Abstract
Preeclampsia (PE) is a pregnancy disorder characterized by high blood pressure and proteinuria that can cause adverse health effects in both mother and fetus. There is no current cure for PE other than delivery of the fetus. While the etiology is unknown, poor placentation of the placenta due to aberrant signaling of growth and angiogenic factors has been postulated as causal factors of PE. In addition, environmental contaminants, such as the metal cadmium (Cd), have been linked to placental toxicity and increased risk of developing PE. Here, we use a translational study design to investigate genomic and epigenomic alterations in both placentas and placental trophoblasts, focused on the angiogenesis-associated transforming growth factor-beta (TGF-β) pathway. Genes within the TGF-β pathway displayed increased expression in both the preeclamptic placenta and Cd-treated trophoblasts. In addition, miRNAs that target the TGF-β pathway were also significantly altered within the preeclamptic placenta and Cd-treated trophoblasts. Integrative analysis resulted in the identification of a subset of Cd-responsive miRNAs, including miR-26a and miR-155, common to preeclamptic placentas and Cd-treated trophoblasts. These miRNAs have previously been linked to PE and are predicted to regulate members of the TGF-β pathway. Results from this study provide future targets for PE treatment.
Keywords: Preeclampsia, TGF-β, JEG-3 cells, gene expression, epigenetics, miRNA
1. Introduction
Preeclampsia (PE) is a pregnancy-related condition characterized by hypertension and proteinuria during gestation, usually including damage to other organ systems, such as the kidneys (Chaiworapongsa et al. 2014). In the United States, PE affects between 5–8% of pregnancies and accounts for approximately 76,000 and 500,000 deaths of women and fetuses worldwide, respectively (Berg et al. 2010). While a precise etiology of PE is unknown, it is postulated that an underlying factor may be poor placentation due to inadequate angiogenesis (Chaiworapongsa et al. 2014; Saito and Nakashima 2014).
An array of ligands and receptors are known to tightly control angiogenesis. For example, growth factors, chemokines, cytokines, and endogenous angiogenesis inhibitors has been observed in the extracellular matrix (ECM) during vascularization (Brooks and Rathmell 2014). Reduced placental blood flow upregulates expression of hypoxia inducible factor 1 alpha (HIF1A), vascular endothelial growth factor (VEGF), VEGF receptor (VEGFR), angiopoeitin-2 (ANGPT2), fibroblast growth factor-3 (FGF-3), nitric oxide synthase (NOS), and transforming growth factors (TGF-α, TGF-β1, TGF-β3) (Harris 2002). However in PE, the expression of anti-angiogenic factors such as fms-like tyrosin kinase-1 (FLT1) and its soluble form (sFLT1) are increased and can then bind to pro-angiogenic factors and inhibit angiogenic signaling (Nikuei et al. 2015). Proper placentation and vascular bed expansion has been found to be dependent on the expression of VEGF (Chaiworapongsa et al. 2014). On the other hand, the TGF-beta superfamily has been observed to oppose trophoblastic migration (Jones et al. 2006), suggesting an intricate balance of growth factor expression that is fundamental for placental health and embryo development (Nikuei et al. 2015; Conti et al. 2013).
Environmental exposure to toxic metals has been linked to placental toxicity/dysregulation causing improper vascularization in the placenta (Esteban-Vasallo et al. 2012; Lazebnik et al. 1989; Pollack et al. 2014). We have recently demonstrated a significant association between elevated levels of placental cadmium (Cd) and increased odds of developing PE (Laine et al. 2015), supporting an existing literature of this relationship (Pollack et al. 2014) Exposure to Cd can occur through multiple sources, including contaminated food and contaminated water sources, as well as cigarette smoke (Satarug et al. 2010). Alterations in the epigenetic landscape and genomic instability are potential detrimental human health effects of Cd exposure. Disruption of genomic stability has been highlighted as a feature of PE, which includes aberrant miRNA expression (Dai et al. 2012; Gao et al. 2012; Gunel et al. 2011; Ishibashi et al. 2012; Lazar et al. 2012; Li et al. 2013; Noack et al. 2011; Wang et al. 2012; Wu et al. 2012; Yang et al. 2011; Zhu et al. 2009) and DNA methylation alterations of the TGF-β pathway (Martin et al. 2015). Additional studies are needed to understand the underlying biology of Cd-associated risk of PE.
In the present study, we used an integrated genomic analysis to examine Cd-driven regulation of the TGF-β pathway in PE, utilizing both clinical samples as well as in vitro experimentation in placental trophoblast cells. Our analysis incorporated placental expression of TGF-β pathway-associated genes and miRNA with placental Cd levels, and Cd-responsive TGF-β pathway-associated genes and miRNA in trophoblasts. This study aimed to examine the direct effects that Cd has on the genome and epigenome of placental cells in order to further elucidate the underlying biology that supports the pathology of PE and to provide future therapeutic targets for treatment.
2. Methods
Placental sample collection
Placentas from a total of 32 women (16 normotensive controls and 16 preeclamptic cases) were included in this study. The women received obstetric care at UNC hospitals and consented to collection of samples at the time of birth as detailed in our previous study (Martin et al. 2015). The American Congress of Obstetricians and Gynecologists diagnosed preeclampsia as sustained de novo hypertension (>140/90 mmHg) and proteinuria (>300mg of protein in a 24 h urine collection or urine protein/creatinine ratio of 0.3mg/dL) after 20 weeks of pregnancy. All preeclamptic women displayed severe features of BP′>160/100 with neurologic dysfunction, renal dysfunction, and/or evidence of HELLP syndrome. Women with confounding conditions such as pre-diabetes, diabetes, and gestational diabetes were excluded from the study. A full-thickness placental biopsy was obtained after delivery, avoiding the periphery and areas of obvious infarction, flash frozen in liquid nitrogen, and subsequently stored at -80C until analysis. Placental Cd concentrations were measured using ICP-MS as detailed (Laine et al. 2015). This research was approved by the Institutional Review Board at the University of North Carolina (#11-2054).
Cell culture methodology
JEG-3 cells, a human placental trophoblast cell line, were used for all cell culture analyses. This cell line maintains characteristics of first trimester trophoblast cells (Matsuo and Strauss 1994) and has been used to assess biological effects of exposures to environmental toxicants (Bergemann et al. 2003; Canettieri et al. 2008; Ding et al. 2011; Koc et al. 2003; Matscheski et al. 2006; Adebambo et al. 2015; Guan et al. 2013; Kummu et al. 2012; Alvarez and Chakraborty 2011). Cells were grown in Dulbecco’s Eagle Minimum Essential Media with 10% FBS, sodium pyruvate, and penicillin/streptomycin at 37°C with 5% CO2. Cd chloride (CdCl2) was dissolved into the media by vortex for final concentrations of 1, 10, and 25 uM.
Cell viability assay
Cells were seeded in 96-well black clear bottom plates at 10,000/mL with a total of 200uL of media. Three-fold dilutions of Cd chloride were carried out starting at 2,000 uM until a final concentration of 0.001uM was reached. Experiments were carried out in biological triplicate. Following a 24 or 48-hour incubation, media was removed and cell viability measured by the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI). Luminescence was measured using a Promega GloMax Microplate Luminometer. IC10 and IC50 concentrations were established and used for subsequent experimentation.
Gene and miRNA expression
For clinical samples, a 0.2 g subsection of placental tissue was cut from each frozen biopsy on dry ice, washed briefly in sterile 1X PBS to remove any residual blood, and homogenized in Buffer RLT with B-mercaptoethanol (Qiagen, Valencia CA). DNA and RNA sequences greater than 18 nucleotides in length were collected using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, Valencia CA) according to manufacturer’s instructions. RNA abundance was analyzed using the Affymetrix GeneChip ® Human Gene 2.0 ST array as described previously (Rager et al. 2014). Data were RMA processed using Partek Genomics Suite 6.4 (St Louis, Missouri). These data are available at the Gene Expression Omnibus (GEO) (GSE73377). For miRNAs, RNA was amplified, labeled, and hybridized on Agilent Human miRNA (8 × 60k) Oligo Microarrays. The green median signal was extracted and quantile normalized using Partek Genomics Suite 6.4 (St Louis, Missouri). These data are available at the Gene Expression Omnibus (GEO).
For the in vitro assays, both large and small RNAs were extracted simultaneously from JEG-3 cells using the Qiagen’s Allprep kit allowing the purification of a single fraction containing all RNA ≥ 18 nucleotides. The quantity of isolated RNA was measured with the Nanodrop 1000 spectrophotometer, and its integrity verified by the Agilent 2100 Bioanalyzer. A total of 200ng of RNA was amplified, labeled, and hybridized on Agilent Human miRNA v.16 (8 × 60k) Oligo Microarrays. The green median signal was extracted and probes that expressed values of 1 deviation from the mean of the negative controls in more than 50% of the samples were removed. Resulting data were quantile normalized using Partek Genomics Suite 6.4 (St Louis, Missouri) and used for further analyses.
Real-time PCR analysis cDNA was generated using either Qiagen’s RT2 First Strand kit or miScript II RT Kit for gene and miRNA analysis, respectively. Quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) performed and assessed using the TGF-β/BMP Signaling Pathway RT2 Profiler PCR Array (Valencia, CA), which tests 84 genes related to TGF-β signal transduction. Raw CT values from the RT2 Profiler PCR Arrays were analyzed for subsequent statistical testing. Validation of miRNAs was completed using Qiagen’s miScript Primer Assays and SYBR Green PCR kits. Experiments were carried out in biological triplicate, and any outliers removed prior to statistical analyses. The miRNA U6 was used as a housekeeping gene.
Statistical Analysis
To assess gene expression and miRNA differences between preeclamptic vs. non-preeclamptic placentas, multivariable models were applied to the 53,637 probes on the Affymetrix GeneChip ® Human Gene 2.0 ST array and the 56,044 probes on the Agilent Human miRNA v.16 (8 × 60k) Oligo Microarrays. These models controlled for gestational age, maternal age and race. Differential RNA expression was statistically defined using a false discovery rate (FDR) corrected q≤0.15. Cd-associated mRNAs were analyzed separately among cases and controls using multivariable regression models. The models controlled for maternal age, gestational age, race, and smoking. Statistical significance was again defined as q≤0.15. A database of miRNAs (n=651) was identified in association with canonical members of the TGF-β pathway involved in signal transduction using TargetScan7.0 (Lewis et al. 2003) and miRBase (Landgraf et al. 2007). Pearson correlation analysis was conducted for miRNAs to determine associations with their target mRNAs. Statistical significance was set at p<0.05.
T-tests were performed to assess statistical significance of the TGF-β Signaling Targets RT2 Profiler PCR Array data using Qiagen’s Data Analysis Portal. CT values of the miRNA validation were used to calculate 2^ (−delta CT) values for cells exposed to 1uM or 10uM CdCl2 for 48-hours and controls and used for analysis of covariance (ANCOVA) to assess for differential miRNA expression. Differentially expressed miRNA transcripts were considered statistically significant if q ≤0.15.
3. Results
3.1 Dysregulation of TGF-β pathway-targeting miRNAs in the preeclamptic placenta
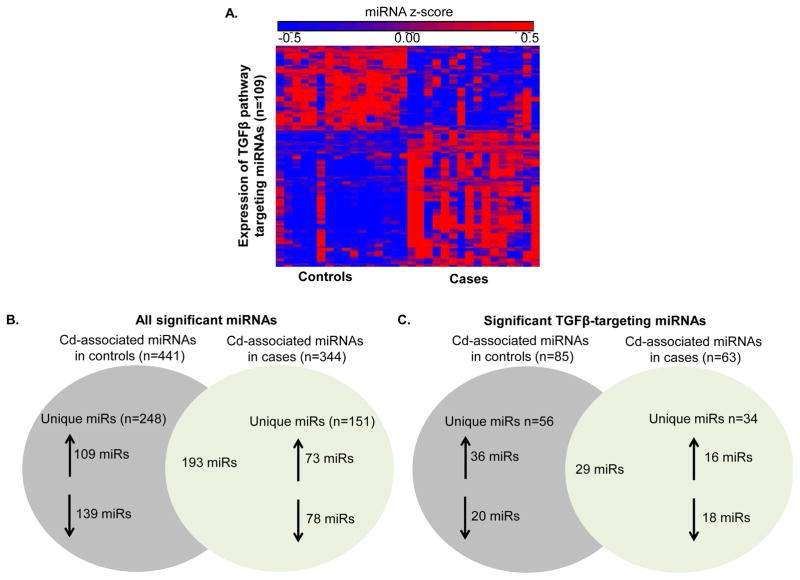
Gene expression was analyzed by genome-wide microarray with a subsequent focused analysis on 84-targeted TGF-β pathway-associated genes for 16 normotensive (control) and 16 preeclamptic (cases) placentas, which resulted in the identification of a total of 31 TGF-β pathway genes that were significantly increased in the preeclamptic placenta (Supplemental Table 1). We set out to assess whether miRNAs may regulate the expression of these TGF-β-associated genes. Of 651 compiled miRNAs that target canonical members of the TGF-β pathway, 109 were identified as differentially expressed in the preeclamptic placenta. These miRNAs tended to be upregulated in the preeclamptic placenta, where 63 miRNAs displayed increased expression and 46 miRNAs displayed decreased expression (Figure 1A, Supplemental Table 2). These identified TGF-β pathway-associated miRNAs were a subset of a broader genome-wide miRNA analysis where a total of 631 out of 861 (73%) tested human miRNAs were differentially expressed in the preeclamptic placenta, 17% (109 out of 631) of which target the TGF-β pathway. Among these miRNAs are 52 that have previously been shown to be altered in preeclampsia (Harapan and Yeni 2015; D. b Chen and W. Wang 2013) (Supplemental Table 2).
Figure 1. miRNA regulation of the TGF-β pathway regulation in the preeclamptic placenta.
A. Differentially expressed TGF-β targeting miRNAs (n=109) from whole-genome miRNA microarray analysis between normotensive (Controls) and preeclamptic (Cases) placentas. B. All significantly expressed miRNAs associated with Cd exposure in Controls (n=441) and Cases (n=344). C. Differentially expressed TGF-β targeting miRNAs in Controls (n=85) and Cases (n=63) associated with Cd exposure.
We next set out to examine the relationship between the TGF-β pathway-associated miRNAs and their target genes of interest. Among the 31 target genes were transforming growth factor beta-1 (TGFB1), transforming growth factor beta receptor 1 and 2 (TGFBR1/2), SMAD family member 2 (SMAD2), SMAD family member 3 (SMAD3), SMAD family member 4 (SMAD4), SMAD specific E3 ubiquitin protein ligase 1 (SMURF1), FBJ murine osteosarcoma viral oncogene homolog (FOS), serpin peptidase inhibitor, clade E, member 1 (SERPINE1), and growth arrest and DNA damage inducible beta (GADD45B). When examining the relationship of TGF-β targeting miRNAs to the target genes of interest, Pearson correlation analysis identified a total of 128 significant miRNA-mRNA relationships; the identified associations were both negative (n=50) and positive (n=78) (Supplemental Table 3). While the relationship between the expression of miRNAs and their target mRNAs is generally expected to have an inverse association, these data highlight the complex mechanisms of miRNA regulation (Supplemental Table 3). Of the 31 TGF-β pathway-associated genes, most (n=23, 74%) displayed a significant association with a TGFβ-associated miRNA suggesting a role for miRNAs as mediators of this pathway.
3.2 Cd-associated dysregulation of TGF-β pathway-targeting miRNAs in the preeclamptic placenta
Next, we performed multivariable regression analysis to test whether miRNA expression in the placenta was associated with placental Cd concentrations. Cd levels were assessed in both normotensive subjects (controls; range: 1.01–10.42 ng/g) and preeclamptics (cases; .35–8.01 ng/g) and examined for their relationships to altered miRNA expression. As a result, 441 and 344 miRNAs were identified in controls and cases, respectively (Figure 1B). miRNAs that target the TGF-β pathway had robust expression changes in relation to Cd levels, with a subset of miRNAs (n=29) common to both cases and controls (Figure 1C). Cd levels were associated with substantial changes in the expression of miRNA targeting the TGF-β pathway in cases and controls, potentially highlighting distinct Cd-induced miRNA regulation that drive the development of PE.
3.3 Cd induces gene expression of the TGF-β pathway members in trophoblast cells
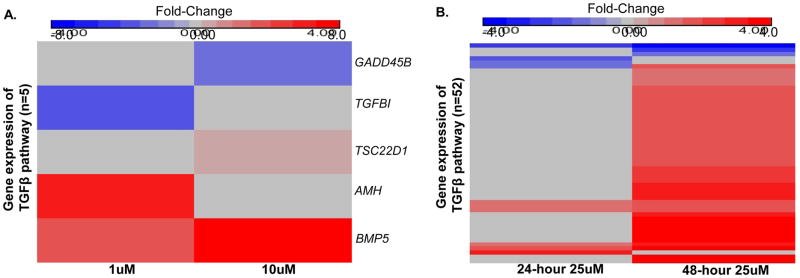
To better understand the relationship between Cd exposure and the regulation of the TGF-β pathway in the placenta, we performed in vitro experiments utilizing JEG-3 cells, a human placental trophoblast cell line. JEG-3 cells were exposed in culture to minimally cytotoxic (<5% cell death) concentrations of 1 and 10uM CdCl2 for 48 hours. These concentrations are similar to previous studies that have used low Cd concentrations (≤1uM) (Adebambo et al. 2015; Guan et al. 2013; Kummu et al. 2012) to evaluate biological disruptions in placental cells as a result of treatment, particularly anti-migratory effects that can influence risk of PE (Alvarez and Chakraborty 2011). Gene expression was analyzed by RT-PCR for 84-targeted TGF-β pathway-associated genes using RNA isolated from untreated JEG-3 cells or JEG-3 cells treated with Cd (Supplemental Table 4). Following a 48-hour incubation, five unique genes were significantly expressed in JEG-3 cells treated with either 1uM or 10uM Cd concentrations compared to untreated cells (Figure 2A), with most (n=3) genes displaying increased expression in response to Cd.
Figure 2. Cd alters the expression of the TGF-β pathway in placental trophoblast cells.
Quantitative PCR results of differentially expressed genes within the TGF-β pathway and corresponding downstream targets in JEG-3 placental cells following treatment with 1uM or 10uM CdCl2 for 48 hours (A) and 25uM CdCl2 for 24 and 48 hours (B).
We next analyzed the effects of Cd treatment on the TGF-β pathway-associated genes using the moderately cytotoxic (<50% cell death) concentration of 25uM CdCl2 for 24 and 48 hours (Figure 2B). This concentration was selected as we aimed to contrast these cellular responses in vitro to PE, a disease attributed to placental toxicity. A total of 52 out of 84 genes tested (62%) displayed significantly altered expression after the 24 or 48-hour Cd treatments (Figure 2B). Most of the significantly changed genes displayed increased expression in response to a 48-hour treatment of Cd. Specifically, at the 48-hour time point 46 out of 49 genes showed an increase in expression, while 6 out of 10 genes were increased after the 24-hour treatment.
3.4 Cd activates the TGF-β pathway targeting miRNAs in trophoblast cells
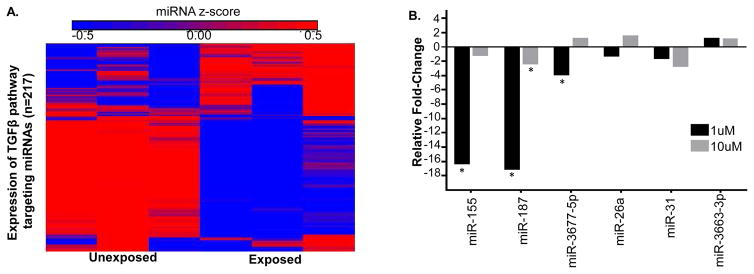
Next, to investigate the regulation of the TGF-β pathway by miRNAs, a genome-wide miRNA microarray analysis was completed using cells treated with 48-hour 25uM CdCl2 since it displayed the most robust genomic response to Cd. The expression levels of 99 out of 651 TGF-β pathway-targeting miRNAs displayed significantly differential expression. The majority of these miRNAs displaying decreased expression (n=64) in relation to Cd (Figure 3A, Supplemental Table 1). These data demonstrate a general inverse relationship between Cd-induced gene expression of the TGF-β pathway and the Cd-induced repression of miRNAs that target the pathway. However, there were both positive (n=36) and negative (n=65) correlations between the expression of TGFB-associated miRNAs and their mRNA targets (Supplemental Table 5). These changes were a subset of a broader genome-wide Cd-induced miRNA response where 466 miRNAs were significantly altered, with 181 upregulated and 285 downregulated (Supplemental Table 1). Similar to the preeclamptic placenta, approximately 20% (99 out of 466) of the altered miRNAs in Cd-treated placental trophoblast cells target the TGF-β pathway.
Figure 3. Expression of miRNAs that regulate the TGF-β pathway in Cd-treated trophoblast cells.
A. Differential expression of TGF-β targeting miRNAs (n=99) from whole-genome miRNA microarray analysis in JEG-3 placental trophoblast cells treated with 25uM CdCl2 for 48 hours. B. Quantitative PCR results of selected miRNAs in JEG-3 cells treated with 1uM or 10uM CdCl2 for 48 hours. miR-155, miR-187*, and miR-3677 were significantly decreased in the 1uM group. The 10uM group expressed significant downregulation miR-187*. Cells treated with 1uM or 10uM CdCl2 were compared to untreated cells to determine fold-change. ANOVA analysis was used to determine significance (*) that was defined as a p-value less than 0.05 and a q-value less than 0.15.
To further examine Cd-responsive miRNAs in trophoblasts treated with the minimally cytotoxic concentrations, a total of six miRNAs were analyzed by quantitative PCR for their expression in JEG-3 cells treated with 1uM or 10uM concentrations of CdCl2 for 48 hours (Figure 3B). These miRNAs were selected because they were TGF-β-associated miRNAs significantly expressed in response to Cd in the preeclamptic placenta (miR-31* and miR-3677-5p), TGF-β-associated miRNAs previously established to be expressed in preeclamptic placenta (miR-26a and miR-155), or miRNAs that had significant expression in JEG-3 cells exposed to 25uM Cd for 48 hours (miR-3663-3p and miR-187*). Following ANOVA analysis, miR-155, miR-187*, and miR-3677-5p were significantly downregulated compared to control in the 1uM group, while miR-187* had significantly decreased expression in the 10uM group compared to control (Figure 3B). Similar to the gene expression data, the minimally cytotoxic Cd concentrations yielded fewer significantly expressed miRNAs in Cd-treated trophoblasts compared to the moderately cytotoxic Cd concentration. Interestingly, the TGFB-associated miR-3677-5p was significantly decreased in the 1 uM group while its predicted gene target bone morphogenetic proteins 5 (BMP5) (Garcia et al. 2011) was substantially increased (fold-change = 3.8) in response to Cd (Figure 2A), suggesting a possible Cd-driven miRNA mechanism for the regulation of the TGF-β pathway.
3.5 A comparison of Cd-associated dysregulation of the TGF-β pathway genes and targeting miRNAs in the preeclamptic placenta and placental trophoblast cells
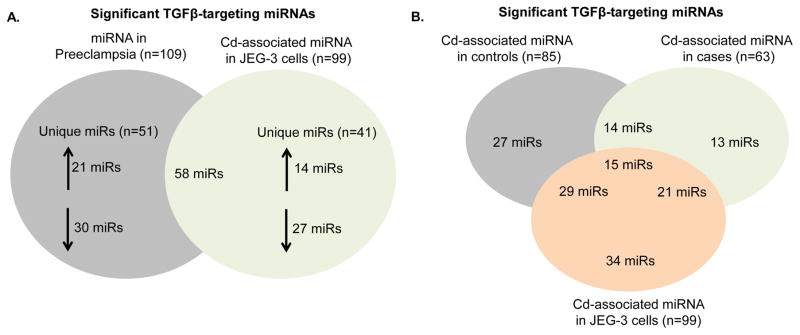
After completing miRNA analysis in both placentas and placental cells, the 109 differentially expressed TGF-β pathway-targeting miRNAs in the preeclamptic placenta and the 99 TGF-β pathway-targeting miRNAs expressed in the 48-hour 25uM Cd-treated trophoblasts were compared. Many (n=58) were common to both groups (Figure 4A). Furthermore, 15 TGF-β pathway-targeting miRNAs were associated with Cd in placental trophoblasts cells, the normotensive placenta, and the preeclamptic placenta (Figure 4B). miRNAs expressed in all three Cd-associated groups generally targeted ligands that activate the TGF-β pathway such as BMP2 and BMP5, as well as TGF-β pathway members responsible for signal transduction such as TGFBR1, SMAD1, SMAD2, and SMAD5. Specifically, miR-26a and miR-155, which target SMAD1 and SMAD2 respectively, were significantly differentially expressed in all three groups, with miR-155 significantly decreased in 48-hour 1uM Cd-treated trophoblast as well. Furthermore, these miRNAs were observed to be associated with PE (Harapan and Yeni 2015), further bolstering a possible mechanism for Cd-associated PE.
Figure 4. Venn diagram of TGF-β pathway targeting miRNAs in control or case placentas and trophoblast cells.
TGF-β-targeting miRNAs uniquely expressed or overlapping among 25uM Cd-treated placental trophoblast cells and the preeclamptic placenta (A). TGF-β-targeting miRNAs uniquely expressed or overlapping among Cd-treated (25uM) placental trophoblast cells, and placentas (cases and controls) in association with Cd.
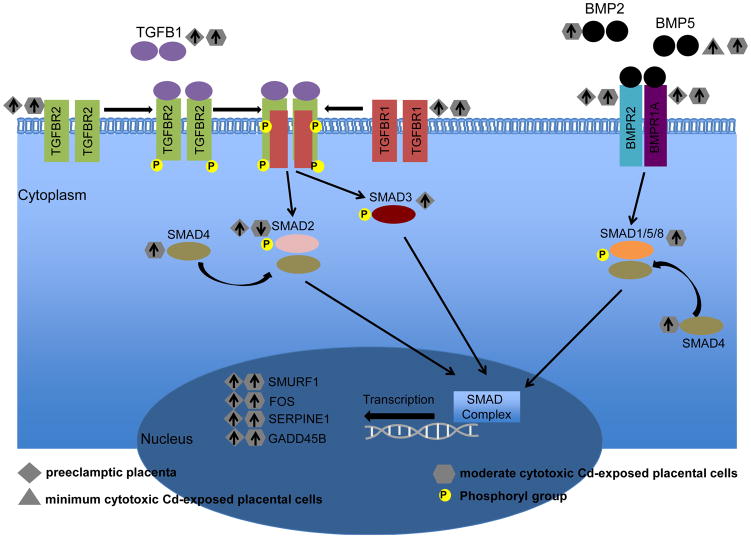
Strong similarities of gene expression involving the TGF-β pathway were also noticeable between placenta and placental cells. Notably, 26 significantly expressed TGF-β pathway genes in Cd-treated placental trophoblast cells were present within the 31 genes altered in the preeclamptic placenta. Among these was TGF-β pathway members TGFBR1, TGFBR2, BMP2, and BMP5, which play key roles in the signal transduction of the TGF-β and BMP signaling pathways, and thus SMAD activation. Moreover, the downstream targets of these pathways, SMURF1, FOS, SERPINE1, and GADD45B, displayed increased expression in placenta and Cd-treated trophoblasts (Figure 5).
Figure 5. Dysregulation of the TGF-β pathway in the preeclamptic placenta and Cd-treated placental trophoblast cells.
Increased (up arrow) or decreased (down arrow) gene expression of canonical members of the TGF-β pathway in preeclamptic placenta and JEG-3 placental trophoblast cells treated with either minimum or moderate cytotoxic CdCl2 concentrations.
4. Discussion
In the present study, we set out to elucidate a potential molecular mechanism by which Cd exposure increases the risk for PE. Using a translational approach that integrates cell culture experimentation with clinical sample analysis, we establish a novel link between the induction of genes within the TGF-β pathway both in the preeclamptic placenta and in Cd-exposed placental cells. Furthermore, we highlight miRNA suppression that may underlie the induction of the TGF-β pathway in response to Cd as an epigenetic mechanism that mediates expression. Investigating the underlying biology of PE and potential environmental influences can provide insight into the specific effects of toxicants on placental molecular phenotypes that increase risk of PE, as well as better treatment options for patients.
One of the main hypotheses for the development of PE is poor angiogenesis in the placenta leading to a hypoxic environment. Numerous growth factors exhibit aberrant maternal and placental expression in preeclamptic patients as compared to women who are normotensive during pregnancy (Nikuei et al. 2015). One family of growth factors is TGF-β, which promotes an anti-migratory signal in trophoblast cells. Here, we analyzed placentas from preeclamptic and normotensive women, as well as JEG-3 placental trophoblast cells treated with Cd, in order to investigate the induction of the TGF-β pathway. TGF-β receptors I and II were among the TGF-β pathway-associated genes that displayed increased expression in relation to PE as well as placental cells exposed to Cd. TGF-β receptors I and II activate the signal transduction of the TGF-β pathway upon the binding of the TGF-β superfamily of ligands. Further studies are needed to reveal how specifically Cd influences the TGF-β pathway in placentas and placental cells.
Currently, there are no treatment options for women with PE except for premature delivery of the placenta/fetus (Chaiworapongsa et al. 2014). Elucidating the molecular events that lead to PE can facilitate the development of safer treatment options. We hypothesized that not only could Cd alter expression of the TGF-β pathway in placentas and in placental trophoblasts, but also that the induction of the TGF-β pathway was regulated through miRNA-mediated mechanisms. We observed significant alterations of miRNAs that target genes associated with TGF-β in both the preeclamptic placenta and JEG-3 cells following treatment of Cd. In support of our findings, related to miRNA dysregulation of the preeclamptic placenta, we identified a total of 52 miRNAs that are dyregulated in the PE placenta and overlap with other studies (D. B. Chen and W. Wang 2013). Among these are miRNAs belonging to the primate-specific miRNA gene cluster (C19MC) imprinted in the placenta including miR-517c, miR-518c, miR-519d, and miR-520h (Noguer-Dance et al. 2010). Moreover, previous studies have elucidated associations of miR-26a and miR-155 with PE, two miRNAs in our study that had significant Cd-altered expression in preeclamptic placenta, normotensive placenta, and JEG-3 cells treated with a moderate Cd concentration. SMAD1 and SMAD2, transcription factors that induce downstream TGF-β pathway genes, are predicted targets of miR-26a and miR-155 respectively and displayed altered gene expression in the preeclamptic placenta and in Cd-treated trophoblasts in this study; highlighting potential focuses for future studies to better understand Cd-mediated regulation of the TGF-β pathway.
While this study provides novel information on Cd-induced expression and epigenetic reprogramming that can further enhance our understanding of the genomic-epigenomic interactions that can drive preeclampsia, it is not without limitations. Further studies utilizing a larger clinical cohort of normotensive and preeclamptic women will be needed to validate these results and identify Cd-specific mechanism for miRNA-mediated regulation of angiogenic pathways, such as TGF-β. In addition, primary placental trophoblast cells should be used in future studies to identify Cd-specific phenotypes of genetic disruption. Furthermore, future studies should also involve a range of cytotoxic concentrations of Cd and other metals to examine the molecular regulation of the TGF-β pathway. Our data suggest higher Cd concentrations may be needed in vitro to observe comparable biological features of the preeclamptic placenta and that a miRNA feedback mechanism may be existent to contest the Cd-induced expression of the TGF-β pathway, highlighting the complexity of metal-induced toxicity in the placenta that can increase risk of PE.
Taken together our results demonstrate that miRNA dysregulation is a feature of PE and is enhanced with Cd exposure. In both the preeclamptic placenta and placental trophoblasts, dysregulated miRNA signaling was associated with altered expression signaling of the TGF-β pathway, which may ultimately diminish trophoblast migratory capabilities. This study provides novel evidence of a relationship between Cd-induced expression and epigenetic reprogramming in the placenta that can further enhance our understanding of the interactions between environmental exposures and genetics that may drive preeclampsia.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Health, specifically the National Institute of Environmental Health Sciences (NIEHS) including R01-ES019315, T32-ES007018, P42-ES005948.
Footnotes
The authors claim no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–480. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116(6):1302–1309. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakashima A. A review of the mechanism for poor placentation in early-onset preeclampsia: the role of autophagy in trophoblast invasion and vascular remodeling. J Reprod Immunol. 2014;101–102:80–88. doi: 10.1016/j.jri.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Brooks SA, Rathmell WK. Uniting Molecular Biomarkers to Advance the Science and Care of Clear Cell Renal Cell Carcinoma. The Journal of OncoPathology. 2014;1(4):45–54. [Google Scholar]
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Nikuei P, Malekzadeh K, Rajaei M, Nejatizadeh A, Ghasemi N. The imbalance in expression of angiogenic and anti-angiogenic factors as candidate predictive biomarker in preeclampsia. Iran J Reprod Med. 2015;13(5):251–262. [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction. 2006;132(2):217–232. doi: 10.1530/rep.1.01076. [DOI] [PubMed] [Google Scholar]
- Conti E, Zezza L, Ralli E, Caserta D, Musumeci MB, Moscarini M, et al. Growth factors in preeclampsia: a vascular disease model. A failed vasodilation and angiogenic challenge from pregnancy onwards? Cytokine Growth Factor Rev. 2013;24(5):411–425. doi: 10.1016/j.cytogfr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Esteban-Vasallo MD, Aragones N, Pollan M, Lopez-Abente G, Perez-Gomez B. Mercury, cadmium, and lead levels in human placenta: a systematic review. Environ Health Perspect. 2012;120(10):1369–1377. doi: 10.1289/ehp.1204952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik N, Kuhnert BR, Kuhnert PM. Zinc, cadmium, and hypertension in parturient women. Am J Obstet Gynecol. 1989;161(2):437–440. doi: 10.1016/0002-9378(89)90538-3. [DOI] [PubMed] [Google Scholar]
- Pollack AZ, Ranasinghe S, Sjaarda LA, Mumford SL. Cadmium and Reproductive Health in Women: A Systematic Review of the Epidemiologic Evidence. Current Environmental Health Reports. 2014;1(2):172–184. doi: 10.1007/s40572-014-0013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine JE, Ray P, Bodnar W, Cable PH, Boggess K, Offenbacher S, et al. Placental Cadmium Levels Are Associated with Increased Preeclampsia Risk. PLoS One. 2015;10(9):e0139341. doi: 10.1371/journal.pone.0139341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118(2):182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Qiu Z, Diao Z, Shen L, Xue P, Sun H, et al. MicroRNA-155 inhibits proliferation and migration of human extravillous trophoblast derived HTR-8/SVneo cells via down-regulating cyclin D1. Placenta. 2012;33(10):824–829. doi: 10.1016/j.placenta.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Gao WL, Liu M, Yang Y, Yang H, Liao Q, Bai Y, et al. The imprinted H19 gene regulates human placental trophoblast cell proliferation via encoding miR-675 that targets Nodal Modulator 1 (NOMO1) RNA Biol. 2012;9(7):1002–1010. doi: 10.4161/rna.20807. [DOI] [PubMed] [Google Scholar]
- Gunel T, Zeybek YG, Akcakaya P, Kalelioglu I, Benian A, Ermis H, et al. Serum microRNA expression in pregnancies with preeclampsia. Genet Mol Res. 2011;10(4):4034–4040. doi: 10.4238/2011.November.8.5. [DOI] [PubMed] [Google Scholar]
- Ishibashi O, Ohkuchi A, Ali MM, Kurashina R, Luo SS, Ishikawa T, et al. Hydroxysteroid (17-beta) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: a novel marker for predicting preeclampsia. Hypertension. 2012;59(2):265–273. doi: 10.1161/HYPERTENSIONAHA.111.180232. [DOI] [PubMed] [Google Scholar]
- Lazar L, Nagy B, Molvarec A, Szarka A, Rigo J., Jr Role of hsa-miR-325 in the etiopathology of preeclampsia. Mol Med Rep. 2012;6(3):597–600. doi: 10.3892/mmr.2012.954. [DOI] [PubMed] [Google Scholar]
- Li P, Guo W, Du L, Zhao J, Wang Y, Liu L, et al. microRNA-29b contributes to pre-eclampsia through its effects on apoptosis, invasion and angiogenesis of trophoblast cells. Clin Sci (Lond) 2013;124(1):27–40. doi: 10.1042/CS20120121. [DOI] [PubMed] [Google Scholar]
- Noack F, Ribbat-Idel J, Thorns C, Chiriac A, Axt-Fliedner R, Diedrich K, et al. miRNA expression profiling in formalin-fixed and paraffin-embedded placental tissue samples from pregnancies with severe preeclampsia. J Perinat Med. 2011;39(3):267–271. doi: 10.1515/jpm.2011.012. [DOI] [PubMed] [Google Scholar]
- Wang W, Feng L, Zhang H, Hachy S, Satohisa S, Laurent LC, et al. Preeclampsia up-regulates angiogenesis-associated microRNA (i.e., miR-17, -20a, and -20b) that target ephrin-B2 and EPHB4 in human placenta. J Clin Endocrinol Metab. 2012;97(6):E1051–1059. doi: 10.1210/jc.2011-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhou H, Lin H, Qi J, Zhu C, Gao Z, et al. Circulating microRNAs are elevated in plasma from severe preeclamptic pregnancies. Reproduction. 2012;143(3):389–397. doi: 10.1530/REP-11-0304. [DOI] [PubMed] [Google Scholar]
- Yang Q, Lu J, Wang S, Li H, Ge Q, Lu Z. Application of next-generation sequencing technology to profile the circulating microRNAs in the serum of preeclampsia versus normal pregnant women. Clin Chim Acta. 2011;412(23–24):2167–2173. doi: 10.1016/j.cca.2011.07.029. [DOI] [PubMed] [Google Scholar]
- Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009;200(6):661, e661–667. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- Martin E, Ray PD, Smeester L, Grace MR, Boggess K, Fry RC. Epigenetics and Preeclampsia: Defining Functional Epimutations in the Preeclamptic Placenta Related to the TGF-beta Pathway. PLoS One. 2015;10(10):e0141294. doi: 10.1371/journal.pone.0141294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H, Strauss JF., 3rd Peroxisome proliferators and retinoids affect JEG-3 choriocarcinoma cell function. Endocrinology. 1994;135(3):1135–1145. doi: 10.1210/endo.135.3.8070357. [DOI] [PubMed] [Google Scholar]
- Bergemann C, Reimer T, Muller H, Hosel A, Briese V, Friese K, et al. Stimulation of hCG protein and mRNA levels in trophoblast tumour cells Jeg3 and BeWo by glycodelin A. Anticancer Res. 2003;23(2A):1107–1113. [PubMed] [Google Scholar]
- Canettieri G, Franchi A, Guardia MD, Morantte I, Santaguida MG, Harney JW, et al. Activation of thyroid hormone is transcriptionally regulated by epidermal growth factor in human placenta-derived JEG3 cells. Endocrinology. 2008;149(2):695–702. doi: 10.1210/en.2007-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Ma L, Wang XZ, Zhang J, Zhao GZ, Wang ZQ, et al. In vitro study on hepatitis B virus infecting human choriocarcinoma JEG3 cells and its mechanism. Intervirology. 2011;54(5):276–281. doi: 10.1159/000324528. [DOI] [PubMed] [Google Scholar]
- Koc S, Kather A, Markert UR, Durst M, Schneider A, Kaufmann AM. Enhancement of immunogenicity of Jeg3 cells by ectopic expression of HLA-A*0201 and CD80. Am J Reprod Immunol. 2003;50(3):243–253. doi: 10.1034/j.1600-0897.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- Matscheski A, Richter DU, Hartmann AM, Effmert U, Jeschke U, Kupka MS, et al. Effects of phytoestrogen extracts isolated from rye, green and yellow pea seeds on hormone production and proliferation of trophoblast tumor cells Jeg3. Horm Res. 2006;65(6):276–288. doi: 10.1159/000092591. [DOI] [PubMed] [Google Scholar]
- Adebambo OA, Ray PD, Shea D, Fry RC. Toxicological responses of environmental mixtures: Environmental metal mixtures display synergistic induction of metal-responsive and oxidative stress genes in placental cells. Toxicol Appl Pharmacol. 2015;289(3):534–541. doi: 10.1016/j.taap.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Sun K, Yang K. The ERK1/2 signaling pathway regulates 11beta-hydroxysteroid dehydrogenase type 2 expression in human trophoblast cells through a transcriptional mechanism. Biol Reprod. 2013;89(4):92. doi: 10.1095/biolreprod.113.110924. [DOI] [PubMed] [Google Scholar]
- Kummu M, Sieppi E, Wallin K, Rautio A, Vahakangas K, Myllynen P. Cadmium inhibits ABCG2 transporter function in BeWo choriocarcinoma cells and MCF-7 cells overexpressing ABCG2. Placenta. 2012;33(10):859–865. doi: 10.1016/j.placenta.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Alvarez MM, Chakraborty C. Cadmium inhibits motility factor-dependent migration of human trophoblast cells. Toxicol In Vitro. 2011;25(8):1926–1933. doi: 10.1016/j.tiv.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Rager JE, Yosim A, Fry RC. Prenatal exposure to arsenic and cadmium impacts infectious disease-related genes within the glucocorticoid receptor signal transduction pathway. Int J Mol Sci. 2014;15(12):22374–22391. doi: 10.3390/ijms151222374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harapan H, Yeni CM. The role of microRNAs on angiogenesis and vascular pressure in preeclampsia: The evidence from systematic review. Egyptian Journal of Medical Human Genetics. 2015;16(4):313–325. [Google Scholar]
- Chen Db, Wang W. Human Placental MicroRNAs and Preeclampsia. Biol Reprod. 2013;88(5):130–130. doi: 10.1095/biolreprod.113.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia DM, Baek D, Shin C, Bell GW, Grimson A, Bartel DP. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat Struct Mol Biol. 2011;18(10):1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DB, Wang W. Human placental microRNAs and preeclampsia. Biol Reprod. 2013;88(5):130. doi: 10.1095/biolreprod.113.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguer-Dance M, Abu-Amero S, Al-Khtib M, Lefevre A, Coullin P, Moore GE, et al. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum Mol Genet. 2010;19(18):3566–3582. doi: 10.1093/hmg/ddq272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.