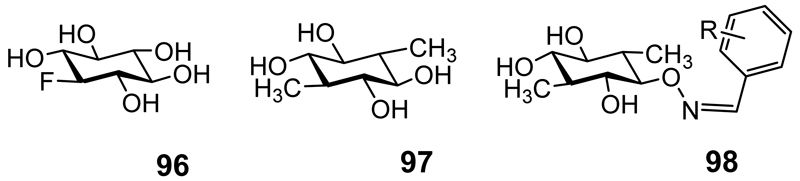
A scyllo-inositol-containing sialyloligosaccharide has been detected in human urine.[36] scyllo-Inositol is not incorporated into phospholipids in mammals.[37] The analogue 1-deoxy-1-fluoro-scyllo-inositol (96 Figure 3) inhibits the incorporation of myo-inositol into phosphatidylinositols.[38] scyllo-Inositol inhibits the incorporation of myo-inositol into lipid-soluble phosphoinositides and water-soluble inositol phosphates in the developing rat conceptus (causing dysmorphogenesis) and also impairs phosphoinositide hydrolysis.[39] scyllo-Inositol is a more potent inhibitor of mycobacterial phosphatidylinositol synthase than of the mammalian equivalents.[40]
Figure 3.
1-deoxy-1-fluoro-scyllo-inositol (96); 1,4-dideoxy-1,4-dimethyl-scyllo-inositol (97); Oxime derivatives of scyllo-inositol (98). R = H or up to 3 hydroxyl substituents on the ring.
The transport of scyllo-inositol into cells in rat kidney slices is an active process that is inhibited by myo-inositol suggesting that both are imported into cells by the same transporter.[41] The uptake of myo-inositol by L1210 leukaemia cells is only partially inhibited by scyllo-inositol, suggesting that myo-inositol may be taken up using two different routes only one of which is inhibited by scyllo-inositol.[42] This is supported by the finding that in rats the transport of myo-inositol through the blood-brain barrier occurs by both simple diffusion and via a specific, saturable transport system that can also transport scyllo-inositol but not other inositol isomers.[43] scyllo-Inositol can also be transported, in competition with myo-inositol, into bovine cardiac cells through a Mg2+-dependent Na+ co-transport process that is both electrogenic and specific: d-chiro-inositol and epi-inositol are weaker competitors of myo-inositol transport.[44] The concentration of myo- and scyllo-inositol in the brain is about 100-fold greater than in the surrounding tissues suggesting that the transport of both into the brain is an active process.[45] Two sodium/myo-inositol transporters, SMIT1 and SMIT2, capable of transporting both myo- and scyllo-inositol into the brain have been identified.[46] The asymmetric distribution of scyllo-inositol (and other metabolites) throughout the vitreous humor implies that the vitreous humor has different roles in different parts of the eye and that it is not just ‘the clear jelly that fills the eyeball’.[47]
At a high (1mM) concentration, scyllo-inositol induces the translocation of the GLUT4 glucose transporter to the plasma membrane in an in vitro model system using rat L6 myotubes.[48] The transport of GLUT4 to the plasma membrane of skeletal muscle cells was observed, also, following the oral administration of scyllo-inositol to mice.[49]
4.4. scyllo-Inositol and Neurological Disorders
Alzheimer’s disease is a common form of dementia for which there is no cure. It is neuropathologically characterized by selective neuronal loss, neurofibrillary tangles and amyloid deposits (insoluble amyloid fibres). The major component of amyloid deposits is amyloid-β (Aβ) a peptide of 39-43 residues, the most amyloidogenic of which has 42 residues (Aβ42). The role of Aβ in the pathogenesis of Alzheimer’s disease has been reviewed and critiqued.[50] The role of scyllo-inositol in Alzheimer’s disease has been the subject of a recent comprehensive review so this aspect of scyllo-inositol science is not reviewed in detail herein.[51] scyllo-Inositol is able to stabilize soluble oligomers of Aβ, the structure of which has been recently described,[52] and prevent the formation of insoluble amyloid fibres.[53] Aβ aggregation is inhibited by scyllo-inositol derivatives with single hydroxyl conservative substitutions (1-deoxy-1-fluoro-scyllo-inositol 96) though not chloro or methoxy single substitutions, but the di-substituted 1,4-dimethyl derivative 97 is also effective.[54] Oxime derivatives such as 98 are also effective at promoting soluble oligomer formation and preventing insoluble fibre formation.[55] These papers mention scyllo-inositol binding to Aβ but the results of in vitro measurements reported in another paper suggests that it does not bind to Aβ42.[56] However, a modelling study suggests that scyllo-inositol can bind to the surface of β-sheet aggregates in a manner that disrupts their lateral stacking into amyloid fibrils.[57] Shorter versions of Aβ containing 25-35 residues transition from isotropic to β-sheet oligomers by the time five molecules are present: scyllo-inositol binds weakly to these oligomers with no adverse effect on the conversion from isotropic to fibrillar conformations.[58] The mechanism of action of scyllo-inositol is unclear.[59] scyllo-Inositol, when dosed in a murine model of Aβ production in combination with R-flurbiprofen (an agent that lowers Aβ production), is not as effective in treating the mice as scyllo-inositol alone.[60]
Another feature of Alzheimer’s disease is the neuronal accumulation of autophagic vacuoles, suggesting that the degradative pathway is dysfunctional in these cells.[61] The accumulation in autophagic vacuoles of Aβ and the enzymes responsible for Aβ production suggests that Aβ may be responsible for the impaired clearance of autophagic vacuoles.[62] In a transgenic mouse model of Alzheimer’s disease treatment with scyllo-inositol caused a decrease in both the size and number of autophagic vacuoles.[63]
In the same mouse model scyllo-inositol, when administered orally, inhibited the aggregation of Aβ and reduced the severity of several of the symptoms of Alzheimer’s disease including impaired cognition, altered synaptic physiology, cerebral Aβ pathology and accelerated mortality.[64] These effects were observed whether the scyllo-inositol was administered before symptoms first appeared or several months after their first appearance. The administration of scyllo-inositol also rescues hippocampal function and restores memory function in animals with pre-existing Aβ oligomers.[65] Some of the symptoms of Alzheimer’s disease can be attributed to the obstruction of blood vessels by amyloid plaques: the administration of scyllo-inositol to model transgenic mice eased both the structural and functional impairment of the cortical microvasculature.[66] The expression levels of the sodium/myo-inositol transporters do not differ between healthy individuals and those with Alzheimer’s disease.[67] A phase 2 clinical trial of scyllo-inositol established a twice daily dose of 250mg as being safe, but the sample size was too small to establish efficacy.[68]
scyllo-Inositol inhibits the aggregation of Aβ in Alzheimer’s disease and also inhibits the neuronal aggregation of α-synuclein, a pathological hallmark of Parkinson’s disease.[69] Likewise, scyllo-inositol reduces the number of neuronal aggregates and inclusions containing polyglutamine-expanded huntingtin protein in Huntington disease and does this by reducing the amount of mutant protein produced.[70] Amyloid deposits can also form in the islets of Langerhans and may contribute to the development of diabetes: scyllo-, myo- and epi-inositols are ineffective in preventing the formation of these amyloid deposits.[71]
In rats seizures induced by pentylenetetrazole were reduced in severity following the administration of scyllo-inositol, suggesting that it may have a role to play in antiepileptic therapy.[72]
4.5. scyllo-Inositol and Diagnostics
Several techniques have been used to measure the amount of scyllo-inositol in both healthy and diseased tissue. Differences in these values may have applications in disease diagnosis. Proton magnetic resonance spectroscopy (1H MRS) has been used to measure the in vivo, ex vivo or in vitro concentrations of many metabolites, particularly in the brain.[73] Many papers have reported the use of 1H MRS to measure the concentration of scyllo-inositol in healthy people (and animals) and those with a variety of diseases. Space constraints preclude herein a detailed discussion of this topic but the papers that have used this technique are listed and briefly summarised in the Supporting Information (SI_2).
Positron emission tomography has been used to establish that [18F]-1-deoxy-1-fluoro-scyllo-inositol does not penetrate the brain of rats or mice following injection into a tail vein but is taken up by human breast cancer xenografts in mice.[74] However, cancer cells injected into the cranium are able to take up [18F]-1-deoxy-1-fluoro-scyllo-inositol to a five-fold greater extent than the surrounding brain tissue.[75] In a human breast cancer model [18F]-1-deoxy-1-fluoro-scyllo-inositol is taken up to a lesser extent than [18F]-2-deoxy-2-fluoro-myo-inositol.[76] These results show that radiotracers may be useful in monitoring inositol uptake in tumours and that inositol transport into cells is not specific for one inositol isomer.
Gas chromatography has been used to establish that the scyllo-inositol content of the sciatic nerve in spontaneous-onset diabetic Chinese hamsters, when compared with healthy equivalents, is reduced at five months age and onwards.[77] scyllo-Inositol levels in the frontal or occipital cortex of unipolar, bipolar and schizophrenic patients, suicide victims and normal controls do not differ.[78] Intracellular scyllo-inositol levels in rat brain extracts have been analysed by micellar electrokinetic chromatography.[79]
4.6. scyllo-Inositol Phosphates
scyllo-Inositol is not incorporated into phospholipids in mammals[37] but scyllo-inositol-containing phosphatidylinositol has been detected in non-mammalian species – see the Supporting Information (SI_2) for details.
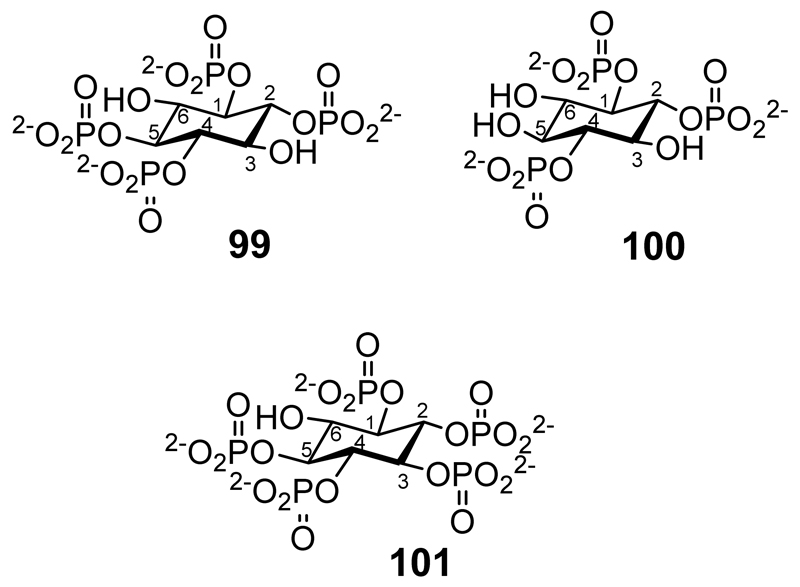
In studies of structure-activity relationships chemically synthesized scyllo-inositol phosphates have been investigated for their ability to compete with myo-inositol phosphates for binding to various receptors and enzymes (Figure 4). dl-scyllo-Inositol 1,2,4-trisphosphate and scyllo-inositol 1,2,4,5-tetrakisphosphate (99) were full agonists at the Ca2+ mobilising inositol 1,4,5-trisphosphate receptor of SH-SY5Y cells, and displaced myo-inositol 1,4,5-trisphosphate from its receptor in bovine adrenal cells.[80] The tetrakisphosphate was also readily metabolised by enzymes with 3-kinase and 5-phosphatase activities. l-scyllo-Inositol 1,2,4-trisphosphate (100) was evaluated for binding to rat type 1, 2 and 3 inositol trisphosphate receptors: it has binding affinities similar to those of Ins(1,4,5)P3 in all three receptor sub-types.[81] scyllo-Inositol 1,2,3,4,5-pentakisphosphate (101) is hydrolyzed by multiple inositol polyphosphate phosphatase but is not dephosphorylated by PTEN or phosphorylated at the 6-position (the equivalent of the myo-inositol 2-position) by pentakisphosphate 2-kinases.[32]
Figure 4.
scyllo-Inositol-1,2,4,5-tetrakisphosphate. (99), l-scyllo-Inositol-1,2,4-trisphosphate (100), scyllo-Inositol-1,2,3,4,5-pentakisphosphate (101).
5. d-chiro-Inositol
5.1. Chemical Synthesis
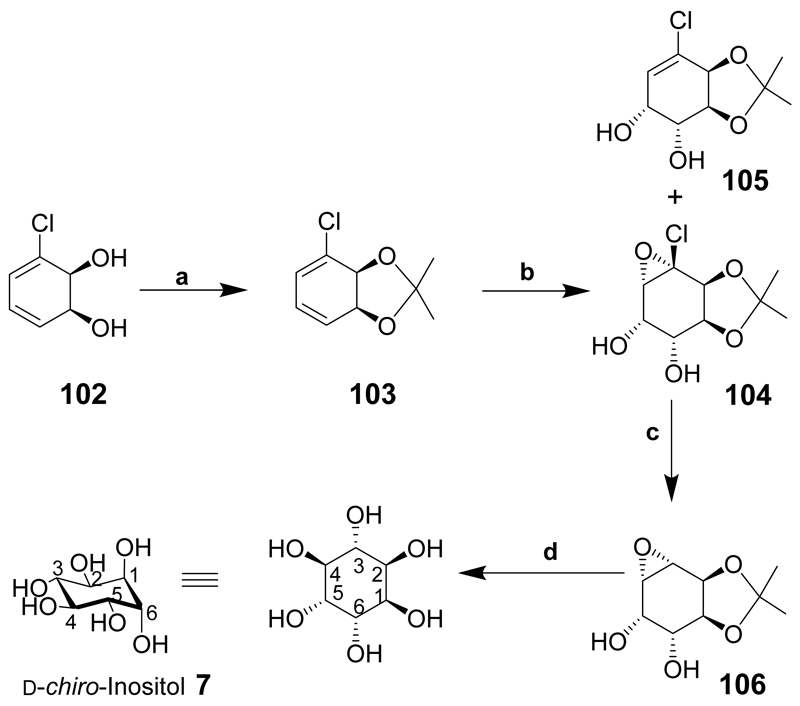
d-chiro-Inositol (7) has been synthesized from a chiral chloro-diol 102 produced by dihydroxylation of chlorobenzene in the presence of Pseudomonas putidia strain39/D (Scheme 13).[82] Since the introduction of the diol was stereoselective, it is a suitable method for the preparation of chiral derivatives such as d-chiro-inositol. Diol 102 as its acetonide 103 was treated with KMnO4 to give an unexpected derivative 104 together with alkene 105 in a reasonable yield. Compound 104 was dehalogenated in the presence of AIBN and tris(trimethylsilyl)silane to give 106 and after a number of different reaction conditions were attempted, the opening of epoxide 106 at reflux temperature in water with catalytic sodium benzoate provided d-chiro-inositol (7).
Scheme 13.
Reaction conditions: (a) 2,2-Dimethoxypropane, PTSA; (b) KMnO4, MgSO4, aqueous acetone, 8:1, ratio of compound 104 to compound 105, 60%; (c) AIBN, tris(trimethylsilyl)silane, toluene, 42%; (d) H2O, sodium benzoate, 77% yield, >95% purity.
5.2. Overview of d-chiro-Inositol Derivatives
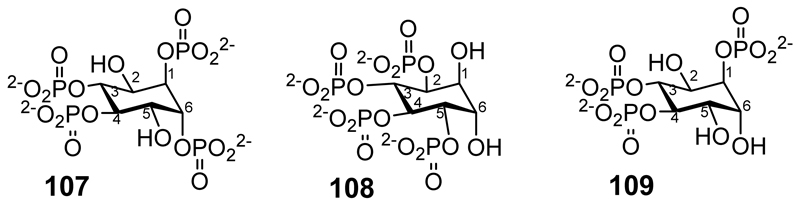
d-chiro-Inositol-1,3,4,6-tetrakisphosphate (107 in Figure 5) has been synthesized from d-pinitol (the 3-O-methyl derivative of d-chiro-inositol) via the 2,5-O-benzyl protected intermediate with phosphorylation of the unprotected hydroxyls followed by deprotection.[83] Also, starting from d-pinitol and proceeding via the 1,6-O-benzyl protected intermediate, the synthesis of d-chiro-inositol 2,3,4,5-tetrakisphosphate (108 Figure 5) has been described using the intermediate d-1,6-di-O-benzyl-chiro-inositol,[84] and d-chiro-inositol-1,3,4-trisphosphate (109) has been synthesized from d-1,2,5-tri-O-benzoyl-3,4-di-O-benzyl-chiro-inositol.[85]
Figure 5.
d-chiro-inositol-1,3,4,6-tetrakisphosphate (107); d-chiro-inositol-2,3,4,5-tetrakisphosphate (108); d-chiro-inositol-1,3,4-trisphosphate (109).
The syntheses of fagopyritols A1 and B1 (galactopyranosyl derivatives of d-chiro-inositol), the biological role of which are discussed in the Supporting Information (SI_2), have been described.[86] Starting from the appropriate penta-O-benzyl-d-chiro-inositol all six isomeric d-galactosaminopyranosyl-d-chiro-inositols have been prepared.[87]
5.3. Biology and Medicine
After myo-inositol, and along with scyllo-inositol, d-chiro-inositol (and its 3-O-methyl derivative, pinitol) is the most studied of the inositols. Pinitol is considered separately below. The non-mammalian biology of d-chiro-inositol and pinitol is discussed in the Supporting Information (SI_2).
The uptake of myo-inositol by a Mg2+-dependent, Na+-inositol co-transport process in cardiac sarcolemmal cells is inhibited by d-chiro-inositol, but to a lesser extent than by scyllo-inositol.[44] In HepG2 liver cells there is a stereospecific myo-inositol/d-chiro-inositol transporter: l-chiro-inositol is not transported.[88] The myo-inositol transporter SMIT2, but not SMIT1, is able to transport d-chiro-inositol into cells:[89] the rat protein does so with high affinity.[90]
In rats d-chiro-inositol is absorbed from the diet, but is neither synthesized endogenously nor produced from myo-inositol.[91] This contradicts earlier work which found that myo-inositol is converted to d-chiro-inositol in a range of rat tissues with little or no conversion in the opposite direction.[92] The first committed step in myo-inositol catabolism is the ring opening catalyzed by myo-inositol oxygenase. This enzyme, isolated from pig kidneys, also catabolizes d-chiro-inositol though at a slower rate than myo-inositol.[93]
Mice genetically engineered to develop folate-resistant neural tube defects in utero can be more effectively treated with d-chiro-inositol than with myo-inositol.[94] d-chiro-Inositol is able to prevent and reverse endothelial dysfunction in rat and rabbit blood vessels.[95]
Some bone-related diseases are caused by excessive bone resorption by osteoclasts that are multinucleated giant cells formed by cell-cell fusion. d-chiro-Inositol has been shown to inhibit cell-cell fusion and the expression of several osteoclastogenic genes by down-regulating nuclear factor of activated T cells c1.[96]
The insecticide DDT, when fed to rats, causes increases in liver weight and hepatic lipids. The co-administration with DDT of 1l-chiro- or 1d-chiro-inositol promotes this effect.[97]
5.4. d-chiro-Inositol, Diabetes, Pregnancy and Polycystic Ovary Syndrome
d-chiro-Inositol is a component, along with galactosamine, of an uncharacterized modulator of insulin function: it stimulates pyruvate dehydrogenase phosphatase [98] and allosterically activates protein phosphatase 2C.[99] A three day fast causes a 20% drop in the amount of d-chiro-inositol in muscle that may contribute to the insulin resistance that occurs after short-term starvation.[100] The fruit from Cucurbita ficifolia, a squash, is used in Asia as an antihyperglycaemic agent. The effectiveness of this treatment is due to the high d-chiro-inositol content.[101] An extract of C. ficifolia, and synthetic d-chiro-inositol, both reduced oxidative stress as measured by changes in the ratio of oxidized to reduced glutathione in murine adipocytes: d-chiro-inositol, but not the plant extract, demonstrated insulin mimetic action.[102] Pumpkin seeds contain several molecules, including d-chiro-inositol, reported to have anti-glycemic effects and the seeds help maintain glycemic control.[103] Insulin prevents damage caused by the synaptic accumulation of amyloid β oligomers and this effect is enhanced by the presence of d-chiro-inositol.[104] d-chiro-Inositol-phosphoglycans move from the foetus to the placenta during pregnancy but diabetic women have lower concentrations of these compounds in the placenta.[105] Insulin resistance is a prominent feature of preeclampsia and d-chiro-inositol levels are increased in this condition and may contribute to insulin resistance.[106]
The urinary excretion of d-chiro-inositol has been measured at 2.1 μmol/day in nondiabetics, but increases six-fold in non-insulin-dependent diabetics and another six-fold in insulin-dependent diabetics.[107] In a rat model of type 1 diabetes and a mouse model of type 2 diabetes the urinary excretion of d-chiro-inositol was much greater than in healthy rats and mice.[108] These studies contradict earlier work showing a lower urinary excretion (and lower muscle content) of d-chiro-inositol in non-insulin-dependent diabetics than in non-diabetics.[109] The transport into cells of d-chiro-inositol by SMIT2 is up-regulated by insulin so the increased urinary excretion of d-chiro-inositol in diabetics may be due to the lack of activity of SMIT2 consequent upon a shortage of insulin.[110] In older non-diabetic adults resistive (strength) training does not influence the urinary excretion of inositols.[111] However, in these older non-diabetic adults higher d-chiro-inositol excretion is linked to a lower activation of skeletal muscle insulin receptor signalling.[112] Low urinary clearance of d-chiro-inositol in men with a wide range of insulin sensitivity is closely related to hyperinsulinemia.[113] Compared with the kidneys from non-diabetic rats those from diabetic rats had a four-fold greater excretion of d-chiro-inositol under normoglycemic conditions with higher excretion under hyperglycemic conditions.[114] Increases in myo-inositol excretion also occurred but were not as great, this despite increased renal expression of both SMIT1 and SMIT2.
Rats suffering from streptozotocin-induced diabetes and diabetic mice have reduced plasma glucose levels when d-chiro-inositol, or a buckwheat concentrate containing high concentrations of d-chiro-inositol, is administered,[115] though the role of d-chiro-inositol in the buckwheat concentrate in the biological response of hepatomas has been questioned.[116] Similarly, insulin-resistant hyperinsulinemic Rhesus monkeys had lower plasma glucose concentrations (and slightly lower insulin concentrations) after being fed a meal with d-chiro-inositol than a meal without d-chiro-inositol.[117] Male Wistar rats suffering from insulin resistance induced by recombinant human growth hormone were treated with d-chiro-inositol which offset the peripheral insulin resistance but not the hepatic insulin resistance.[118] Glucosamine induces peripheral and hepatic insulin resistance in rats, but pre-treatment with d-chiro-inositol prevented the induced peripheral insulin resistance.[119] In mice suffering from streptozotocin-induced diabetes chronic treatment with d-chiro-inositol prevents autonomic and somatic neuropathy.[120] When infused into streptozotocin-induced diabetic rats a d-chiro-inositol-glycan mediator of insulin action normalizes plasma glucose at a dose equivalent to insulin without inducing hypoglycaemia.[121] d-chiro-Inositol has been found to inhibit hepatic glucose output suggesting that this may be the mechanism by which d-chiro-inositol exerts its antidiabetic effect.[122]
The uptake of insulin by rat L6 myotubes is stimulated by 1d-chiro-, 1l-chiro-, epi- and muco-inositol and may be associated with the translocation of the glucose transporter 4 protein to the plasma membrane: allo- and scyllo-inositols are less potent stimulants.[48]
Intrauterine growth restriction in piglets results in low weight at birth. These low weight piglets have significantly higher plasma concentrations of myo-inositol and d-chiro-inositol than their larger littermates. Since both myo-inositol and d-chiro-inositol have been associated with glucose intolerance and insulin resistance in adults it has been suggested that impaired glucose metabolism during foetal development may be a contributing factor to the development of type 2 diabetes in adulthood.[123]
The administration of d-chiro-inositol in combination with myo-inositol, folic acid and manganese to women during the second trimester of pregnancy results, after thirty days, in significantly lower cholesterol, low density lipoprotein, triglyceride and glycemia compared with controls.[124]
Polycystic ovary syndrome (PCOS) afflicts 5% to 10% of women of reproductive age making it the most common gynecological disorder. It is characterised by hyperandrogenism, chronic anovulation (irregular menstrual cycles), and polycystic ovaries. Common complications of PCOS include obesity, glucose intolerance and insulin resistance. The general features of PCOS (definition, prevalence, aetiology, pathophysiology, clinical features, adverse health consequences, assessment and management) have been recently reviewed.[125]
The deficiency of a putative d-chiro-inositol-containing phosphoglycan that mediates insulin action has been tested by the administration of d-chiro-inositol in the hope that this would replenish the mediator stores and improve insulin sensitivity in PCOS sufferers.[126] It was found that the action of insulin was increased with consequent improved ovulatory function, and lowered serum androgen concentrations, blood pressure and plasma triglyceride concentrations. However, dietary supplementation with a combination of myo-inositol and d-chiro-inositol resulted in improved oocyte and embryo quality as well as better pregnancy rates in PCOS sufferers undergoing IVF treatment than in those PCOS sufferers whose diet was supplemented with just d-chiro-inositol.[127] This supports the finding that increasing the d-chiro-inositol dosage progressively worsens oocyte quality and the ovarian response [128] and that myo-inositol is better able to improve oocyte quality in intracytoplasmic sperm injection cycles than d-chiro-inositol.[129] It has been hypothesized that the activity of the epimerase that converts myo-inositol to d-chiro-inositol is enhanced in the ovaries of PCOS sufferers and that the consequent deficiency of myo-inositol is responsible for the poor oocyte quality.[130] PCOS sufferers taking myo-inositol and d-chiro-inositol in a physiological ratio have an improved metabolic (i.e. lipid) profile thus reducing the risk of cardiovascular disease.[131] Both myo-inositol and d-chiro-inositol are effective in improving ovarian function and metabolism in PCOS sufferers but myo-inositol has a greater effect on the metabolic profile while d-chiro-inositol is better able to reduce hyperandrogenism.[132] The treatment of 48 patients affected by PCOS and menstrual irregularities with d-chiro-inositol and folic acid resulted in statistically significant improvements in several measures of ovarian function and metabolic function.[133] In PCOS sufferers metformin increases the insulin-stimulated release of the putative d-chiro-inositol-containing phosphoglycan that mediates insulin action:[134] there is a selective impairment in PCOS sufferers between insulin action and the release of the mediator.[135] PCOS has been associated with a degree of oxidative stress: the administration of d-chiro-inositol to PCOS sufferers prevents the oxidation of protein thiol groups in the follicular fluid.[136] In obese hyperinsulinemic PCOS sufferers the administration of d-chiro-inositol improves insulin sensitivity and hormonal parameters.[137]
In the previous paragraph there are a couple of references to a ‘putative d-chiro-inositol-containing phosphoglycan’. The structure of this compound is currently unknown: we have been unable to find any evidence that it even contains a phosphate moiety, the presence of which is implied by it being called a phosphoglycan. The fact that the assay for this compound is based on its ability to activate pyruvate dehydrogenase phosphatase suggests that it may be the modulator of insulin function mentioned above that contains d-chiro-inositol and galactosamine.[98]
PCOS sufferers have higher d-chiro-inositol urinary clearance rates than non-sufferers: urinary clearance is inversely correlated with insulin sensitivity and is a good independent predictor of insulin resistance and compensatory hyperinsulinemia.[138] The number of PCOS sufferers reporting irregular menstrual cycles decreases with increasing duration of d-chiro-inositol treatment.[139]
The use of d-chiro-inositol (and other insulin-sensitizing agents) in PCOS has been the subject of several reviews.[140]
5.5. d-chiro-Inositol Phosphates
Glycosylphosphatidylinositols (GPIs) containing d-chiro-inositol have been reported to exist in electric rays (Torpedo species),[141] bovine liver[142] and Entamoeba histolytica trophozoites.[143] Also, d-chiro-inositol-containing phosphoglycan derivatives that are mediators of insulin action have been reported to be derived from d-chiro-inositol-containing GPI-anchors.[144] However, these reports may reflect the method of isolating the GPI and d-chiro-inositol: the isomerization of myo-inositol in GPIs to d-chiro-inositol occurs upon acidic hydrolysis of GPI-anchored proteins.[145] Recent reviews of GPIs state that they contain myo-inositol and make no mention of d-chiro-inositol.[146] Chemically synthesized GPIs containing d-chiro-inositol are cleaved by GPI-specific phospholipase D but not by phosphatidylinositol-specific phospholipase C.[147]
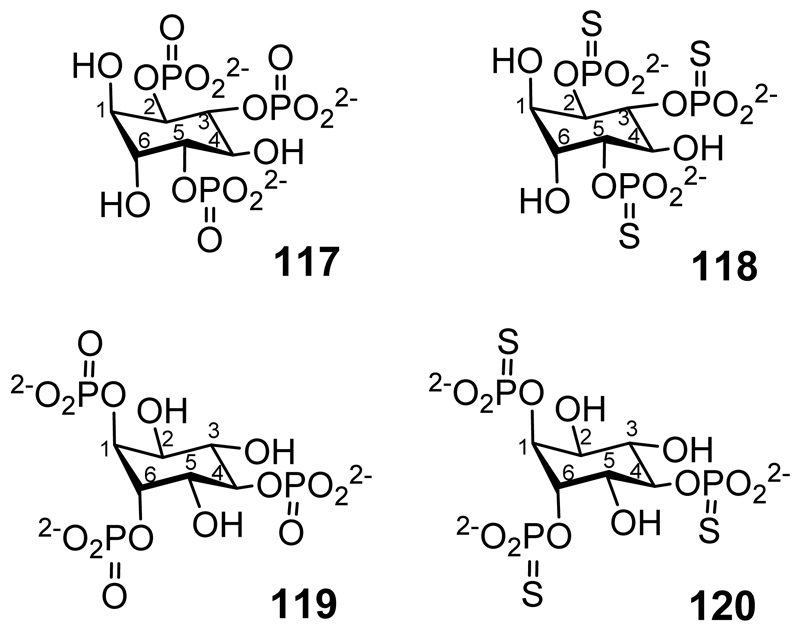
d-chiro-Inositol 1,3,4,6-tetrakisphosphate (107) is a full agonist of the inositol trisphosphate receptor in two cell lines, but the enantiomer is inactive.[83] With an IC50 of 1.5μM d-chiro-inositol 2,3,4,5-tetrakisphosphate (108) is a potent inhibitor of Ins(3,4,5,6)P4 1-kinase/Ins(1,3,4)P3 5/6 kinase but its enantiomer is more than 20-fold less active.[84] The release of calcium from saponin-permeabilized rat basophilic leukaemia cells is inhibited by d-chiro-inositol 1,3,4-trisphosphate (109) with EC50 = 4.2μM while the enantiomer has EC50 = 120μM.[85]
5.6. Pinitol
Pinitol is the 3-O-methyl derivative of d-chiro-inositol. The insulin-like effects of d-chiro-inositol described above are mimicked by pinitol in streptozotocin-induced diabetic mice, causing decreases in hyperglycaemia and plasma glucose concentrations.[148] Another study with streptozotocin-induced diabetic rats found that after treatment with pinitol the levels of blood glucose, total cholesterol, triglycerides, free fatty acids and both low–density lipoprotein and very low-density lipoprotein cholesterol were all significantly reduced, but that high density lipoprotein cholesterol levels increased.[149]
The oral administration of pinitol to obese human subjects with diet-treated type 2 diabetes or glucose intolerance resulted in no change to glucose production, insulin-mediated glucose disposal, or the rate of appearance in the plasma of free fatty acids or glycerol, but did increase the amount of pinitol in the plasma.[150] However, another study has reported that postprandial blood glucose is reduced in patients with type 2 diabetes when given pinitol 60 minutes prior to a meal.[151] Patients with type 2 diabetes that was poorly controlled by hypoglycaemic drugs were treated with pinitol at a dosage of 20mg kg-1 day-1 for twelve weeks after which they had decreased fasting and postprandial glucose levels but unchanged lipid profiles and adipocytokine levels.[152] A 6g dose of pinitol, when coadministered with glucose, reduced serum glucose and insulin at 45 and 60 minutes compared with controls.[153] In non-diabetic humans the administration of pinitol one hour prior to an oral glucose tolerance test did not alter glucose or insulin levels, nor did it alter the activation of the skeletal muscle insulin receptor.[154]
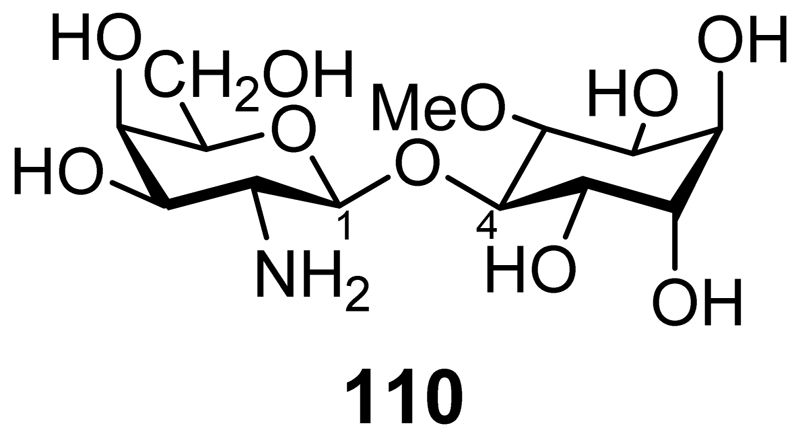
A pinitol galactoside (pinitol β-1,4-galatosamine, INS-2, Figure 6, 110) isolated from beef liver has insulin mimetic properties as shown by its ability to decrease elevated blood glucose in streptozotocin diabetic rats and the stimulation of glucose incorporation into glycogen in hepatoma cells in the presence of insulin.[155] In MIN6 β cells INS-2 stimulates insulin secretion and in isolated mouse islets it potentiates glucose stimulated insulin secretion.[156] It does this through a mechanism that involves the stimulation of the protein phosphatase 2C mediated inhibition of ATP sensitive potassium channels.
Figure 6.
INS-2 Pinitol β-1,4-galatosamine
Pinitol slightly inhibits the formation of foam cells (lipid-laden macrophages) and significantly reduces the release of tumor necrosis factor α, monocyte chemoattractant protein-1, interleukin-1β and interleukin-8.[157] It also suppresses inflammation- and carcinogen-induced activation of NF-κB leading to the reduced expression of a number of genes involved in proliferation, apoptosis, invasion and angiogenesis.[158] Pinitol reduces osteoclastogenesis by inhibiting the receptor activator of NF-κB ligand (RANKL).[159] By reducing the cell surface expression of αvβ3 integrin and inhibiting focal adhesion kinase phosphorylation, c-Src kinase activity and NF-κB activation pinitol inhibits prostate cancer metastasis.[160] In rats pinitol has a protective effect against chemically-induced liver damage.[161]
6. l-chiro-Inositol
6.1. Chemical Synthesis
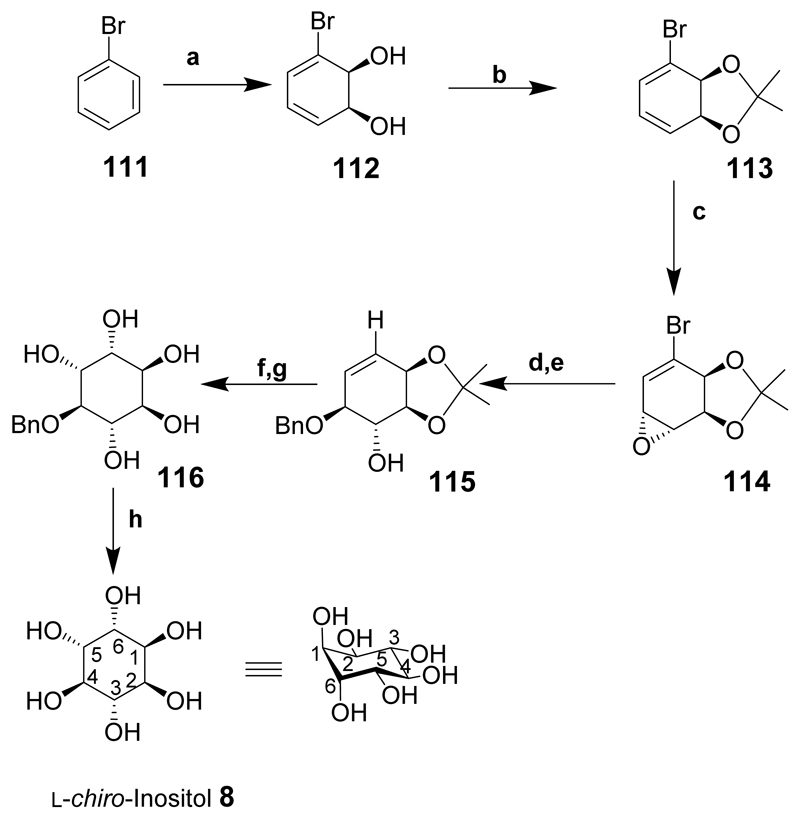
The synthesis of l-chiro-inositol (8) by the microbial oxidation of bromobenzene has been described (Scheme 14).[162] Bromobenzene (111) was oxidised in the presence of toluene dioxygenase from E. coli to give diol 112. The enantioselective dihydroxylation and subsequent easy steps make this route to chiral l-chiro-inositol (8) particularly attractive. Protection of diol 112 as an acetonide 113 and epoxidation provided the required intermediate 114. The epoxide ring was opened with benzyl alcohol in the presence of a Lewis acid to give compound 115. cis-Dihydroxylation and acid hydrolysis of the acetonide provided 4-O-benzyl-l-chiro-inositol (116) and deprotection (over a palladium catalyst) gave l-chiro-inositol (8).
Scheme 14.
Reaction conditions: (a) Toluene dioxygenase; (b) 2,2-dimethoxypropane, TsOH, rt; (c) MCPBA, CH2Cl2, 96%; (d) PhCH2OH, BF3:Et2O, –10°C, 85%; (e) n-Bu3SnH, AIBN, THF, 78%; (f) OsO4, acetone, H2O, NMO, 75%; (g) HCl, EtOH, 79%; (h) 10% Pd/C, H2, H2O, 81%, (30% overall yield from 114).
6.2. Biology
Lithium potentiates the epileptogenic effects of cholinergic agents, e.g. pilocarpine. This effect of lithium can be reversed by the co-administration of myo-inositol but not l-chiro-inositol.[163]
At a concentration of 0.1mM l-chiro-inositol is as efficient as 100nM insulin in promoting GLUT4-dependent glucose uptake by rat L6 myotubes.[48] Between nondiabetics and non-insulin-dependent diabetics there was little difference in the urinary excretion of l-chiro-inositol, but excretion increased just over three-fold, to 0.51 μmol/day, in insulin-dependent diabetics.[107]
The non-mammalian biology of l-chiro-inositol is discussed in the Supporting Information (SI_2).
6.3. l-chiro-Inositol Phosphates
l-chiro-Inositol-2,3,5-trisphosphate (117 in Figure 7)[164] and l-chiro-inositol-2,3,5-trisphosphorothioate (118) have been synthesized from quebrachitol, the 2-O-methyl derivative of l-chiro-inositol.[165] l-chiro-Inositol-1,4,6-trisphosphate (119) and the trisphosphorothioate (120) have also been synthesized, as have l-chiro-inositol-1,3,4,6-tetrakisphosphate (121 in Figure 8),[83] 1l-chiro-inositol-2,3,4,5-tetrakisphosphate (122)[84] and l-chiro-inositol-1,3,4-trisphosphate (123).[85]
Figure 7.
l-chiro-inositol-2,3,5-trisphosphate (117) l-chiro-inositol-2,3,5-trisphosphorothioate (118) l-chiro-inositol-1,4,6-trisphosphate (119) l-chiro-inositol-1,4,6-trisphosphorothioate (120).
Figure 8.
l-chiro-inositol-1,3,4,6-tetrakisphosphate (121); l-chiro-inositol-2,3,4,5-tetrakisphosphate (122); l-chiro-inositol-1,3,4-trisphosphate (123).
l-chiro-Inositol-2,3,5-trisphosphate (117)[164] binds to the inositol trisphosphate receptor, inhibits inositol 1,4,5-trisphosphate 5-phosphatase and inositol 1,4,5-trisphosphate 3-kinase, and is a full agonist at the Ca2+ mobilising receptor in SH-SY5Y cells.[166] l-chiro-Inositol-2,3,5-trisphosphorothioate (118) is a partial agonist for the release of intracellular calcium from saponin-permeabilised platelets,[167] and is a potent inhibitor of inositol 1,4,5-trisphosphate 5-phosphatase and inositol 1,4,5-trisphosphate 3-kinase.[168] Both compounds non-competitively inhibit phosphatidylinositol 3-kinase.[169]
The inhibition of inositol (1,4,5)P3/(1,3,4,5)P4-polyphosphate 5-phosphatase by l-chiro-inositol-1,4,6-trisphosphate (119 in Figure 7) and its trisphosphorothioate analogue (120) has been described.[170] These same two compounds also have a significant effect on the kinetics of a small chloride channel in the sarcoplasmic reticulum from skeletal muscle.[171]
l-chiro-Inositol-1,3,4,6-tetrakisphosphate (121, in Figure 8),[83] l-chiro-inositol-2,3,4,5-tetrakisphosphate (122)[84] and l-chiro-inositol-1,3,4-trisphosphate (123),[85] when compared with their d-chiro-inositol equivalents, are less potent inhibitors/agonists of the inositol trisphosphate receptor, Ins(3,4,5,6)P4 1-kinase/Ins(1,3,4)P3 5/6 kinase, and the release of calcium from saponin-permeabilized rat basophilic leukaemia (RBL) cells, respectively. The synthesis of l-chiro-Ins(1,2,3,4,5,6)P6 and its subsequent degradation to other l-chiro-inositol phosphates by phytases has been described.[172]
6.4. Quebrachitol
The 2-O-methyl derivative of l-chiro-inositol is known as quebrachitol. As discussed in SI_2 it is naturally occurring in some plants and apicomplexan parasites. Quebrachitol has a mildly sweet taste but is not a substitute for glucose in reducing diabetic hypoglycaemia: in doses large enough to taste as sweet as cane sugar it causes colic and diarrhoea.[173] Quebrachitol inhibits platelet activating factor receptor binding to rabbit platelets (IC50 = 42.2μM).[174] Quebrachitol has a role to play in protecting cells due to it having antioxidant and free radical scavenging properties.[175] Acute gastric lesions can be caused by ethanol and indomethacin. Quebrachitol protects against the effects of these drugs though there is an inverse dose response: the smaller the dosage of quebrachitol the larger the protective effect,[176] although one explanation may be that the larger doses are themselves causing gastrointestinal upset. The protective effect of quebrachitol is due to mechanisms that involve nitric oxide release and/or the activation of K+-ATP channels.
7. epi-Inositol
7.1. Chemical Synthesis
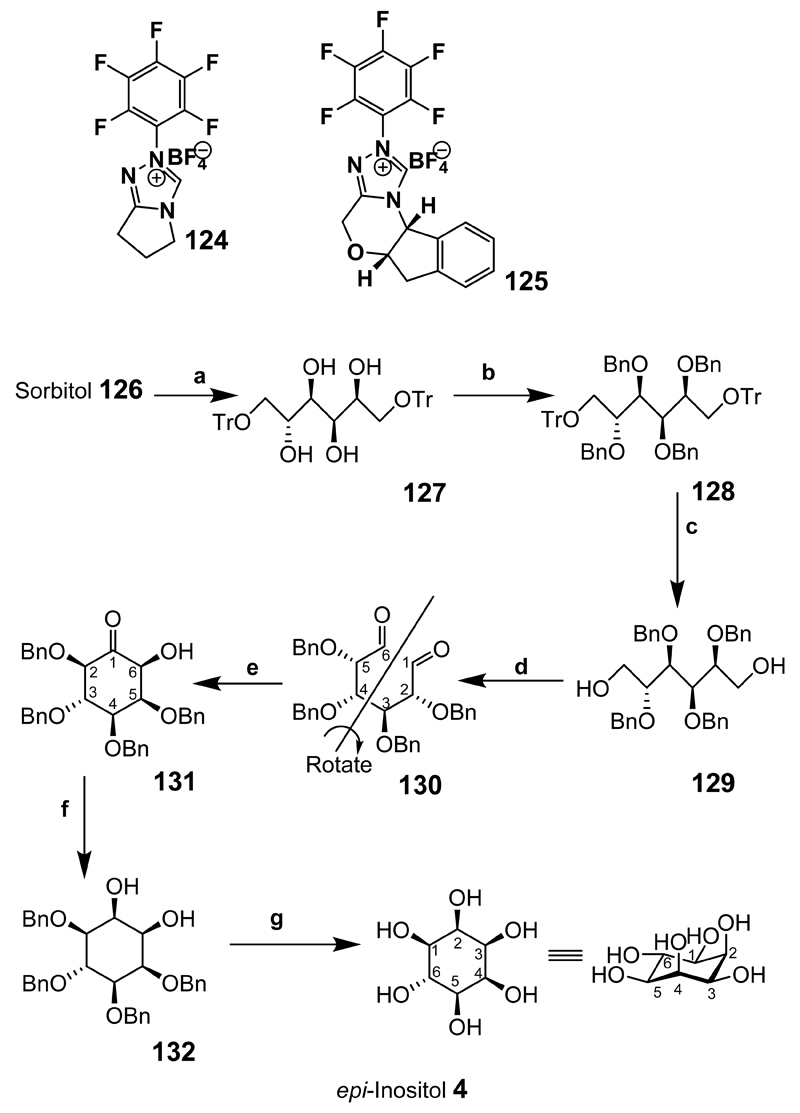
Recently, allo- and epi-inositols have been synthesized using carbohydrate dialdehyde intermediates (Scheme 15).[177] In the presence of N-heterocyclic carbene catalysts such as 124 and 125 the dialdehyde derivatives gave cyclic acyloin products. The cyclisation of the dialdehyde intermediates synthesised from readily available starting materials is therefore worth highlighting, because the limits of the chemical cyclisation using these catalysts have not been widely explored. epi-Inositol was synthesized from sorbitol (126) by a protection/deprotection strategy of tritylation 127, benzylation 128 and detritylation 129. Oxidation of the diol under Swern oxidative conditions gave the dialdehyde 130. Since dialdehyde 130 is not symmetrical, treatment with catalysts 124 or 125 led to the formation of two products in which compound 131 provided the desired precursor to epi-inositol (4). The other isomer (not shown) was problematic to purify and most likely decomposed on silica. Compound 131 was then reduced with sodium borohydride to give 132 and the benzyl groups removed by hydrogenolysis to give epi-inositol (4) in 78% yield from ketone 131.
Scheme 15.
Reaction conditions: epi-Inositol Synthesis: (a) TrCl, pyr. Reflux, 1.5 h, 93%; (b) BnBr, NaH, Bu4NI, THF, 25°C, 6 h, reflux 19 h, 85%; (c) CH2Cl2-MeOH (2:1), TFA, 18 h, 79%; (d) (i) (COCl)2, DMSO, CH2Cl2, –78°C, 25 min; (ii) Et3N, –78°C to 25°C, 1.5 h, 88%; (e) Catalyst 124 or 125, Et3N, 14%; (f) EtOH, NaBH4, 1 h, reflux; (g) PdCl2, EtOH, H2, 78% for steps f and g.
7.2. Biology and Medicine
Lithium modulates the in vivo response to serotonergic and cholinergic stimulants probably through a common phosphoinositide signal transduction pathway.[178] Seizures induced by serotonergic (2,5-dimethoxy-4-iodoprenyl-2-aminopropane) and cholinergic (pilocarpine) receptor agonists in rats with either acutely or chronically high levels of lithium were largely blocked by epi-inositol but with less effect than myo-inositol.[178] These results for pilocarpine have been confirmed with the finding that epi-inositol is less potent but as effective as myo-inositol in reversing the effects of lithium; scyllo- and l-chiro-inositol were less effective or inactive.[179] In a dose-dependent fashion pilocarpine is toxic to retinal ganglion cells with the toxicity being potentiated by lithium but blocked by epi- and myo-inositols.[180] The lithium-induced suppression of neuronal firing in the hypothalamic suprachiasmatic nucleus can be reversed by myo-inositol but not epi-inositol.[181] myo-Inositol, but not epi-inositol, reverses the effect of lithium by increasing the rate of spreading of neuronal growth cones.[182] Lithium is a classical inhibitor of the phosphoinositide pathway and is teratogenic. The teratogenicity can be overcome by equimolar myo-inositol but not epi-inositol.[183]
In yeast epi-inositol has no effect on inositol monophosphatase but does inhibit the expression of the INO1 gene (encoding inositol-1-phosphate synthase) resulting in lower conversion of glucose-6-phosphate to d-myo-inositol 3-phosphate than might otherwise be the case.[184] epi-Inositol thus reverses the lithium-induced increase in INO1 expression. The daily intraperitoneal injection of epi-inositol into rats reduced anxiety levels compared to controls and was more effective than myo-inositol.[185]
In cardiac sarcolemmal vesicles the transport of myo-inositol into cells is through a Mg2+-dependent, Na+-inositol co-transport process that is inhibited by epi-inositol.[44] epi- And scyllo-inositols have been shown to be more potent inhibitors of mycobacterial phosphatidylinositol synthase than of mammalian analogues.[40] At a concentration of 0.1mM epi-inositol is as efficient as 100nM insulin in promoting GLUT4-dependent glucose uptake by rat L6 myotubes.[48] epi-Inositol has an initial impact on the aggregation of amyloid-β associated with Alzheimer’s disease but is unable to maintain that impact over time.[64]
The non-mammalian biology of epi-inositol is discussed in the Supporting Information (SI_2).
7.3. epi-Inositol Phosphates
The synthesis of epi-inositol 1,4,5-trisphosphate has been described: the compound shows poor affinity for the inositol 1,4,5-trisphosphate receptor.[186]
8. neo-Inositol
8.1. Chemical Synthesis
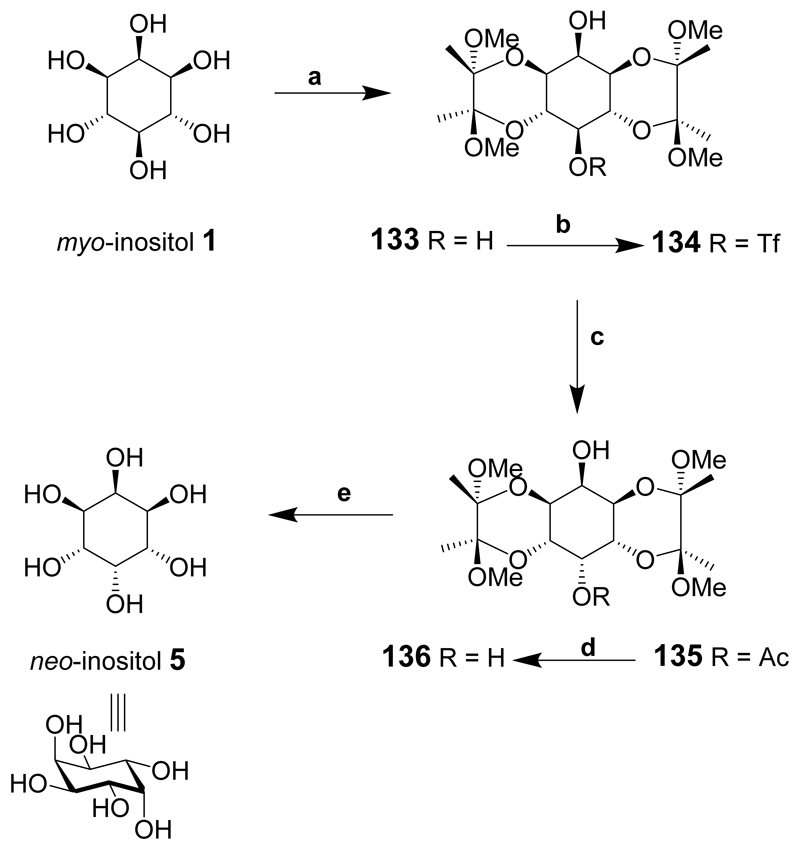
A multi-gram good-yielding simple synthesis of neo-inositol from myo-inositol in five steps has been reported (Scheme 16).[187] The synthesis relies on the selective protection of two pairs of trans-1,2-diols at the 1- and 6-positions and the 3- and 4-positions of myo-inositol using butane-2,3-diacetal (BDA) protecting groups. The hydroxyl groups at positions 2- and 5- of diol 133 are axial and equatorial respectively. The difference in reactivity between C-2-OH and C-5-OH enabled selective triflation of the 5-hydroxyl in good yield at low temperature to give 134. Clean inversion of configuration at C-5 was only accomplished in dimethylacetamide (DMA) and caesium acetate at elevated temperature to give 135 since mixed products were observed using dimethylformamide (DMF). Deacylation of 135 was accomplished using catalytic sodium methoxide and the resulting diol 136 precipitated from the solution. The BDA protecting groups were removed using refluxing aqueous acetic acid and the product recrystallized from boiling water to provide pure neo-inositol (5) in a good overall yield from compound 1.
Scheme 16.
Reagents and conditions: (a) Butanedione, MeOH, CH(OMe)3, (±)-10-camphorsulfonic acid, reflux; (b) Trifluoromethanesulphonic anhydride, pyridine, CH2Cl2, –78°C to rt; (c) 50:1 dimethylacetamide-water, 50°C; (d) NaOMe, MeOH, reflux; (e) 4:1 AcOH-water, reflux.
neo-Inositol is the least water-soluble of the inositol isomers due to its unusually stable crystal structure.[188]
8.2. Biology
An enzyme in a calf brain extract that converts d-glucose 6-phosphate into d-myo-inositol 3-phosphate is also capable, though at a much slower rate, of converting d-mannose 6-phosphate into l-neo-inositol 1-phosphate.[189] neo-Inositol has also been found to be formed by the action of an epimerase isolated from bovine brain.[190]
The non-mammalian biology of neo-inositol is discussed in the Supporting Information (SI_2).
8.3. neo-Inositol Phosphates
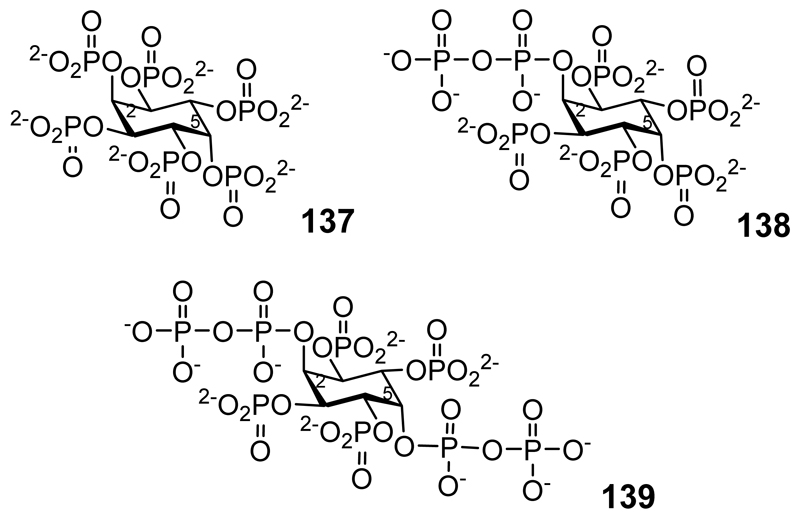
Although originally identified as myo-inositol phosphates [191] a subsequent reassessment of the data revealed that trophozoites in Entamoeba histolytica contain neo-inositol hexakisphosphate (137 in Figure 9), 2-diphospho-neo-inositol 1,3,4,5,6-pentakisphosphate (138), and 2,5-bisdiphospho-neo-inositol 1,3,4,6-tetrakisphosphate (139).[192] The synthesis of neo-Ins(1,2,3,4,5,6)P6 (137) and subsequent degradation to other neo-inositol phosphates by phytases has been described.[172]
Figure 9.
neo-inositol hexakisphosphate (137) 2-diphospho-neo-inositol 1,3,4,5,6-pentakisphosphate (138), 2,5-bisdiphospho-neo-inositol 1,3,4,6-tetrakisphosphate (139).
9. muco-Inositol
9.1. Chemical Synthesis
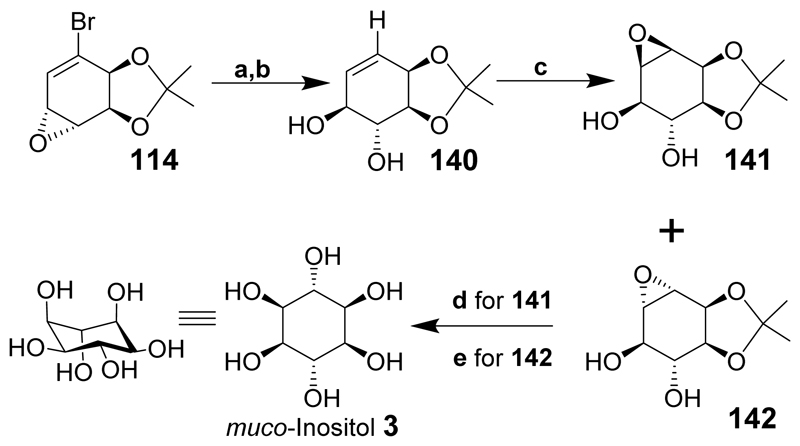
muco-Inositol was first synthesized in the 1930s[193] and the fascinating historical background describing why the publication of the experimental parts of the paper was delayed is also explained.[194] More recently muco-inositol has been chemo-enzymatically synthesized starting from bromobenzene (Scheme 17).[162] Bromobenzene (111) was converted to the bromo-epoxide (114) as described for l-chiro-inositol. Ring-opening of epoxide 114 furnished the required hydroxyl stereochemistry and was accomplished in excellent yield using dilute potassium hydroxide. Radical dehalogenation gave the trans-diol 140 and epoxidation of the double bond gave compounds 141 and (142) in a 1.8:1 ratio and good yield. Different conditions for acid hydrolysis of compounds 141 and 142 using (d) and (e) respectively, afforded muco-inositol (3) in high yield.
Scheme 17.
Reaction conditions: (a) 10% aqueous KOH, H2O, DME, 87%; (b) n-Bu3SnH, AIBN, THF, 90%; (c) MCPBA, CH2Cl2, 71%; (d) 10% aqeous H2SO4, 78%; (e) Amberlyst A-27, H2O, 89%.
1l-chiro-Inositol has been selectively epimerized to muco-inositol derivatives.[195] muco-Inositol oligomers have been synthesized starting from 1-bromo-2,3-dihydroxycyclohexa-4,6-diene.[196] Derivatives of muco-inositol have been used as the starting point for the synthesis of derivatives of epi- and cis-inositols.[197]
9.2. Biology
At a concentration of 0.1mM muco-inositol is as efficient as 100nM insulin in promoting GLUT4-dependent glucose uptake by rat L6 myotubes.[48] l-myo-Inositol-1-phosphate synthase isolated from human fetal brain and adult rat brain is able to catalyse the conversion of galactose 6-phosphate to muco-inositol 1-phosphate.[198] Whether this reaction occurs in vivo is unknown. The non-mammalian biology of muco-inositol is discussed in the Supporting Information (SI_2).
10. allo-Inositol
10.1. Chemistry
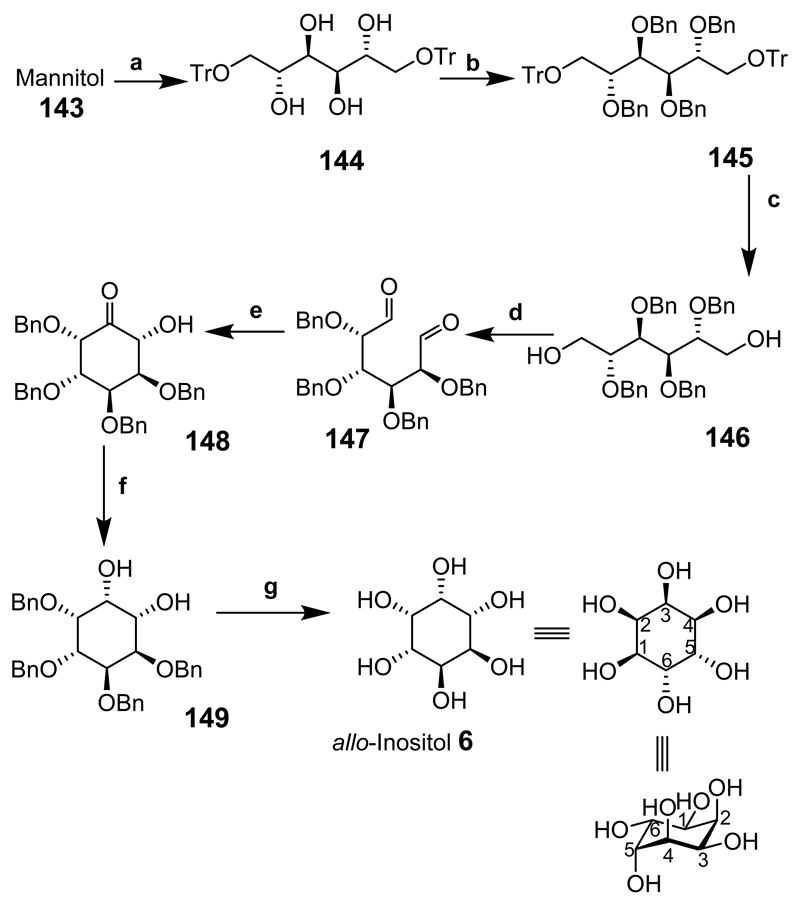
allo-Inositol has been synthesized using carbohydrate dialdehyde intermediates in a similar manner to epi-inositol (Scheme 18).[177] This route is attractive for reasons similar to those discussed for epi-inositol. Mannitol (143) was tritylated at the 1- and 6-positions to provide 144 then benzylation of the remaining hydroxyl groups gave the fully blocked intermediate 145. Acid hydrolysis of the trityl groups gave 146 followed by oxidation of the diol to give dialdehyde 147 showing characteristic symmetry in the 1H NMR. Compound 147 was then treated with catalyst 124 or 125 over a 24 h period in the presence of triethylamine to provide the ring-closed product 148. Hydroxyketone 148 was reduced to give a single product 149 and the benzyl groups were removed by hydrogenolysis (in the presence of a palladium catalyst) in high yield to give allo-inositol (6).
Scheme 18.
Reagents and conditions: allo-Inositol Synthesis: (a) TrCl, pyr. Reflux, 1.5 h, 97%; (b) BnBr, NaH, Bu4NI, THF, 25°C, 6 h, reflux 19 h, 90%; (c) CH2Cl2-MeOH (2:1), TFA, 18 h, 86%; (d) (i) (COCl)2, DMSO, CH2Cl2, –78°C, 25 min; (ii) Et3N, –78°C to 25°C, 1.5 h, 99%; (e) Catalyst 124 or 125 Et3N, 54%; (f) EtOH, NaBH4, 1 h, reflux; (g) PdCl2, EtOH, H2, 81% for 2 steps f and g.
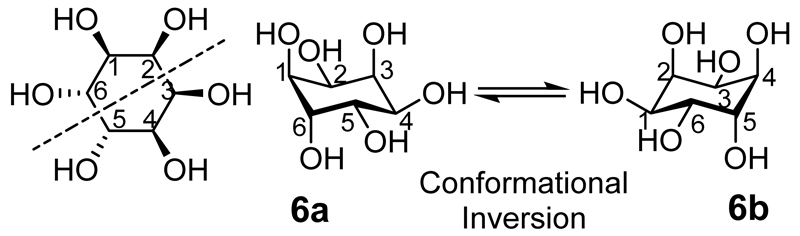
Among the inositols allo-inositol is unique in that, although the Mills projection appears to show a plane of symmetry, when the ring inverts the conformational isomers are also enantiomers (Figure 10):[199] allo-inositol does not have a plane of symmetry. Because interconversion between conformational isomers (6a and 6b) is rapid, allo-inositol exists as an optically inactive racemic mixture despite being chiral. Therefore, it is likely impossible, at least at room temperature, to observe or separate the two enantiomers[200] and optical rotations have also not been formally assigned. It is possible, however, to synthesize allo-inositol derivatives from suitably protected myo-inositol derivatives and characterize protected or partially-protected derivatives of the two enantiomers that are conformationally stable or fixed.[201] Such compounds or their phosphorylated derivatives might possess interesting biological activities.
Figure 10.
The black dashed line indicates an apparent plane of symmetry that does not really exist: the hydroxyl on one side of the line is equatorial while the equivalent hydroxyl on the other side of the line is axial. The lack of any plane of symmetry in allo-inositol means that the conformational isomers are also enantiomers.
10.2. Biology
allo-Inositol has been found to be an inhibitor of amyloid-β aggregation [202] and, at a concentration of 1mM, it is as efficient as 100nM insulin in promoting GLUT4-dependent glucose uptake by rat L6 myotubes but has little effect at 0.1mM.[48] It is not known, for either of these activities, if one particular conformational isomer evokes these effects.
The non-mammalian biology of allo-inositol is discussed in the Supporting Information (SI_2).
11. cis-Inositol
11.1. Chemistry
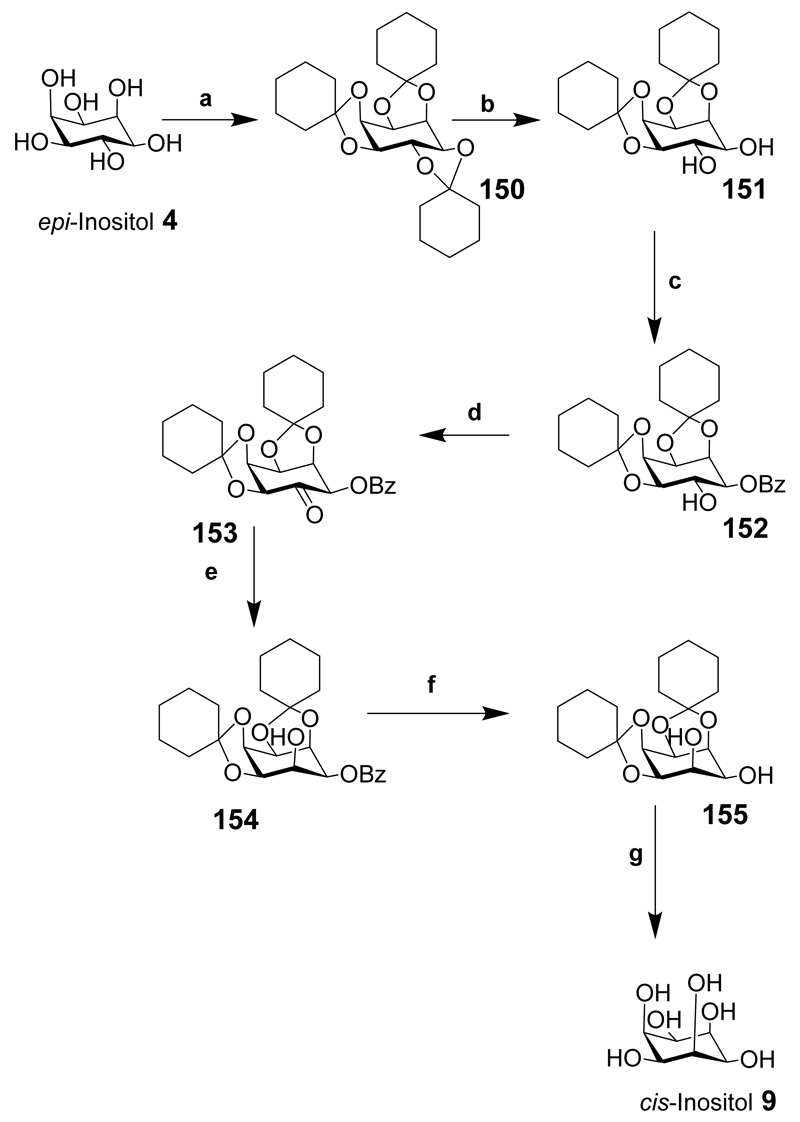
cis-Inositol is available via a one step hydrogenation of tetrahydroxyquinone, but with complex chromatography required.[203] cis-Inositol has also been synthesised from epi-inositol in a seven step process with a 25% yield (Scheme 19).[204] The good yield of the seven transformations from epi-inositol makes it a suitable starting point for the synthesis of this rare inositol. epi-Inositol (4) was fully protected as the tricyclohexylidene derivative (150). Acid catalysed hydrolysis of the 5,6-trans cyclohexylidene derivative (150) exposed the 5,6-diol to give 151 and benzoylation gave a mixture of products; however, only 152 was required. Swern oxidation of the 6-OH of 152 gave 153 but, interestingly, other oxidation procedures failed. The ketone was reduced stereoselectively and in near quantitative yield using sodium borohydride to provide 154, that was debenzoylated in near quantitative yield to give 155, and acidic hydrolysis of the cyclohexylidene protecting groups gave cis-inositol (9). From a historical perspective this synthesis still holds today although some of the yields could be improved using new methods. Others have inverted the orientation of one or two hydroxyl groups of a suitably protected inositol by conversion to a triflate, or oxidised a hydroxyl group to give a ketone then inverted the stereochemistry at the carbon centre, as described in Schemes 3 and 6.[205]
Scheme 19.
Reaction conditions: (a) Cyclohexanone, benzene, reflux, PTSA, 59%; (b) Light petroleum, benzene, PTSA/EtOH 71%; (c) Pyridine, benzoyl chloride, 70-75°C, 5 h, 51%; (d) Benzene, DMSO, Ac2O 17 h, 62%; (e) Chloroform/methanol NaBH4, 2h, 96%; (f) Sodium, dry MeOH, 99%; (g) 80% acetic acid, heat, 76%.
11.2. Biology
As far as we have been able to determine cis-inositol is not known to occur naturally and we have been unable to find any literature describing biological roles for it.
12. myo-Inositol
Although this review is focused primarily upon the inositol isomers other than myo, we felt it of interest to mention a few of the more recent papers in the myo-inositol area, especially where they feature chemistry that might be applied more widely. A number of reviews up to 2010 have covered the preparation of biologically important myo-inositol derivatives.[9, 206] This section briefly reviews what we consider to be the most active areas since 2010 and does not attempt to deal, with two exceptions, with reports of any work on myo-inositol based phospholipids, but we briefly describe the biological activity of other myo-inositol derivatives.
12.1. myo-Inositol 1,4,5,6-Tetrakisphosphate
Much recent interest has centered upon the higher inositol polyphosphates and new developments have revealed that higher myo-inositol phosphates play cellular roles in previously unexplored areas. In one recent highlight of note a link was found with the histone deacetylase (HDAC) enzyme family that catalyses the acetylation and deacetylation of lysine residues in histones which are involved in regulating gene expression. Thus, the assembly of a deacetylase activation domain (DAD)-HDAC3-(SMRT/NCOR2) (nuclear receptor co-repressor 2) complex is regulated by d-myo-inositol 1,4,5,6-tetrakisphosphate (Ins(1,4,5,6)P4) (156 in Figure 11) during complex assembly with Ins(1,4,5,6)P4 binding in a specific deep basically charged cleft.[207] While precise SAR studies on specificity are as yet unavailable, it has since been shown that this is a more general mode of regulating HDACs since the NuRD complex, which contains HDAC1, also requires inositol phosphates for activity.[208] Phosphorylated myo-inositol derivatives containing four to six phosphates have been reviewed.[209]
Figure 11.
12.2. myo-Inositol Pentakisphosphate and Hexakisphosphate Derivatives
The higher phosphorylated myo-inositol derivatives include isomers of myo-inositol pentakisphosphate, for example myo-inositol 1,3,4,5,6-pentakisphosphate (157) where the metal ion co-ordination properties of Ins(1,3,4,5,6)P5 have recently been explored.[210] Ins(1,3,4,5,6)P5 (157) and related derivatives were recently synthesized using a route from the myo-inositol 1,3,5-orthobenzoate leading regioselectively to the precursor 2-O-benzoyl-myo-inositol (158) via a 1,2-bridged 2′-phenyl-1′,3′-dioxolan-2′-ylium ion.[211] Substituted pentakisphosphate derivatives are of interest and 2-O-benzyl-myo-inositol 1,3,4,5,6-pentakisphosphate, 2-O-Bn-Ins(1,3,4,5,6)P5 (159), showed anticancer activity in xenograft studies and also is a nanomolar inhibitor of 3-phosphoinositide-dependent protein kinase 1 (PDK1).[212] 2-O-Bn-Ins(1,3,4,5,6)P5 is more potent than Ins(1,3,4,5,6)P5 and also inhibits the mammalian target of rapamycin (mTOR) in vitro. Compounds such as Ins(1,3,4,5,6)P5 [213] and Ins(1,2,3,4,5,6)P6 [214] were already known to possess anticancer properties. How Ins(1,3,4,5,6)P5 and 2-O-Bn-Ins(1,3,4,5,6)P5 apparently enter cells is a matter of some debate, but visualization of fluorescent derivatives has provided some clues.[215] Thus, Ins(1,3,4,5,6)P5 was modified at the 2-position to provide 2-aminoethyl-myo-inositol 1,3,4,5,6-pentakisphosphate that was then conjugated to a fluorescein derivative to give the fluorescent FAM-Ins(1,3,4,5,6)P5 (160). This ligand enabled direct visualisation of the compound, which was taken up apparently by non-specific endocytosis by H1229 tumor cells, supporting previous data that Ins(1,3,4,5,6)P5 can be taken up by some cell types.[216] Another fluorescent multiply-phosphorylated myo-inositol derivative was synthesized by conjugating 2-aminoethyl-Ins(1,4,5)P3 with fluorescein isothiocyanate to give (FITC)-Ins(1,4,5)P3 (161). This was used to develop a high-throughput fluorescence polarization (FP) assay and to uncover the thermodynamics of Ins(1,4,5)P3 binding to the Ins(1,4,5)P3-binding-core and N-terminal domain.[217] (FITC)-Ins(1,4,5)P3 Is a high-affinity weak partial agonist, possibly due to the large hydrophobic FITC moiety that may block interaction between the Ins(1,4,5)P3 binding domain and the N-terminal domain.
12.3. Synthesis of Diphosphoinositol Phosphate Derivatives
Perhaps the current most topical myo-inositol derived chemical targets are diphosphoinositol phosphates, that have numerous functions including the regulation of insulin sensitivity and weight gain,[218] the control of telomere length,[219] apoptosis[220] and vesicle trafficking.[221] Diphosphoinositol phosphates were identified independently in 1993 by two separate groups[222] and the first synthesis was published shortly thereafter.[223] These compounds are difficult to isolate from cells due to their high negative charge density, tiny quantities and the lack of a UV active chromophore. New and improved methods are necessary to synthesise the pyrophosphate linkage regiospecifically in the presence of other phosphate groups and to address initial approaches to the design and exploitation of the first useful analogues. The biochemistry of these naturally occurring diphosphoinositol phosphates has been the subject of a number of reviews[224] and will not be discussed here.
Ten step syntheses of 1d-diphosphoinositol 2,3,4,5,6-pentakisphosphate (177) (1PP-InsP5) and 3d-diphosphoinositol 1,2,4,5,6-pentakisphosphate (178) (3PP-InsP5) have been reported.[225] The synthesis had a number of new chemical transformations or modifications of existing reactions that had not previously been used in inositol chemistry. myo-Inositol was transformed into the fully blocked orthobenzoate intermediate in two steps from myo-inositol. Partial reduction of the orthoester 162 with DIBAL-H exposed the 5-hydroxyl group that was then p-methoxybenzylated. Careful acidic hydrolysis produced the meso-derivative 2,4,5,6-tetra-O-p-methoxybenzyl myo-inositol (163). These transformations allowed the desymmetrisation of the 1,3-diol with a chiral reagent separating into the required 1d- and 3d- derivatives (Scheme 20). Interestingly, the chirality is derived from the protecting groups on the P(III) reagent 164 used to monophosphitylate the diol. The products are then oxidised to give both 1- and 3-phosphorylated derivatives 165 and 166. Both derivatives were isolated from the mixture, by crystallisation for 166 and chromatography for 165 and in high diastereoselectivity. All the p-methoxybenzyl groups were cleaved under different acidic conditions than previous used, to provide the pentahydroxy- compounds (not shown). Phosphitylation of the remaining five hydroxyl groups using reagent 167 then oxidation gave the myo-inositol hexakisphosphate derivatives 168 and 169.
Scheme 20.
Reaction conditions: (a) DIBAL-H (2.7 eq), CH2Cl2, –78°C; (b) PMB-Cl, NaH, DMF; (c) PTSA (cat), MeOH/H2O, 5 min., reflux; (d) compound 163 (3 equivalents), mix with reagent 164, 5-p-F-Ph-1H-tetrazole, CH3CN, then, 0°C, mCPBA; 1:1 mixture of 165 and 166, separated by chromatography or recrystallization and yield based upon consumption of P(III) reagent (164). (e) TFA (2.5%) in CHCl3; (f) P(III) reagent 167 (10 eq), 4,5-dicyanoimidazole (15 eq), 0°C, CH3CN, then MCPBA (10 eq).
The pyrophosphate motif was prepared by base-induced elimination of one base-labile protecting group of chiral phosphate triester 170 to eliminate cinnamonitrile, exposing a negative charge and masked using a trimethylsilyl (TMS) equivalent derived from N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA). A second elimination of cinnamonitrile then occurred followed by re-masking as above to give 171 (Scheme 21). Intermediates such as 171 can also be generated from the elimination of other protecting groups followed by TMS masking in a similar manner. 171 Can be used for the synthesis of the pyrophosphate motif via other suitable derivatives. Methanolysis of the TMS group of 171 provided the monophosphate as the DBU salt 172 and the phosphate monoester was phosphitylated using reagent 173 to give a P(III)-P(V) intermediate that was oxidised to give compound 174 (general intermediate) and specifically provide crystalline products (175) and (176) from 168 and 169. This is the first time a P(III)-P(V) inositol pyrophosphate derivative has been reported, and the P(III)-P(V) bond is discussed in detail in a publication from the nucleoside field.[226] The benzyl and o-xylylene groups of 175 and 176 were deprotected by hydrogenolysis unveiling the phosphorylated derivatives 1d-diphosphoinositol 2,3,4,5,6-pentakisphosphate (177) (1PP-InsP5) and 3d-diphosphoinositol 1,2,4,5,6-pentakisphosphate (178) (3PP-InsP5) after crystallisation (Scheme 22).
Scheme 21.
Reaction conditions: Pyrophosphate formation- (a) DBU, then BSTFA in CH3CN to give intermediate 171; (b) TFA in Methanol then remove solvent; (c) P(III) reagent 173, 1H-tetrazole/CH3CN, then MCPBA. B = DBU.
Scheme 22.
Reaction conditions: (a) H2, palladium black or PtO2, 80 bar, tert-BuOH/H2O, 4/1 to 1/1, NaHCO3, 3 h, recrystallise product.
Similar methods were used to synthesize 4-PP-InsP5 and 6-PP-InsP5 (Schemes 23 and 24). The meso-silylated orthoester 179 was prepared in two steps from myo-inositol [225] allowing the 4- and 6-hydroxyl groups to be mono-phosphorylated (using the phosphitylating reagent 164) that are then oxidised to the chiral mono-phosphates at the 4- and 6-position, 180 and 181 respectively. The two diastereoisomers were then separated by chromatography and identified using X-ray diffraction. Acidic hydrolysis first removed the silicon protecting group then the orthoformate exposing the remaining five hydroxyl groups that were phosphitylated using reagent 167 and the intermediate was then oxidised to give the fully protected hexakisphosphate 182 and 183. The pyrophosphate linkage was achieved as described in Scheme 21 and the benzyl and o-xylylene groups of compounds 184 and 185 were deprotected and the product subjected to anion exchange chromatography providing the final compounds (4-PP-InsP5 186 and 6-PP-InsP5 187) after seven steps.
Scheme 23.
Reaction conditions: Reagent 164, (1 eq) 179 (3 eq), DCI CH3CN, 0°C, tert-BuOOH, (5.5 M in nonane), 45% for (180), 36% for (181) and resolved by chromatography and re-crystallisation (high purity), yield based upon consumption of reagent 164; (b) PTSA (cat), MeOH/CH2Cl2, recrystallize; (c) reagent 167, DCI, 0°C, CH3CN, 0°C, MCPBA, recrystallise.
Scheme 24.
Reaction conditions: (a) see Scheme 21; (b) H2, palladium black or PtO2, 80 bar, tert-BuOH/H2O, 4/1 to 1/1, NaHCO3, 3 h, recrystallize product.
A different chemical strategy was used to synthesise 5PP-InsP4 (Scheme 25).[227] The suitably protected compound 188 made in one step was selectively benzylated at the 2-hydroxyl position to give 189. Phosphitylation with the P(III) reagent 190 and oxidation gave the fully blocked derivative 191. Careful deprotection of the di-BDA acetal protecting groups provided the tetrol 192 that was phosphitylated with reagent 173 and oxidation of the product gave 193. Two compounds, 5PP-Ins(1,3,4,6)P4 (195) and 2-O-Bn-5PP-Ins(1,3,4,6)P4 (196) were provided from this single meso intermediate 194. Compounds 195 and 196 were made by removing both cyanoethyl groups in the presence of DBU (in a similar method presented in Scheme 21) followed by temporary protection with TMS group (using BSTFA protection strategy) then methanolysis and acidification to provide the phosphate monoester. Phosphitylation of the phosphate monoester using reagent 173 at the 5-position gave a P(V)-P(III) phosphate-phosphite intermediate and oxidation then provided the pyrophosphate intermediate 194 that was deprotected by hydrogenolysis to give 5PP-Ins(1,3,4,6)P4 (195). 2-O-Bn-5PP-Ins(1,3,4,6)P4 (196) Was prepared from crude 194 containing DBU that inhibited the hydrogenolysis of the 2-O-benzyl group. All final compounds were purified by ion exchange chromatography.
Scheme 25.
Reaction conditions: (a) BnBr, NaH, DMF; (b) reagent 190, CH2Cl2, 5-Ph-1H-tetrazole, then MCPBA, –40°C→rt; (c) 90% TFA(aq), 1:1; (d) reagent 173, CH2Cl2, 5-Ph-1H-tetrazole; (e) MCPBA, –40°C→rt; (f) DBU then BSTFA; (g) TFA, MeOH; (h) reagent 173, CH2Cl2, 5-Ph-1H-tetrazole; (i) MCPBA, –40°C→rt; (j) Pd(OH)2, H2, tert-BuOH, H2O (196 was prepared in the presence of DBU to inhibit O-benzyl deprotection).
12.4. The First Synthesis of d-1,5- and d-3,5-Diphosphoinositol 1,2,4,6-Tetrakisphosphate InsP8
The benzylidene derivative (Scheme 26, 197) was prepared in three steps from myo-inositol. The 5-hydroxyl group (shown with axial stereochemistry) was phosphitylated with the bis-fluorenylmethyl phosphoamidite (198) and the intermediate was oxidized. The benzylidene acetal was cleaved under acidic conditions resulting in a meso-1,3-diol 199 that was desymmetrised with the C2-symmetric P(III) reagent 164 then oxidation gave a mixture of 1- and 3- phosphorylated derivatives 200 and 201 that were isolated in high diastereoisomeric purity. Acidic hydrolysis of the p-methoxybenzyl groups gave the corresponding tetraols which were phosphitylated with reagent 167 and oxidized to provide the fully blocked hexakisphosphates 202 and 203 in which the absolute configuration was not established at this stage, but the diastereoisomeric purity of these compounds was high. The bis-fluorenylmethyl and bis-chiral auxiliary phosphate protecting groups were removed under basic conditions and replaced with TMS groups as previously described in Scheme 20. Methanolysis cleaved the TMS groups and monoprotonation of the phosphate monoesters gave suitable phosphitylation precursors to form the bis-diphospho- derivative at the 1- and 5-positions. Thus, phosphitylation of the bis-1,5-phosphate monoesters with reagent 173 and oxidation afforded the 1,5- and 3,5-bis-diphosphates (Scheme 27), 204 and 205. Hydrogenolysis provided the enantiomeric compounds 206 and 207, although configurationally unassigned, albeit containing a 10% impurity of InsP7.[228] The synthesis of compounds 206 and 207 was the second method for the formation of a bis-pyrophosphate. Previously, the synthesis of myo-inositol 2,5-PP-1,3,4,6-tetrakisphosphate had been described.[229] However, there was no indication of the purity of the final compound, critical for establishing the usefulness of the methodology for the synthesis of other inositol pyrophosphate derivatives. Furthermore, myo-inositol 2,5-PP-1,3,4,6-tetrakisphosphate was only used as a standard to analyse other higher phosphorylated molecules and seemed impure using metal dye detection HPLC.
Scheme 26.
Reaction conditions: (a) reagent 198, tert-BuOOH, 1 h, (b) PTSA, CH2Cl2, MeOH, Δ, 10 min; (c) reagent 164, 1H-tetrazole, CH3CN, then MCPBA; (d) 5% TFA, CHCl3, 4 h, recrystallize; (e) reagent 167, DCI, CH3CN, then MCPBA.
Scheme 27.
Reaction conditions: (a) See Scheme 21 for reaction conditions; (b) Pd/C, H2 (180 bar), tertBuOH, H2O, NaHCO3.
In the first practical synthesis of an InsP8 (Schemes 26 and 27), a pair of diphosphorylated myo-inositol enantiomers diphosphoinositol tetrakisphosphates 1,5-PP-Ins(2,3,4,6)P4 (natural enantiomer) and 3,5-PP-Ins(1,2,4,6)P4 (unnatural enantiomer) was synthesised.[228] The absolute configuration was proven by X-ray crystallography of the kinase domain of human diphosphoinositol pentakisphosphate kinase 2 (PPIP5K2) with each enantiomer separately soaked in the protein.
12.5. Synthesis of Diphosphoinositol Analogues
The first diphosphoinositol analogues to be designed replaced the diphosphoinositol linkage at position-5 by a phosphonoacetate group, with the other hydroxyl positions decorated with phosphate monoesters.[230] Two other analogues were synthesized, either benzylated at position-2 or left unmodified, both with a phosphonoacetate at the 5-position. Diol 188 was easily prepared from myo-inositol in large amounts and regioselective acylation at the 5-position gave the protected phosphonoacetate derivative 208 (Scheme 28). The butane diacetal (BDA) groups were then removed under acidic conditions and phosphitylation of the hydroxyl groups with reagent 173, then oxidation of the products gave the fully blocked compound 209. All benzyl groups were removed in one step to give the target compound, 5-PA-InsP5 (210), in excellent yield. Diol 188 was a key starting intermediate for the synthesis of two further analogues. Selective benzylation of the axial 2-hydroxy group and esterification at the 5-hydroxy then gave phosphonoacetate 211. Acidic hydrolysis for a short period of time exposed the remaining hydroxyl groups that were then phosphorylated to provide the fully blocked compound (not shown). Hydrogenolysis for a short period, importantly in the presence of triethylammonium bicarbonate (TEAB), removed the benzyl phosphate protecting groups to give compound 212. Longer hydrogenolysis in the absence of TEAB under pressure removed the benzyl group blocking position-2 to provide compound 213. These compounds have fewer charges and are non-hydrolyzable even though they possess an ester group between the inositol ring and the phosphonate group and were good surrogates for the natural product. Indeed, they were successfully co-crystallized with the kinase PPIP5K2. The binding of 5-PP-InsP5 to the kinase domain of PPIP5K2 is mimicked by 5-PA-InsP5, and analogues were phosphorylated by the enzyme. PA-InsPs are thus promising candidates for further studies into the biology of PP-InsPs[230] and are already finding biological application.[231]
Scheme 28.
Reaction conditions: (a) (BnO)2P(O)CH2CO2H, DCC, CH2Cl2, 73%; (b) TFA, H2O, 5 min, up to 62%; (c) reagent 173, 5-Ph-1H-tetrazole, CH2Cl2, then 3-chloroperoxybenzoic acid, CH2Cl2, up to 93%; (d) H2, Palladium hydroxide/carbon, MeOH, H2O, 50 psi, 92%; (e) BnBr, NaH, DMF, 55%; (f) (BnO)2P(O)CH2CO2H, EDAC, DMAP, CH2Cl2, 95%; (g) H2, Palladium hydroxide/carbon, MeOH, TEAB(aq) 79%; (h) H2, Palladium hydroxide/carbon, MeOH, H2O, 50 psi, 79%.
A second type of diphosphoinositol analogue, a derivative with a methylene bisphosphonate derivative at the 5-position and the remaining five hydroxyl groups substituted with phosphate monoesters, was synthesized from an isopropylidene derivative (214) (Scheme 29).[232]
Scheme 29.
Reaction conditions: (a) Benzyl((bis(benzyloxy)phosphoryl)methyl)-phosphonochloridate, KHMDS, THF, –78°C → rt, O/N; (b) Sodium methoxide, MeOH, rt, O/N, then p-toluenesulfonic acid, H2O, (CH3)2CO, O/N; (c) reagent 167, MeCN, 1H-tetrazole, 0°C→rt, 1.5 days, then 3-chloroperoxybenzoic acid, MeCN, 0°C→rt, 3 h; (d) H2, Palladium black, NaHCO3, t-BuOH/H2O, rt, O/N; (e) NH3(aq) conc, 4 days, then H+-Dowex.
The 5-hydroxyl group of the isopropylidene derivative 214 was phosphonylated to give 215 and the remaining hydroxyl groups were unveiled by debenzoylation and acidic hydrolysis of the two trans-isopropylidene groups then gave 216. Phosphitylation of the pentahydroxy derivative using reagent 167 was followed by oxidation to the pentakisphosphate 217 and deprotection of all benzyl groups by hydrogenolysis gave the desired derivative 218 in good yield. Compound 215 was subjected to acidic hydrolysis to give 219 then subjected to phosphitylation using reagent 167 then oxidation gave 220. Hydrogenolysis removed all the benzyl and o-xylylene groups, and the target compounds 221 and 222 were obtained after deprotection. The synthesis of analogues of 5PP-Ins(1,2,3,4,6)P5 (not shown), such as compound 218, is an important step in elucidating the mechanism of its natural parent pyrophosphate, associated with cellular functions such as repair of damaged DNA.[233] Critically, 218 exhibited only slightly different pKas to 5PP-Ins(1,2,3,4,6)P5 and importantly, was resistant to hydrolysis.[232] 5PP-Ins(1,2,3,4,6)P5 binds the PH-domain of Akt or protein kinase Bα (PKBα) and stabilises the complex preventing its phosphorylation on threonine-308 by 3-phosphoinositide-dependent protein kinase (PDK1), as monitored by a phosphospecific antibody. Potent inhibition of PKB phosphorylation was demonstrated.[232] If the 2-phosphate was removed (221 and 222) there was improved inhibition of PKBα and the presence of the aromatic moiety of 221 increased inhibition significantly. The methylenephosphonate moiety provides a suitable substitute for the parent pyrophosphate derivative.[232] 218 Was also used to compare the relative affinities of InsP6 and 5PP-InsP5 for CK2 (casein kinase II) phosphorylation.[234]
Several analogues possessing a methylene bisphosphonate at the 1d-position with phosphate groups at the 2-, 3-, 5- and 6-positions and a variety of substituents at the 4-position have been reported (Scheme 30).[235] The 1d-butanoate ester of enantiomerically pure 223 (commercially available) was carefully removed unveiling the 1-hydroxy group (224) and phosphonylation gave the methylene bisphosphonate 225.[232] After phosphonylation the butanoate ester at position-4 either remained intact or was removed using base to give 226. The isopropylidene acetals of compounds 225 and 226 were hydrolyzed under acidic conditions to give tetraol 227 and pentaol 228 that were phosphorylated using reagent 167 as described previously to give 229 and 230, respectively. Deprotection by hydrogenolysis gave 231 and 232 and further deprotection of the butanoate ester of 231 with base gave the target methylenebisphosphonate tetrakisphosphate 233.
Scheme 30.
Reagents and conditions: (a) DIPE, MeOH, 14 h 36°C; (b) (bis(benzyloxyphosphoryloxy)methyl) phosphoryl chloride, DBU, 1H-tetrazole, CH2Cl2, 0°C→rt, O/N; (c) DIBAL-H, CH2Cl2, –78°C, 6 min; (d) TFA-MeOH-CH2Cl2, 0°C, 3h; (e) Reagent 167, 5-Ph-1H-tetrazole, CH2Cl2, 0°C→rt, 18 h, then MCPBA oxidation, 78°C→rt, 3h; (f) H2, Palladium black, NaHCO3, H2O-tBuOH, rt, O/N; (g) NH3(aq) conc., rt, 4 days, then Dowex H+ form.
Similarly to 5PCP-InsP5 (218), 1PCP-InsP5 was also evaluated for PKBα inhibition.[235] 233 Was a potent inhibitor of the phosphorylation of PKBα by PDK1 and similarly, 220 and 221 were around three-fold less potent. 218, 221 And 222 were also tested against the PP-InsP metabolising enzyme, human diphosphoinositol polyphosphate phosphohydrolase-1 (hDIPP1).[236] Using Ap5A (diadenosine pentakisphosphate) as a substrate in the presence of hDIPP1, 218 and 5PP-InsP5 showed similar competitive inhibition. However, 232 has a 136-fold lower Ki value but 218 is approximately two-fold lower compared to its natural parent 1PP-InsP5 (167) and in line with the substrate preference for 1PP-InsP5.[237] Removing the 2-phosphate to give 233 provides a further four-fold enhancement of inhibition compared to 232 demonstrating that removal of a negative charge increases inhibition of hDIPP1. 231 Has similar inhibition properties to 232 in the low nanomolar region and may be a useful candidate to produce a lipophilic analogue for in vivo studies, in a similar way as 253.
12.6. Replacement of the 5-Phosphate of Ins(1,4,5)P3 with Bioisosteres
Animal cells generally express mixtures of the three subtypes of inositol 1,4,5-trisphosphate receptor. Recent data have provided the first structure-activity analyses of key Ins(1,4,5)P3 analogues using homogenous populations of each mammalian Ins(1,4,5)P3R subtype.[238] Other myo-inositol phosphate analogues have been synthesized that may provide new mechanistic insights and structure-activity relationship information for the inositol trisphosphate receptor. Inositol 1,4,5-trisphosphate analogues in which the 5-phosphate was replaced with less acidic bioisosteres have been synthesized.[239] The chiral protected 1,4-bisphosphate 234 in (Scheme 31) was prepared using a nine step procedure that exposes the 5-hydroxyl group for the introduction of a bioisostere to replace the 5-phosphate. Two compounds were prepared from the versatile 1,4-bisphosphate intermediate 234 having a methylphosphonate and a sulphate at the 5-position. The methylphosphonate group was incorporated at the 5-position of the inositol ring using bis[6-(trifluoromethyl)benzotriazol-1-yl]-methylphosphonate. Hydrogenolysis in the presence of a catalyst then removed all benzyl groups in excellent yield to give 235. The sulphate group was introduced at the 5-hydroxy position using sulphur trioxide in pyridine, and hydrogenolysis removed all benzyl groups to give the 5-modified sulphate product 236. The 5-carboxylate was prepared by oxidative cleavage of the 5-O-allyl derivative 237 and the remaining benzyl groups were deprotected by hydrogenolysis to give 238. For the final modified compound, a slightly different intermediate (239 in Scheme 32) was used. Desilylation, then phosphitylation and oxidation provided 1,4-bisphosphorylated derivative 240. Oxidative removal of the p-methoxybenzyl group then phosphitylation and oxidation provided fully blocked derivative 241. The protected 5-phosphate group contains one OBn and one OMe protective group and the benzyl groups were deblocked by hydrogenolysis to yield 242. Compounds 235 and 242 were found to be weak Ins(1,4,5)P3 receptor antagonists in the low millimolar range.
Scheme 31.
Reagents and conditions: (a) Bis[6-trifluoromethyl)benzotriazol-1-yl)]methylphosphonate, pyridine, then BnOH, deprotect using palladium black, H2, H2O, NaHCO3; (b) SO3, pyr.; then tert-butanol, H2O, NaHCO3; (c) RuCl3.H2O, CCl4, NaIO4, MeCN, H2O; deprotection for (b) and (c) as for (a).
Scheme 32.
Reagents and conditions: (a) TBAF in THF; then reagent 173, CH2Cl2, 1H-tetrazole, then MCPBA; (b) ceric ammonium nitrate, MeCN, H2O; then (MeO)(BnO)PNiPr2, CH2Cl2, 1H-tetrazole; then MCPBA; (c) palladium black, H2, tert-butanol, H2O, NaHCO3.
12.7. Inositol Phosphate Ligands that Uncovered the Capture Site of PPIP5K2
In addition to the substrate analogues designed for the kinase PPIP5K2,[230] a number of inositol phosphate analogues (Figure 12) modified with aromatic substituents at position 2- of InsP5, such as 2-O-benzyl myo-inositol 1,3,4,5,6-pentakisphosphate (159), were found to stimulate significantly the ATPase activity of the kinase PPIP5K2, although 2-aminoethyl Ins(1,3,4,5,6)P5 (243) had no effect and the 2-O-butanoate derivative (244) was weak.[227] Surprisingly, however, compounds modified at both 2- and 5-positions showed significantly enhanced activity and it was particularly unexpected that 2-O-Bn-5-PA-InsP4 (212) should be a more effective activator of ATPase activity than the two natural substrates, InsP6 and the pyrophosphate PP-InsP5. 2,5-Di-O-benzyl-myo-inositol 1,3,4,6-tetrakisphosphate (245) was even more effective.
Figure 12.
Inositol phosphate ligands evaluated at PPIP5K2; compound 245 is the most potent ligand.
Earlier work on the phosphonoacetate derivative 5-PA-InsP5 (210) soaked with PPIP5K2 indicated unresolved structural information. When crystals were re-soaked at a higher concentration an additional ligand binding-site was detected, located near to the entrance to the catalytic pocket and only one of the two sites could be occupied at any time. When PPIP5K2 was then soaked in the presence of 2-O-Bn-5-PA-InsP4 (212) or 2,5-di-O-Bn-myo-inositol-1,3,4,6-tetrakisphosphate (245) both compounds were exclusively located in the second ligand-binding site (named the substrate capture site). The aromatic nature of the functional group especially at position-2 was critical in revealing the second site. This second site facilitates substrate capture prior to transfer into the catalytic pocket and reveals a so-called “catch and-pass” reaction mechanism, the first to be observed in a small molecule kinase. This second binding site offers new opportunities for targeted drug design and 245, particularly with its reduced phosphate quotient, offers an interesting initial lead for further optimisation.
12.8. Photoactivated myo-Inositol Lipid Derivatives
Although this section primarily focuses on soluble myo-inositol phosphates, a few examples of recent work on myo-inositol lipid derivatives are noteworthy. A photoactivatable and membrane permeable phospholipid derivative of phosphatidylinositol 3,4,5-trisphosphate PtdIns(3,4,5)P3 (253) has been designed.[240] The structure of the product was characterised by a photolabile C8-PtdIns(3,4,5)P3-7-diethylamino-4-methylenehydroxycoumarin derivative (253) located on the 3-phosphate, with butanoate groups on the 2- and 6-hydroxyl groups and acetoxymethyl groups blocking the remaining negative charged phosphate groups. The synthesis of 253 (in Scheme 33) is a useful addition to the biological toolkit since it is the first membrane-permeable caged fluorescent phosphoinositol lipid. The monobutanoate 247 was prepared as described previously from 246.[235] Phosphitylation using 248 then oxidation gave 249; selective removal of the trans-isopropylidene under mild acidic conditions and further phosphitylation and oxidation using reagent 198 gave derivative 250. Acidic hydrolysis of the cis-isopropylidene exposed the 1,2-diol that was then reprotected as a butane acetal and partial hydrolysis provided the 2-butanoate derivative. Phosphitylation at the 1-position with reagent 251 then oxidation provided the fully blocked precursor 252 that could be made into the target lipophilic analogue. Selective removal of the 9H-fluorenylmethyl groups in the presence of piperidine exposed the phosphate monoesters that were then reprotected as acetoxymethyl esters to give 253 albeit in low yield.
Scheme 33.
Reagents and conditions: (a) EtiPr2N-MeOH, (1:4), 36°C, 11 h, 91%; (b) Reagent 248, 4,5-dicyanoimidazole, CH2Cl2, 0°C, to rt, 20 min., then AcOOH, –18°C, to rt, 1 h, 70%; (c) Formic acid-CH2Cl2 (7:3), rt, 3.5 h; (d) Reagent 198, 4,5-dicyanoimidazole, CH2Cl2/MeCN, 4:1, rt, 1 h, then AcOOH, –18°C, to rt, 1.5 h, 48% (over 2 steps); (e) Formic acid/CH2Cl2, 95:5, 4.5 h; (f) 1,1-Dimethoxybutane, CH2Cl2, jandajel pyridinium trifluoroacetate, rt, 23 h, Dowex 50WX8, H+, 1 h; (g) Reagent 251 4,5-dicyanoimidazole, CH2Cl2-MeCN, rt, 30 min, then AcOOH, –18°C, to rt, 30 min, 46% (over 3 steps); (h) CH2Cl2, piperidine, rt, 1 h; (i) bromomethyl acetate, EtiPr2N, MeCN, rt, 10 h, 16% (over 2 steps).
The lipophilic compound crosses the cell membrane allowing enzyme hydrolysis of the esters whilst the molecule is unmasked to reveal its hydroxyl groups and phosphate units to give 254 which remains unmetabolized. Uncaging of 254 by photolysis reveals diC8-PtdIns(3,4,5)P3 with which cellular events such as PH-domain translocation and the induction of membrane ruffling can be observed under a suitable microscope.
12.9. Solid Phase Synthesis of myo-Inositol C8-Phospholipids
A new solid phase synthesis strategy for the production of inositol phospholipid analogues has been developed over the last few years.[241] This had not previously been achieved. A suitable linker was found to be the benzylidene acetal derivative (using the Wang alcohol resin),[242] easily introduced and removed from the inositol ring and cleaved from the solid support, avoiding tedious purification steps. A suitable inositol precursor such as 255 was also sought that could be resolved and blocked with protective groups that could be introduced and removed as required and then phosphorylated and deprotected in a strategy loosely based upon the protecting groups previously employed elsewhere.[243] The protective groups employed were the acetal at the 2,3-positions (256) (cyclohexylidene was then replaced with a benzylidene derivative 257 on the Wang resin) and TBDPS (tert-butyldiphenylsilyl) was located at the 1-position. The less toxic MEM (methoxyethoxymethyl) and benzoyl protecting groups were also used at positions 4-, 5- and 6- in 257 to synthesise phosphatidylinositol analogues containing a short C-8 chain or aminopropyl derivative that made them water soluble. Furthermore, regioselective benzylidene ring-opening using DIBAL-H exposes the 3-hydroxy group of 258 in readiness for phosphorylation at this position. The synthesis of the C-8 phospholipid 259 (Scheme 34) is discussed to illustrate the use of solid support methodology of inositol lipid synthesis. These methods can also be adapted for the synthesis of water soluble inositol phosphates and other derivatives.
Scheme 34.
Reagents and conditions: (a) BzCl in CHCl3, pyr. –40°C, 1 h; (b) MEM-Cl, Hünigs base, CHCl3, Δ, 48h; (c) 65% HCOOH, in MeOH, rt, 48h; (d) 4-(dimethoxymethyl)phenol, CH2Cl2, PPTS, rt, 24 h, then 40 °C for 4 h; (e) Wang alcohol resin, DIAD, triphenylphosphine, THF, rt, 48 h; (f) DIBAL-H, CH2Cl2, −78°C → −30°C, 3 h; (g) reagent 173, DCI, CH2Cl2, CH3CN, 24 h, then peracetic acid, −30°C → rt, 1 h; (h) TASF in DMF, 32 h; (i) glycerol diester phosphoramidite, DCI, CH2Cl2, MeCN, 24 h, then peracetic acid −30°C → rt, 1 h; (j) DDQ, CH2Cl2, H2O; (k) TMSBr, rt, 1h.
The chiral derivative (255 in Scheme 34) was used as a starting material to deliver the required intermediate, having a cyclohexylidene at positions d-2- and d-3- and the TBDPS group at 1-. Selective benzoylation followed by MEM protection then provided 256. The cyclohexylidene acetal was removed and replaced with a benzylidene acetal attached to the Wang resin in a three step process to provide compound 257. DIBAL-H cleaved the benzylidene derivatives and benzoyl groups to give the 2-O-benzyl- intermediate (258) and expose the 3-hydroxy for further phosphorylation. Phosphorylation at 3-, 4- and 5-hydroxyl groups, and deprotection of the silicon protecting group allowed the introduction of the lipid derivative at the 1-hydroxyl. Removal of the compounds from the resin using DDQ for the 2-benzylated compounds provided the pure derivatives. Full deprotection of the individual compounds using TMSBr provided the final compound 259 in quantitative yield (Scheme 34). This strategy has the advantage of preparing a number of water soluble diC8-phospholipid derivatives in relatively few steps without the need for tedious purification that can be the most time consuming process in a synthesis. The intermediates are also stable when stored and further compounds can be synthesized by producing large amounts of intermediate on solid phase, making these methods versatile and time-saving.
12.10. Plasmanylinositols
There are few molecular analyses of inositol phospholipid composition across species. Mammalian inositol phospholipids (260 in Figure 14) mainly consist of the inositol ring and a phosphodiester motif with a diacylglycerol backbone for phosphatidylinositol. Phosphatidylinositol can be further decorated with up to three phosphate groups located at the 3-, 4- and 5-positions. A phosphodiester linkage located at the 1-position is attached to a molecule of glycerol with a stearoyl ester (C-16) at the sn-1 position and an arachidonic ester (C-20:4) at the sn-2 on the glycerol backbone which accounts for most of the total phospholipid. Unusual myo-inositol lipids such as 261 have recently been identified in the slime mould Dictyostelium discoideum.[244] They contain an ether linkage at the sn-1 position and an unsaturated ester (11-Z-octadecenoyl ester) at the sn-2 position of the glycerol-phosphodiester structure, and are designated “plasmanylinositols”. These compounds respond to cell stimuli via chemoattractants and their levels are controlled by kinases and phosphatases such as PI-3-kinase and PTEN. Establishing their functions and potential wider distribution will be a future challenge.
Figure 14.
The structure of a mammalian phospholipid (260) compared to the newly discovered plasmanylinositol (261) found in Dictyostelium.
12.11. Biphenyl Phosphate Derivatives
myo-Inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) stimulates Ca2+ release from intracellular stores via the Ins(1,4,5)P3 receptor. This can also be achieved by various regioisomeric inositol polyphosphates and their analogues, including those based on non myo-core inositol structures that bind in various modes. Recent data have provided structure-activity analyses of homologous populations of all mammalian receptor subtypes.[238] Additionally, there has been much interest in synthetic glyconucleotides based upon the naturally-occurring adenophostins, where the pharmacophore presented to the receptor is comprised of a combination of a phosphorylated carbohydrate and a pendant group that can be a nucleotide or other motif.[245] Recent data have also focussed upon receptor subtypes in concert with adenophostin analogues.[246]
There has always been the challenge to design simple synthetic modulators of the Ins(1,4,5)P3 receptor not based upon inositol that would mimic a myo-inositol polyphosphate. Since the publication of the first antagonist lead, biphenyl 2,3′,4,5′,6-pentakisphosphate BiPh(2,3′,4,5′,6)P5, (262 in Figure 15),[247] which also exhibits other biological activities,[248] similar compounds have been reported that explore the growing potential of benzene polyphosphates in cell signalling applications. Thus, a non-carbohydrate/inositol phosphate Ins(1,4,5)P3 receptor agonist, based upon a biphenyl structure and decorated with phosphates, biphenyl 3,3′,4,4′,5,5′-hexakisphosphate, BiPh(3,3′,4,4′,5,5′)P6 (263), has been synthesized that surprisingly releases Ca2+.[249] Interestingly, by relocating a phosphate group at the 3- and 3′-positions on each ring back one carbon the resulting compound BiPh(2,2′,4,4′,5,5′)P6 (264) then acts as a full antagonist. Increasing the length of the connection between the two polyphosphorylated head groups of 264 by two carbon and two oxygen atoms to give 265, retains the antagonist effect with slightly less potency than 264.[249] While such compounds are obviously not membrane permeant and are thus limited as investigative cellular tools they do offer a radically new template and, with such surprising activities, may offer through optimisation a new and simple approach to explore the design of badly needed selective receptor modulators. More understanding of their mode of action is required.
Figure 15.
The structures of benzene polyphosphates showing agonist behavior and antagonist behavior at the Ins(1,4,5)P3 receptor.
Additionally, such benzene polyphosphates have been shown to be good mimics at myo-inositol phospholipid headgroup binding sites and, moving on from the initial report,[250] a recent co-crystal structure reported with the lipid phosphatase SHIP2 may offer clues for drug design at lipid phosphatases of potential therapeutic interest.[251]
13. Summary and Outlook
The signal transduction field, one of the most active in biology, provides rich opportunities for interrogation through chemistry. myo-Ins(1,4,5)P3 has enjoyed pre-eminent status in Ca2+ signalling for many years and signals in virtually all mammalian cells through the Ins(1,4,5)P3R ion channel, a ubiquitous system regulating processes ranging from transcription and cell division to memory and learning. There are also now the emerging complementary nucleotide messengers nicotinic acid adenine dinucleotide 2'-phosphate (NAADP), cyclic adenosine 5'-diphosphate ribose (cADPR) and adenosine 5'-diphosphate ribose (ADPR). Together, they regulate the majority of cellular functions, often with spatial and temporal coordination. While Ins(1,4,5)P3, cADPR and NAADP act via mobilization of intracellular Ca2+ many fundamental aspects of the newer signalling pathways are still unknown.
The pathways based upon myo-inositol, including phospholipids and soluble inositol polyphosphates, have now enjoyed more than 30 years of interrogation and development. Ins(1,4,5)P3 and other inositol phosphates and phosphoinositides are regulated by specific kinases and phosphatases; several signaling pathways are dysregulated in disease, placing some of these enzymes, for example PI-3 kinase (PI3K) at the forefront of drug discovery. However, even many years after the discovery of Ins(1,4,5)P3 the fundamental question of exactly how IP3 binding leads to Ca2+ release remains essentially unanswered and there are still no truly effective and selective Ins(1,4,5)P3R antagonists - a continuing challenge for medicinal chemistry. Further surprises in this area are clearly still in store. Recently, an Ins(1,4,5,6)P4 stereoisomer emerged as a co-regulator of histone deacetylases.[207, 208] Acetylation and deacetylation of histones is an epigenetic mechanism regulating chromatin organisation and gene expression, and deacetylase inhibitors are promising clinical cancer drugs. Targeting the inositol polyphosphate binding site, for example, might offer exciting new therapeutic approaches. Higher inositol polyphosphates (IP5 to IP8) have been a developing theme over recent years and also offer rich potential. Ins(1,3,4,5,6)P5 has anticancer properties and IP6 is linked to chromatin remodelling, RNA editing, mRNA export and DNA repair. Ubiquitous in eukaryotic cells, but not found in bacteria, IP6 is also a cofactor in the activation of bacterial toxins (e.g. from Clostridium difficile), a mechanism that would be pertinent to target synthetically. IP5 and IP6 are phosphorylated to the still higher diphosphoinositol phosphates IP7 and IP8 that have been linked to vesicular trafficking, apoptosis, DNA repair, telomere maintenance, stress responses, neurological function and immune responses. IP7 and IP8, representing the most crowded 3D-array of phosphates in nature, are at the cutting-edge of current biological interest, with few available tools and few real targets understood. However, progress is being made in the design of both parent molecules and their non-hydrolysable analogues [225–228, 230, 232, 235] and early promise from the application of the latter.[231] Recent work in this area has also illustrated how for the kinase PPIP5K2 identification of a second binding site might allow chemical modulation of the active site and identified a simple substituted inositol polyphosphate lead.[227] Another very recent and cutting edge advance has been the identification of the inositol hexakisphosphate kinase IP6K2, as a mediator of cancer cell migration and metastasis, via IP7 synthesis, suggesting that IP6K2 could be a novel cancer drug target.[252] These are just some examples as to how the biological range based exclusively upon the myo-inositol isomer has expanded and continues to develop since the initial discovery of Ins(1,4,5)P3 in 1983. Given this and the comparative paucity of information about the distribution and possible roles of many of the other eight inositol isomers and their numerous potential polyphosphates, as discussed here, further and wider research in the inositol field is warranted. While the field based upon the myo-isomer is not slowing down one aim of this review is to stimulate a greater interest in the other eight isomers and, importantly given the vast literature now available on myo-inositol, the armamentarium of chemical techniques and tools available now that could be applied elsewhere is very large. The time is now ripe for a concerted excursion beyond the myo-isomer. A recent stimulus has been provided.[11]
As stated in the Introduction the work cited describes effects across a very wide area of biology and biochemistry. As such it is difficult to draw any general conclusions about the roles of the “other” inositols beyond saying that they are very diverse, and too few of these roles are yet known to support the drawing of any wide conclusions. Unsurprisingly, the different isomers have different effects (relative to each other) in different systems. Large amounts of these isomers are readily available through the numerous chemical syntheses described herein. We have tried to concentrate both on highly practical routes for synthesis, mindful that this is a prime feature for biological investigations and indeed early attempts to explore medical applications (as for scyllo and d-chiro inositols), but have not neglected the roles of interest and elegance. Many of the papers cited compare the effects of one or more of the less common inositol isomers with myo-inositol in some particular role, but none compares all nine isomers in the same situation. The phosphorylated forms of most of the inositol isomers are widespread in the environment where they form a large but poorly understood pool of environmental phosphate that is derived mainly from plants.[11] Some of these phosphorylated inositol isomers have been chemically synthesised and tested in assays against various enzymes and receptors to find possible biological roles.[e.g. 32, 80, 84, 164] Obviously, such derivatives with different stereochemistry or regiochemistry of substitution can merely just act on systems controlled by myo-inositol derivatives, but the hope would be to find unique activities. The higher inositol polyphosphate field is an emerging area of likely seminal importance in signaling, largely unexplored structurally, and PP-InsP binding sites could be attractive new drug targets. One has to point to these emerging myo-inositol pyrophosphates and their complexity as an area of great current chemical and biological activity and potential, but also note that neo-inositol IP7 and IP8 pyrophosphates are already known from protozoa, but are still of unknown function.[192] There are thus tantalising hints of wider activity already in evidence.
Although lipid work is not really described here there is a vast literature available based around the design of drug-like compounds to block PI3K. Disappointing PI3K inhibitor selectivity, however, underlies the search for alternative targets, e.g. amongst promising inositol phospholipid phosphatases. SHIP enzymes, for example, are linked to human diseases, particularly diabetes, cancer and obesity. The recent structure of a SHIP2:head group surrogate complex with a simple inositol polyphosphate surrogate identified an active site loop for targeting inhibition and specificity [251] and earlier work had demonstrated that a simple benzene tetrakisphosphate could be crystallized with the Akt-PKB PH domain.[250] The structure of the Akt PH domain in complex with Ins(1,3,4,5)P4 was used to design more drug-like compounds moving away from the phospholipid headgroup using computational virtual screening techniques.[253] To illustrate how this has also developed for other related potential drug targets based upon second messengers mobilizing Ca2+, but which is substantially beyond the scope of this review, one can point to the discovery of the drug-like inhibitors e.g. SAN 2589 and 4825 blocking cardiac ADP-ribosyl cyclase, [254] that produces the messenger cADPR from NAD, and the considerable success of the NED-19 class of compound where, using computational virtual screening techniques an organic molecule that mimics NAADP and of real biological application was discovered to modulate the activity of the messenger NAADP.[255] Indeed, also, the simple NAADP derivative BZ194 has shown activity in T cells [256], autoimmune disease in vivo [257] and in vivo in the heart.[258] These are promising advances for the idea of pharmacological intervention in second messenger signalling and it should be clear that all of these techniques and ideas that move away from parent polyphosphate structures to more simple and drug-like compounds are in principle applicable to myo-inositol polyphosphates and their various targets, and obviously, perhaps more widely in the inositol series. Still more of interest from this general area is the observation that non-inositol based polyphosphates, e.g. benzene polyphosphates to date, can act as both agonists and antagonists at Ins(1,4,5)P3R. How this functions remains still to be properly understood, but the potential is clear. This is a general area of considerable promise and chemistry in concert with structural biology has huge potential contributions to make here.
Exploration will be greatly assisted by the development of new and/or efficient methods for synthesizing the inositols, their analogues and derivatives, providing that they are practical routes. These areas benefit enormously from the large amount of work that has been carried out on designed derivatives and tools to investigate myo-inositol phosphates in particular, for example fluorescently tagged and caged derivatives, and such techniques, refined substantially now since the mid-1980s, will make it much easier to access the tools required to explore the biology of other inositol isomers and their phosphate derivatives.
Supplementary Material
Figure 13.
Caged C8-PtdIns(3,4,5)P3 (253) protected with acetoxymethyl groups that are enzymatically hydrolyzed to give a photolabile derivative (254) prior to uncaging.
Acknowledgments
We thank the Wellcome Trust for long term support of our work in this area. BVLP is a Wellcome Trust Senior Investigator (Grant 101010).
Glossary
- Aβ
amyloid-β
- ADPR
adenosine 5'-diphosphate ribose
- cADPR
cyclic adenosine 5'-diphosphate ribose
- AcOH
acetic acid
- AIBN
azobisisobutyronitrile
- BDA
butane diacetal
- Bn
benzyl
- BSTFA
N,O-bis(trimethylsilyl)trifluoroacetamide
- BzCl
benzoyl chloride
- CK2
casein kinase II
- DBU
(1,8-diazabicyclo[5.4.0]undec-7-ene).
- DCC
N,N-dicyclohexylcarbodiimide
- DCI
4,5-dicyanoimidazole
- DDQ
2,3-Dichloro5,6-dicyano-1,4-benzoquinone
- DIBAL-H
diisobutylaluminium hydride
- DIPE
N,N-diisopropylethylamine
- DMAP
N,N-dimethylamino pyridine
- DME
dimethoxyethane
- DMF
N,N-dimethylformamide
- DMSO
dimethylsulfoxide
- EDAC
1-ethyl-3-(3-dimethlaminopropyl)carbodiimide
- Fm
fluorenylmethyl
- HDAC
histone deacetylase
- hDIPP1
human diphosphoinositol polyphosphate phosphohydrolase 1
- KHDMS
potassium bis(trimethylsilyl)amide
- KOBz
potassium benzoate
- GLUT4
glucose transporter type 4
- GPI
glycosylphosphatidylinositol
- IP6K2
inositol hexakisphosphate kinase 2
- MCPBA
3-chloroperoxybenzoic acid
- MeCN
acetonitrile
- MEM-Cl
methoxyethoxymethyl chloride
- MeOH
methanol
- MRS
Magnetic Resonance Spectroscopy
- NAADP
nicotinic acid adenine dinucleotide 2'-phosphate
- NCOR2
nuclear receptor co-repressor 2
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMO
N-methylmorpholine-N-oxide
- O/N
overnight
- PDK1
3-phosphoinositide-dependent protein kinase
- PMB-Cl
p-methoxybenzyl chloride
- PI3K
Phosphoinositide 3-kinase
- PKBα
protein kinase B α
- PPTS
pyridinium p-toluenesulfonate
- PTSA
p-toluenesulfonic acid
- RANKL
receptor activator of nuclear factor kappa-B ligand
- SHIP2
SH-2 domain containing inositol 5-phosphatase 2
- TASF
tris(dimethylamino)sulfonium difluorotrimethyl silicate
- TEAB
triethylammonium bicarbonate
- TFA
trifluoroacetic acid
- Tf
trifluoromethansulphonyl
- Tf2O
trifluoromethanesulfonic anhydride
- THF
tetrahydrofuran
- TIPS-Cl
triisopropylsilyl chloride
- TMS
trimethylsilyl
- TMSBr
bromotrimethylsilane
- TFSA
trifluoromethylsulfonic acid
- TrCl
trityl chloride
- UV
ultra violet
- XEP
o-xylyene
Biographies
Barry Potter is Professor of Biological & Medicinal Chemistry at Oxford University and Wellcome Trust Senior Investigator, with interests in signal transduction and anticancer drug discovery. He studied Chemistry at Oxford, also earning a DPhil and a DSc. After postdocs in Oxford and at a Max-Planck-Institute in Göttingen he was Lecturer at Leicester University, then moved to Bath University as Full Professor and in 2015 returned to Oxford. He has brought drugs to many clinical trials and cofounded Sterix Ltd. He was elected to the UK Academy of Medical Sciences, Academia Europaea and has won four RSC medals, the GSK International Achievement Award and the 2015/16 RSC-BMCS Lectureship. He was Investigator of the Year at the 2012 European Life Science Awards.

Mark Thomas has a B.Sc. degree (biochemistry, 1989, Wales), a Ph.D. degree (protein chemistry and enzymology, 1992, London) and a M.Sc. degree (computer science, 2000, Bristol). He has academic experience in protein chemistry, enzymology, crystallization and crystallography, and both academic and industrial experience in computational drug discovery, design and development. He is a named author on thirty-eight published papers and five patents.

Stephen Mills was born and brought up in Oldham near Manchester, England. He obtained an MPhil at the University of Leicester in 1991 and a PhD at the University of Bath in 1994 with Professor Barry V. L. Potter. After a short break, Stephen returned to continue scientific research in 2000. His research interests include the synthesis of new types of compound that modulate the inositol 1,4,5-trisphosphate receptor and the inhibition and characterization of the inositol 5-phosphatase enzyme family. He is a named author of more than twenty-five papers.

Footnotes
Turtle motif reprinted (adapted) with permission from Inositol Phosphates and Derivatives, ACS Symposium Series 463 (1991), cover, Allen B Reitz ed. Copyright (1991) American Chemical Society.
Contributor Information
Dr Mark P Thomas, Department of Pharmacy & Pharmacology, University of Bath, Claverton Down, Bath, BA2 7AY, UK.
Dr Stephen J Mills, Department of Pharmacy & Pharmacology, University of Bath, Claverton Down, Bath, BA2 7AY, UK.
Prof Dr Barry V L Potter, Department of Pharmacology, University of Oxford, Mansfield Road, Oxford, OX1 3QT, UK.
References
- [1].a) Michell RH. Biochem Soc Symp. 2007;74:223–246. doi: 10.1042/BSS0740223. [DOI] [PubMed] [Google Scholar]; b) Michell RH. Nat Rev Mol Cell Biol. 2008;9:151–161. doi: 10.1038/nrm2334. [DOI] [PubMed] [Google Scholar]; c) Michell RH. Adv Enzyme Regul. 2011;51:84–90. doi: 10.1016/j.advenzreg.2010.10.002. [DOI] [PubMed] [Google Scholar]
- [2].a) Parys JB, De Smedt H. Adv Exp Med Biol. 2012;740:255–279. doi: 10.1007/978-94-007-2888-2_11. [DOI] [PubMed] [Google Scholar]; b) Shears SB, Ganapathi SB, Gokhale NA, Schenk TMH, Wang H, Weaver JD, Zaremba A, Zhou Y. Subcell Biochem. 2012;59:389–412. doi: 10.1007/978-94-007-3015-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Saiardi A. Adv Biol Regul. 2012;52:351–359. doi: 10.1016/j.jbior.2012.03.002. [DOI] [PubMed] [Google Scholar]
- [4].Boss WF, Im YJ. Annu Rev Plant Biol. 2012;63:409–429. doi: 10.1146/annurev-arplant-042110-103840. [DOI] [PubMed] [Google Scholar]
- [5].Alcázar-Román AR, Wente SR. Chromosoma. 2008;117:1–13. doi: 10.1007/s00412-007-0126-4. [DOI] [PubMed] [Google Scholar]
- [6].Tsui MM, York JD. Adv Enzyme Regul. 2010;50:324–337. doi: 10.1016/j.advenzreg.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Valluru R, Van den Ende W. Plant Sci. 2011;181:387–400. doi: 10.1016/j.plantsci.2011.07.009. [DOI] [PubMed] [Google Scholar]; b) Valluru R, Van den Ende W. Plant Sci. 2012;185-186:340–341. [Google Scholar]
- [8].Antonsson B. Biochim Biophys Acta. 1997;1348:179–186. doi: 10.1016/s0005-2760(97)00105-7. [DOI] [PubMed] [Google Scholar]
- [9].a) Potter BVL, Lampe D. Angew Chem Int Ed Engl. 1995;34:1933–1972. [Google Scholar]; Angew Chem. 1995;107:2085–2125. [Google Scholar]; b) Irvine RF, Schell MJ. Nature Rev Mol Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]; c) Best MD, Zhang H, Prestwich GD. Nat Prod Rep. 2010;27:1403–1430. doi: 10.1039/b923844c. [DOI] [PubMed] [Google Scholar]; d) Hatch AJ, York JD. Cell. 2010;143:1030. doi: 10.1016/j.cell.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Chakraborty A, Kim S, Snyder SH. Sci Sig. 2011;4:re1. doi: 10.1126/scisignal.2001958. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Gillaspy GE. New Phytol. 2011;192:823–839. doi: 10.1111/j.1469-8137.2011.03939.x. [DOI] [PubMed] [Google Scholar]; g) Michell RH. Adv Enzyme Regul. 2011;51:84–90. doi: 10.1016/j.advenzreg.2010.10.002. [DOI] [PubMed] [Google Scholar]; h) Lee J-Y, Kim Y-r, Park J, Kim S. Ann New York Acad Sci. 2012;1271:68–74. doi: 10.1111/j.1749-6632.2012.06725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Croze ML, Soulage CO. Biochimie. 2013;95:1811–1827. doi: 10.1016/j.biochi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- [10].Agranoff BW. J Biol Chem. 2009;284:21121–21126. doi: 10.1074/jbc.X109.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Turner BL, Cheesman AW, Godage HY, Riley AM, Potter BVL. Environ Sci Technol. 2012;46:4994–5002. doi: 10.1021/es204446z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Turner BL, Cheesman AW, Godage HY, Riley AM, Potter BVL. Environ Sci Technol. 2013;47:1774–1775. doi: 10.1021/es204446z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Turner BL, Richardson AE, Mullaney E, editors. Inositol phosphates: linking agriculture and the environment. CABI Publishing; 2006. [Google Scholar]; b) Barker CJ, editor. Inositol phosphates and lipids: methods and protocols. Humana Press; 2010. [Google Scholar]; c) Majumder AL, Biswas BB, editors. Biology of inositols and phosphoinositides. Springer; 2010. [Google Scholar]; d) Biswas BB, Biswas S, editors. Myo-inositol phosphates, phosphoinositides, and signal transduction. Springer; 2011. [Google Scholar]; e) Falasca M, editor. Phosphoinositides and disease. Springer; 2012. [Google Scholar]; f) Bleasdale JE, Eichberg J, Hause G, editors. Inositol and phosphoinositides: metabolism and regulation. Humana Press; 2013. [Google Scholar]; g) Kerr WG, Fernandes S, editors. Inositol phospholipid signaling in physiology and disease. Wiley-Blackwell; 2013. [DOI] [PubMed] [Google Scholar]; h) Rocha H, Cardoso M, editors. Inositol: synthesis, functions and clinical implications. Nova Science Publishers Inc; 2013. [Google Scholar]
- [14].Cosgrove DJ, Irving GCJ. Studies in Organic Chemistry 4: Inositol phosphates; their chemistry, biochemistry and physiology. Elsevier; 1980. [Google Scholar]
- [15].Sureshan KM, Shashidhar MS, Praveen T, Das T. Chem Rev. 2003;103:4477–4503. doi: 10.1021/cr0200724. [DOI] [PubMed] [Google Scholar]
- [16].Schedler DJA, Baker DC. Carbohydr Res. 2004;339:1585–1595. doi: 10.1016/j.carres.2004.03.030. [DOI] [PubMed] [Google Scholar]
- [17].Duchek J, Adams DR, Hudlicky T. Chem Rev. 2011;111:4223–4258. doi: 10.1021/cr1004138. [DOI] [PubMed] [Google Scholar]
- [18].Kılbaş A, Balci M. Tetrahedron. 2011;67:2355–2389. [Google Scholar]
- [19].Chung S-K, Kwon Y-U. Bioorg Med Chem Lett. 1999;9:2135–2140. doi: 10.1016/s0960-894x(99)00348-0. [DOI] [PubMed] [Google Scholar]
- [20].Takahashi H, Kittaka H, Ikegami S. J Org Chem. 2001;66:2705–2716. doi: 10.1021/jo001575h. [DOI] [PubMed] [Google Scholar]
- [21].Podeschwa M, Plettenburg O, vom Brocke J, Block O, Adelt S, Altenbach H-J. Eur J Org Chem. 2003;10:1958–1972. [Google Scholar]
- [22].Kornienko A, d’Alarcao M. Tetrahedron Asymmetry. 1999;10:827–829. [Google Scholar]
- [23].Luchetti G, Ding K, d’Alarcao M, Kornienko A. Synthesis. 2008:3142–3147. doi: 10.1055/s-2008-1067260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Luchetti G, Ding K, Kornienko A, d’Alarcao M. Synthesis. 2008:3148–3154. doi: 10.1055/s-2008-1067259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kornienko A, d’Alarcao M. Tetrahedron Lett. 1997;38:6497–6500. [Google Scholar]
- [26].Gigg J, Gigg R, Payne S, Conant R. Carbohydr Res. 1985;142:132–134. [Google Scholar]
- [27].Anderson RC, Wallis ES. J Am Chem Soc. 1948;70:2931–2935. doi: 10.1021/ja01189a030. [DOI] [PubMed] [Google Scholar]
- [28].Sarmah MP, Shashidhar MS. Carbohydr Res. 2003;338:999–1001. doi: 10.1016/s0008-6215(03)00052-1. [DOI] [PubMed] [Google Scholar]
- [29].Sun Y, Zhang G, Hawkes CA, Shaw JE, McLaurin J, Nitz M. Bioorg Med Chem. 2008;16:7177–7184. doi: 10.1016/j.bmc.2008.06.045. [DOI] [PubMed] [Google Scholar]
- [30].Chung S-K, Kwon Y-U, Chang Y-T, Sohn K-H, Shin J-H, Park K-H, Hong B-J, Chung I-H. Bioorg Med Chem. 1999;7:2577–2589. doi: 10.1016/s0968-0896(99)00183-2. [DOI] [PubMed] [Google Scholar]
- [31].Kwon Y-U, Im J, Choi G, Kim Y-S, Choi KY, Chung S-K. Bioorg Med Chem Lett. 2003;13:2981–2984. doi: 10.1016/s0960-894x(03)00629-2. [DOI] [PubMed] [Google Scholar]
- [32].Riley AM, Trusselle M, Kuad P, Borkovec M, Cho J, Choi JH, Qian X, Shears SB, Spiess B, Potter BVL. ChemBioChem. 2006;7:1114–1122. doi: 10.1002/cbic.200600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].a) Fleury PF, Courtois JE, Jouannet AL. Bull Soc Chim Biol. 1951;33:1885–1893. [PubMed] [Google Scholar]; b) Helleu C. Bull Soc Chim Biol. 1957;39:633–640. [PubMed] [Google Scholar]
- [34].a) Posternak T, Schopfer WH, Kaufmann-Boetsch B, Edwards S. Helv Chim Acta. 1963;46:2676–2685. [Google Scholar]; b) Sherman WR, Stewart MA, Simpson PC, Goodwin SL. Biochemistry. 1968;7:819–824. doi: 10.1021/bi00842a040. [DOI] [PubMed] [Google Scholar]; c) Sherman WR, Stewart MA, Kurien MM, Goodwin SL. Biochim Biophys Acta. 1968;158:197–205. doi: 10.1016/0304-4165(68)90131-1. [DOI] [PubMed] [Google Scholar]
- [35].Groenen PMW, Merkus HMWM, Sweep FCGJ, Wevers RA, Janssen FSM, Steegers-Theunissen RPM. Ann Clin Biochem. 2003;40:79–85. doi: 10.1258/000456303321016213. [DOI] [PubMed] [Google Scholar]
- [36].Parkkinen J. FEBS Lett. 1983;163:10–13. doi: 10.1016/0014-5793(83)81151-x. [DOI] [PubMed] [Google Scholar]
- [37].Fenili D, Brown M, Rappaport R, McLaurin J. J Mol Med. 2007;85:603–611. doi: 10.1007/s00109-007-0156-7. [DOI] [PubMed] [Google Scholar]
- [38].McPhee F, Downes CP, Lowe G. Biochem J. 1991;277:407–412. doi: 10.1042/bj2770407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].a) Strieleman PJ, Connors MA, Metzger BE. Diabetes. 1992;41:989–997. doi: 10.2337/diab.41.8.989. [DOI] [PubMed] [Google Scholar]; b) Strieleman PJ, Metzger BE. Teratology. 1993;48:267–278. doi: 10.1002/tera.1420480310. [DOI] [PubMed] [Google Scholar]; c) Wentzel P, Wentzel CR, Gäreskog MB, Eriksson UJ. Teratology. 2001;63:193–201. doi: 10.1002/tera.1034. [DOI] [PubMed] [Google Scholar]
- [40].Salman M, Lonsdale JT, Besra GS, Brennan PJ. Biochim Biophys Acta. 1999;1436:437–450. doi: 10.1016/s0005-2760(98)00151-9. [DOI] [PubMed] [Google Scholar]
- [41].Takenawa T, Tsumita T. Biochim Biophys Acta. 1974;373:490–494. doi: 10.1016/0005-2736(74)90028-5. [DOI] [PubMed] [Google Scholar]
- [42].Moyer JD, Malinowski N, Napier EA, Strong J. Biochem J. 1988;254:95–100. doi: 10.1042/bj2540095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].a) Spector R. J Neurochem. 1976;27:229–236. doi: 10.1111/j.1471-4159.1976.tb01569.x. [DOI] [PubMed] [Google Scholar]; b) Spector R. Neurochem Res. 1988;13:785–787. doi: 10.1007/BF00971603. [DOI] [PubMed] [Google Scholar]
- [44].Rubin LJ, Hale CC. J Mol Cell Cardiol. 1993;25:721–731. doi: 10.1006/jmcc.1993.1084. [DOI] [PubMed] [Google Scholar]
- [45].a) Palmano KP, Whiting PH, Hawthorne JN. Biochem J. 1977;167:229–235. doi: 10.1042/bj1670229. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Michaelis T, Helms G, Merboldt K-D, Hänicke W, Bruhn H, Frahm J. NMR Biomed. 1993;6:105–109. doi: 10.1002/nbm.1940060116. [DOI] [PubMed] [Google Scholar]
- [46].a) Kwon HM, Yamauchi A, Uchida S, Preston AS, Garcia-Perez A, Burg MB, Handler JS. J Biol Chem. 1992;267:6297–6301. [PubMed] [Google Scholar]; b) Hager K, Hazama A, Kwon HM, Loo DDF, Handler JS, Wright EM. J Membr Biol. 1995;143:103–113. doi: 10.1007/BF00234656. [DOI] [PubMed] [Google Scholar]; c) Inoue K, Shimada S, Minami Y, Morimura H, Miyai A, Yamauchi A, Tohyama M. Neuroreport. 1996;7:1195–1198. doi: 10.1097/00001756-199604260-00020. [DOI] [PubMed] [Google Scholar]; d) Coady MJ, Wallendorff B, Gagnon DG, Lapointe J-Y. J Biol Chem. 2002;277:35219–35224. doi: 10.1074/jbc.M204321200. [DOI] [PubMed] [Google Scholar]; e) Bourgeois F, Coady MJ, Lapointe J-Y. J Physiol. 2005;563:333–343. doi: 10.1113/jphysiol.2004.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Locci E, Scano P, Rosa MF, Nioi M, Noto A, Atzori L, Demontis R, De-Giorgio F, d,Aloja E. PLoS One. 2014;9:e97773. doi: 10.1371/journal.pone.0097773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yap A, Nishiumi S, Yoshida K, Ashida H. Cytotechnology. 2007;55:103–108. doi: 10.1007/s10616-007-9107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yamashita Y, Yamaoka M, Hasunuma T, Ashida H, Yoshida K. J Agric Food Chem. 2013;61:4850–4854. doi: 10.1021/jf305322t. [DOI] [PubMed] [Google Scholar]
- [50].a) DaSilva KA, Shaw JE, McLaurin J. Exp Neurol. 2010;223:311–321. doi: 10.1016/j.expneurol.2009.08.032. [DOI] [PubMed] [Google Scholar]; b) Karran E, Mercken M, De Strooper B. Nature Rev Drug Disc. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]; c) Gilbert BJ. J Clin Pathol. 2013;66:362–366. doi: 10.1136/jclinpath-2013-201515. [DOI] [PubMed] [Google Scholar]
- [51].Ma K, Thomason LAM, McLaurin J. Adv Pharmacol. 2012;64:177–212. doi: 10.1016/B978-0-12-394816-8.00006-4. [DOI] [PubMed] [Google Scholar]
- [52].a) Gu L, Liu C, Guo Z. J Biol Chem. 2013;288:18673–18683. doi: 10.1074/jbc.M113.457739. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Tay WM, Huang D, Rosenberry TL, Paravastu AK. J Mol Biol. 2013;425:2494–2508. doi: 10.1016/j.jmb.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].a) McLaurin J, Franklin T, Chakrabartty A, Fraser PE. J Mol Biol. 1998;278:183–194. doi: 10.1006/jmbi.1998.1677. [DOI] [PubMed] [Google Scholar]; b) McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE. J Biol Chem. 2000;275:18495–18502. doi: 10.1074/jbc.M906994199. [DOI] [PubMed] [Google Scholar]
- [54].a) Sun Y, Zhang G, Hawkes CA, Shaw JE, McLaurin J, Nitz M. Bioorg Med Chem. 2008;16:7177–7184. doi: 10.1016/j.bmc.2008.06.045. [DOI] [PubMed] [Google Scholar]; b) Hawkes CA, Deng L-H, Shaw JE, Nitz M, McLaurin J. Eur J Neurosci. 2010;31:203–213. doi: 10.1111/j.1460-9568.2009.07052.x. [DOI] [PubMed] [Google Scholar]
- [55].Shaw JE, Chio J, Dasgupta S, Lai AY, Mo GCH, Pang F, Thomason LAM, Yang AJ, Yip Cm, Nitz M, McLaurin J. ACS Chem Neurosci. 2012;3:167–177. doi: 10.1021/cn2000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Guo J-P, Yu S, McGeer PL. J Alzheimers Dis. 2010;19:1359–1370. doi: 10.3233/JAD-2010-1331. [DOI] [PubMed] [Google Scholar]
- [57].a) Li G, Rauscher S, Baud S, Pomès R. J Phys Chem B. 2012;116:1111–1119. doi: 10.1021/jp208567n. [DOI] [PubMed] [Google Scholar]; b) Li G, Pomès R. J Phys Chem B. 2013;117:6603–6613. doi: 10.1021/jp311350r. [DOI] [PubMed] [Google Scholar]
- [58].Bleiholder C, Do TD, Wu C, Economou NJ, Bernstein SS, Buratto SK, Shea J-E, Bowers MT. J Am Chem Soc. 2013;135:16926–16937. doi: 10.1021/ja406197f. [DOI] [PubMed] [Google Scholar]
- [59].Sinha S, Du Z, Maiti P, Klärner F-G, Schrader T, Wang C, Bitan G. ACS Chem Neurosci. 2012;3:451–458. doi: 10.1021/cn200133x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Aytan N, Choi J-K, Carreras I, Kowall NW, Jenkins BC, Dedeoglu A. Exp Neurol. 2013;250:228–238. doi: 10.1016/j.expneurol.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nixon RA, Yang D-S. Neurobiol Dis. 2011;43:38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee J-H, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, et al. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lai AY, McLaurin J. Biochim Biophys Acta. 2012;1822:1629–1637. doi: 10.1016/j.bbadis.2012.07.003. [DOI] [PubMed] [Google Scholar]
- [64].McLaurin J, Kierstead ME, Brown ME, Hawkes CA, Lambermon MHL, Phinney AL, Darabie AA, Cousins JE, French JE, Lan MF, Chen F, et al. Nature Med. 2006;12:801–808. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- [65].a) Townsend M, Cleary JP, Mehta T, Hofmeister J, Lesne S, O’Hare E, Walsh DM, Selkoc DJ. Ann Neurol. 2006;60:668–676. doi: 10.1002/ana.21051. [DOI] [PubMed] [Google Scholar]; b) Fenili D, Brown M, Rappaport R, McLaurin J. J Mol Med. 2007;85:603–611. doi: 10.1007/s00109-007-0156-7. [DOI] [PubMed] [Google Scholar]
- [66].Dorr A, Sahota B, Chinta LV, Brown ME, Lai AY, Ma K, Hawkes CA, McLaurin J, Stefanovic B. Brain. 2012;135:3039–3050. doi: 10.1093/brain/aws243. [DOI] [PubMed] [Google Scholar]
- [67].Fenili D, Weng Y-Q, Aubert I, Nitz M, McLaurin J. PLoS One. 2011;6:e24032. doi: 10.1371/journal.pone.0024032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Salloway S, Sperling R, Keren R, Porsteinsson AP, van Dyck CH, Tariot PN, Gilman S, Arnold D, Abushakra S, Hernandez C, Crans G, et al. Neurology. 2011;77:1253–1262. doi: 10.1212/WNL.0b013e3182309fa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Vekrellis J, Xilouri M, Emmanouilidou E, Stefanis L. J Neurochem. 2009;109:1348–1362. doi: 10.1111/j.1471-4159.2009.06054.x. [DOI] [PubMed] [Google Scholar]
- [70].Lai AY, Lan CP, Hasan S, Brown ME, McLaurin J. J Biol Chem. 2014;289:3666–3676. doi: 10.1074/jbc.M113.501635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang H, Raleigh DP. PLoS One. 2014;9:e104023. doi: 10.1371/journal.pone.0104023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Nozadze M, Mikautadze E, Lepsveridze E, Mikeladze E, Kuchiashvili N, Kiguradze T, Kikvidze M, Solomonia R. Seizure. 2011;20:173–176. doi: 10.1016/j.seizure.2010.10.008. [DOI] [PubMed] [Google Scholar]
- [73].a) Bonavita S, Di Salle F, Tedeschi G. Eur J Radiol. 1999;30:125–131. doi: 10.1016/s0720-048x(99)00051-0. [DOI] [PubMed] [Google Scholar]; b) Di Costanzo A, Trojsi F, Tosetti M, Giannatempo GM, Nemore F, Piccirillo M, Bonavita S, Tedeschi G, Scarabino T. Eur J Radiol. 2003;48:146–153. doi: 10.1016/j.ejrad.2003.08.009. [DOI] [PubMed] [Google Scholar]; c) Rae CD. Neurochem Res. 2014;39:1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- [74].Vasdev M, Chio J, van Oosten EM, Nitz M, McLaurin J, Vines DC, Houle S, Reilly RM, Wilson AW. Chem Commun. 2009:5527–5529. doi: 10.1039/b913317h. [DOI] [PubMed] [Google Scholar]
- [75].McLarty K, Moran MD, Scollard DA, Chan C, Sabha N, Mukherjee J, Guha A, McLaurin J, Nitz M, Houle S, Wilson AA, et al. Nucl Med Biol. 2011;38:953–959. doi: 10.1016/j.nucmedbio.2011.02.017. [DOI] [PubMed] [Google Scholar]
- [76].Carroll L, Perumal M, Vasdev N, Robins E, Aboagye EO. Bioorg Med Chem Lett. 2012;22:6148–6150. doi: 10.1016/j.bmcl.2012.08.022. [DOI] [PubMed] [Google Scholar]
- [77].Hirano F, Tanaka H, Okamoto K, Makino Y, Inaba M, Nomura Y, Fukawa E, Miura T, Tani T, Makino I. Diabetes Res Clin Pract. 1995;28:151–159. doi: 10.1016/0168-8227(95)01091-q. [DOI] [PubMed] [Google Scholar]
- [78].Shapiro J, Belmaker RH, Biegon A, Seker A, Agam G. J Neural Transm. 2000;107:603–607. doi: 10.1007/s007020070081. [DOI] [PubMed] [Google Scholar]
- [79].Tzeng H-F, Chen J-Y, Huang S-W, Wang Y-J, Yang C-S. Electrophoresis. 2007;28:1221–1228. doi: 10.1002/elps.200600683. [DOI] [PubMed] [Google Scholar]
- [80].Wilcox RA, Safrany ST, Lampe D, Mills SJ, Nahorski SR, Potter BVL. Eur J Biochem. 1994;223:115–124. doi: 10.1111/j.1432-1033.1994.tb18972.x. [DOI] [PubMed] [Google Scholar]
- [81].Nerou EP, Riley AM, Potter BVL, Taylor CW. Biochem J. 2001;355:59–69. doi: 10.1042/0264-6021:3550059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mandel M, Hudlicky T, Kwart LD, Whited GM. J Org Chem. 1993;58:2331–2333. [Google Scholar]
- [83].Liu C, Davis RJ, Nahorski SR, Ballereau S, Spiess B, Potter BVL. J Med Chem. 1999;42:1991–1998. doi: 10.1021/jm980733i. [DOI] [PubMed] [Google Scholar]
- [84].Liu C, Riley AM, Yang X, Shears SB, Potter BVL. J Med Chem. 2001;44:2984–2989. doi: 10.1021/jm000553k. [DOI] [PubMed] [Google Scholar]
- [85].Tegge W, Denis GV, Ballou CE. Carbohydr Res. 1991;217:107–116. doi: 10.1016/0008-6215(91)84121-t. [DOI] [PubMed] [Google Scholar]
- [86].Cid MB, Alfonso F, Martín-Lomas M. Carbohydr Res. 2004;339:2303–2307. doi: 10.1016/j.carres.2004.06.021. [DOI] [PubMed] [Google Scholar]
- [87].Marnera G, d’Alarcao M. Carbohydr Res. 2006;341:1105–1116. doi: 10.1016/j.carres.2006.03.031. [DOI] [PubMed] [Google Scholar]
- [88].Ostlund RE, Jr, Seemayer R, Gupta S, Kimmel R, Ostlund EL, Sherman WR. J Biol Chem. 1996;271:10073–10078. doi: 10.1074/jbc.271.17.10073. [DOI] [PubMed] [Google Scholar]
- [89].Coady MJ, Wallendorff B, Gagnon DG, Lapointe J-Y. J Biol Chem. 2002;277:35219–35224. doi: 10.1074/jbc.M204321200. [DOI] [PubMed] [Google Scholar]
- [90].Aouameur R, Da Cal S, Bissonnette P, Coady MJ, Lapointe J-Y. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1300–G1307. doi: 10.1152/ajpgi.00422.2007. [DOI] [PubMed] [Google Scholar]
- [91].Lin X, Ma L, Gopalan C, Ostlund RE., Jr Br J Nutr. 2009;102:1426–1434. doi: 10.1017/S0007114509990456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].a) Pak Y, Huang LC, Lilley KJ, Larner J. J Biol Chem. 1992;267:16904–16910. [PubMed] [Google Scholar]; b) Pak Y, Hong Y, Kim S, Piccariello T, Farese RV, Larner J. Mol Cells. 1998;8:301–309. [PubMed] [Google Scholar]
- [93].a) Arner RJ, Prabhu KS, Thompson JT, Hildenbrandt GR, Liken AD, Reddy CC. Biochem J. 2001;360:313–320. doi: 10.1042/0264-6021:3600313. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Brown PM, Caradoc-Davies TT, Dickson JM, Cooper GJS, Loomes KM, Baker EN. Acta Cryst. 2006;F62:811–813. doi: 10.1107/S1744309106028144. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Brown PM, Caradoc-Davies TT, Disckson JMJ, Cooper GJS, Loomes KM, Baker EN. Proc Natl Acad Sci USA. 2006;103:15032–15037. doi: 10.1073/pnas.0605143103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Cogram P, Tesh S, Tesh J, Wade A, Allan G, Greene NDE, Copp AJ. Hum Reprod. 2002;17:2451–2458. doi: 10.1093/humrep/17.9.2451. [DOI] [PubMed] [Google Scholar]
- [95].Nascimento NRF, Lessa LMA, Kerntopf MR, Sousa CM, Alves RS, Queiroz MGR, Price J, Heimark DB, Larner J, Du X, Brownlee M, et al. Proc Natl Acad Sci USA. 2006;103:218–223. doi: 10.1073/pnas.0509779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yu J, Choi S, Park E-S, Shin B, Yu J, Lee SH, Takami M, Kang JS, Meong H, Rho J. J Clin Immunol. 2012;32:1360–1371. doi: 10.1007/s10875-012-9722-z. [DOI] [PubMed] [Google Scholar]
- [97].Okazaki Y, Setoguchi T, Katayama T. Biosci Biotechnol Biochem. 2006;70:2766–2770. doi: 10.1271/bbb.60257. [DOI] [PubMed] [Google Scholar]
- [98].a) Pak Y, Larner J. Biochem Biophys Res Commun. 1992;184:1042–1047. doi: 10.1016/0006-291x(92)90696-i. [DOI] [PubMed] [Google Scholar]; b) Larner J, Huang LC, Schwartz CFW, Oswald AS, Shen T-Y, Kinter M, Tang G, Zeller K. Biochem Biophys Res Commun. 1988;151:1416–1426. doi: 10.1016/s0006-291x(88)80520-5. [DOI] [PubMed] [Google Scholar]; c) Larner J. IUBMB Life. 2001;51:139–148. doi: 10.1080/152165401753544205. [DOI] [PubMed] [Google Scholar]; d) Larner J. Int J Experimental Diab Res. 2002;3:47–60. doi: 10.1080/15604280212528. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Larner J, Brautigan DL, Thorner MO. Mol Med. 2010;16:543–551. doi: 10.2119/molmed.2010.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Brautigan DL, Brown M, Grindrod S, Chinigo G, Kruszewski A, Lukasik SM, Bushweller JH, Horal M, Keller S, Tamura S, Heimark DB, et al. Biochemistry. 2005;44:11067–11073. doi: 10.1021/bi0508845. [DOI] [PubMed] [Google Scholar]
- [100].Shashkin PN, Huang LC, Larner J, Vandenhoff GE, Katz A. Int J Exp Diab Res. 2002;3:163–169. doi: 10.1080/15604280214280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Xia T, Wang Q. J Pharm Pharmacol. 2006;58:1527–1532. doi: 10.1211/jpp.58.10.0014. [DOI] [PubMed] [Google Scholar]
- [102].a) Roman-Ramos R, Almanza-Perez JC, Fortis-Barrera A, Angeles-Mejia S, Banderas-Dorantes TR, Zamilpa-Alvarez A, Diaz-Flores M, Jasso I, Blancas-Flores G, Gomez J, Alarcon-Aquilar FJ. Am J Chin Med. 2012;40:97–110. doi: 10.1142/S0192415X12500085. [DOI] [PubMed] [Google Scholar]; b) Fortis-Barrera Á, Alarcón-Aguilar FJ, Banderas-Dorantes T, Díaz-Flores M, Román-Ramos R, Cruz M, García-Macedo R. J Pharm Pharmacol. 2013;65:1563–1576. doi: 10.1111/jphp.12119. [DOI] [PubMed] [Google Scholar]
- [103].Adams GG, Imran S, Wang S, Mohammad A, Kok MS, Gray DA, Channell GA, Harding SE. Crit Rev Food Sci Nutr. 2014;54:1322–1329. doi: 10.1080/10408398.2011.635816. [DOI] [PubMed] [Google Scholar]
- [104].Pitt J, Thorner M, Brautigan D, Larner J, Klein WL. FASEB J. 2013;27:199–207. doi: 10.1096/fj.12-211896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].a) Scioscia M, Siwetz M, Fascilla F, Huppertz B. Placenta. 2012;33:882–884. doi: 10.1016/j.placenta.2012.07.007. [DOI] [PubMed] [Google Scholar]; b) Scioscia M, Pesci A, Zamboni G, Huppertz B, Resta L. Arch Gynecol Obstet. 2013;288:459–460. doi: 10.1007/s00404-013-2729-8. [DOI] [PubMed] [Google Scholar]
- [106].Scioscia M, Nigro M, Montagnani M. J Reprod Immunol. 2014;101–102:140–147. doi: 10.1016/j.jri.2013.05.006. [DOI] [PubMed] [Google Scholar]
- [107].Ostlund RE, Jr, McGill JB, Herskowitz I, Kipnis DM, Santiago JV, Sherman WR. Proc Natl Acad Sci USA. 1993;90:9988–9992. doi: 10.1073/pnas.90.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kawa JM, Przybylski R, Taylor CG. Exp Biol Med. 2003;228:907–914. doi: 10.1177/153537020322800806. [DOI] [PubMed] [Google Scholar]
- [109].Kennington AS, Hill CR, Craig J, Bogardus C, Raz I, Ortmeyer HK, Hansen BC, Romero G, Larner J. New Engl J Med. 1990;323:373–378. doi: 10.1056/NEJM199008093230603. [DOI] [PubMed] [Google Scholar]
- [110].Lin X, Ma L, Fitzgerald RL, Ostlund RE., Jr Arch Biochem Biophys. 2009;481:197–201. doi: 10.1016/j.abb.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Campbell WW, Joseph LJO, Ostlund RE, Jr, Anderson RA, Farrell PA, Evans WJ. Int J Sport Nutr Exerc Metab. 2004;14:430–442. doi: 10.1123/ijsnem.14.4.430. [DOI] [PubMed] [Google Scholar]
- [112].Stull AJ, Thyfault JP, Haub MD, Ostlund RE, Jr, Campbell WW. Metab Clin Expr. 2008;57:1545–1551. doi: 10.1016/j.metabol.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Villeneuve M-C, Ostlund RE, Jr, Baillargeon J-P. Metab Clin Expr. 2009;58:62–68. doi: 10.1016/j.metabol.2008.08.007. [DOI] [PubMed] [Google Scholar]
- [114].Chang HH, Chong B, Phillips AR, Loomes KM. Exp Biol Med. 2015;240:8–14. doi: 10.1177/1535370214543064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].a) Ortmeyer HK, Huang LC, Zhang L, Hansen BC, Larner J. Endocrinology. 1993;132:646–651. doi: 10.1210/endo.132.2.8425484. [DOI] [PubMed] [Google Scholar]; b) Kawa JM, Taylor CG, Przybylski R. J Agric Food Chem. 2003;51:7287–7291. doi: 10.1021/jf0302153. [DOI] [PubMed] [Google Scholar]; c) Yao Y, Shan F, Bian J, Chen F, Wang M, Ren G. J Agric Food Chem. 2008;56:10027–10031. doi: 10.1021/jf801879m. [DOI] [PubMed] [Google Scholar]
- [116].Curran JM, Stringer DM, Wright B, Taylor CG, Przybylski R, Zahradka P. J Agric Food Chem. 2010;58:3197–3204. doi: 10.1021/jf903890c. [DOI] [PubMed] [Google Scholar]
- [117].Ortmeyer HK, Larner J, Hansen BC. Obes Res. 1995;3(Suppl. 4):605S–608S. doi: 10.1002/j.1550-8528.1995.tb00232.x. [DOI] [PubMed] [Google Scholar]
- [118].Sugimoto M, Takeda N, Hattori J, Yoshino K, Nakashima K, Okumura S, Ishimori M, Yasuda K. Endocr J. 1999;46(Suppl):S51–S53. doi: 10.1507/endocrj.46.suppl_s51. [DOI] [PubMed] [Google Scholar]
- [119].Yoshino K, Takeda N, Sugimoto M, Nakashima K, Okumura S, Hattori J, Sasaki A, Kawachi S, Takami K, Takami R, Yasuda K. Metabolism. 1999;48:1418–1423. doi: 10.1016/s0026-0495(99)90153-1. [DOI] [PubMed] [Google Scholar]
- [120].Farias VX, Macêdo FHP, Oquendo MB, Tomé AR, Báo SN, Cintra DOS, Santos CF, Albuquerque AAC, Heimark DB, Larner J, Fonteles MC, et al. Diabetes Obes Metab. 2011;13:243–250. doi: 10.1111/j.1463-1326.2010.01344.x. [DOI] [PubMed] [Google Scholar]
- [121].Fonteles MC, Huang LC, Larner J. Diabetalogia. 1996;39:731–734. doi: 10.1007/BF00418546. [DOI] [PubMed] [Google Scholar]
- [122].Whiting L, Danaher RN, Ruggiero K, Lee CC, Chaussade C, Mulvey T, Phillips A, Loomes KM. Horm Metab Res. 2013;45:394–397. doi: 10.1055/s-0032-1330016. [DOI] [PubMed] [Google Scholar]
- [123].Nissen PM, Nebel C, Oksbjerg N, Bertram HC. J Biomed Biotechnol. 2011 doi: 10.1155/2011/378268. Article ID 378268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Malvasi A, Casciaro F, Minervini MM, Kosmas I, Mynbaev OA, Pacella E, Monti Condesnitt V, Creanza A, Di Renzo GC, Tinelli A. Eur Rev Med Pharmacol Sci. 2014;18:270–274. [PubMed] [Google Scholar]
- [125].Shannon M, Wang Y. J Midwifery Women’s Health. 2012;57:221–230. doi: 10.1111/j.1542-2011.2012.00161.x. [DOI] [PubMed] [Google Scholar]
- [126].a) Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. N Engl J Med. 1999;340:1314–1320. doi: 10.1056/NEJM199904293401703. [DOI] [PubMed] [Google Scholar]; b) Iuorno MJ, Jakubowicz DJ, Baillargeon J-P, Dillon P, Guan RD, Allan G, Nestler JE. Endocr Pract. 2002;8:417–423. doi: 10.4158/EP.8.6.417. [DOI] [PubMed] [Google Scholar]; c) Cheang KI, Baillargeon J-P, Essah PA, Ostlund RE, Jr, Apridonize T, Islam L, Nestler JE. Metab Clin Expr. 2008;57:1390–1397. doi: 10.1016/j.metabol.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Colazingari S, Treglia M, Najjar R, Bevilacqua A. Arch Gynecol Obstet. 2013;288:1405–1411. doi: 10.1007/s00404-013-2855-3. [DOI] [PubMed] [Google Scholar]
- [128].Isabella R, Raffone E. J Ovarian Res. 2012;5:14. doi: 10.1186/1757-2215-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Unfer V, Carlomagno G, Rizzo P, Raffone E, Roseff S. Eur Rev Med Pharmacol Sci. 2011;15:452–457. [PubMed] [Google Scholar]
- [130].Carlomagno G, Unfer V, Roseff S. Fertil Steril. 2011;95:2515–2516. doi: 10.1016/j.fertnstert.2011.05.027. [DOI] [PubMed] [Google Scholar]
- [131].a) Nordio M, Proietti E. Eur Rev Med Pharmacol Sci. 2012;16:575–581. [PubMed] [Google Scholar]; b) Minozzi M, Nordio M, Pajalich R. Eur Rev Med Pharmacol Sci. 2013;17:537–540. [PubMed] [Google Scholar]
- [132].Pizzo A, Laganà AS, Barbaro L. Gynecol Endocrinol. 2013;30:205–208. doi: 10.3109/09513590.2013.860120. [DOI] [PubMed] [Google Scholar]
- [133].Laganà AS, Barbaro L, Pizzo A. Arch Gynecol Obstet. 2015;291:1181–1186. doi: 10.1007/s00404-014-3552-6. [DOI] [PubMed] [Google Scholar]
- [134].Baillargeon J-P, Iuorno MJ, Jakubowicz DJ, Aprodonidze T, He N, Nestler JE. J Clin Endocrinol Metab. 2004;89:242–249. doi: 10.1210/jc.2003-030437. [DOI] [PubMed] [Google Scholar]
- [135].Baillargeon J-P, Iuorno MJ, Apridonidze T, Nestler JE. Metab Syndr Relat Disord. 2010;8:127–136. doi: 10.1089/met.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].a) De Leo V, La Marca A, Cappelli V, Stendardi A, Focarelli R, Musacchio MC, Piomboni P. Minerva Ginecol. 2012;64:531–538. [PubMed] [Google Scholar]; b) Piomboni P, Focarelli R, Capaldo A, Stendardi A, Cappelli V, Cianci A, La Marca A, Luddi A, De Leo V. J Assist Reprod Genet. 2014;31:1269–1276. doi: 10.1007/s10815-014-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Genazzani AD, Santagni S, Rattighieri E, Chierchia E, Despini G, Marini G, Prati A, Simoncini T. Gynecol Endocrinol. 2014;30:438–443. doi: 10.3109/09513590.2014.897321. [DOI] [PubMed] [Google Scholar]
- [138].a) Baillargeon J-P, Diamanti-Kandarakis E, Ostlund RE, Jr, Apridonidze T, Iuorno JM, Nestler JE. Diabetes Care. 2006;29:300–305. doi: 10.2337/diacare.29.02.06.dc05-1070. [DOI] [PubMed] [Google Scholar]; b) Baillargeon J-P, Nestler JE, Ostlund RE, Apridonidze T, Diamanti-Kandarakis E. Hum Reprod. 2008;23:1439–1446. doi: 10.1093/humrep/den097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].La Marca A, Grisendi V, Dondi G, Sighinolfi G, Cianci A. Gynecol Endocrinol. 2015;31:52–56. doi: 10.3109/09513590.2014.964201. [DOI] [PubMed] [Google Scholar]
- [140].a) Seli E, Duleba AJ. Curr Diab Rep. 2004;4:69–75. doi: 10.1007/s11892-004-0014-8. [DOI] [PubMed] [Google Scholar]; b) Galazis N, Galazi M, Atiomo W. Gynecol Endocrinol. 2011;27:256–262. doi: 10.3109/09513590.2010.538099. [DOI] [PubMed] [Google Scholar]; c) Galletta M, Grasso S, Vaiarelli A, Roseff SJ. Eur Rev Med Pharmacol Sci. 2011;15:1212–1214. [PubMed] [Google Scholar]; d) Saha L, Kaur S, Saha PK. Fundam Clin Pharmacol. 2012;26:54–62. doi: 10.1111/j.1472-8206.2010.00916.x. [DOI] [PubMed] [Google Scholar]; e) Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Cochrane Database Syst Rev. 2012;16:1–164. doi: 10.1002/14651858.CD003053.pub5. [DOI] [PubMed] [Google Scholar]; f) Bizzarri M, Carlomagno G. Eur Rev Med Pharmacol Sci. 2014;18:1896–1903. [PubMed] [Google Scholar]; g) Unfer V, Porcaro G. Expert Rev Clin Pharmacol. 2014;7:623–631. doi: 10.1586/17512433.2014.925795. [DOI] [PubMed] [Google Scholar]
- [141].Futerman AH, Low MG, Ackermann KE, Sherman WR, Silman I. Biochem Biophys Res Commun. 1985;129:312–317. doi: 10.1016/0006-291x(85)91439-1. [DOI] [PubMed] [Google Scholar]
- [142].Pak Y, Larner J. Biochem Biophys Res Commun. 1992;184:1042–1047. doi: 10.1016/0006-291x(92)90696-i. [DOI] [PubMed] [Google Scholar]
- [143].McCoy JJ, Mann BJ, Vedvick TS, Pak Y, Heimark DB, Petri WA., Jr J Biol Chem. 1993;268:24223–24231. [PubMed] [Google Scholar]
- [144].Farese RV, Standaert ML, Yamada K, Huang LC, Zhang C, Cooper DR, Wang Z, Yang Y, Suzuki S, Toyata T, Larner J. Proc Natl Acad Sci USA. 1994;91:11040–11044. doi: 10.1073/pnas.91.23.11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Taguchi R, Yamazaki J, Tsutsui Y, Ikezawa H. Arch Biochem Biophys. 1997;342:161–168. doi: 10.1006/abbi.1997.9991. [DOI] [PubMed] [Google Scholar]
- [146].a) Kinoshita T, Fujita M, Maeda Y. J Biochem. 2008;144:287–294. doi: 10.1093/jb/mvn090. [DOI] [PubMed] [Google Scholar]; b) Maeda Y, Kinoshita T. Prog Lipid Res. 2011;50:411–424. doi: 10.1016/j.plipres.2011.05.002. [DOI] [PubMed] [Google Scholar]; c) Tsai Y-H, Liu X, Seeberger PH. Angew Chem Int Ed Engl. 2012;51:11438–11456. doi: 10.1002/anie.201203912. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2012;124:11604–11623. [Google Scholar]
- [147].Bonilla JB, Cid MB, Contreras F-X, Goñi FM, Martín-Lomas M. Chem Eur J. 2006;12:1513–1528. doi: 10.1002/chem.200500833. [DOI] [PubMed] [Google Scholar]
- [148].Bates SH, Jones RB, Bailey CJ. Br J Pharmacol. 2000;130:1944–1948. doi: 10.1038/sj.bjp.0703523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Geethan PKMA, Prince PSM. J Biochem Mol Toxicol. 2008;22:220–224. doi: 10.1002/jbt.20218. [DOI] [PubMed] [Google Scholar]
- [150].Davis A, Christiansen M, Horowitz JF, Klein S, Hellerstein MK, Ostlund RE., Jr Diabetes Care. 2000;23:1000–1005. doi: 10.2337/diacare.23.7.1000. [DOI] [PubMed] [Google Scholar]
- [151].Kang M-J, Kim J-I, Yoon S-Y, Kim JC, Cha I-J. J Med Food. 2006;9:182–186. doi: 10.1089/jmf.2006.9.182. [DOI] [PubMed] [Google Scholar]
- [152].Kim MJ, Yoo KH, Kim JH, Seo YT, Ha BW, Kho JH, Shin YG, Chung CH. Diabetes Res Clin Pract. 2007;77:S247–S251. doi: 10.1016/j.diabres.2007.01.066. [DOI] [PubMed] [Google Scholar]
- [153].Hernández-Mijares A, Bañuls C, Peris JE, Monzó N, Jover A, Bellod L, Victor VM, Rocha M. Food Chem. 2013;141:1267–1272. doi: 10.1016/j.foodchem.2013.04.042. [DOI] [PubMed] [Google Scholar]
- [154].Stull AJ, Wood KV, Thyfault JP, Campbell WW. Horm Metab Res. 2009;41:381–386. doi: 10.1055/s-0028-1128140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Larner J, Price JD, Heimark D, Smith L, Rule G, Piccariello T, Fonteles MC, Pontes C, Vale D, Huang L. J Med Chem. 2003;46:3283–3291. doi: 10.1021/jm030071j. [DOI] [PubMed] [Google Scholar]
- [156].Lazarenko R, Geisler J, Bayliss D, Larner J, Li C. Mol Cell Endocrinol. 2014;387:1–7. doi: 10.1016/j.mce.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Choi M-S, Lee W-H, Kwon E-Y, Kang MA, Lee M-K, Park YB, Jeon S-M. J Med Food. 2007;10:594–601. doi: 10.1089/jmf.2006.220. [DOI] [PubMed] [Google Scholar]
- [158].Sethi G, Ahn KS, Sung B, Aggarwal BB. Mol Cancer Ther. 2008;7:1604–1614. doi: 10.1158/1535-7163.MCT-07-2424. [DOI] [PubMed] [Google Scholar]
- [159].Liu S-C, Chuang S-M, Tang C-H. Int Immunophamacol. 2012;12:494–500. doi: 10.1016/j.intimp.2012.01.002. [DOI] [PubMed] [Google Scholar]
- [160].Lin T-H, Tan T-W, Tsai T-H, Chen C-C, Hsieh T-F, Lee S-S, Liu H-H, Chen W-C, Tang C-H. Int J Mol Sci. 2013;14:9790–9802. doi: 10.3390/ijms14059790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Magielse J, Arcoraci T, Breynaert A, van Dooren I, Kanyanga C, Fransen E, Van Hoof V, Vlietinck A, Apers S, Pieters L, Hermans N. J Ethnopharmacol. 2013;146:250–256. doi: 10.1016/j.jep.2012.12.039. [DOI] [PubMed] [Google Scholar]
- [162].Brammer LE, Jr, Hudlicky T. Tetrahedron: Asymmetry. 1998;9:2011–2014. [Google Scholar]
- [163].Kofman O, Sherman WR, Katz V, Belmaker RH. Psychopharmacol. 1993;110:229–234. doi: 10.1007/BF02246978. [DOI] [PubMed] [Google Scholar]
- [164].Liu C, Nahorski SR, Potter BVL. J Chem Soc Chem Commun. 1991:1014. [Google Scholar]
- [165].Liu C, Al-Hafidh J, Westwick J, Potter BVL. Bioorg Med Chem. 1994;2:253–257. doi: 10.1016/s0968-0896(00)82168-9. [DOI] [PubMed] [Google Scholar]
- [166].Safrany ST, Wilcox RA, Liu C, Potter BVL, Nahorski SR. Eur J Pharmacol. 1992;226:265–272. doi: 10.1016/0922-4106(92)90071-3. [DOI] [PubMed] [Google Scholar]
- [167].a) Safrany ST, Wilcox RA, Liu C, Dubreuil D, Potter BVL, Nahorski SR. Mol Pharmacol. 1993;43:499–503. [PubMed] [Google Scholar]; b) Liu C, Al-Hafidh J, Westwick J, Potter BVL. Bioorg Med Chem. 1994;2:253–257. doi: 10.1016/s0968-0896(00)82168-9. [DOI] [PubMed] [Google Scholar]
- [168].Safrany ST, Mills SJ, Liu C, Lampe D, Noble NJ, Nahorski SR, Potter BVL. Biochemistry. 1994;33:10763–10769. doi: 10.1021/bi00201a025. [DOI] [PubMed] [Google Scholar]
- [169].Ward SG, Mills SJ, Liu C, Westwick J, Potter BVL. J Biol Chem. 1995;270:12075–12084. doi: 10.1074/jbc.270.20.12075. [DOI] [PubMed] [Google Scholar]
- [170].a) Hansbro PM, Foster PS, Liu C, Potter BVL, Denborough MA. Biochem Biophys Res Commun. 1994;200:8–15. doi: 10.1006/bbrc.1994.1407. [DOI] [PubMed] [Google Scholar]; b) Safrany ST, Mills SJ, Liu C, Lampe D, Noble NJ, Nahorski SR, Potter BVL. Biochemistry. 1994;33:10763–10769. doi: 10.1021/bi00201a025. [DOI] [PubMed] [Google Scholar]
- [171].Kourie JI, Foster PS, Dulhunty AF. J Membrane Biol. 1997;157:147–158. doi: 10.1007/s002329900224. [DOI] [PubMed] [Google Scholar]
- [172].Adelt S, Podeschwa M, Dallmann G, Altenbach H-J, Vogel G. Bioorg Chem. 2003;31:44–67. doi: 10.1016/s0045-2068(02)00523-0. [DOI] [PubMed] [Google Scholar]
- [173].McCance RA, Lawrence RD. Biochem J. 1933;27:986–989. doi: 10.1042/bj0270986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Moharam BA, Jantan I, Jalil J, Shaari K. Molecules. 2010;15:7840–7848. doi: 10.3390/molecules15117840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].a) Kim AR, Zou YN, Park TH, Shim KH, Kim MS, Kim ND, Kim JD, Bae SJ, Choi JS, Chung HY. Phytother Res. 2004;18:1–7. doi: 10.1002/ptr.1358. [DOI] [PubMed] [Google Scholar]; b) Nobre Júnior HV, Cunha GMA, Moraes MO, Luciana MFD, Oliveira RA, Maia FD, Nogueira MAS, Lemos TLG, Rao VS. Food Chem Toxicol. 2006;44:1544–1551. doi: 10.1016/j.fct.2006.04.002. [DOI] [PubMed] [Google Scholar]
- [176].de Olinda TM, Lemos TLG, Machado LL, Rao VS, Santos FA. Phytomed. 2008;15:327–333. doi: 10.1016/j.phymed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- [177].Stockton KP, Greatrex BW, Taylor DK. J Org Chem. 2014;79:5088–5096. doi: 10.1021/jo500645z. [DOI] [PubMed] [Google Scholar]
- [178].Williams MB, Jope RS. Brain Res. 1995;685:169–178. doi: 10.1016/0006-8993(95)00395-7. [DOI] [PubMed] [Google Scholar]
- [179].a) Kofman O, Sherman WR, Katz V, Belmaker RH. Psychopharmacol. 1993;110:229–234. doi: 10.1007/BF02246978. [DOI] [PubMed] [Google Scholar]; b) Patishi Y, Belmaker RH, Bersudsky Y, Kofman O. Biol Psychiatry. 1996;39:829–832. doi: 10.1016/0006-3223(95)00574-9. [DOI] [PubMed] [Google Scholar]; c) Richards MH, Belmaker RH. J Neural Transm. 1996;103:1281–1285. doi: 10.1007/BF01271188. [DOI] [PubMed] [Google Scholar]; d) Belmaker RH, Agam G, van Calker D, Richards MH, Kofman O. Neuropsychopharmacol. 1998;9:220–232. doi: 10.1016/S0893-133X(98)00017-7. [DOI] [PubMed] [Google Scholar]
- [180].Vorwerk CK, Simon P, Gorla M, Katowicz W, Zurakowski D, Levin LA, Dryer EB. Invest Opthalmol Vis Sci. 1999;40:813–816. [PubMed] [Google Scholar]
- [181].Mason R, Biello SM. Neurosci Lett. 1992;144:135–138. doi: 10.1016/0304-3940(92)90734-o. [DOI] [PubMed] [Google Scholar]
- [182].Shaltiel G, Dalton EC, Belmaker RH, Harwood AJ, Agam G. Bipolar Disord. 2007;9:281–289. doi: 10.1111/j.1399-5618.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- [183].a) Busa WB, Gimlich RL. Dev Biol. 1989;132:315–324. doi: 10.1016/0012-1606(89)90228-5. [DOI] [PubMed] [Google Scholar]; b) Becchetti A, Whitaker M. Development. 1997;124:1099–1107. doi: 10.1242/dev.124.6.1099. [DOI] [PubMed] [Google Scholar]
- [184].Shaldubina A, Ju S, Vaden DL, Ding D, Belmaker RH, Greenberg ML. Mol Psychiatry. 2002;7:174–180. doi: 10.1038/sj.mp.4000965. [DOI] [PubMed] [Google Scholar]
- [185].a) Einat H, Elkabaz-Schwortz Z, Cohen H, Kofman O, Belmaker RH. Int J Neuropsychopharmacol. 1998;1:31–34. doi: 10.1017/S1461145798001035. [DOI] [PubMed] [Google Scholar]; b) Bersudsky Y, Einat H, Stahl Z, Belmaker RH. Curr Psychiat Rep. 1999;1:141–147. doi: 10.1007/s11920-999-0023-z. [DOI] [PubMed] [Google Scholar]; c) see, also, Shaldubina A, Einat H, Bersudsky Y, Belmaker RH. Neuropsychiatr Dis Treat. 2005;1:189–190. doi: 10.2147/nedt.1.2.189.61052.
- [186].Ballereau S, Guédat P, Poirier SN, Guillemette G, Spiess B, Schlewer G. J Med Chem. 1999;42:4824–4835. doi: 10.1021/jm991084t. [DOI] [PubMed] [Google Scholar]
- [187].Riley AM, Jenkins DJ, Potter BVL. Carbohydr Res. 1998;314:277–281. [Google Scholar]
- [188].Angyal SJ, Craig DC. Carbohydr Res. 1994;263:149–154. doi: 10.1016/0008-6215(94)00157-x. [DOI] [PubMed] [Google Scholar]
- [189].Sherman WR, Goodwin SL, Gunnell KD. Biochemistry. 1971;10:3491–3499. doi: 10.1021/bi00795a002. [DOI] [PubMed] [Google Scholar]
- [190].Hipps PP, Holland WH, Sherman WR. Biochem Biophys Res Commun. 1977;77:340–346. doi: 10.1016/s0006-291x(77)80202-7. [DOI] [PubMed] [Google Scholar]
- [191].Martin J-B, Bakker-Grunwald T, Klein G. Eur J Biochem. 1993;214:711–718. doi: 10.1111/j.1432-1033.1993.tb17972.x. [DOI] [PubMed] [Google Scholar]
- [192].Martin J-B, Laussmann T, Bakker-Grunwald T, Vogel G, Klein G. J Biol Chem. 2000;275:10134–10140. doi: 10.1074/jbc.275.14.10134. [DOI] [PubMed] [Google Scholar]
- [193].Dangschat G, Fisher HOL. Naturwissenschaften. 1939;27:756–757. [Google Scholar]
- [194].Dangschat G, Fischer HOL, MacDonald DL. Carbohydr Res. 1987;164:343–355. [Google Scholar]
- [195].Miethchen R, Sowa C, Frank M, Michalik M, Reinke H. Carbohydr Res. 2002;337:1–9. doi: 10.1016/s0008-6215(01)00281-6. [DOI] [PubMed] [Google Scholar]
- [196].Freeman S, Hudlicky T. Bioorg Med Chem Lett. 2004;14:1209–1212. doi: 10.1016/j.bmcl.2003.12.050. [DOI] [PubMed] [Google Scholar]
- [197].Espelie KE, Anderson L. Carbohydr Res. 1976;46:53–66. doi: 10.1016/s0008-6215(00)83530-2. [DOI] [PubMed] [Google Scholar]
- [198].Adhikari J, Majumder AL. Ind J Biochem Biophys. 1988;25:408–412. [PubMed] [Google Scholar]
- [199].Murthy PPN. Subcell Biochem. 2006;39:1–19. doi: 10.1007/0-387-27600-9_1. [DOI] [PubMed] [Google Scholar]
- [200].Abraham RJ, Byrne JJ, Griffiths L, Koniotou R. Magn Reson Chem. 2005;43:611–624. doi: 10.1002/mrc.1611. [DOI] [PubMed] [Google Scholar]
- [201].a) Sureshan KM, Watanabe Y. SynLett. 2004:493–496. [Google Scholar]; b) Sureshan KM, Miyasou T, Watanabe Y. Carbohydr Res. 2004;339:1551–1555. doi: 10.1016/j.carres.2004.03.031. [DOI] [PubMed] [Google Scholar]
- [202].Nitz M, Fenili D, Darabie AA, Wu L, Cousins JE, McLaurin J. FEBS J. 2008;275:1663–1674. doi: 10.1111/j.1742-4658.2008.06321.x. [DOI] [PubMed] [Google Scholar]
- [203].Angyal SJ, Odier L, Tate ME. Carbohydr Res. 1995;266:143–146. [Google Scholar]
- [204].Angyal SJ, Hickman RJ. Carbohydr Res. 1971;20:97–104. doi: 10.1016/s0008-6215(00)84952-6. [DOI] [PubMed] [Google Scholar]
- [205].a) Chung S-K, Kwon Y-U. Bioorg Med Chem Lett. 1999;9:2135–2140. doi: 10.1016/s0960-894x(99)00348-0. [DOI] [PubMed] [Google Scholar]; b) Takahashi H, Kittaka H, Ikegami S. J Org Chem. 2001;66:2705–2716. doi: 10.1021/jo001575h. [DOI] [PubMed] [Google Scholar]
- [206].a) Conway SJ, Miller GJ. Nat Prod Rep. 2007;24:687–707. doi: 10.1039/b407701f. [DOI] [PubMed] [Google Scholar]; b) Best MD, Zhang H, Prestwich GD. Nat Prod Rep. 2010;27:1403–1430. doi: 10.1039/b923844c. [DOI] [PubMed] [Google Scholar]
- [207].Watson PJ, Fairall L, Santos GM, Schwabe JWR. Nature. 2012;481:335–340. doi: 10.1038/nature10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Millard CJ, Watson PJ, Celardo I, Gordiyenko Y, Cowley SM, Robinson CV, Fairall L, Schwabe JWR. Mol Cell. 2013;51:57–67. doi: 10.1016/j.molcel.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].a) Irvine RF, Schell MJ. Nature Rev Mol Cell Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]; b) Shears SB, Ganapathi SB, Gokhale NA, Schenk TMH, Wang H, Weaver JD, Zaremba A, Zhou Y. Subcell Biochem. 2012;59:389–412. doi: 10.1007/978-94-007-3015-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Veiga N, Torres J, Macho I, Gómes K, Godage HY, Riley AM, Potter BVL, González G, Kremer C. Dalton Trans. 2013;42:6021–6032. doi: 10.1039/c2dt31807e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].a) Godage HY, Riley AM, Woodman TJ, Potter BVL. Chem Commun. 2006:2989–2991. doi: 10.1039/b605392k. [DOI] [PubMed] [Google Scholar]; b) Godage HY, Riley AM, Woodman TJ, Thomas MP, Mahon MF, Potter BVL. J Org Chem. 2013;78:2275–2288. doi: 10.1021/jo3027774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Falasca M, Chiozzotto D, Godage HY, Mazzoletti M, Riley AM, Previdi S, Potter BVL, Broggini M, Maffucci T. Br J Cancer. 2010;102:104–114. doi: 10.1038/sj.bjc.6605408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Piccolo E, Vignati S, Maffucci T, Innominato PF, Riley AM, Potter BVL, Pandolfi PP, Broggini M, Iacobelli S, Innocenti P, Falasca M. Oncogene. 2004;23:1754–1765. doi: 10.1038/sj.onc.1207296. [DOI] [PubMed] [Google Scholar]
- [214].Vucenik I, Shamsuddin AM. Nutr Cancer. 2006;55:109–125. doi: 10.1207/s15327914nc5502_1. [DOI] [PubMed] [Google Scholar]
- [215].Riley AM, Windhorst S, Lin H-Y, Potter BVL. ChemBioChem. 2014;15:57–67. doi: 10.1002/cbic.201300583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].a) Piccolo E, Vignati S, Maffucci T, Innominato PF, Riley AM, Potter BVL, Pandolfi PP, Broggini M, Iacobelli S, Innocenti P, Falasca M. Oncogene. 2004;23:1754–1765. doi: 10.1038/sj.onc.1207296. [DOI] [PubMed] [Google Scholar]; b) Maffucci T, Piccolo E, Cumashi A, Iezzi M, Riley AM, Saiardi A, Godage HY, Rossi C, Broggini M, Iacobelli S, Potter BVL, et al. Cancer Res. 2005;65:8339–8349. doi: 10.1158/0008-5472.CAN-05-0121. [DOI] [PubMed] [Google Scholar]
- [217].Ding Z, Rossi AM, Riley AM, Rahman T, Potter BVL, Taylor CW. Mol Pharmacol. 2010;77:995–1004. doi: 10.1124/mol.109.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR, Maag D, Kim S, Huang AS, Dailey MJ, Saleh M, et al. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].York SJ, Armbruster BN, Greenwell P, Petes TD, York JD. J Biol Chem. 2005;280:4264–4269. doi: 10.1074/jbc.M412070200. [DOI] [PubMed] [Google Scholar]
- [220].a) Morrison BH, Bauer JA, Kalvakolanu DV, Lindner DJ. J Biol Chem. 2001;276:24965–24970. doi: 10.1074/jbc.M101161200. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nagata E, Luo HR, Saiardi A, Bae B-I, Suzuki N, Snyder SH. J Biol Chem. 2005;280:1634–1640. doi: 10.1074/jbc.M409416200. [DOI] [PubMed] [Google Scholar]
- [221].a) Fleischer B, Xie J, Mayrleitner M, Shears SB, Palmer DJ, Fleisher S. J Biol Chem. 1994;269:17826–17832. [PubMed] [Google Scholar]; b) Ye W, Ali N, Bembeneck ME, Shears SB, Lafer EM. J Biol Chem. 1995;270:1564–1568. [PubMed] [Google Scholar]
- [222].a) Menniti FS, Miller RN, Putney JW, Jr, Shears SB. J Biol Chem. 1993;268:3850–3856. [PubMed] [Google Scholar]; b) Stephens L, Radenberg T, Thiel U, Vogel G, Khoo K-H, Dell A, Jackson TR, Hawkins PT, Mayr GW. J Biol Chem. 1993;268:4009–4015. [PubMed] [Google Scholar]
- [223].Falck JR, Reddy KK, Ye J, Saady M, Mioskowski C, Shears SB, Tan Z, Safrany S. J Am Chem Soc. 1995;117:12172–12175. [Google Scholar]
- [224].a) Shears SB. Mol Pharmacol. 2009;76:236–252. doi: 10.1124/mol.109.055897. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wilson MSC, Livermore TM, Saiardi A. Biochem J. 2013;452:369–379. doi: 10.1042/BJ20130118. [DOI] [PubMed] [Google Scholar]; c) Thomas MP, Potter BVL. FEBS J. 2014;281:14–33. doi: 10.1111/febs.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Capolicchio S, Thakor DT, Linden A, Jessen HJ. Angew Chem Int Ed Engl. 2013;52:6912–6916. doi: 10.1002/anie.201301092. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2013;125:7050–7054. [Google Scholar]
- [226].Cremosnik GS, Hofer A, Jessen HJ. Angew Chem Int Ed Engl. 2014;53:286–289. doi: 10.1002/anie.201306265. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2014;126:290–294. [Google Scholar]
- [227].Wang H, Godage HY, Riley AM, Weaver JD, Shears SB, Potter BVL. Chem Biol. 2014;21:689–699. doi: 10.1016/j.chembiol.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Capolicchio S, Wang H, Thakor DT, Shears SB, Jessen HJ. Angew Chem Int Ed Engl. 2014;53:9508–9511. doi: 10.1002/anie.201404398. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2014;126:9662–9665. [Google Scholar]
- [229].Lin H, Fridy PC, Ribeiro AA, Choi JH, Barma DK, Vogel G, Flack JR, Shears SB, York JD, Mayr GW. J Biol Chem. 2009;284:1863–1872. doi: 10.1074/jbc.M805686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Riley AM, Wang H, Weaver JD, Shears SB, Potter BVL. Chem Commun. 2012;48:11292–11294. doi: 10.1039/c2cc36044f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Pulloor NK, Nair S, Kostic AD, Bist P, Weaver JD, Riley AM, Tyagi R, Uchil PD, York JD, Snyder SH, García-Sastre A, et al. PLoS Pathog. 2014;10:e1003981. doi: 10.1371/journal.ppat.1003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Wu M, Dul BE, Trevisan AJ, Fiedler D. Chem Sci. 2013;4:405–410. doi: 10.1039/C2SC21553E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Saiardi A, Resnick AC, Snowman AM, Wendland B, Snyder SH. Proc Natl Acad Sci USA. 2005;102:1911–1914. doi: 10.1073/pnas.0409322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Rao F, Cha J, Xu J, Xu R, Vandiver MS, Tyagi R, Tokhunts R, Koldobskiy MA, Fu C, Barrow R, Wu M, et al. Mol Cell. 2014;54:119–132. doi: 10.1016/j.molcel.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Wu M, Chong LS, Capolicchio S, Jessen HJ, Resnick AC, Fiedler D. Angew Chem Int Ed Engl. 2014;53:7192–7197. doi: 10.1002/anie.201402905. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2014;126:7320–7325. [Google Scholar]
- [236].Safrany ST, Caffrey JJ, Yang X, Bembenek ME, Moyer MB, Burkhart WA, Shears SB. EMBO J. 1998;17:6599–6607. doi: 10.1093/emboj/17.22.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].a) Lonetti A, Szijgyarto Z, Bosch D, Loss O, Azevedo C, Saiardi A. J Biol Chem. 2011;286:31966–31974. doi: 10.1074/jbc.M111.266320. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kilari RS, Weaver JD, Shears SB, Safrany ST. FEBS Lett. 2013;587:3464–3470. doi: 10.1016/j.febslet.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Saleem H, Tovey SC, Rahman T, Riley AM, Potter BVL, Taylor CW. PLoS One. 2013;8:e54877. doi: 10.1371/journal.pone.0054877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Keddie NS, Ye Y, Aslam T, Luyten T, Bello D, Garnham C, Bultynch G, Galione A, Conway SJ. Chem Commun. 2011;47:242–244. doi: 10.1039/c0cc03003a. [DOI] [PubMed] [Google Scholar]
- [240].a) Mentel M, Laketa V, Subramanian D, Gillandt H, Schultz C. Angew Chem Int Ed Engl. 2011;50:3811–3814. doi: 10.1002/anie.201007796. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2011;123:3895–3898. [Google Scholar]; b) Höglinger D, Nadler A, Schultz C. Biochim Biophys Acta. 2014;1841:1085–1096. doi: 10.1016/j.bbalip.2014.03.012. [DOI] [PubMed] [Google Scholar]
- [241].Bru M, Kotkar SP, Kar N, Köhn M. Chem Sci. 2012;3:1893–1902. [Google Scholar]
- [242].Wang S-S. J Am Chem Soc. 1973;95:1328–1333. doi: 10.1021/ja00785a602. [DOI] [PubMed] [Google Scholar]
- [243].Kubiak RJ, Bruzik KS. J Org Chem. 2003;68:960–968. doi: 10.1021/jo0206418. [DOI] [PubMed] [Google Scholar]
- [244].Clark J, Kay RR, Kielkowska A, Niewczas I, Fets L, Oxley D, Stephens LR, Hawkins PT. EMBO J. 2014;33:2188–2200. doi: 10.15252/embj.201488677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].a) Rossi AM, Sureshan KM, Riley AM, Potter BVL, Taylor CW. Brit J Pharmacol. 2010;161:1070–1085. doi: 10.1111/j.1476-5381.2010.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sureshan KM, Riley AM, Thomas MP, Tovey SC, Taylor CW, Potter BVL. J Med Chem. 2012;55:1706–1720. doi: 10.1021/jm201571p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Saleem H, Tovey SC, Riley AM, Potter BVL, Taylor CW. PLoS One. 2013;8:e58027. doi: 10.1371/journal.pone.0058027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Vandeput F, Combettes L, Mills SJ, Backers K, Wohlkönig A, Parys JB, De Smedt H, Missiaen L, Dupont G, Potter BVL, Erneux C. FASEB J. 2007;21:1481–1491. doi: 10.1096/fj.06-7691com. [DOI] [PubMed] [Google Scholar]
- [248].Mills SJ, Vandeput F, Trusselle MN, Safrany ST, Erneux C, Potter BVL. ChemBioChem. 2008;9:1757–1766. doi: 10.1002/cbic.200800104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Mills SJ, Luyten T, Erneux C, Parys JB, Potter BVL. MESSENGER. 2012;1:167–181. doi: 10.1166/msr.2012.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Mills SJ, Komander D, Trusselle MN, Safrany ST, van Aalten DMF, Potter BVL. ACS Chem Biol. 2007;2:242–246. doi: 10.1021/cb700019r. [DOI] [PubMed] [Google Scholar]
- [251].Mills SJ, Persson C, Cozier G, Thomas MP, Trésaugues L, Erneux C, Riley AM, Nordlund P, Potter BVL. ACS Chem Biol. 2012;7:822–828. doi: 10.1021/cb200494d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Rao F, Xu J, Fu C, Cha JY, Gadalla MM, Xu R, Barrow JC, Snyder SH. Proc Natl Acad Sci USA. 2015;112:1773–1778. doi: 10.1073/pnas.1424642112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Mahadevan D, Powis G, Mash EA, George B, Gokhale VM, Zhang S, Shakalya K, Du-Cuny L, Berggren M, Ali MA, Jana U, et al. Mol Cancer Ther. 2008;7:2621–2632. doi: 10.1158/1535-7163.MCT-07-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Kannt A, Sicka K, Kroll K, Kadereit D, Gögelein H. Naunyn-Schmiedberg’s Arh Pharmacol. 2012;385:717–727. doi: 10.1007/s00210-012-0750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, et al. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Dammermann W, Zhang B, Nebel M, Cordiglieri C, Odoardi F, Kirchberger T, Kawakami N, Dowden J, Schmid F, Dornmair K, Hohenegger M, et al. Proc Natl Acad Sci USA. 2009;106:10678–10683. doi: 10.1073/pnas.0809997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Cordiglieri C, Odoardi F, Zhang B, Nebel M, Kawakami N, Klinkert WEF, Lodygin D, Lühder F, Breunig E, Schild D, Ulaganathan VK, et al. Brain. 2010;133:1930–1943. doi: 10.1093/brain/awq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Nebel M, Schwörer AP, Warszta D, Siebrands CC, Limbrock A-C, Swarbrick JM, Fliegert R, Weber K, Bruhn S, Hohenegger M, Geisler A, et al. J Biol Chem. 2013;288:16017–16030. doi: 10.1074/jbc.M112.441246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.