Abstract
This study examined potential prevention of music-induced temporary threshold shift (TTS) in normal-hearing participants. A dietary supplement composed of β-carotene, vitamins C and E, and magnesium was assessed using a randomized, placebo-controlled, double-blind study design. Dosing began 3 days prior to the music exposure with the final dose consumed approximately 30-min pre-exposure. There were no group differences in post-exposure TTS or music-induced decreases in distortion product otoacoustic emission (DPOAE) amplitude. Transient tinnitus was more likely to be reported by the treatment group, but there were no group differences in perceived loudness or bothersomeness. All subjects were monitored until auditory function returned to pre-exposure levels. Taken together, this supplement had no effect on noise-induced changes in hearing. Recommendations for future clinical trials are discussed.
Keywords: temporary threshold shift, β-carotene, vitamin C, vitamin E, magnesium, digital audio player, distortion product otoacoustic emission, tinnitus
Introduction
Noise-induced hearing loss (NIHL) and noise-induced tinnitus are the top two disabilities for returning military personnel [US Department of Veterans Affairs, 2014] and NIHL is one of the top two occupational illnesses [US Bureau of Labor Statistics, 2014]. Protection against NIHL is currently accomplished using engineering controls that reduce hazardous sound levels of equipment at the source, administrative controls that reduce exposure time to hazardous sounds, and hearing protection devices (HPDs) such as earplugs and earmuffs that attenuate the intensity of hazardous sound exposures at the user level [OSHA, 1983]. Collectively, these measures can reduce noise exposure, but user error during HPD insertion, poor compliance with recommendations for HPD use, financial cost of equipment modification, and other difficulty with implementation of noise control measures decrease overall effectiveness [Davies et al., 2012; Okpala, 2007; Suter, 2012].
One of the potential strategies that might improve hearing loss prevention outcomes is the attenuation of metabolic stress of the inner ear via therapeutic compounds, as oxidative stress plays a key role in the development of NIHL. In animal models, reduction of noise-induced metabolic stress has resulted in successful protection against NIHL using a variety of agents with antioxidant action [for detailed review, see Le Prell and Bao, 2012]. A number of these agents have also reduced acquired hearing loss secondary to aminoglycoside antibiotics and cisplatin ototoxicity [see reviews by Abi-Hachem et al., 2010; Campbell and Le Prell, 2012; Poirrier et al., 2010]. Currently, there are no FDA-approved therapeutics for the prevention of hearing loss in humans; however, some promising clinical test data have emerged [for review, see Le Prell and Lobarinas, 2015]. For example, human temporary threshold shift (TTS) induced by exposure to broadband noise was reduced by the administration of 10-day pre-noise magnesium (Mg) supplement [Attias et al., 2004]. Similarly, a vitamin B12 supplement was found to reduce TTS induced by narrow-band noise exposure, with 8 days of pre-noise treatment [Quaranta et al., 2004]. More recently, Quaranta et al. [2012] reported that alpha lipoic acid reduced TTS induced by pure tone exposure with 10 days of pre-noise dosing. Protection was achieved only using the 10-day pre-noise dosing strategy; no reductions in TTS were achieved with 1-hour pre-noise dosing. Finally, Staffa et al. [2014] reported reductions in TTS induced by unilateral exposure to narrow-band noise using 30-day pre-treatment with coenzyme Q10, in addition to 8 other active agents including lactium, melatonin, choline, Ginkgo biloba, and vitamins E, B1, B6, and B12. When the treatment period was shorter (7 days pre-noise) and the supplement included only coenzyme Q10, TTS induced by white noise was not reduced although noise-induced decreases in distortion product otoacoustic emission (DPOAE) amplitude were ameliorated [Fetoni et al., 2009].
In contrast to the above outcomes from laboratory-based clinical studies, clinical trials that have assessed the prevention of TTS after exposure to real-world noise have provided less promising outcomes. These real-world trials were completed using populations exposed to noise at a discotheque [Kramer et al., 2006], steel [Lin et al., 2010] and textile [Doosti et al., 2014] factories, and military weapons training exercises [Le Prell et al., 2011c; Lindblad et al., 2011]. Exposures were variable among nights at the discotheque, with average exposures being as low as 92 dBA for some participants and as high as 103 dB for others; differences in noise exposure could have introduced significant variability into observed TTS in N-acetylcysteine (NAC) and placebo-treated participants [Kramer et al., 2006]. TTS in factory workers assigned to the placebo condition has been less than 3 dB [Doosti et al., 2014; Lin et al., 2010], and thus reductions in TTS with NAC or ginseng have been small, even if statistically significant. Finally, there was no reliable TTS in placebo-treated subjects or cohorts treated with NAC [Lindblad et al., 2011] or a combination of β-carotene, vitamins C and E, and Mg [Le Prell et al., 2011c], thus eliminating the opportunity to assess protection at the group level. Taken together, the successful use of several antioxidant agents for reducing TTS in humans tested in laboratory models starkly contrasts with the failure to demonstrate reliable protection in trials conducted in real-world settings.
One potential solution to the challenge of screening drugs for otoprotective benefit in humans was proposed by Le Prell et al. [2012] based on the development of a laboratory-based music exposure paradigm incorporating a personal music player. This model represents a real-world ‘noise’ administered within a laboratory setting. The current study assessed the potential prevention of TTS by a dietary supplement using this laboratory-based music exposure. The selection of the active agents within the dietary supplement was motivated by previous studies in mice and guinea pigs showing pre-noise dosing efficacy for NIHL and TTS prevention with the combination of β-carotene, vitamins C and E, and Mg [Le Prell et al., 2011a, b; Le Prell et al., 2007; Tamir et al., 2010].
Materials and Methods
Participants
Advertisements recruiting participants between the ages of 18–31 years were posted at multiple locations on the University campus, inviting young adults with normal hearing to participate in a study on prevention of temporary changes in hearing after listening to music on a digital audio player. A written informed consent packet was reviewed with each prospective participant, including disclosure of data related to inner ear neural damage observed in noise-exposed mice after robust TTS [Kujawa and Liberman, 2006, 2009]. It is essential to disclose unknown risks, as the boundary at which synaptopathy emerges in animals and humans is unknown [Le Prell et al., 2012; Spankovich et al., 2014]. Since this study was completed, it has become clear that smaller TTS insults are not always accompanied by synaptic loss and decreased auditory brainstem response amplitude [Fernandez et al., 2015; Hickox and Liberman, 2014; Jensen et al., 2015]. Ethically, these unknown boundaries must be transparent to all participants in any study expected to produce TTS.
Following written informed consent, a brief health history was completed using a paper survey. Health exclusionary criteria included history of gastrointestinal disorders, bleeding disorders, neurological disorders/frequent headaches, allergy or hypersensitivity to colorant yellow dye No. 5 (tartrazine), and pregnancy. All female participants were required to complete a pregnancy test with negative results. Audiological testing was then conducted to determine if the participant met the criteria for normal hearing for the study (defined below). Participants were asked to avoid loud sound for 48 h prior to any scheduled hearing tests at baseline and during the study, and they were asked not to consume any dietary supplements or aspirin-based products for 48 h preceding study day 1 [the first day of dosing with their assigned clinical trial material (CTM)] and the remainder of the study period. All protocols and procedures were approved by the required Investigational Review Boards, and the study was conducted under the supervision of an NIH-selected data safety monitoring board. This trial was posted on clinicaltrials.gov (NCT00808470), and the CTM and the trial were under the oversight of the US Food and Drug Administration (FDA) (Investigational New Drug application No. 116027, allowed to proceed 8/31/2012).
Audiometric Screening Procedures
Screening procedures included hearing and tinnitus surveys as previously described [Le Prell et al., 2012]. Otoscopy was performed to ensure normal tympanic membrane anatomy and the presence of clear, unobstructed ear canals. Tympanometry was performed using a Grason-Stadler (Eden Prairie, Minn., USA) GSI 38 immittance measurement device that was in compliance with ANSI S3.39 and IEC 601-1 criteria and which was professionally calibrated prior to study onset. Normal middle ear function was defined as tympanometric configurations with middle ear pressure values from −140 to +40 daPa, peak compensated static acoustic admittance (peak Ytm; +200 daPa as the ear canal referent) values from 0.3 to 1.8 ml, and acoustic equivalent volume values from 0.8 to 2.1 cm3. Middle ear pressure values were required to be within these limits as part of the inclusion criteria. Failure in either otoscopy or tympanometry precluded participation in the study, and no additional audiometric testing was completed for participants who failed to meet either criterion.
Audiometric threshold measurements were collected using a GSI 61 diagnostic audiometer with Etymotic Research Inc. (Elk Grove Village, Ill., USA) ER3A insert earphones (calibrated annually according to ANSI 3.6 1996) in a double-walled sound-treated test booth [meeting ANSI/ASA S3.1–1999 (R2008) specifications]. Pure-tone air conduction thresholds were obtained using a modified Hughson-Westlake procedure for test frequencies of 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz with the left ear always tested first. The initial presentation level was 30 dB HL, after which the intensity was decreased in 10-dB steps until the participant failed to respond. Presentation levels were increased in 2-dB steps after each missed tone presentation, and decreased by 6 dB after correct detection responses [following Le Prell et al., 2012; Spankovich et al., 2014]. Ascending runs using 2-dB increments were repeated three times, and threshold was operationally defined as the lowest sound level at which responses were obtained on two out of three ascending runs. Reliability was assessed using repeated tests at 2 and 8 kHz in each ear, and responses were deemed reliable if the difference between test and retest thresholds was <5 dB, a criterion previously used by Fausti et al. [1999]. Bone conduction pure-tone audiometry was performed for test frequencies of 0.25, 0.5, 1, 2, 3, and/or 4 kHz if air conduction threshold values at these frequencies were between 15 and 25 dB HL. Inclusion criteria included: (1) air conduction thresholds no worse than 25 dB HL from 0.25 to 8 kHz, (2) threshold asymmetry <15 dB at all test frequencies, and (3) air-bone gaps <10 dB if air conduction threshold was >15 dB HL but <25 dB HL, and (4) production of repeatable thresholds at 2 and 8 kHz within the screening session. Participants that passed the audiometric screening were invited to proceed in the study.
There were no DPOAE amplitude criteria for study enrollment; however, DPOAE amplitude was measured at the baseline visit. DPOAE measurements were completed using a Mimosa HearID system (Mimosa Acoustics Inc., Champaign, Ill., USA), in combination with an ER10C microphone-earphone assembly (Etymotic Research Inc.). The probe assembly was coupled to the participant’s ear with a disposable foam ear tip and calibrated in the ear using the HearID in-ear calibration protocol. Responses were elicited with two simultaneously presented ‘primary’ tones (frequencies f1 and f2) at an f2/f1 ratio of 1.2, with f2 frequencies of 2, 3, 4, 6, and 8 kHz. Measures of DPOAE response growth (input-output) with increasing stimulus level were obtained at each of the six f2 frequencies, with L1 ranging from 25 to 65 dB SPL, and L2 being 10 dB quieter than L1. During testing, stimulus levels decreased in 5-dB steps within each frequency. DPOAE amplitudes (2f1–f2) and adjacent noise floors were averaged using a simplified stopping rule, with all tests averaged over 10 s. The DPOAE protocol specifically followed a previous protocol that was developed for the purposes of measuring the effects of noise exposure on DPOAE responses in workers exposed to occupational noise insult [Goldman et al., 2006], and is the same protocol used previously to establish decreases in DPOAE amplitude after music player use [Le Prell et al., 2012].
Randomization
Participants that met the eligibility criteria returned to the laboratory prior to their scheduled music exposure dates for randomization to one of the treatment conditions and to pick up masked study supplies; study supplies were stored in a locked refrigerator until they were dispensed to the participant. Supplies were dispensed up to 7 days prior to the scheduled music exposure after participants were randomized to the treatment or control condition using a double-blind process. Participants received a bottle containing either the dietary supplement or placebo tablets and were given a treatment log for recording CTM consumption; the treatment log indicated their specific treatment dates, which began 3 days prior to their scheduled music exposure study date. The log form reminded participants to refrain from taking the fourth daily dose of CTM until they were at the study site, so that the study team could observe and confirm that this dose was consumed. The remaining tablets and the supply log were returned to the study team on the day of the exposure, after consuming the last daily dose. Treatments were randomized into blocks of 4 such that within the first 4 consecutive bottles there were 2 placebo assignments and 2 active treatment assignments. Females were randomized from the top half of the list proceeding downwards, and males were randomized from the bottom half of the list proceeding upwards, such that there were approximately equal numbers of males and females within the two treatment conditions. Participants who were eligible to enroll were compensated USD 15 per hour for their time following randomization. In accordance with IRB-approved study guidelines, participants were free to withdraw from the study at any time, and were compensated for their time up to the point at which they withdrew.
Treatments
Micronutrient treatment was a combination of β-carotene (18 mg), vitamin C (500 mg ascorbic acid), vitamin E (305 mg α-tocopherol acetate), and Mg (287.26 mg magnesium citrate and 6.5 stearate). Dosing with the active agents was the same as in a previous study [Le Prell et al., 2011c], although the CTM were chewable mint-flavored tablets rather than the capsule formulation used in the earlier investigation. Placebo tablets were inactive and identical in appearance to the active tablets. All tablets were manufactured by Patheon Pharmaceuticals (Cincinnati, Ohio, USA) using good manufacturing practices. Active agent concentration and stability were confirmed using analytic techniques performed by the contract manufacturer. The full daily dose required consumption of 6 tablets/day; there were no post-music treatments beyond the 4 days of dosing, which ended on the day of the music exposure.
Study Procedures: Pre-Music
On the day of the music exposure, participants answered a brief series of questions regarding recent noise exposure and any current pre-exposure tinnitus. If present, tinnitus was rated on a visual analogue scale from 1 to 10 for both loudness and bothersomeness with additional questions describing its subjective qualities. Conventional pure-tone air conduction threshold testing was then completed as a measure of individual reliability across test dates (screening vs. pre-music baseline) and to establish pre-music baseline hearing sensitivity for both ears. Pre-music DPOAE testing was completed on both ears, and was followed by the music listening period. The pre-music audiogram was used to calculate music-induced threshold change and to monitor recovery to pre-music baseline.
Study Procedures: Music Exposure
Participants had the option to select between a ‘pop music playlist’ and a ‘rock music playlist’, pre-loaded onto two iPod® Classic (Apple Inc., Cupertino, Calif., USA) devices. All songs were digitally modified as described in Le Prell et al. [2011d] and were delivered as in Le Prell et al. [2012]; the purpose of the modification was to normalize starting level and reduce amplitude excursions across each song and across the playlist. The listening level required to induce a small TTS using these playlists was established as approximately 100-dBA in-ear sound level in an earlier investigation [Le Prell et al., 2012]. After adjusting for free-field equivalent sound levels using a conservative 5-dB adjustment, the corresponding level of free-field sound (on which risk estimates are calculated) is approximately 95 dBA; a value corresponding to 100% of the OSHA daily permissible exposure limit (PEL) [OSHA, 1983]. More precise measurement, where the individual transfer function for each participant’s ear is measured and used to calculate an individual exposure free-field equivalent, typically results in equivalent free-field levels of 5–15 dB less than the measured in-ear level [Berger et al., 2009, Bradley et al., 1987; Levey et al., 2011; Rice et al., 1987; Skrainar et al., 1987; Turunen-Rise et al., 1991; Worthington et al., 2009].
Music was presented via 6i isolator™ earbuds (ER6I; Etymotic Research Inc.) as in Le Prell et al. [2012]. To calibrate the iPod® devices, sound levels were measured with the ER6I earbuds inserted into Type 4157 Artificial Ear Simulators (Brüel & Kjær, Denmark) using the ER6I-15SM 3-flange earphone inserts to provide a tight seal within the external DB2012 ear simulator. Spectral data were sampled virtually continuously (at 0.001-ms intervals) using the PULSE system (version 12.5, Brüel & Kjær) throughout the 4-hour playlists. The data samples entered a multibuffer that automatically exported average sound levels (sum of 1/3-octave bands from 20 Hz to 20 kHz) for the previous 64-second interval at 1-second intervals.
The ER6I earphones were selected for this study, in part, because of their availability in two tip sizes (ER6I-15SM; ER6I-18), allowing them to fit securely into the ear canal for all participants. Clean tips were placed on the earphones for each participant. Immediately prior to the music listening period, participants were instructed not to adjust the volume, pause, stop the music, or skip songs, and the lock button on the iPod was used to prevent any inadvertent changes. Prior to the music exposure, participants were reminded they could withdraw from the study at any time during the music listening period if they were uncomfortable or for any other reason. They were given instructions that they could read, write, study, send text messages, use a laptop (sound muted), or engage in any other quiet activity, and that they could visit the restroom as needed, as long as the earbuds stayed in place and the music continued to play. The participants were also instructed that they should not sleep during the listening period. Participants were checked on at 30-min intervals to ensure compliance with the study procedures during the 4-hour listening period.
Post-Music Functional Tests: Audiometry and DPOAE Tests
Immediately after the 4-hour music playlist ended, participants were surveyed to determine if they had developed any tinnitus symptoms, and they were asked how the music level compared to their own normal listening level for music players. After the exposure ended, conventional pure-tone post-music threshold assessments began 15 min after the exposure and were repeated at 1 h and 15 min, 2 h and 15 min, and 3 h and 15 min post-music. DPOAE amplitudes were measured immediately following each pure-tone threshold assessment. Each timed data collection series ended with a survey for any current tinnitus symptoms. The pure-tone threshold assessments, DPOAE amplitude measurements, and tinnitus questionnaires were repeated the next day and 1 week later to ensure all subjects had complete functional recovery.
Statistical Analyses
Statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) software version 21 (IBM, Armonk, N.Y., USA) and SAS software (SAS Institute Inc., Cary, N.C., USA). During the initial masked analysis of the outcomes for reporting to the DSMB, independent two-tailed t tests were used to assess the potential threshold differences between right and left ears, males and females, and pre-treatment hearing for participants randomized to the two treatment groups, at each frequency. In addition, as per the statistical analysis plan, independent two-tailed t tests were used to evaluate primary outcome of TTS at 4,000 Hz, and secondary outcomes of TTS at other frequencies. Repetition of independent t tests inflates the likelihood of type I statistical errors, but there were no statistically significant differences between the two treatment groups on any functional measure at any of the tested frequencies. Unmasking was performed subsequent to DSMB review of the analyses and outcomes.
Results
Participants Included
A total of 97 volunteers were screened (ages 18–28; mean = 21.64 years, SD = 2.414); 72 enrolled in the study and were randomized to a treatment condition. Of these 72 randomized subjects, 70 completed the study (32 males and 38 females). For the remaining 27 volunteers who were not included in the study, 8 were excluded due to abnormal otoscopy or tympanometry, 13 were excluded due to thresholds outside of the study criteria, 4 who were eligible to participate failed to schedule return appointments for the music listening period and were not randomized to a study arm, 1 withdrew due to illness on the day of exposure, and 1 withdrew on the day of exposure after learning they had not been compliant with the treatment protocol as they took only 1 tablet per day, instead of the requested 6 tablets per day. There was a second participant who similarly consumed only 1 tablet per day; that participant completed the study and the data were analyzed using an intention to treat analysis. This participant received tablets containing the active agent.
Screening and Pre-Music Baseline
Right ears and left ears were compared within the screening and pre-music baseline conditions. There were no statistically significant differences between right and left ears at any test frequency or between the screening and pre-music conditions. Therefore, right and left ears were combined for subsequent analyses. There was a small but statistically significant threshold difference at 3,000 Hz (p < 0.05) and 4,000 Hz (p < 0.05) for the screening thresholds of males versus females. These small threshold differences were approximately 2–3 dB. These small differences are not clinically relevant; however, they parallel the small differences observed in several previous studies with other college student cohorts, with each study cohort showing males to have slightly higher (poorer) average thresholds [Le Prell et al., 2012; Spankovich et al., 2014]. Male and female thresholds were averaged in all subsequent analyses given the small differences. Thresholds were then compared between the placebo condition and the micronutrient treatment group to confirm that the groups were equivalent after randomization. There were no statistically significant differences at any frequency. The remainder of this report discusses the primary and secondary outcomes of interest in this clinical trial.
Temporary Threshold Shift
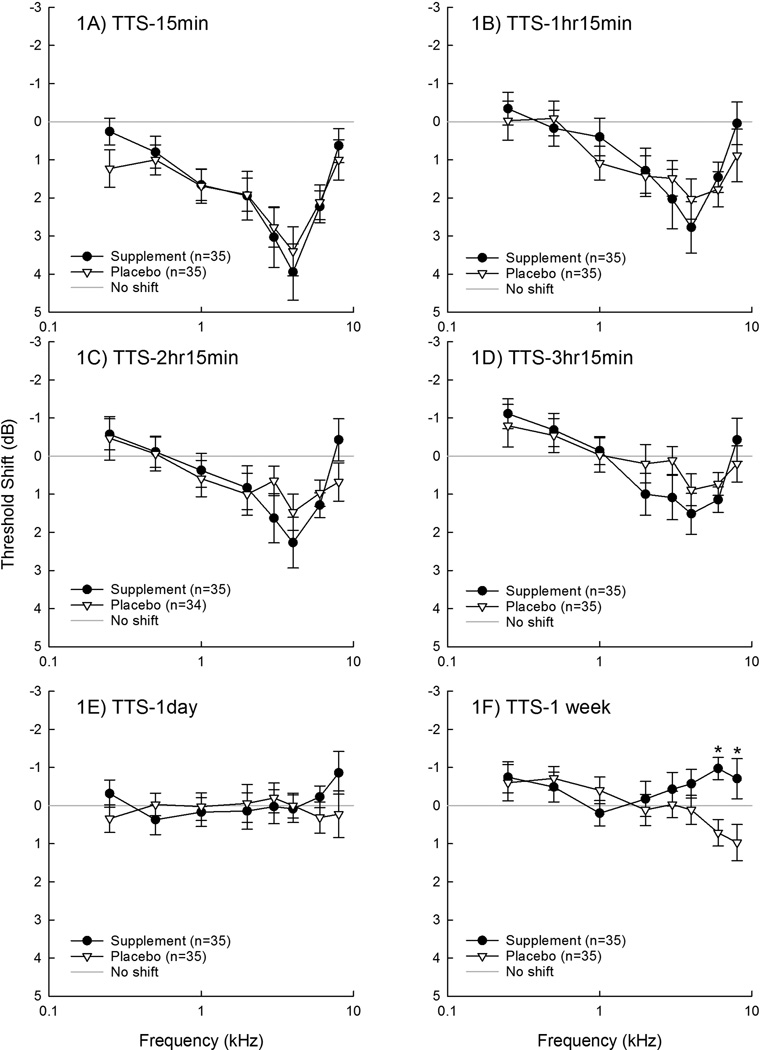
There was no reduction in TTS as a function of treatment at any of the tested frequencies, including the primary outcome frequency of 4 kHz, at the 15-min test time when TTS was the most robust (fig. 1a). There were no statistically significant differences at any frequency at any test time through the 24-hour test time (fig. 1b–d), at which complete recovery was observed. Additional planned secondary analyses included changes in pure-tone average thresholds (PTA) at frequencies important for speech reception (0.5, 1, and 2 kHz) and frequencies commonly affected by noise (3, 4, and 6 kHz). Consistent with the single-frequency outcomes, there were no statistically significant differences between groups for these PTA analyses (not shown).
Figure 1.
A-1F. Change from the baseline threshold (mean+SEM) measured immediately prior to music exposure was measured at 15 min post-music (1A), 1 hour and 15 min post music (1B), 2 hours and 15 min post music (1C), 3 hours and 15 min post music (1D), 1 day post music (1E), and 1 week post music (1F). Subjects that had threshold differences outside of the expected test-retest reliability criteria were retested 1 week later. All subjects were confirmed to return to their pre-study functional baseline. *Asterisks indicate statistically significant group difference (p<0.05) at 6 and 8 kHz at 1-week post music time.
The only statistically reliable difference observed was at the 1-week post-music test time, where there was a statistically significant difference in ‘TTS’ at 6 and 8 kHz (fig. 1e). Thresholds measured in the supplement group averaged ~1 dB better than their respective pre-music thresholds at 6 and 8 kHz, whereas thresholds measured in the placebo group averaged ~1 dB worse than their respective pre-music thresholds at 6 and 8 kHz. This observed difference at the 1-week test time appeared to be directly related to variable performance of several participants across the screening, pre-music, and final 1-week test. Several participants had larger than expected (>5 dB) deviations from frequency to frequency for the 1-week post-music test relative to pre-music baseline tests. With some participants performing better than pre-music baseline and others performing poorer than pre-music baseline, the average differences from their baseline were approximately 1 dB in either direction.
When any participant produced final 1-week test thresholds that were seemingly poorer than those measured at the pre-music baseline (i.e., outside of expected test-retest reliability), the thresholds measured at 1-week post-music were compared with the thresholds measured at the screening test. In addition, the DPOAE amplitude data were reviewed as a correlate of objective outer hair cell functional recovery. All data were forwarded for review by the supervising physician (P.J.A.), and the participant was asked to return for an additional follow-up audiometric test session with one of the licensed audiologists on the study team (C.S., S.K.G., E.L.). Any additional tests outside of the final 1-week study examination were required to be reported to the supervising IRBs. All participants were confirmed to have returned to baseline at the 1-week test or at additional follow-up testing if required.
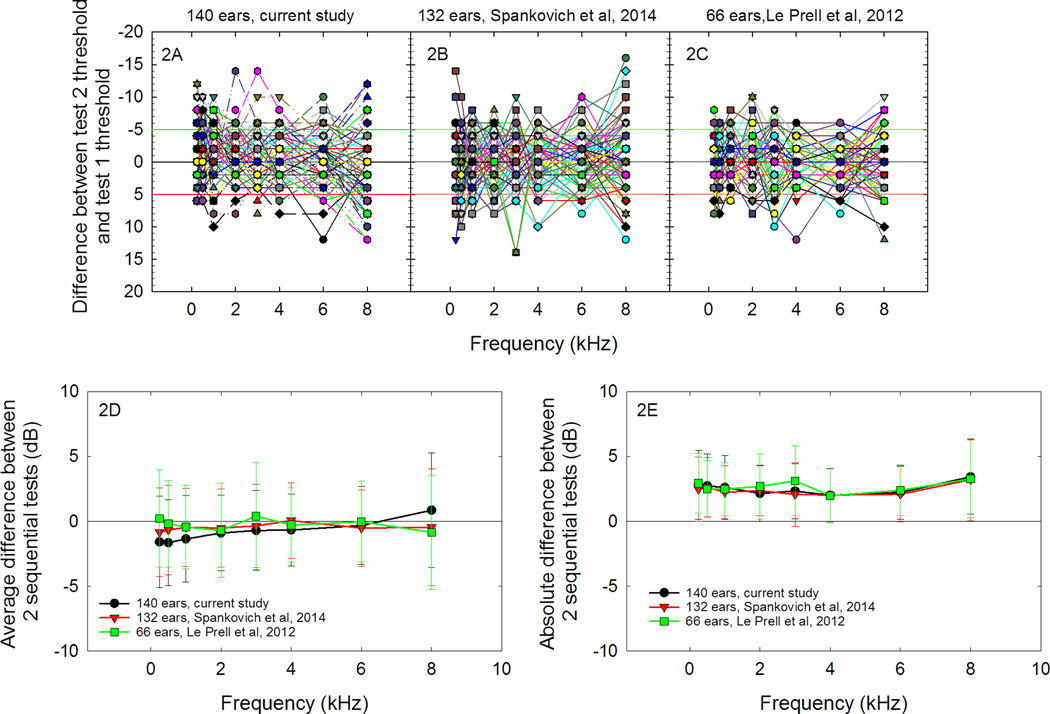
The issue of test-retest reliability of the subjects from session to session was a major issue, even with the requirement that participants be consistent within ±5 dB within the first test session. Figure 2a illustrates test-retest differences between the screening test thresholds and the pre-music baseline test thresholds measured in the current study; these test-retest data are collected in the absence of any experimental sound exposure. The lack of consistency from test to test by a subset of the subjects is consistent with test-retest data for different participant cohorts that volunteered for two previous investigations that also used the current 2-dB step size protocol (2B, 2C), with these test-retest data collected in the absence of any experimental sound exposure or other manipulation. When test-retest data are averaged across participants (2D), the average difference from test to test is negligible as participants are evenly distributed with some who perform better on the second test than the first test, and others who perform worse on the second test than the first test. However, when looking at the absolute value of the test to test differences (2E), it becomes clear that the average participant will vary approximately 3-dB from test to test, with 8 kHz being the most variable (at least within the 0.25- to 8-kHz conventional test battery). Because the overlapping data points make it difficult to visually resolve the total number of symbols at any one location, test-retest histogram data are provided in table 1.
Figure 2.
Variability from test 1, completed at screening, to test 2, completed prior to the music exposure, is shown in Figure 2A. Negative values indicate performance was better on the second pre-music test; the green line indicates the conventional −5 dB reliability assumption. Positive values indicate performance was poorer on the second pre-music test; the red line indicates the conventional +5 dB reliability assumption. Reliability of the 70 subjects shown here was consistent with that reported for 66 subjects tested by Spankovich et al. [2014] (Fig. 2B) and that of the 33 subjects tested by Le Prell et al. [2012] (Fig. 2C). Because some symbols are hidden behind others, data value frequencies are listed in table 1. The average test-retest difference is near zero (Fig. 2D) as there are equal numbers of subjects that perform better, versus those that perform more poorly, on the second test. The average of the absolute value of the test to test difference is ~3-dB with standard deviations indicating that the majority of subjects are consistent within 5–6 dB from test to test.
Table 1.
| A. Number of Ears and Percent of Ears with Specific Test-Retest Differences at Each Test Frequency across 140 Ears (Illustrated in Figure 2A). | ||||||||
|---|---|---|---|---|---|---|---|---|
| 250 | 500 | 1000 | 2000 | 3000 | 4000 | 6000 | 8000 | |
| −16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| −14 | 0 | 0 | 0 | 1 (1) | 1 (1) | 0 | 0 | 0 |
| −12 | 2 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (1) |
| −10 | 4 (3) | 3 (2) | 1 (1) | 0 | 1 (1) | 1 (1) | 1 (1) | 1 (1) |
| −8 | 4 (3) | 6 (4) | 6 (4) | 1 (1) | 0 | 1 (1) | 2 (1) | 4 (3) |
| −6 | 10 (7) | 13 (9) | 11 (8) | 10 (7) | 7 (5) | 7 (5) | 5 (4) | 3 (2) |
| −4 | 25 (18) | 21 (15) | 26 (19) | 16 (11) | 20 (14) | 15 (11) | 10 (7) | 8 (6) |
| −2 | 26 (19) | 33(24) | 25 (18) | 34 (24) | 33 (24) | 34 (24) | 41 (29) | 32 (23) |
| 0 | 37 (26) | 38 (27) | 43 (31) | 44 (26) | 37 (26) | 50 (36) | 34 (24) | 22 (16) |
| 2 | 23 (16 | 17 (12) | 21 (15) | 27 (20) | 28 (20) | 21 (15) | 36 (26) | 27 (19) |
| 4 | 7 (5) | 6 (4) | 1 (1) | 5 (8) | 11 (8) | 8 (6) | 6 (4) | 17 (12) |
| 6 | 2 (1) | 3 (2) | 4 (3) | 1 (1) | 1 (1) | 2 (1) | 3 (2) | 14 (10) |
| 8 | 0 | 0 | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 5 (4) |
| 10 | 0 | 0 | 1 (1) | 0 | 0 | 0 | 0 | 3 (2) |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) | 2 (1) |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B. Number of Ears and Percent of Ears with Specific Test-Retest Differences at Each Test Frequency across 132 Ears (Spankovich et al., 2014; illustrated in Figure 2B). | ||||||||
|---|---|---|---|---|---|---|---|---|
| 250 | 500 | 1000 | 2000 | 3000 | 4000 | 6000 | 8000 | |
| −16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) |
| −14 | 1 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) |
| −12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) |
| −10 | 2 (1) | 1 (1) | 0 | 0 | 1 (1) | 0 | 2 (1) | 3 (2) |
| −8 | 0 | 0 | 2 (1) | 1 (1) | 1 (1) | 1 (1) | 2 (1) | 3 (2) |
| −6 | 5 (4) | 11 (8) | 6 (4) | 8 (6) | 6 (4) | 2 (1) | 2 (1) | 8 (6) |
| −4 | 23 (16) | 22 (16) | 12 (9) | 15 (11) | 14 (10) | 14 (10) | 16 (11) | 11 (8) |
| −2 | 31 (22) | 27 (19) | 39 (28) | 37 (26) | 25 (18) | 27 (19) | 30 (21) | 27 (19) |
| 0 | 39 (28) | 34 (24) | 37 (26) | 30 (21) | 50 (36) | 47 (34) | 45 (32) | 32 (23) |
| 2 | 18 (13) | 22 (16) | 23 (16) | 29 (21) | 25 (18) | 24 (17) | 24 (17) | 20 (14) |
| 4 | 8 (6) | 8 (6) | 6 (4) | 6 (4) | 6 (4) | 10 (7) | 6 (4) | 13 (9) |
| 6 | 2 (1) | 3 (2) | 4 (3) | 4 (3) | 2 (1) | 5 (4) | 4 (3) | 7 (5) |
| 8 | 2 (1) | 2 (1) | 3 (2) | 2 (1) | 0 | 0 | 1 (1) | 2 (1) |
| 10 | 0 | 2 (1) | 0 | 0 | 0 | 2 (1) | 0 | 2 (1) |
| 12 | 1 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1) |
| 14 | 0 | 0 | 0 | 0 | 2 (1) | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. Number of Ears and Percent of Ears with Specific Test-Retest Differences at Each Test Frequency across 66 Ears (Le Prell et al., 2012; illustrated in Figure 2C). | ||||||||
|---|---|---|---|---|---|---|---|---|
| 250 | 500 | 1000 | 2000 | 3000 | 4000 | 6000 | 8000 | |
| −16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| −14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| −12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| −10 | 0 | 0 | 0 | 2 (1) | 0 | 0 | 0 | 1 (1) |
| −8 | 1 (1) | 0 | 1 (1) | 1 (1) | 2 (1) | 0 | 0 | 4 (3) |
| −6 | 5 (4) | 6 (4) | 5 (4) | 4 (3) | 6 (4) | 2 (1) | 3 (2) | 7 (5) |
| −4 | 7 (5) | 11 (8) | 9 (6) | 6 (4) | 5 (4) | 8(6) | 7 (5) | 8 (6) |
| −2 | 12 (9) | 4 (3) | 11 (8) | 18 (13) | 9 (6) | 15 (11) | 17 (12) | 10 (7) |
| 0 | 13 (9) | 20 (14) | 18 (13) | 17 (12) | 17 (12) | 23 (16) | 17 (12) | 20 (14) |
| 2 | 15 (11) | 17 (12) | 12 (9) | 10 (7) | 10 (7) | 13 (9) | 10 (7) | 4 (3) |
| 4 | 5 (4) | 5 (4) | 8 (6) | 2 (1) | 9 (6) | 3 (2) | 8 (6) | 5 (4) |
| 6 | 5 (4) | 1 (1) | 2 (1) | 5 (4) | 4 (3) | 1 (1) | 3 (2) | 5 (4) |
| 8 | 3 (2) | 2 (1) | 0 | 1 (1) | 2 (1) | 0 | 1 (1) | 0 |
| 10 | 0 | 0 | 0 | 0 | 2 (1) | 0 | 0 | 1 (1) |
| 12 | 0 | 0 | 0 | 0 | 0 | 1 (1) | 0 | 1 (1) |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
DPOAE Amplitude
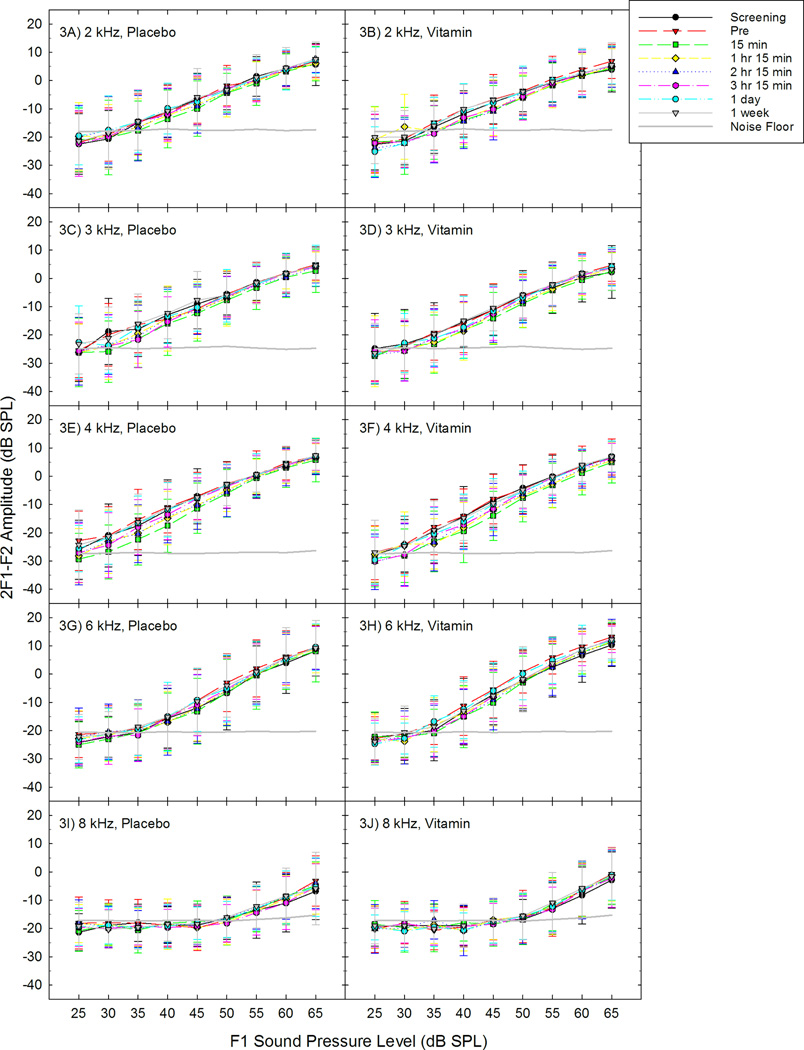
DPOAE amplitude was reliably decreased after noise exposure at 2, 3, 4, and 6 kHz, for f1 levels ranging from 35 to 55 dB SPL (table 2). These data are consistent with frequencies and levels at which changes were observed in the previous study using this exposure [Le Prell et al., 2012]. At lower f1 sound levels, an evoked DPOAE response does not exceed the noise floor of the system, and at higher f1 sound levels, no noise-induced change in DPOAE amplitude is evident. The amount of change in DPOAE amplitude after music exposure did not reliably vary as a function of treatment, however (fig. 3). Complete recovery of the DPOAE amplitude functions was observed in both the placebo and the treatment group.
Table 2.
Change in DPOAE following music exposure
| F1 dBSPL | Assignment | 2 kHz | 3 kHz | 4 kHz | 6 kHz | 8 kHz |
|---|---|---|---|---|---|---|
| 65 | Placebo | NS | NS | NS | NS | NS |
| Supplement | ||||||
| 60 | Placebo | NS | NS | NS | NS | NS |
| Supplement | p=.002 | p=.001 | ||||
| 55 | Placebo | p=.034 | NS | NS | NS | NS |
| Supplement | p=.045 | p<.001 | p=.005 | p=.02 | ||
| 50 | Placebo | p=.005 | p=.045 | p<.001 | NS | NF |
| Supplement | p=.004 | p<.001 | p=.005 | p=.01 | ||
| 45 | Placebo | p=.013 | p=.004 | p<.001 | p=.01 | NF |
| Supplement | p<.001 | p=.001 | p<.001 | p=.01 | ||
| 40 | Placebo | NS | p=.01 | p<.001 | NS | NF |
| Supplement | p=.01 | p=.01 | p<.001 | |||
| 35 | Placebo | NF | p<.001 | p<.001 | NF | NF |
| Supplement | p=.01 | p=.005 | ||||
| 30 | Placebo | NF | NF | NF | NF | NF |
| Supplement | ||||||
| 25 | Placebo | NF | NF | NF | NF | NF |
| Supplement |
Legend: NF=Noise Floor
NS=No significant difference
Figure 3.
Distortion product otoacoustic emission (DPOAE) amplitude was assessed before and after music exposure for subjects; screening, pre-music baseline, 15 min-post music, 1 hour and 15 min post music, 2 hours and 15 min post music, 3 hours and 15 min post music, 1 day post music, and 1 week post music, are shown for the placebo and the supplement groups at 2 kHz (3A, 3B), 3 kHz (3C, 3D), 4 kHz (3E, 3F), 6 kHz (3G, 3H), and 8 kHz (3I, 3J). Noisefloor data are averaged across all subjects and all test times with a single noise floor value shown for each frequency. There was no effect of treatment assignment on the change in DPOAE amplitude.
Tinnitus
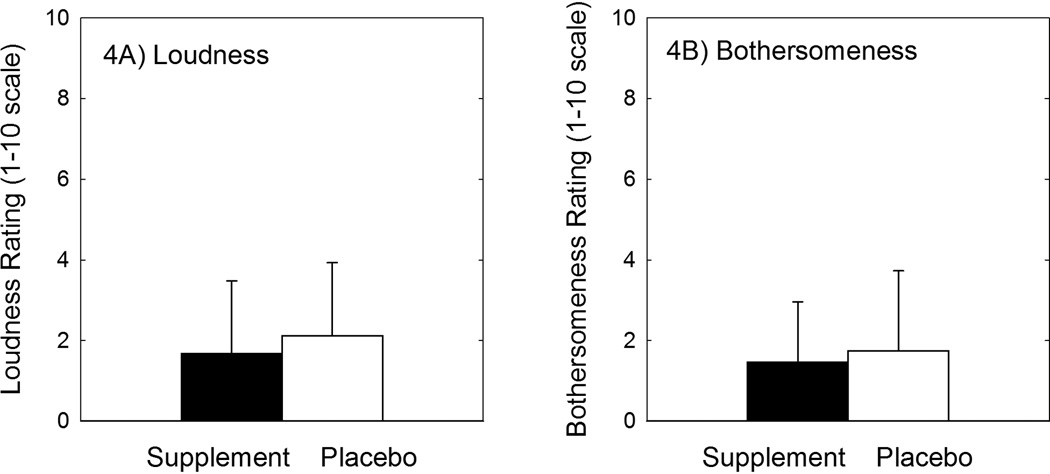
There were a total of 19 participants (54.3%) in the treatment group who reported tinnitus at one or more test times after the music exposure, compared to a total of 9 participants (25.7%) in the placebo group. The Fisher’s exact probability associated with the 2 by 2 contingency table of percentages was 0.02, suggesting that the proportion of participants reporting tinnitus and related symptoms was not equal in the treatment and placebo groups, with tinnitus more likely to be reported by individuals in the micronutrient treatment group. Although tinnitus was more likely to be reported, there was no difference in the perceived severity of the symptoms. Among participants reporting tinnitus, average tinnitus loudness was rated as 1.68 ± 1.80 by those in the micronutrient treatment group versus an average loudness rating of 2.12 ± 1.81 by those in the placebo group (fig. 4a). Average bothersomeness was rated as 1.47 ± 1.49 by those in the micronutrient treatment group versus an average bothersomeness rating of 1.75 ± 1.98 by those in the placebo group (fig. 4b). Given that both loudness and bothersomeness were rated on a visual analogue scale from 1 to 10, the loudness and bothersomeness scores were at the lower end of the scale.
Figure 4.
Among participants reporting tinnitus, there was no reliable difference in the average tinnitus loudness rating (4A) or bothersomeness rating (4B). There were a total of 19 participants (54.3%) in the treatment group who reported tinnitus at one or more test times after the music exposure, compared to a total of 9 participants (25.7%) in the placebo group; there were significantly more participants reporting tinnitus in the supplement group than in the placebo group.
Adverse Events
There were 12 adverse events reported (7 GI events such as stomach ache, vomiting, diarrhea; 5 non-GI events including fever, aches, chills, headaches, sore throat, pain in lower jaw, dizziness, and tinnitus). Of the 7 GI events, 3 were reported by participants in the micronutrient treatment group and 4 by participants in the placebo group. The rate of occurrence of GI events was not statistically different between groups. For the 5 non-GI events, 1 was reported by a participant consuming placebo versus 4 by participants in the experimental group.
Listening Habits: A Brief Comment
Although this study was not designed to provide precise information regarding participants’ normal music listening level, we did collect qualitative ratings of how the music level compared with their typical music listening habits and self-reported information on listening habits. With respect to the perceived intensity level of the study music exposure, 46 participants (66%) reported the music was ‘a lot louder’ than they typically listen to, 19 participants (27%) reported it was ‘a little bit louder’ than they typically listen to, 3 participants (4%) reported it was equal to their ‘typical’ listening level, and 2 participants (3%) reported they do not typically use music players. Given the significant attention devoted to potentially risky listening habits during music player use by adolescents and young adults, it is interesting that only 4% of the participants reported listening to music at sound levels similar to those presented here, a sound exposure level that reaches 100% of the OSHA-mandated PEL after 4 h. Most participants listened for shorter periods of time per day and reported listening at lower sound levels. With regard to typical listening duration, 5 participants (7%) reported that they do not use music players on a daily basis, 29 participants (41%) reported less than 1 h of use per day, 29 participants (41%) reported 1–2 h of use per day, 4 participants (6%) reported 3–5 h of use per day, and 3 participants (4%) reported 5–8 h of use per day. With exposures of 90-dBA sound levels for 8 h/day 5 days/week throughout an anticipated 40-year career duration (i.e., 100% OSHA-allowed daily noise dose), some 21–29% are at excess risk of material hearing loss [see Pelton, 2001, table 9–1, p. 187]. Music player use was relatively frequent for some participants in this study. With respect to typical listening frequency, 8 participants (11%) reported that they use music players less than 1 day per week, 14 participants (20%) reported 1–2 days of use per week, 31 participants (44%) reported 3–5 days of use per week, and 12 participants (17%) reported 5–7 days of use per week.
Discussion
This study assessed the potential efficacy of a micronutrient therapy for the prevention of music-induced temporary hearing loss. A reliable music-induced TTS with the largest TTS measured at 4 kHz was observed, with smaller TTS measured at 3 and 6 kHz. The average TTS was smaller, and the variability was increased, in the current cohort of 70 participants relative to a previous report with a sample of 12 subjects [Le Prell et al., 2012]. The average TTS at 4 kHz (3.7 ± 4.6 dB across all participants in both conditions, with individual TTS measurements ranging from −4 dB to 20 dB) differed from the average change at 4 kHz in the initial pilot study, which was 6.3 dB (SD = 3.9 dB), with a range of 0–14 dB [Le Prell et al., 2012]. The finding of a smaller average TTS as well as the increased variability observed here has important implications for clinical trial design if future investigations using a similar model are to be adequately powered to detect potential changes in TTS.
A key issue to be considered as part of any future investigation assessing potential TTS prevention using an otoprotective drug agent and this music exposure paradigm is the clinical relevance of the prevention of small threshold changes. The 4-dB average change is not clinically significant, raising questions about the clinical importance of any observed prevention of this change in future trials. While positive outcomes provide important proof-of-concept confirmation that the drug has the potential to act on the inner ear and prevent noise-induced threshold shift, prevention of small changes may not be sufficient evidence of clinical efficacy to support a new drug application, or widespread application of an existing drug; those decisions will ultimately be made by the FDA or other regulatory agencies that review potential label claims (for discussion of regulatory process, see Lynch et al., 2016). As an alternative analysis plan, one could prospectively plan to assess potential reductions in participants with larger TTS changes, as in the preliminary report by Kil et al. (2014). In a different study that assessed prevention of permanent threshold shift (PTS), Kopke et al. [2015] used this analysis approach to assess a potential reduction in the rate of PTS using NAC as a potential therapeutic agent. In the study presented here, there were 20 ears out of 140 tested (14% of the total participant pool) where TTS at 4 kHz was 10 dB or greater at the 15-min test time. Results from these 20 ears provide some data on which prospective study power calculations could be based.
The negative finding related to prevention of TTS using the dietary supplement was unexpected given that TTS has been successfully reduced by these active agents in guinea pigs at times extending from 1 h post-noise to 7 days post-noise [Le Prell et al., 2011a], and PTS has also been reduced in mice and guinea pigs receiving this combination of active agents [Le Prell et al., 2011b, 2007; Tamir et al., 2010]. Although lack of benefit for this combination for the purpose of TTS prevention in humans must be acknowledged, other possible explanations for this finding should be considered. The first possibility that could underlie the current negative outcome is participant compliance. Although participants largely reported good compliance with the treatment protocol (with the exception of the 2 participants who reported consumption of only 1 tablet per day), it is possible that compliance with treatment requirements of 6 tablets/day was less complete than that reported by the participants verbally and on their treatment diaries. The only dose that was consumed under the supervision of the study team was the dose consumed on the day of music dosing. A number of comments by participants regarding a ‘chalky’ taste and texture that was highly unpleasant by the 6th tablet were provided in the treatment log or made verbally at the final dose, and it is thus possible that compliance was less than that reported on paper by the individual subjects.
A second and seemingly more likely possibility that could underlie the current negative outcomes is failure to achieve a therapeutic dose. A previous study with 2 days of treatment with these active agents delivered in a capsule form produced robust increases in the levels of β-carotene, vitamin C, and vitamin E in blood samples when assessed 2 h after the final dose (with Mg levels not increased) [see Le Prell et al., 2011c]. It is possible that the tablet form did not have the same bioavailability; and it is also possible that even if the achieved increases were comparable, they were not therapeutically sufficient. Although it was not feasible to include blood samples in the current investigation, we strongly advocate the inclusion of appropriate treatment biomarkers in any future investigation. In future studies, higher daily doses of vitamins C and E could be delivered without exceeding the US Institute of Medicine upper daily limits, although the Mg dose used here approached the upper daily intake limit recommended for healthy adults and thus should not be increased [Institute of Medicine, 2004a, b]. As an alternative to higher dosing, longer-term pre-music dosing might yield benefits that were not achieved with the current dosing strategy. Vitamin levels clearly continue to increase over multiple days or even weeks [Levine et al., 1996], suggesting longer duration dosing strategies may ultimately be the most effective. Long-term (8-year) dosing with a micronutrient formulation similar to that used here (500 mg vitamin C, 400 IU vitamin E, and 15 mg β-carotene, and also including 80 mg zinc oxide and 2 mg cupric oxide) was used in the age-related macular degeneration study (AREDS) to reduce risk of development of age-related eye disease, indicating longer dosing periods with the vitamin active agents should be safe for healthy populations [Chew et al., 2013; Lewis and Marchell, 2006; Lutkenhoner, 2011]. Future studies should incorporate blood samples not only to confirm increased plasma levels of the active agents as a measure of compliance with dosing and bioavailability, but also so as to allow correlations between specific active agents and potential treatment effects within participants. Analysis of blood samples would also provide important insight into the potential interactions between baseline nutritional status and supplement benefit. There are suggestions that positive health outcomes with dietary supplements will be limited to those individuals with nutritional deficits [Saposnik, 2011].
A third possibility that could underlie negative outcomes is reduction of antioxidant properties of the tablets from ionizing radiation during X-ray analysis of the CTM during the shipping process. The clinical supplies were manufactured in Cleveland, Ohio, and shipped to Toronto, Canada, where they were bottled and encoded for blinded administration; then, they were shipped to Gainesville, Fla., for use in this trial. Subsequent to the unmasking of the results, the bottling company communicated that shipments across the USA-Canadian border are routinely, but not always, X-rayed to verify package contents. Unfortunately such inspections are not documented, and it cannot be confirmed whether the CTM were X-rayed during the shipping process. Some irradiation conditions have been shown to reduce antioxidant properties of nutrients in food and supplement products [Fan and Sokorai, 2007, 2008; Nemtanu and Brasoveanu, 2016], with other irradiation dosing having no effect [Saroj et al., 2007] or even a positive effect resulting in increased antioxidant activity [Hussein et al., 2011]. Unpublished data indicate that X-ray of rat chow supplemented with ACEMg at the Karolinska Institute prior to allowing it into the animal quarters resulted in greatly reduced antioxidant properties of the vitamins [M. Ulfendahl, pers. obs.]. Better understanding of the effects of radiation on antioxidant properties is an urgent issue since many food supplies stored for emergency use by governmental and non-governmental organizations to deal with global catastrophes are routinely X-rayed to reduce bacterial content for storage as well as potential X-ray inspection during shipping. The effect of such X-rays on nutrient value and vitamins are not well understood.
The lack of benefit in the current experimental investigation of acute changes in hearing contrasts with potential long-term positive effects of dietary nutrients that have been suggested based on associations (correlations) observed in epidemiological data sets. A variety of epidemiological data have been interpreted to suggest possible benefits for different dietary nutrients with respect to hearing [for review, see Le Prell and Spankovich, 2013]. Analysis of the cross-sectional National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002 revealed a statistically significant association between overall dietary quality and hearing, with better high-frequency PTAs reliably associated with better diets after adjusting for other risk factors [Spankovich and Le Prell, 2013]. Furthermore, this effect had a statistically significant interaction with noise, with previous noise exposure being associated with more severe hearing loss as a function of poorer diet, after adjusting for other risk factors [Spankovich and Le Prell, 2014]. Choi et al. [2014] used a different analytic approach, including nutrient-specific analysis. They reported higher intakes of β-carotene, vitamin C, and Mg, as estimated by 24-hour dietary recall and supplement use, were associated with lower (better) speech PTAs and high-frequency PTAs in 20- to 69-year-olds [Choi et al., 2014]. Recent epidemiological data are consistent in that higher intakes of β-carotene, β-cryptoxanthin, and folate, whether total or from diet, were associated with lower risk of self-reported hearing loss in female participants enrolled in the Nurses’ Health Study II [Curhan et al., 2015]. However, the opposite relationship was reported for higher vitamin C intake, with higher intake associated with higher risk of self-reported hearing loss [Curhan et al., 2015]. For the vitamin C analysis in this study, the comparison specifically included women taking less than 75 mg/day, versus those taking 1,000 mg/day, primarily in the form of supplements. Experimental data addressing the extent to which causal relationships underlie these association-based data are needed. Data are limited; however, consistent with the notion that healthy eating lifestyle may have long-term hearing benefits, long-term (3-year) supplementation with vitamin B9 (folic acid) slowed the small but progressive changes in auditory function observed in a control cohort (1.0 vs. 1.7 dB shift over 3 years) [see Durga et al., 2007]. Additional empirical testing is needed to clarify relationships among the duration of the dosing, the effects of participant diet, differences between dietary nutrient intake and supplement use, and the possibility that higher doses resulting in increased serum levels influence the risk of hearing loss.
Summary and Conclusions
We tested the potential effect of a micronutrient dietary supplement containing β-carotene, vitamins C and E, and Mg on the prevention of TTS after music player use in healthy individuals with normal hearing. The clinical supplies were manufactured using good manufacturing practices, with the stability of all four active agents assessed at multiple times to assure that the active agents were stable and available at the intended doses. The extent to which stability of the experimental CTM shipped to Canada for bottling and back to the USA for use in the study may have differed from that of the CTM maintained at the manufacturing site is not known. With 3 days of pre-music dosing and dosing on the day of the music exposure at the clinical trial site, there was no reduction in the observed TTS, and there was no improvement in the rate of recovery of the music-induced TTS. The lack of protection observed here contrasts with multiple positive outcomes in animal studies as well as benefit inferred from correlations observed in epidemiological data. Although our results show a lack of TTS prevention, it is possible that the lack of benefit is the result of inadequate dosing, dose duration, or compromised antioxidant properties during the transfer of the supplies for bottling and use in the study. Inclusion of biomarkers as a measure of compliance and bioavailability would have been useful, and such metrics are strongly advocated for any future investigation for the prevention of TTS using novel therapeutic compounds. Future studies should carefully consider study power given that the average TTS changes were small. The potential prevention of the small changes in TTS induced by the exposure presented here (approximately 100% of the OSHA PEL) may not be sufficient evidence of clinical efficacy to support new health claims, but positive outcomes would certainly provide important proof that a drug has the potential to protect the inner ear.
Acknowledgments
The project was supported by U01 DC 008423 from the National Institutes of Health - National Institute on Deafness and Other Communication Disorders (NIH-NIDCD) awarded to the University of Michigan (J.M.M.), with subcontracts awarded to the University of Florida (C.G.L.) and Southern Illinois University School of Medicine (K.C.M.K.). Clinical trial material, including active tablets and matched placebo, were provided by Hearing Health Sciences Inc. Josef Miller is a founding member of Hearing Health Sciences Inc., holds stock in the company, and serves as the Chief Scientific Consultant. We thank the members of the NIH-selected data safety monitoring board, as well as the late Gordon Hughes at the NIH, for helpful feedback and suggestions throughout their oversight of these studies. We are grateful to Sharon Kujawa who served as a paid consultant for discussions related to DPOAE protocol development and TTS safety considerations. Finally, we thank Diana Guercio, Victoria Ledon, Lindsay May, Erika Ortiz, and Caitlin Simmons, who consented and tested subjects at the University of Florida, and Susan DeRemer at the University of Michigan, who provided assistance with IRB applications. We also gratefully acknowledge the contributions of Jim Wyatt, Marcello Pineiro, and Robert Trepanier at Brüel and Kjær, who were instrumental in developing iPod® calibration protocols.
References
- Abi-Hachem RN, Zine A, Van De Water TR. The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent Pat CNS Drug Discov. 2010;5:147–163. doi: 10.2174/157488910791213121. [DOI] [PubMed] [Google Scholar]
- Attias J, Sapir S, Bresloff I, Reshef-Haran I, Ising H. Reduction in noise-induced temporary threshold shift in humans following oral magnesium intake. Clin Otolaryngol. 2004;29:635–641. doi: 10.1111/j.1365-2273.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- Berger EH, Megerson SC, Stergar ME. Personal music players: are we measuring sound levels correctly? ASHA Leader. 2009;14:14–17. [Google Scholar]
- Bradley R, Fortnum H, Coles R. Patterns of exposure of schoolchildren to amplified music. Br J Audiol. 1987;21:119–125. doi: 10.3109/03005368709077784. [DOI] [PubMed] [Google Scholar]
- Campbell KCM, Le Prell CG. Potential therapeutic agents. Semin Hearing. 2012;33:97–113. [Google Scholar]
- Chew EY, Clemons TE, Agron E, Sperduto RD, Sangiovanni JP, Kurinij N, Davis MD Age-Related Eye Disease Study Research Group. Long-term effects of vitamins C and E, beta-carotene, and zinc on age-related macular degeneration: AREDS report No. 35. Ophthalmology. 2013;120:1604–1611. doi: 10.1016/j.ophtha.2013.01.021. e1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y-H, Miller JM, Tucker KL, Hu H, Park SK. Antioxidant vitamins and magnesium and the risk of hearing loss in the us general population. Am J Clin Nutr. 2014;99:148–155. doi: 10.3945/ajcn.113.068437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curhan SG, Stankovic KM, Eavey RD, Wang M, Stampfer MJ, Curhan GC. Carotenoids, vitamin A, vitamin C, vitamin E, and folate and risk of self-reported hearing loss in women. Am J Clin Nutr. 2015;102:1167–1175. doi: 10.3945/ajcn.115.109314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HW, Louie A, Nahid M, Shoveller J. Potential barriers to engineered noise control in food and beverage manufacturing in British Columbia, Canada: a qualitative study. Int J Audiol. 2012;51(suppl 1):S43–S50. doi: 10.3109/14992027.2011.633936. [DOI] [PubMed] [Google Scholar]
- Doosti A, Lotfi Y, Moossavi A, Bakhshi E, Talasaz AH, Hoorzad A. Comparison of the effects of n-acetyl-cysteine and ginseng in prevention of noise induced hearing loss in male textile workers. Noise Health. 2014;16:223–227. doi: 10.4103/1463-1741.137057. [DOI] [PubMed] [Google Scholar]
- Durga J, Verhoef P, Anteunis LJ, Schouten E, Kok FJ. Effects of folic acid supplementation on hearing in older adults: a randomized, controlled trial. Ann Intern Med. 2007;146:1–9. doi: 10.7326/0003-4819-146-1-200701020-00003. [DOI] [PubMed] [Google Scholar]
- Fan X, Sokorai KJ. Effects of ionizing radiation on sensorial, chemical, and microbiological quality of frozen corn and peas. J Food Prot. 2007;70:1901–1908. doi: 10.4315/0362-028x-70.8.1901. [DOI] [PubMed] [Google Scholar]
- Fan X, Sokorai KJ. Retention of quality and nutritional value of 13 fresh-cut vegetables treated with low-dose radiation. J Food Sci. 2008;73:S367–S372. doi: 10.1111/j.1750-3841.2008.00871.x. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Henry JA, Helt WJ, Phillips DS, Frey RH, Noffsinger D, Larson VD, Fowler CG. An individualized, sensitive frequency range for early detection of ototoxicity. Ear Hear. 1999;20:497–505. doi: 10.1097/00003446-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PW, Lall K, Liberman MC, Kujawa SG. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35:7509–7520. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetoni AR, Garzaro M, Ralli M, Landolfo V, Sensini M, Pecorari G, Mordente A, Paludetti G, Giordano C. The monitoring role of otoacoustic emissions and oxidative stress markers in the protective effects of antioxidant administration in noise-exposed subjects: a pilot study. Med Sci Monit. 2009;15:PR1–PR8. [PubMed] [Google Scholar]
- Goldman B, Sheppard L, Kujawa SG, Seixas NS. Modeling distortion product otoacoustic emission input/output functions using segmented regression. J Acoust Soc Am. 2006;120:2764–2776. doi: 10.1121/1.2258871. [DOI] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol. 2014;111:552–564. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein SZ, Yusoff KM, Makpol S, Yusof YA. Antioxidant capacities and total phenolic contents increase with gamma irradiation in two types of Malaysian honey. Molecules (Basel) 2011;16:6378–6395. doi: 10.3390/molecules16086378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Dietary reference intakes (DRIS): Tolerable Upper Intake Levels (UL), Elements. Bethesda: Food and Nutrition Board, Institute of Medicine, National Academies of Science; 2004a. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes (DRIS): Tolerable upper intake levels (UL), vitamins. Bethesda: Food and Nutrition Board, Institute of Medicine, National Academies of Science; 2004b. [Google Scholar]
- Jensen JB, Lysaght AC, Liberman MC, Qvortrup K, Stankovic KM. Immediate and delayed cochlear neuropathy after noise exposure in pubescent mice. PLoS One. 2015;10:e0125160. doi: 10.1371/journal.pone.0125160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil J, Lynch ED, Griffiths S, Lobarinas E, Spankovich C, Antonelli PJ, Le Prell CG. Efficacy of SPI-1005 for prevention of noise-induced hearing loss: Phase 2 clinical trial results. Otolaryngol Head Neck Surg. 2014;151:83–84. [Google Scholar]
- Kopke R, Slade MD, Jackson R, Hammill T, Fausti S, Lonsbury-Martin B, Sanderson A, Dreisbach L, Rabinowitz P, Torre P3rd, Balough B. Efficacy and safety of n-acetylcysteine in prevention of noise induced hearing loss: a randomized clinical trial. Hear Res. 2015;323:40–50. doi: 10.1016/j.heares.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Kramer S, Dreisbach L, Lockwood J, Baldwin K, Kopke RD, Scranton S, O’Leary M. Efficacy of the antioxidant n-acetylcysteine (NAC) in protecting ears exposed to loud music. J Am Acad Audiol. 2006;17:265–278. doi: 10.3766/jaaa.17.4.5. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after ‘temporary’ noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Bao J. Prevention of noise-induced hearing loss: potential therapeutic agents. In: Le Prell CG, Henderson D, Fay RR, Popper AN, editors. Noise-Induced Hearing Loss: Scientific Advances, Springer Handbook of Auditory Research. New York: Springer Science + Business Media; 2012. pp. 285–338. [Google Scholar]
- Le Prell CG, Dell S, Hensley BN, Hall JWI, Campbell KCM, Antonelli PA, Green GE, Miller JM, Guire K. Digital music exposure reliably induces temporary threshold shift (TTS) in normal hearing human subjects. Ear Hear. 2012;33:e44–e58. doi: 10.1097/AUD.0b013e31825f9d89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Dolan DF, Bennett DC, Boxer PA. Nutrient treatment and achieved plasma levels: reduction of noise-induced hearing loss at multiple post-noise test times. Transl Res. 2011a;158:54–70. doi: 10.1016/j.trsl.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Gagnon PM, Bennett DC, Ohlemiller KK. Nutrient-enhanced diet reduces noise-induced damage to the inner ear and hearing loss. Transl Res. 2011b;158:38–53. doi: 10.1016/j.trsl.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med. 2007;42:1454–1463. doi: 10.1016/j.freeradbiomed.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Johnson A-C, Lindblad A-C, Skjönsberg A, Ulfendahl M, Guire K, Green GE, Campbell KCM, Miller JM. Increased vitamin plasma levels in Swedish military personnel treated with nutrients prior to automatic weapon training. Noise Health. 2011c;13:432–443. doi: 10.4103/1463-1741.90317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Lobarinas E. Strategies for assessing antioxidant efficacy in clinical trials. In: Miller JM, Le Prell CG, Rybak LP, editors. Oxidative Stress in Applied Basic Research and Clinical Practice: Free Radicals in ENT Pathology. New York: Humana Press; 2015. pp. 163–192. [Google Scholar]
- Le Prell CG, Spankovich C. Noise-induced hearing loss: detection, prevention and management. In: Kirtane MV, de Souza CE, Sanna M, Devaiah AK, editors. Otology and Neurotology. Stuttgart: Thieme; 2013. pp. 268–284. [Google Scholar]
- Le Prell CG, Yang Q, Harris J. Modification of digital music files for use in human temporary threshold shift studies. J Acoust Soc Am Ex Lett. 2011d;130:EL142–EL146. doi: 10.1121/1.3630017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey S, Levey T, Fligor BJ. Noise exposure estimates of urban MP3 player users. J Speech Lang Hear Res. 2011;54:263–277. doi: 10.1044/1092-4388(2010/09-0283). [DOI] [PubMed] [Google Scholar]
- Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DK, Marchell TC. Safety first: a medical amnesty approach to alcohol poisoning at a us university. Int J Drug Policy. 2006;17:329–338. [Google Scholar]
- Lin CY, Wu JL, Shih TS, Tsai PJ, Sun YM, Ma MC, Guo YL. N-acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269:42–47. doi: 10.1016/j.heares.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Lindblad AC, Rosenhall U, Olofsson A, Hagerman B. The efficacy of n-acetylcysteine to protect the human cochlea from subclinical hearing loss caused by impulse noise: a controlled trial. Noise Health. 2011;13:392–401. doi: 10.4103/1463-1741.90293. [DOI] [PubMed] [Google Scholar]
- Lutkenhoner B. Auditory signal detection appears to depend on temporal integration of subthreshold activity in auditory cortex. Brain Res. 2011;1385:206–216. doi: 10.1016/j.brainres.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Lynch ED, Kil J, Le Prell CG. Human clinical studies in noise-induced hearing loss. In: Le Prell CG, Lobarinas L, Fay RR, Popper AN, editors. Translational Research in Audiology and the Hearing Sciences, Springer Handbook of Auditory Research. New York: Springer + Business Media; 2016. in press. [Google Scholar]
- Nemtanu MR, Brasoveanu M. Impact of electron beam irradiation on quality of sea buckthorn (Hippophae rhamnoides l.) oil. J Sci Food Agric. 2016;96:1736–1744. doi: 10.1002/jsfa.7280. [DOI] [PubMed] [Google Scholar]
- Okpala NCE. Knowledge and attitude of infantry soldiers to hearing conservation. Mil Med. 2007;172:520–522. doi: 10.7205/milmed.172.5.520. [DOI] [PubMed] [Google Scholar]
- OSHA. 29 cfr 1910.95. Occupational noise exposure; hearing conservation amendment; final rule, effective 8 March 1983. Washington: US Department of Labor, Occupational Safety and Health Administration; 1983. [Google Scholar]
- Pelton HK. Hearing conservation. In: Dobie RA, editor. Medical-Legal Evaluation of Hearing Loss. 2. San Diego: Singular; 2001. pp. 184–208. [Google Scholar]
- Poirrier AL, Pincemail J, Van Den Ackerveken P, Lefebvre PP, Malgrange B. Oxidative stress in the cochlea: an update. Curr Med Chem. 2010;17:3591–3604. doi: 10.2174/092986710792927895. [DOI] [PubMed] [Google Scholar]
- Quaranta N, Dicorato A, Matera V, D’Elia A, Quaranta A. The effect of alpha-lipoic acid on temporary threshold shift in humans: a preliminary study. Acta Otorhinolaryngol Ital. 2012;32:380–385. [PMC free article] [PubMed] [Google Scholar]
- Quaranta A, Scaringi A, Bartoli R, Margarito MA, Quaranta N. The effects of ‘supra-physiological’ vitamin B12 administration on temporary threshold shift. Int J Audiol. 2004;43:162–165. doi: 10.1080/14992020400050022. [DOI] [PubMed] [Google Scholar]
- Rice CG, Rossi G, Olina M. Damage risk from personal cassette players. Br J Audiol. 1987;21:279–288. doi: 10.3109/03005368709076420. [DOI] [PubMed] [Google Scholar]
- Saposnik G. The role of vitamin B in stroke prevention: a journey from observational studies to clinical trials and critique of the VITAmins To Prevent Stroke (VITATOPS) Stroke. 2011;42:838–842. doi: 10.1161/STROKEAHA.110.608356. [DOI] [PubMed] [Google Scholar]
- Saroj SD, Hajare S, Shashidhar R, Dhokane V, Sharma A, Bandekar JR. Radiation processing for elimination of salmonella typhimurium from inoculated seeds used for sprout making in India and effect of irradiation on germination of seeds. J Food Prot. 2007;70:1961–1965. doi: 10.4315/0362-028x-70.8.1961. [DOI] [PubMed] [Google Scholar]
- Skrainar SF, Royster LH, Berger EH, Pearson RG. The contribution of personal radios to the noise exposure of employees at one industrial facility. Am Ind Hyg Assoc J. 1987;48:390–395. doi: 10.1080/15298668791384913. [DOI] [PubMed] [Google Scholar]
- Spankovich C, Griffiths SK, Lobarinas E, Morgenstein KE, de la Calle S, Ledon V, Guercio D, Le Prell CG. Temporary threshold shift after impulse-noise during video game play: laboratory data. Int J Audiol. 2014;53(suppl 2):S53–S65. doi: 10.3109/14992027.2013.865844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spankovich C, Le Prell CG. Healthy diets, healthy hearing: national health and nutrition examination survey, 1999–2002. Int J Audiol. 2013;52:369–376. doi: 10.3109/14992027.2013.780133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spankovich C, Le Prell CG. Associations between dietary quality, noise, and hearing: data from the national health and nutrition examination survey, 1999–2002. Int J Audiol. 2014;53:796–809. doi: 10.3109/14992027.2014.921340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffa P, Cambi J, Mezzedimi C, Passali D, Bellussi L. Activity of coenzyme q 10 (q-ter multicomposite) on recovery time in noise-induced hearing loss. Noise Health. 2014;16:265–269. doi: 10.4103/1463-1741.140499. [DOI] [PubMed] [Google Scholar]
- Suter AH. Engineering controls for occupational noise exposure the best way to save hearing. Sound Vibration. 2012;46:24–32. [Google Scholar]
- Tamir S, Adelman C, Weinberger JM, Sohmer H. Uniform comparison of several drugs which provide protection from noise induced hearing loss. J Occup Med Toxicol. 2010;5:26–32. doi: 10.1186/1745-6673-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen-Rise I, Flottorp G, Tvete O. A study of the possibility of acquiring noise-induced hearing loss by the use of personal cassette players (walkman) Scand Audiol Suppl. 1991;34:133–144. [PubMed] [Google Scholar]
- US Bureau of Labor Statistics. Employer-reported workplace injuries and illnesses - 2013. usdl-14-2183, 2014. [Google Scholar]
- US Department of Veterans Affairs. Vba annual benefits report fiscal year 2013. 2014. [Google Scholar]
- Worthington DA, Siegel JH, Wilber LA, Faber BM, Dunckley KT, Garstecki DC, Dhar S. Comparing two methods to measure preferred listening levels of personal listening devices. J Acoust Soc Am. 2009;125:3733–3741. doi: 10.1121/1.3125798. [DOI] [PMC free article] [PubMed] [Google Scholar]