Abstract
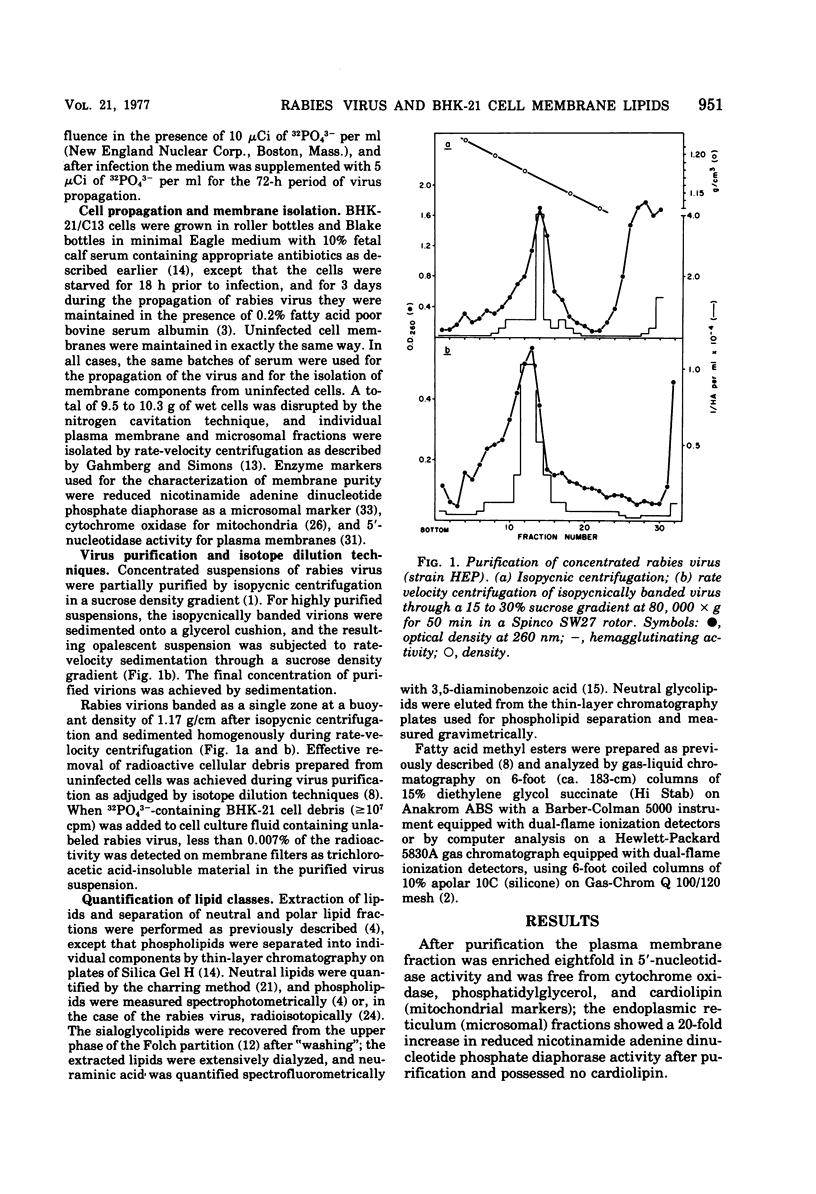
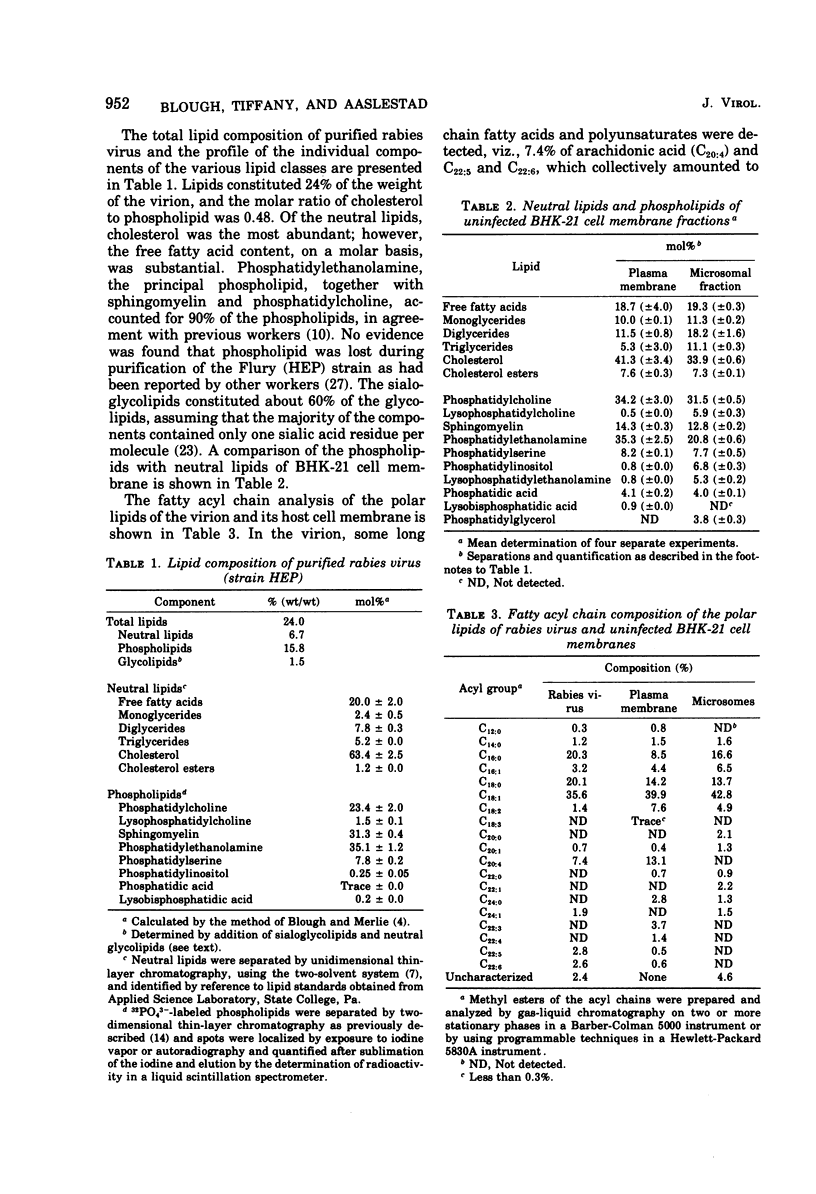
The lipid composition of highly purified Flury strain of rabies virus (HEP) propagated in BHK-21 cells in a chemically defined medium was observed to be 6.7% neutral lipids, 15.8% phospholipids, and 1.5% glycolipids. In the virion, phosphatidylethanolamine, phosphatidylcholine, and sphingomyelin were the most abundant phospholipids, accounting for 90% of the total, and the molar ratio of cholesterol to phospholipid was 0.48. Uninfected BHK-21 cell membranes were obtained by nitrogen cavitation techniques and separated by density gradient centrifugation, and the membranes were assayed for purity using 5'-nucleotidase, cytochrome oxidase, and reduced nicotinamide adenine dinucleotide phosphate diaphorase activities. Lipids of the plasma membrane were enriched in cholesterol, phosphatidylcholine, and phosphatidylethanolamine. In contrast, membranes of the endoplasmic reticulum were enriched in phosphatidylcholine, but contained smaller amounts of phosphatidylethanolamine and sphingomyelin. Comparison of the fatty acyl chains of virus and membranes from uninfected cells revealed the virion to have the lowest ratio of C18:1 to C18:0 (1.771), compared with values of about 3.0 for the plasma membrane and endoplasmic reticulum. Total polyenoic fatty acids were enriched in the plasma membrane, whereas the virus contained higher amounts of total saturates than either of the two membrane preparations. Analysis of the polar and neutral lipid fractions as well as the acyl chain analysis suggests the virion has a lipid composition that is intermiediate to that of the plasma membrane and endoplasmic reticulum and is consistent with the view that numerous viral particles are synthesized de novo by not utilizing a preexisting membrane template. From the ratio of cholesterol to phospholipid of 0.48, we calculated that 1.92 X 10(5) molecules of lipid would cover 4.14 X 10(4) nm2 in the form of a bilayer. Considerations of the molecular dimensions of the rabies envelope (total surface area, 5 X 10(4) nm2) as a bilayer suggest that some penetration of lipids by envelope proteins (M and G) is necessary.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaslestad H. G., Urbano C. Nucleotide composition of the ribonucleic acid of rabies virus. J Virol. 1971 Dec;8(6):922–924. doi: 10.1128/jvi.8.6.922-924.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C., Blough H. A. Composition of the neutral lipids of bovine meilbomian secretions. J Lipid Res. 1976 Jul;17(4):373–376. [PubMed] [Google Scholar]
- Blough H. A., Merlie J. P. The lipids of incomplete influenza virus. Virology. 1970 Mar;40(3):685–692. doi: 10.1016/0042-6822(70)90213-8. [DOI] [PubMed] [Google Scholar]
- Blough H. A. Newly synthesised lipids incorporated into influenza virus membranes. Nature. 1974 Sep 27;251(5473):333–335. doi: 10.1038/251333a0. [DOI] [PubMed] [Google Scholar]
- Blough H. A., Tiffany J. M. Theoretical aspects of structure and assembly of viral envelops. Curr Top Microbiol Immunol. 1975;70:1–30. doi: 10.1007/978-3-642-66101-3_1. [DOI] [PubMed] [Google Scholar]
- Blough H. A., Weinstein D. B., Lawson D. E., Kodicek E. The effect of vitamin A on myxoviruses. II. Alterations in the lipids of influenza virus. Virology. 1967 Nov;33(3):459–466. doi: 10.1016/0042-6822(67)90121-3. [DOI] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer H., Kulas H. P., Schneider L. G., Schlumberger H. D. The lipid composition of rabies virus. Z Naturforsch C. 1973 Jan-Feb;28(1):90–93. doi: 10.1515/znc-1973-1-216. [DOI] [PubMed] [Google Scholar]
- Eger R., Compans R. W., Rifkin D. B. The organization of the proteins of vesicular stomatitis virions: labeling with pyridoxal phosphate. Virology. 1975 Aug;66(2):610–615. doi: 10.1016/0042-6822(75)90233-0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gahmberg C. G., Simons K. Isolation of plasma membrane fragments from BHK21 cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(2):176–182. doi: 10.1111/j.1699-0463.1970.tb04284.x. [DOI] [PubMed] [Google Scholar]
- Gallaher W. R., Blough H. A. Synthesis and turnover of lipids in monolayer cultures of BHK-21 cells. Arch Biochem Biophys. 1975 May;168(1):104–114. doi: 10.1016/0003-9861(75)90233-7. [DOI] [PubMed] [Google Scholar]
- HESS H., ROLDE E. FLUOROMETRIC ASSAY OF SIALIC ACID IN BRAIN GANGLIOSIDES. J Biol Chem. 1964 Oct;239:3215–3220. [PubMed] [Google Scholar]
- Hummeler K., Koprowski H., Wiktor T. J. Structure and development of rabies virus in tissue culture. J Virol. 1967 Feb;1(1):152–170. doi: 10.1128/jvi.1.1.152-170.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Choppin P. W. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969 Jun;38(2):255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- Kuwert E., Wiktor T. J., Sokol F., Koprowski H. Hemagglutination by rabies virus. J Virol. 1968 Dec;2(12):1381–1392. doi: 10.1128/jvi.2.12.1381-1392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McSharry J. J., Wagner R. R. Lipid composition of purified vesicular stomatitis viruses. J Virol. 1971 Jan;7(1):59–70. doi: 10.1128/jvi.7.1.59-70.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A. R., Vernon S. K., Dobkin M. B., Rubin B. A. Characterization of subviral components resulting from treatment of rabies virus with tri(n-butyl) phosphate. J Gen Virol. 1972 Jan;14(1):33–48. doi: 10.1099/0022-1317-14-1-33. [DOI] [PubMed] [Google Scholar]
- Renkonen O., Gahmberg C. G., Simons K., Käriäinen L. The lipids of the plasma membranes and endoplasmic reticulum from cultured baby hamster kidney cells (BHK21). Biochim Biophys Acta. 1972 Jan 17;255(1):66–78. doi: 10.1016/0005-2736(72)90008-9. [DOI] [PubMed] [Google Scholar]
- Renkonen O., Käriäinen L., Pettersson R., Oker-Blom N. The phospholipid composition of Uukuniemi virus, a non-cubical tick-borne arbovirus. Virology. 1972 Dec;50(3):899–901. doi: 10.1016/0042-6822(72)90443-6. [DOI] [PubMed] [Google Scholar]
- SMITH L. A study of some oxidative enzymes of baker's yeast. Arch Biochem Biophys. 1954 Jun;50(2):285–298. doi: 10.1016/0003-9861(54)90044-2. [DOI] [PubMed] [Google Scholar]
- Schlesinger H. R., Wells H. J., Hummeler K. Comparison of the lipids of intracellular and extracellular rabies viruses. J Virol. 1973 Nov;12(5):1028–1030. doi: 10.1128/jvi.12.5.1028-1030.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol F., Kuwert E., Wiktor T. J., Hummeler K., Koprowski H. Purification of rabies virus grown in tissue culture. J Virol. 1968 Aug;2(8):836–849. doi: 10.1128/jvi.2.8.836-849.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol F., Schlumberger H. D., Wiktor T. J., Koprowski H. Biochemical and biophysical studies on the nucleocapsid and on the RNA of rabies virus. Virology. 1969 Aug;38(4):651–665. doi: 10.1016/0042-6822(69)90184-6. [DOI] [PubMed] [Google Scholar]
- Sokol F., Stancek D., Koprowski H. Structural proteins of rabies virus. J Virol. 1971 Feb;7(2):241–249. doi: 10.1128/jvi.7.2.241-249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C. S., Bodansky O. Subcellular localization and properties of 5'-nucleotidase in the rat liver. J Biol Chem. 1967 Feb 25;242(4):694–699. [PubMed] [Google Scholar]
- Vernon S. K., Neurath A. R., Rubin B. A. Electron microscopic studies on the structure of rabies virus. J Ultrastruct Res. 1972 Oct;41(1):29–42. doi: 10.1016/s0022-5320(72)90036-6. [DOI] [PubMed] [Google Scholar]
- WALLACH D. F., KAMAT V. B. PLASMA AND CYTOPLASMIC MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA. Proc Natl Acad Sci U S A. 1964 Sep;52:721–728. doi: 10.1073/pnas.52.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]



