Abstract
Background
Atypical antipsychotics such as olanzapine cause metabolic side effects leading to obesity and insulin resistance. The underlying mechanisms remain elusive. In this study we investigated the effects of chronic treatment of olanzapine on the fatty acid composition of plasma in mice.
Methods
Twenty 8-week female Balb/c mice were randomly assigned to two groups: the OLA group and the control group. After treatment with olanzapine (10 mg/kg/day) or vehicle intraperitoneally for 8 weeks, fasting glucose, insulin levels and oral glucose tolerance test were determined. Effects on plasma fatty acid profile and plasma indices of D5 desaturase, D6 desaturase and SCD1 activity were also investigated.
Results
Chronic administration of olanzapine significantly elevated fasting glucose and insulin levels, impaired glucose tolerance, but did not increase body weight. Total saturated fatty acids and n-6 polyunsaturated fatty acids were significantly increased and total monounsaturated fatty acids were significantly decreased, while total n-3 polyunsaturated fatty acids showed no prominent changes. Chronic olanzapine treatment significantly up-regulated D6 desaturase activity while down-regulating D5 desaturase activity. Palmitic acid (C16:0), dihomo-γ-linolenic acid (C20:3n-6) and D6 desaturase were associated with an increase probability of insulin resistance, whereas nervonic acid (C24:1) and SCD1 were significantly associated with a lower insulin resistance probability.
Conclusions
All results indicated that such drug-induced effects on fatty acid profile in plasma were relevant for the metabolic adverse effects associated with olanzapine and possibly other antipsychotics. Further studies are needed to investigate geneticand other mechanisms to explain how plasma fatty acids regulate glucose metabolism and affect the risk of insulin resistance.
Introduction
Olanzapine is a widely used second generation antipsychotic (SGA) drug for the treatment of schizophrenia with a low propensity for neurological side effects[1]. However, it is frequently associated with serious metabolic side effects, such as dyslipidemia and insulin resistance (IR). Weight gain is frequently observed with olanzapine treatment in pre-clinical studies and in clinical settings[2–4]. However, the risk of metabolic side effects such as IR may be independent of weight gain[5]. Numerous studies have documented metabolic changes in the absence of substantial weight gain in clinical patients[6]. Furthermore, studies with healthy subjects showed that treatment with olanzapine caused significant metabolic impairments in the absence of weight gain[7–10]. Despite this body of evidence, the mechanisms underlying olanzapine-induced dyslipidemia and insulin resistance remain elucidated.
The fatty acids (FAs) in particular are highly associated with obesity, IR, and type 2 diabetes[11–15]. Several lines of evidence suggest that FAs are involved in the pathogenesis of IR via a reduction of insulin sensitivity and the promotion of pancreatic beta cell apoptosis and dysfunction[16–18]. Different FAs have distinct effects on insulin sensitivity, beta cell function andtissue inflammation[19]. In particular, n-6 polyunsaturated fatty acids (PUFAs) and saturated fatty acids (SFAs) (especially arachidonicacid and palmitic acid) can be viewed as pro-inflammatory molecules, whereas n-3 PUFAs (especially eicosapentaenoic acid and docosahexaenoic acid) can be viewed as anti-inflammatorymolecules[20].
The FA profile in blood partly reflects dietary FA intake, but it is also strongly determined by the endogenous FA metabolism[21]. The D5 desaturase (D5D) and D6 desaturase (D6D) catalyze the rate-limiting steps in the conversion of linoleic acid and α-linolenic acid into longchain n-6 and n-3PUFAs, whereas the stearoyl-CoAdesaturase-1 (SCD1) is required for the conversion of SFAs into monounsaturated fatty acids (MUFAs)[22]. By introducing a double bond into the FA chain, desaturaseenzymes have important consequences on thechemical structure of FAs and subsequently on their function. In fact, the activity of all three desaturaseshas been implicated in the development of certain metabolic diseases such as insulin resistance and type 2 diabetes[23–25].
The FA profile can be used as an indicator of disease risk[26]. An altered FA profile and estimated activities of desaturases have been associated with insulin resistance, type 2 diabetes[27], obesity[28], hypertriglyceridemia[29] and cardiovascular disease[30]. Free FAs concentrations are decreased following olanzapine treatment in clinical and animal studies[9]. However, previous basic and clinical studies also suggest that antipsychotics may augment PUFAs biosynthesis[31]. To our knowledge, there has not yet been an evaluation of the effect of chronic olanzapine treatment on the metabolic parameters and FA profile in Balb/c mice or human. Therefore, the primary aim of the current study was to establish, for the first time, the plasma FA profile in Balb/c mice after chronic treatment of olanzapine and to assess their associations with insulin resistance.
Material and Method
Animals
Twenty 8-week female Balb/c mice weighing 20–25g (Huafukang, Beijing, China) were purchased and maintained under a 12-hour light/dark cycle (lights on at 08:00 h) at 22±1°C. Animals were housed individually and allowed free access to food and water. Prior to the experiments, animals were routinely acclimated (>1 week) tolaboratory conditions to reduce potential stress effects during experiments. The animal facilities and protocol were performed in accordance with the guidelines of the Chinese Council on Animal Care and approved by the Institutional Animal Care and Use Committee of the Tongji Medical College, Huazhong University of Science and Technology (Permit Number:XH-B20150422).The number of mice was theminimum necessary to obtain significant results and inagreement with the triple R spirit for reduction of the number of animals used. Body weight was routinely recorded. Mice were anesthetized by isoflurane (Sigma, USA) and sacrificed at 9:00 am after 8 weeks of olanzapine treatment. Blood was collected into EDTA-coated tubes and plasma was isolated by centrifugation at 1,800×g for 10 min at 4°C. All samples were stored at –80°C until further analysis. All efforts were made to minimize suffering.
The primary study endpoints were the fasting insulin level and the homeostatic model assessment of insulin resistance (HOMA-IR) index. The secondary endpoint was the oral glucose tolerance test (OGTT).
Drug
Mice were randomly assigned to two groups: the OLA group and the control group. The OLA group received olanzapine intraperitoneally (10mg/kg/day) in the morning (08:00–08:40h) for 8 weeks, while the control group received drug vehicle. All injection volumes were 10ml/kg.
Olanzapine (Sigma, USA) was dissolved in0.1 N HCl in distilled water, adjusting to pH 6.0 with 1 N NaOH, and adding distilled water to reach the desired concentration. Vehicle was similarly pH-adjusted, distilled water. The solutions were stored at 4°C and protected from light degradation. At the time of drug administration, body weight was measured.
OGTT
OGTT was performed on the last day of the chronic treatment. Mice were food restricted for 14h prior to the OGTT. Mice received olanzapine one hour prior to the start of the OGTT. During the OGTT, basal blood samples and glucose measurements were obtained and then glucose was given via oral gavage (1.5g glucose/kg). Blood samples were collected via a tail snip at 30, 60, 90, and 120 min and glucose levels were determined. Blood glucose levels at 30, 60, 90, 120 min were determined via a tail snip method. Individual glucose measurements at the 5 time points during the OGTT were integrated to generate a single area under the curve (AUC) value.
Plasma glucose and insulin
Fast glucose concentrations were determined using a hand-held glucometer (One Touch Ultra). Fast insulin levels were measured using commercially available kits (ELISA, Crystal Chem Inc., IL, USA) according to the manufacturer’s instructions. All analyses were performed by a technician blinded to treatment.
Insulin resistance
To determine insulin resistance in mice, we calculated the HOMA-IR index on the last day of every week. This equation takes into account the product of both fasting levels of glucose (expressed as mmol/L) and insulin (μU/mL) at 60 minutes post-olanzapine treatment and divides by a constant of 22.5 ([I0 x G0]/22.5), where I0 and G0 are fasting insulin and glucose. A larger calculated HOMA-IR value denotes greater insulin resistance.
Fatty acid analysis
The plasma FA profile was determined by gas chromatography with a previous derivatization to their corresponding fatty acid methyl esters[31]. Briefly, fatty acids were trans-esterified and analysed using a TSQ 8000 gas chromatography system (Thermo Fisher Scientific, USA). Fatty acid identification was based on retention times of authenticated fatty acid methyl ester standards (Sigma, USA). Results were expressed as weight percent of total fatty acids (mg fatty acid/100mg fatty acids). We calculated total SFAs, MUFAs, n-3 and n-6 PUFAs. Desaturase activity was estimated indirectly using FA product/precursor ratios[32]. D5 Desaturase activity was calculated as the ratio of C20:4n-6/C20:3n-6, D6 Desaturase activity was calculated as the ratio of C20:3n-6/C18:2n-6, and SCD1 activity was calculated asthe ratio of C18:1/C18:0.
Statistics
All data are expressed as the mean±SD. To calculate statistical significance, Student’s t-test was used because our hypothesis is to test differences between two groups, the OLA group and the control group. The relationship between plasma FA composition, estimated desaturase activities and fasting glucose, insulin, HOMA-IR was determined by Spearman’s rank order correlation analysis. For allanalyses, statistical significance was determined at a P<0.05. Analyses were performed with SPSS version 15.0 (SPSS Inc.,Chicago, IL, USA).
Results
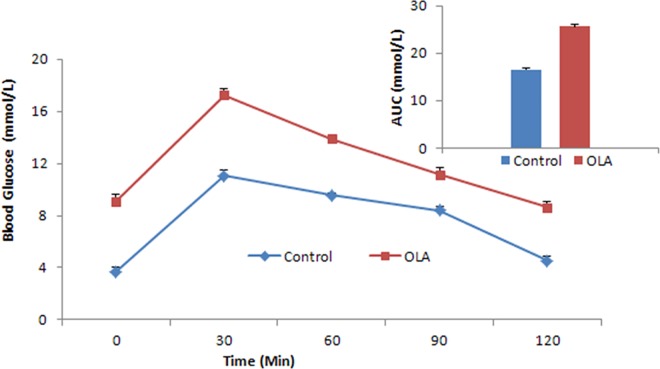
Mice in the OLA group exhibited a significant elevation in fasting glucose and insulin levels after 8-week treatment of olanzapine. In addition, a significant increase was evidenced in HOMA-IR index, suggesting an insulin resistance state in the OLA group. However, no significant change in body weight was observed after chronic olanzapine treatment compared to the control group (Table 1). After 8 weeks of olanzapine treatment, we challenged animals with an oral glucose tolerance test (Fig 1). The OLA group displayed significantly elevated fasting glucose and insulin levels after 14h of food restriction. Glucose levels were significantly increased compared to the control group throughout the OGTT. Additionally, the AUC was significantly increased by chronic olanzapine treatment (16.56mmol/L vs 25.59mmol/L, p<0.001), suggesting decreased whole-body insulin sensitivity. These results (detailed in S1 Data) demonstrate that chronically olanzapine administration induces insulin resistance without the change in body weight in mice.
Table 1. Mean value of weight, fasting glucose, insulin and HOMA-IR scores in the control group and the OLA group.
| Variables | The control group | The OLA group | ||||
|---|---|---|---|---|---|---|
| Baseline | 8-week treatment | p | Baseline | 8-week treatment | p | |
| Weight(g) | 21.27±0.38 | 21.43±0.35 | 0.3266 | 21.73±0.98 | 21.88±0.92 | 0.7257 |
| G0 | 3.32±0.27 | 3.51±0.25 | 0.1151 | 3.49±0.39 | 8.37±0.48 | <0.001 |
| I0 | 23.35±0.43 | 23.25±0.68 | 0.6829 | 23.33±0.76 | 31.31±1.02 | <0.001 |
| HOMA-IR | 3.44±0.26 | 3.62±0.24 | 0.1305 | 3.62±0.44 | 11.64±0.71 | <0.001 |
G0, fasting glucose levels(mmol/L); I0, fasting insulin levels(μU/ml); HOMA-IR, homeostasis model assessment of insulin resistance(μU·mmol)/(ml·L).
Values are represented as mean±SD.
Fig 1. Effects of chronic olanzapine treatment on glucose and AUC(top right) levels in femaleBalb/c mice in the oral glucose tolerance test.
Glucose levels of the OLA group were significantly elevated compared to the control group throughout the OGTT. The AUC of the OLA group was also significantly increased (p<0.001). AUC: area under the curve; OGTT: oral glucose tolerance test. * indicates different from the control group, p<0.001.
Plasma FAs composition are shown in Table 2.The plasma total SFAs (32.18% and 29.21%, p<0.001), and total n-6 PUFAs (29.34%and 26.10%, p<0.01) were both respectively higher in the OLA group compared to the control group. However, total MUFAs (34.60% and 40.88%, p<0.001) were significantly decreased in the OLA group compared to the control group. Total n-3 PUFAs showed no significant difference between two groups. In the OLA group, we observed increased palmitic acid (C16:0) and heptadecanoic acid (C17:0) levels when compared to the control group (20.29% and 18.74%, 0.70% and 0.52%). Linoleic acid (C18:2n-6), γ-linolenic acid (C18:3n-6), and dihomo-γ-linolenic acid (C20:3n-6) were statistically significantly increased in the plasma of OLA group as compared to the control group (22.1% and 19.46%, 0.55% and 0.47%, 1.26% and 0.90%), while eicosadienoic acid (C20:2n-6) was significantly decreased (0.77% and 1.01%). Moreover, in the OLA group myristoleic acid (C14:1), pentadecanoic acid (C15:1), palmitoleic acid (C16:1) and heptadecanoic acid (C17:1) were significantly decreased compared to the control group (0.09% and 0.14%, 0.02% and 0.03%, 4.72% and 6.85%, 0.39% and 0.44%), whereas nervonic acid (C24:1) was increased (0.07% and 0.05%). Our results (detailed in S1 Data) showed significantly decreased plasma activity of D5 desaturase and increased plasma activity of D6 desaturase in the OLA group compare to the control group. However, the plasma activity of SCD1 was similar in both groups.
Table 2. Fatty acid composition and estimated desaturase activities in mice after treatment.
| Fatty acid | The control group | The OLA group | p value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| C14:0, % | 2.12 | 0.27 | 2.01 | 0.17 | 0.2797 |
| C15:0, % | 0.23 | 0.03 | 0.25 | 0.04 | 0.1776 |
| C16:0, % | 18.74 | 0.75 | 20.29 | 1.02 | 0.0011 |
| C17:0, % | 0.52 | 0.06 | 0.70 | 0.10 | 0.0001 |
| C18:0, % | 8.12 | 0.87 | 7.86 | 0.99 | 0.5289 |
| C20:0, % | 0.35 | 0.09 | 0.32 | 0.05 | 0.3286 |
| C22:0, % | 0.06 | 0.02 | 0.07 | 0.01 | 0.6426 |
| C24:0, % | 0.07 | 0.03 | 0.09 | 0.01 | 0.0751 |
| Total SFAs, % | 30.21 | 0.99 | 31.58 | 1.76 | 0.0462 |
| C14:1, % | 0.14 | 0.03 | 0.09 | 0.02 | 0.0002 |
| C15:1, % | 0.03 | 0.01 | 0.02 | 0.01 | 0.0023 |
| C16:1, % | 6.85 | 1.05 | 4.72 | 0.32 | 0.0000 |
| C17:1, % | 0.44 | 0.04 | 0.39 | 0.03 | 0.0123 |
| C18:1, % | 29.59 | 2.76 | 27.68 | 1.16 | 0.0591 |
| C20:1, % | 2.53 | 0.54 | 2.46 | 0.70 | 0.8144 |
| C22:1, % | 0.25 | 0.06 | 0.26 | 0.06 | 0.6124 |
| C24:1, % | 0.05 | 0.02 | 0.07 | 0.02 | 0.0114 |
| Total MUFAs, % | 39.88 | 1.85 | 35.70 | 0.95 | 0.0000 |
| C18:3n-3, % | 0.83 | 0.22 | 0.70 | 0.13 | 0.1074 |
| C20:5n-3, % | 0.51 | 0.16 | 0.53 | 0.08 | 0.6909 |
| C22:5n-3, % | 0.69 | 0.20 | 0.64 | 0.05 | 0.4356 |
| C22:6n-3, % | 1.76 | 0.31 | 2.03 | 0.28 | 0.0502 |
| Total n-3 PUFAs, % | 3.79 | 0.80 | 3.90 | 0.33 | 0.6986 |
| C18:2n-6, % | 19.46 | 1.21 | 22.01 | 2.55 | 0.0104 |
| C18:3n-6, % | 0.47 | 0.03 | 0.55 | 0.07 | 0.0031 |
| C20:2n-6, % | 1.01 | 0.09 | 0.77 | 0.12 | 0.0001 |
| C20:3n-6, % | 0.90 | 0.17 | 1.26 | 0.07 | 0.0000 |
| C20:4n-6, % | 3.82 | 0.46 | 3.56 | 0.39 | 0.1938 |
| C22:4n-6, % | 0.44 | 0.06 | 0.39 | 0.09 | 0.1568 |
| Total n-6 PUFAs, % | 26.10 | 1.42 | 28.54 | 2.20 | 0.0085 |
| D5D(C20:4n-6/C20:3n-6) | 4.32 | 0.69 | 2.83 | 0.21 | 0.0000 |
| D6D(C20:3n-6/C18:2n-6) | 0.05 | 0.01 | 0.06 | 0.01 | 0.0050 |
| SCD1(C18:1/C18:0) | 3.65 | 0.23 | 3.56 | 0.37 | 0.5255 |
D5D, D5 desaturase; D6D, D6 desaturase; SCD1, stearoyl coenzyme A desaturase-1.
Table 3 shows significant associations between FA proportions and estimated desaturase activities and fasting glucose, insulin levels and HOMA-IR in the OLA group (detailed in S1 Data). In the OLA group, plasma palmitic acid (C16:0) composition and dihomo-γ-linolenic acid (C20:3n-6) were positively correlated with insulin and IR, but not with glucose. Plasma heptadecanoic acid (C17:0), pentadecanoic acid (C15:1) and linoleic acid (C18:2n-6) were inversely correlated with insulin, but not with glucose or IR. Plasma nervonic acid (C24:1) was inversely with glucose and IR, but not with insulin. For estimated desaturase activities, D6 desaturase was positively associated with insulin and IR, whereas SCD1 was inversely associated with insulin and IR. D5 desaturase showed non-significant associations with glucose, insulin and IR.
Table 3. Spearman rank correlation coefficients between plasma fatty acids, estimated desaturase activities and fasting glucose, insulin and HOMA-IR in the OLA mice.
| Fasting glucose | Fasting insulin | HOMA-IR | |
|---|---|---|---|
| Fatty acids | |||
| C14:0 | -0.49 | 0.45 | -0.16 |
| C15:0 | -0.38 | -0.72 | -0.64 |
| C16:0# | 0.38 | 0.65* | 0.68* |
| C17:0# | -0.28 | -0.68* | -0.57 |
| C18:0 | 0.31 | 0.79 | 0.70 |
| C20:0 | -0.50 | -0.65 | -0.80 |
| C22:0 | -0.63 | -0.37 | -0.79 |
| C24:0 | -0.54 | 0.68 | -0.14 |
| C14:1# | -0.28 | 0.21 | -0.27 |
| C15:1# | 0.08 | -0.72* | -0.28 |
| C16:1# | 0.26 | 0.26 | 0.18 |
| C17:1# | -0.08 | -0.38 | -0.29 |
| C18:1 | 0.49 | 0.54 | 0.65 |
| C20:1 | -0.29 | -0.79 | -0.68 |
| C22:1 | -0.66 | -0.52 | -0.89 |
| C24:1# | -0.68* | -0.02 | -0.66* |
| C18:3n-3 | -0.45 | -0.46 | -0.61 |
| C20:5n-3 | 0.54 | 0.47 | 0.69 |
| C22:5n-3 | -0.04 | -0.48 | -0.33 |
| C22:6n-3 | 0.80 | 0.16 | 0.78 |
| C18:2n-6# | -0.27 | -0.83* | -0.61 |
| C18:3n-6# | 0.12 | -0.22 | -0.18 |
| C20:2n-6# | -0.29 | 0.07 | -0.40 |
| C20:3n-6# | 0.40 | 0.71* | 0.68* |
| C20:4n-6 | 0.13 | 0.82 | 0.54 |
| C22:4n-6 | -0.18 | 0.53 | 0.02 |
| D5D(C20:4n-6/C20:3n-6) # | 0.04 | 0.64 | 0.42 |
| D6D(C20:3n-6/C18:2n-6) # | 0.42 | 0.76* | 0.75* |
| SCD1(C18:1/C18:0) | -0.32 | -0.82* | -0.73* |
D5D, D5desaturase; D6D, D6desaturase; SCD1,stearoyl coenzyme A desaturase-1; HOMA-IR, homeostasis model assessment-insulin resistance.
#fatty acids and estimated desaturase activities that show significant difference between the OLA group and the control group.
*P<0.05.
Discussion
The current study showed that chronic olanzapine treatment induces a significant increase of fasting glucose and insulin levels and insulin resistance in female Balb/c mice without weight gain. Mice in the OLA group showed higher levels of total SFAs and n-6 PUFAs and lower levels of total MUFAs than those in the control group. Particularly, the levels of FAs (C16:0), (C17:0), (C24:1), (C18:2n-6), (C18:3n-6) and (C20:3n-6) were higher, and the levels of FAs (C14:1), (C15:1), (C16:1), (C17:1) and (C20:2n-6) were lower in the OLA group compared to the control group. In contrast, the levels of total n-3 PUFAs showed no significant differences between the two groups. We also found that palmitic acid (C16:0), dihomo-γ-linolenic acid (C20:3n-6) and D6 desaturase were associated with an increase probability of IR, whereas nervonic acid (C24:1) and SCD1 were significantly associated with a lower IR probability.
Previous work has demonstrated that chronic treatment of SGAs frequently induces excessive weight gain and obesity in schizophrenic patients and animals[33]. However, in the present study we observed no significant weight gain in mice following chronic olanzapine treatment. In agreement with our results, Karen L.Teff et al[10] reported that olanzapine causes significant elevations in postprandial insulin, glucagon-like peptide 1, and glucagon coincident with insulin resistance in the absence of weight gain in healthy subjects. Moreover, decreased body weight has previously been observed in rats following chronic olanzapine or paliperidone treatment at specific dose[34]. Furthermore, numerous reports have documented hyperglycemia and new-onset type 2 diabetes in the absence of substantial weight gain inSGA-treated patients[6].
In the present study, we showed that chronic olanzapine treatment significantly increased total SFAs and decreased total MUFAs. Additionally, palmitic acid, the main contributor of total SFAs, was significantly increased and positively correlated with IR, in agreement with recent studies showing associations with impaired insulin sensitivity[35–36]. Many epidemiological reports suggested that diets high in saturated fats are associated with insulin resistance and an increased prevalence of type 2 diabetes[37]. In particular, elevated levels of SFAs induce inflammation, which results in insulin resistance via several pathways involving diacylglycerol-mediated protein kinase C activation or Toll-like receptors[38–39]. On the other hand, data are emerging which support that monounsaturated fatty acids have protective effects against saturated fat mediated toxicity[38–40]. Moreover, cultured cells incubated in high concentrations of saturated fatty acids exhibited impaired insulin signaling[41]. Interestingly, the addition of a monounsaturated fatty acid, such as oleate or palmitoleate, to palmitate in the incubation media can attenuate the deleterious effects of palmitate on insulin signaling[38]. The significant difference in the SFAs proportion between the OLA group and the control group that we observed was small(median 31.58% vs. 30.21%). However, the significant difference in the MUFAs proportion was obvious (median 35.70% vs. 39.88%). The biological relevance was unclear. It is possible that the deleterious effects of a high saturated fatty acid profile may be predominately mediatedvia impairments in other tissues (e.g. adipose, liver, vascular) and/or secondary to resulting pro-inflammatory/stress responses in these other tissues. Moreover, our results suggest that the higher proportion of SFAs and lower proportion of MUFAs in the OLA group may be caused by endogenous transformation of SFAs to MUFAs, as the calculated activity of SCD-1 was slightly lower in the OLA group.
At an exploratory level of significance we also found higher levels of the essential fatty acid linoleic acid (C18:2n-6) and total n-6 PUFAs in the OLA group. These n-6 PUFAs are linked to inflammatory signaling, insulin resistance and type 2 diabetes risk[42]. Therefore a linoleic acid (C18:2n-6) poor diet might have the beneficial effect of less arachidonic acid (C20:4n-6) production and anti-inflammatory eicosanoid synthesis[43]. In the current study, we found no significant difference in n-3 fatty acids between the OLZ groups and the control group. Consistent with our findings, Robert K. McNamara et al. showed that chronic treatment with olanzapine and quetiapine did not significantly up-regulate plasma indices of n-3 PUFAs biosynthesis[44]. Also in agreement with previous reports, n-3 PUFAs were not associated with the worsening of hyperglycemia or the risk of insulin resistance[36].
The FA profile in blood and tissues partly reflectsdietary FA intake, but it is also strongly determined bythe endogenous FA metabolism. The fatty acid-modifying enzymes for which connections to insulin resistance and type 2 diabetes have been shown include D5 desaturase, D6 desaturase and SCD[22]. Clinical studies have found that chronic treatment with risperidone or olanzapine significantly increase plasma indices of D6 desaturase activity[45]. Similarly, our results showed that chronic treatment of olanzapine significantly stimulated the activity of D6 desaturase. Moreover, the activity of D6 desaturase was found to positively correlate with insulin resistance, which was in agreement with previous studies[26, 46]. Our results also exhibited that chronic olanzapine treatment significantly decreased the activity of D5 desaturase. However, we did not observe significant difference in the activity of SCD1 between the two groups. Although correlations between the activity of D5 desaturase and SCD1 and insulin resistance were shown in previous studies[22, 26], we were unable to reproduce these observations, probably due to the different study animals.
Recently, it has been postulated that potential alterations in the metabolic pathway of PUFAs synthesis could constitute a fundamental trigger in the initiation and propagation of metabolic abnormalities, such as IR[26, 47]. Numerous previous studies reported that SGAs medications up-regulate long-chain PUFA biosynthesis in rats[44], and PUFAs reduce SCD1 mRNA expression at the level of transcription and mRNA stability[48–50]. SCD1 is of particular interest because it is the rate-limiting step in the transformation of pro-inflammatory SFAs to MUFAs[22]. In the present study, we also found that chronic treatment of olanzapine significantly increased n-6 PUFAs. Additionally, total SFAs was increased and total MUFAs was decreased in the OLA group. As SCD1 is the rate-limiting step in the transformation of SFAs to MUFAs, it is important to account for PUFAs in future antipsychotic studies. It is therefore possible that, at least in part, the side effects of chronic olanzapine treatment maybe related to changes in the n-6 PUFAs. Future studies with larger samples and different antipsychotic medications are needed to address this important question.
This study has three notable limitations. First, olanzapine has a shorter plasma half-life in rats (~2.5h) than in humans. However, previous studies showed that 15 days of i.p. olanzapine at 6 mg/kg body weight in rats produced a mean plasma concentration of 12.0±4.9 ng/ml at 3h after the last daily dose, which is comparable to the plasma concentration (above 9 ng/ml) that had a greater likelihood of clinical response[51]. Moreover, numerous reports showed that chronic daily treatment with olanzapine induced significant changes in adiposity and lipid metabolismin female rats[34,44,52]. Nevertheless, without the plasma olanzapine concentration data it remains possible that greater changes in FA profile may have been observed with a different mode of administration. Second, this study examined the insulin resistance in mice with OGTT test rather than clamp technique, which is the “gold-standard” technique. However, Heidi N. Boyda et al showed a high degree of correlation between results obtained with the GTT and the HIEC in rats treated with SGA drugs[53]. The OGTT test in this study may predict, at least in part, the insulin resistance state in mice. Third, due to the limitation of the lab, we put animals in adjacent rooms. However, throughout the experiment mice were treated in the same way, except in adjacent rooms. Nevertheless, in view of experiment design it would be better if the mice were put in the same room.
In conclusion, our study shows that chronic olanzapine treatment induced a significant increase in fasting glucose and insulin levels and insulin resistance without body weight gain. Chronic treatment of olanzapine significantly increased total SFAs and total n-6 PUFAs, while decreased total MUFAs. Olanzapine also significantly up-regulated D6 desaturase activity while down-regulated D5 desaturase activity. Our study also find that palmitic acid (C16:0), dihomo-γ-linolenic acid (C20:3n-6) and D6 desaturase were associated with an increase probability of IR, whereas nervonic acid (C24:1) and SCD1 were significantly associated with a lower IR probability. The present data provide further support for olanzapine-mediated perturbation of fatty acid profile in plasma as a molecular mechanism involved in antipsychotic-associated metabolic adverse effects. Further studies are needed to investigate genetic and other mechanisms to explain how plasma fatty acids regulate glucose metabolism and affect the risk of IR.
Supporting Information
(XLSX)
Acknowledgments
The work was supported by the National Natural Science Foundation of China under Grant No. 81573509. We acknowledge the technical assistance of Xiaohui Liu and her metabolic assay platform in Tsinghua University in the metabolic experiments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from the Natural Science Foundation of China (http://www.nsfc.gov.cn/) (Grant 81573509) awarded to WY Li. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. 10.1056/NEJMoa051688 [DOI] [PubMed] [Google Scholar]
- 2.Shobo M, Yamada H, Mihara T, Kondo Y, Irie M, Harada K, et al. Two models for weight gain and hyperphagia as side effects of atypical antipsychotics in male rats: validation with olanzapine and ziprasidone. Behav Brain Res. 2011;216(2):561–568. 10.1016/j.bbr.2010.08.046 [DOI] [PubMed] [Google Scholar]
- 3.Cooper GD, Pickavance LC, Widling JP, Halford JC, Goudle AJ. A parametric analysis of olanzapine-induced weight gain in female rats. Psychopharmacology (Berl). 2005;181(1):80–89. [DOI] [PubMed] [Google Scholar]
- 4.Baptista T, ElFakih Y, Uzcategui E, Sandia I, Talamo E, Araujo de Baptista E, et al. Pharmacological management of atypical antipsychotic-induced weight gain. CNS Drugs. 2008;22(6):477–495. [DOI] [PubMed] [Google Scholar]
- 5.Hahn M, Chintoh A, Giacca A, Xu L, Lam L, Mann S, et al. Atypical antipsychotics and effects of muscarinic, serotonergic, dopaminergic and histaminergic receptor binding on insulin secretion in vivo: an animal model. Schizophr Res. 2011;131(1–3):90–95. 10.1016/j.schres.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 6.Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19Suppl 1:1–93. [DOI] [PubMed] [Google Scholar]
- 7.Sacher J, Mossaheb N, Spindelegger C, Klein N, Geiss-Granadia T, Sauermann R, et al. Effects of olanzapine and ziprasidone on glucose tolerance in healthy volunteers. Neuropsychopharmacology.2008;33(7):1633–1641. 10.1038/sj.npp.1301541 [DOI] [PubMed] [Google Scholar]
- 8.Vidarsdottir S, de Leeuw van Weenen JE, Frolich M, Roelfsema F, Romijn JA, Pijl H. Effects of olanzapine and haloperidol on the metabolic status of healthy man. J Clin Endocrinol Metab. 2010;95(1):118–125. 10.1210/jc.2008-1815 [DOI] [PubMed] [Google Scholar]
- 9.Albaugh VL, Singareddy R, Mauger D, Lynch CJ. A double blind,placebo-controlled, randomized crossover study of the acute metabolic effects of olanzapine in healthy volunteers. PLoS One. 2011;6(8):e22662 10.1371/journal.pone.0022662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-Induced Insulin Resistance and Postprandial Hormonal Dysregulation Independent of Weight Gain or Psychiatric Disease. Diabetes. 2013;62:3232–3240. 10.2337/db13-0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan CY, Virtue S, Murfitt S, Roberts LD, Phua YH, Dale M, et al. Adiposetissue fatty acid chain length and mono-unsaturation increases with obesity and insulin resistance. Sci Rep. 2016;6:23873 10.1038/srep23873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrient. 2016;8(3):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Xu S, Wei S, Deng Y, Li Y, Yang F, et al. Comparative Proteomic Study of Fatty Acid-treated Myoblasts Reveals Role of Cox-2 in Palmitate-induced Insulin Resistance. Sci Rep. 2016;6:21454 10.1038/srep21454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han E, Yun Y, Kim G, Lee YH, Wang HJ, Lee BW, et al. Effects of Omega-3 Fatty Acid Supplementation on Diabetic Nephropathy Progression in Patients with Diabetes and Hypertriglyceridemia. PLoS One. 2016;11(5):e0154683 10.1371/journal.pone.0154683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salto LM, Bu L, W. Beeson WL, Firek A, Cordero-MacIntyre Z, De Leon M. The Ala54Thr Polymorphism of the Fatty Acid Binding Protein 2 Gene Modulates HDL Cholesterol in Mexican-Americans with Type 2 Diabetes. Int J Environ Res Public Health. 2016;13(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diatbete. 2002;51(1):7–18. [DOI] [PubMed] [Google Scholar]
- 17.Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, et al. A Sustained Increase in Plasma Free Fatty Acids Impairs Insulin Secretion in Nondiabetic Subjects Genetically Predisposed to Develop Type 2 Diabetes. Diabetes. 2003;52(10):2461–2474. [DOI] [PubMed] [Google Scholar]
- 18.Roden M. Does endurance training protect from lipotoxicity? Diabetes. 2012; 61(10):2397–2399. 10.2337/db12-0662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara T, Kimura I, Inoue D, Ichimura A, Hirasawa A. Free fatty acid receptors and their role in regulation of energy metabolism. Reviews of physiology, biochemistry and pharmacology. 2013;164:77–116. 10.1007/112_2013_13 [DOI] [PubMed] [Google Scholar]
- 20.Sears B, Perry M. The role of fatty acids in insulin resistance.Lipids in Health and Disease. 2015;14:121 10.1186/s12944-015-0123-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjermo H, Riserus U. Role of hepatic desaturases in obesity-related metabolic disorders. CurrOpinClinNutrMetab Care. 2010;13:703–708. [DOI] [PubMed] [Google Scholar]
- 22.Kroger J, Schulze MB. Recent insights into the relation of D5 desaturase and D6 desaturase activity to the development of type 2 diabetes.CurrOpinLipidol. 2012;23:4–10. [DOI] [PubMed] [Google Scholar]
- 23.Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, et al. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. NutrMetabCardiovasc Dis. 2008;18:503–510. [DOI] [PubMed] [Google Scholar]
- 24.Chow LS, Li S, Eberly LE, Seaquist ER, Eckfeldt JH, Hoogeveen RC, et al. Estimated plasma stearoyl co-A desaturase-1 activity and risk of incident diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Metabolism. 2013;62:100–108. 10.1016/j.metabol.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs S, Schiller K, Jansen EH, Boeing H, Schulze MB, Kroger J. Evaluation of various biomarkers as potential mediators of the association between D5 desaturase, D6 desaturase, and stearoyl-CoA desaturase activity and incident type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition–Potsdam Study. Am J ClinNutr. 2015;102:155–164. [DOI] [PubMed] [Google Scholar]
- 26.Mayneris-Perxachs J, Guerendiain M, Castellote Al, Estruch R, Covas MI, Fito M, et al. Plasma fatty acid composition, estimated desaturase activities, and their relation with the metabolic syndrome in a population at high risk of cardiovascular disease. Clinical Nutrition. 2014;33:90–97. 10.1016/j.clnu.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 27.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes.Prog Lipid Res. 2009;48:44–51. 10.1016/j.plipres.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warensjö E, Öhrvall M, Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. NutrMetabCardiovasc Dis. 2006;16:128–136. [DOI] [PubMed] [Google Scholar]
- 29.Paillard F, Catheline D, Duff FL, Bouriel M, Deugnier Y, Pouchard M, et al. Plasma palmitoleic acid, a product of stearoyl-coAdesaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. NutrMetabCardiovasc Dis. 2008;18:436–440. [DOI] [PubMed] [Google Scholar]
- 30.Erkkilä A, de Mello VD, Risérus U, Laaksonen DE. Dietary fatty acids and cardiovascular disease: an epidemiological approach. Prog Lipid Res. 2008;47:172–187. 10.1016/j.plipres.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 31.McNamara RK, Able JA,Jandacek R, Rider T, Tso P. Chronic Risperidone Treatment Preferentially Increases Rat Erythrocyte and Prefrontal Cortex Omega-3 Fatty Acid Composition: Evidence for Augmented Biosynthesis. Schizophr Res. 2009;107(2–3):150–157. 10.1016/j.schres.2008.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayneris-Perxachs J, Guerendiain M, Castellote AI, Estruch R, Covas MI, Fito M, et al. Plasma fatty acid composition, estimated desaturase activities, andtheir relation with the metabolic syndrome in a population at highrisk of cardiovascular disease. ClinNutr. 2014;33(1):90–97. [DOI] [PubMed] [Google Scholar]
- 33.Henderson DC. Weight gain with atypical antipsychotics: evidence and insights. J Clin Psychiatry.2007; 68:18–26. [PubMed] [Google Scholar]
- 34.McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Atypical Antipsychotic Medications Increase Postprandial Triglyceride and Glucose Levels in Male Rats: Relationship with Stearoyl-CoA Desaturase Activity. Schizophr Res. 2011;129(1):66–73. 10.1016/j.schres.2011.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma W, Wu JH, Wang Q, Lemaitre RN, Mukamal KJ,Djousse L, et al. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study. Am J ClinNutr. 2015;101:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lankinen MA, Stančáková A, Uusitupa M, Ågren J, Pihlajamäki J, Kuusisto J, et al. Plasma fatty acids as predictors of glycaemia and type 2 diabetes. Diabetologia. 2015;58(11):2533–2544. 10.1007/s00125-015-3730-5 [DOI] [PubMed] [Google Scholar]
- 37.Hu FB, van Dam RM, Liu S. Diet and risk of Type II diabetes: the role of types of fat and carbohydrate. Diabetologia. 2001; 44(7):805–817. 10.1007/s001250100547 [DOI] [PubMed] [Google Scholar]
- 38.Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, et al. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem. 2008;283(17):11107–11116. 10.1074/jbc.M708700200 [DOI] [PubMed] [Google Scholar]
- 39.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate -induced insulin resistance in myotubes. J Biol Chem. 2006;281(37):26865–26875. 10.1074/jbc.M513304200 [DOI] [PubMed] [Google Scholar]
- 40.Pardo V, González-Rodríguez A, Guijas C,Balsinde J, Valverde AM. Opposite Cross-Talk by Oleate and Palmitate on Insulin Signaling in Hepatocytes through Macrophage Activation.J Biol Chem. 2015;290(18):11663–11677. 10.1074/jbc.M115.649483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch BiochemBiophys. 2003;419(2):101–109. [DOI] [PubMed] [Google Scholar]
- 42.Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. European journal of pharmacology. 2011;668Suppl 1:S50–58. [DOI] [PubMed] [Google Scholar]
- 43.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr and Metab. 2012; 2012:539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Differential Effects of Antipsychotic Medications on Polyunsaturated Fatty Acid Biosynthesis in Rats: Relationship with Liver Delta6-Desaturase Expression. Schizophr Res. 2011;129(1):57–65. 10.1016/j.schres.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaddurah-Daouk R, McEvoy J, Baillie RA, Lee D, Yao JK, Doraiswamy PM, et al. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol Psychiatry. 2007;12(10):934–945. 10.1038/sj.mp.4002000 [DOI] [PubMed] [Google Scholar]
- 46.Sethom MM, Fares S, Feki M, Hadj-Taieb S, Elasmi M, Omar S, et al. Plasma fatty acids profile and estimated elongase and desaturases activities in Tunisian patients with the metabolic syndrome. Prostaglandins LeukotEssent Fatty Acids. 2011;85(3):137–141. [DOI] [PubMed] [Google Scholar]
- 47.Araya J, Rodrigo R, Pettinelli P, Arava AV, Poniachik J, Videla LA. Decreased liver fatty acid delta-6 and delta-5 desaturase activity in obese patients.Obesity (Silver Spring). 2010;18(7):1460–1463. [DOI] [PubMed] [Google Scholar]
- 48.Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res. 1999;40:1549–1558. [PubMed] [Google Scholar]
- 49.Sessler AM, Kaur N, Palta JP, Ntambi JM. Regulation of stearoyl-CoA desaturase 1 mRNA stability by polyunsaturated fatty acids in 3T3-L1 adipocytes. J Biol Chem. 1996;271:29854–29858. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–376. 10.1146/annurev.nutr.24.121803.063211 [DOI] [PubMed] [Google Scholar]
- 51.Aravagiri M, Teper Y, Marder SR. Pharmacokinetics and tissue distribution of olanzapine in rats. Biopharm Drug Dispos. 1999;20(8):369–377. [DOI] [PubMed] [Google Scholar]
- 52.Mann S, Chintoh A, Giacca A, Fletcher P, Nobrega J, Hahn M, et al. Chronic olanzapine administration in rats: effect of route of administration on weight, food intake and body composition.PharmacolBiochemBehav. 2013;103(4):717–722. [DOI] [PubMed] [Google Scholar]
- 53.Boyda HN, Procyshyn RM, Pang CC, Hawkes E, Wong D, Jin CH, et al. Metabolic side-effects of the novel second-generation antipsychotic drugs asenapine and iloperidone: a comparison with olanzapine.PLoS One. 2013;8(1):e53459 10.1371/journal.pone.0053459 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.