Abstract
Liquid Extraction Surface Analysis (LESA) is a new, high throughput tool for ambient mass spectrometry. A solvent droplet is deposited from a pipette tip onto a surface and maintains contact with both the surface and the pipette tip for a few seconds before being re-aspirated. The technique is particularly suited to the analysis of trace materials on surfaces due to its high sensitivity and low volume of sample removal. In this work, we assess the suitability of LESA for obtaining detailed chemical profiles of fingerprints, oral fluid and urine, which may be used in future for rapid medical diagnostics or metabolomics studies. We further show how LESA can be used to detect illicit drugs and their metabolites in urine, oral fluid and fingerprints. This makes LESA a potentially useful tool in the growing field of fingerprint chemical analysis, which is relevant not only to forensics but also to medical diagnostics. Finally, we show how LESA can be used to detect the explosive material RDX in contaminated artificial fingermarks.
1. Introduction
The relatively recent inception of ambient mass spectrometry methods such as desorption electrospray ionisation (DESI) [1, 2], plasma-assisted desorption ionisation (PADI) [3], direct analysis in real time (DART) [4] and paper spray mass spectrometry [5] has provided an exciting new capability for the high throughput analysis of many types of sample. Because the techniques generally do not require any form of sample preparation and can be carried out under ambient conditions, there has been considerable interest in their adoption for the analysis of biological fluids [6–8].
Liquid Extraction Surface Analysis (LESA) [9–13] is a new tool in surface mass spectrometry, which also operates under ambient conditions and has recently become commercially available. The technique involves the deposition of a solvent droplet from a pipette tip onto a surface. The solvent droplet maintains contact with both the surface and the pipette tip for a defined time period (a few seconds) before being re-aspirated. The resulting sample can either be directly electrosprayed or subjected to further manipulation before mass spectrometry analysis. The technique consumes only a very small volume of sample, making it minimally destructive and suitable for the analysis of trace materials on surfaces. Because the analysis takes place under ambient conditions and does not require sample preparation, high throughput screening of samples is possible. In contrast to other ambient analysis techniques mentioned above, the process of surface sampling and ionisation are decoupled, offering opportunities for superior quantification. Whilst LESA has shown promise for the profiling of proteins, lipids, drugs and metabolites in tissue and blood [10–13], its potential application to the chemical profiling of other biological fluids has not yet been considered.
Chemical profiling of biological fluids is a growing research area, with applicability to metabolomics, medical diagnostics, toxicologyand roadside drug testing. For these applications a variety of biological matrices may be used or have been proposed for use, with complementary features. In medical diagnostics, urine is widely used for the detection of disease and for therapeutic drug monitoring [14]. In the forensics field, oral fluid is becoming the matrix of choice for roadside testing due to its non-invasive nature, but is rapidly gaining acceptance as a suitable matrix for medical diagnostics [15–16].
Another biological material of interest is a latent fingerprint. The field of fingerprint chemical analysis is also a rapidly growing research area, starting from a recent paradigm shift in the forensic community for using fingerprints for more than just their ridge detail [21–29]. It has been previously shown that the chemical composition relates to the age of a latent fingerprint [28], the sex of the donor [24], their drug habits or medical history [17–20], the way they interact with development reagents [21] and might even be used to confirm their identity using their amino acid profile [29] or to verify their location on questioned documents [23]. This may be useful when a fingerprint is smudged or details of the donor are not contained within the fingerprint database. Many techniques have been proposed for these applications, including spectroscopic techniques [30], which lack the selectivity of mass spectrometry methods; chromatography based approaches [22,29] which require considerable sample preparation and consume at least an entire fingerprint; and vacuum based methods [20–25], which have lower sample throughput and have been shown to degrade fingerprint chemistry [31].
Here, we show how LESA coupled to high resolution mass spectrometry can be used to rapidly detect a wide array of molecules, including ingested drugs and their metabolites, amino acids, fatty acids, peptides and other endogenous compounds in urine, oral fluid and fingerprint. The wide array of compounds detected from a small sample make the technique an attractive approach for diagnostic assays of the future from any of these matrices.
We also show how LESA can detect an explosive material in contaminated fingerprints. Finally, we will demonstrate how fingerprint analysis using LESA can be carried out in conjunction with higher resolution imaging methods (in this case Matrix Assisted Laser Desorption Ionisation Mass Spectrometry (MALDI)), for a more detailed review of compound distribution.
2. Experimental
Sample collection
Latent fingerprints were obtained from an individual attending a drug and alcohol treatment service to receive treatment for drug dependence. “Natural” fingerprints were deposited onto glass microscope slides at a pressure between 400–1000 g and barcoded, before being shipped to the laboratory for analysis. Two corresponding oral fluid samples were collected from the patient using a Quantisaltm collection kit, Alere Toxicology, UK. One oral fluid sample was screened for amphetamines, benzodiazepines, cannabis, cocaine, methadone and opiates, using a standard immunoassay drug screening procedure at LGC Forensics, Teddington, Middlesex. The second was reserved for LESA analysis. A urine sample was also collected from the patient, using an LGC Forensics urine collection kit. Immediately prior to analysis, 0.2 μL drops of the oral fluid solution and the urine were spotted onto separate glass slides. The fingerprints, second oral fluid sample and the urine were stored in a fridge at 2–5°C and then allowed to reach room temperature prior to analysis. Reference spectra were collected from standards of cocaine, methylecgnonine (EME), benzoylecgonine (BZE), methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), 2-ethyl-5-methyl-3,3-diphenylpyrroline (EMDP), heroin and 6-acetylmorphine (6-AM) immediately prior to analysis. Drug and metabolite standards were purchased from Sigma Aldrich Limited, UK. A favourable ethical opinion for sample collection and analysis was granted from the UK National Research Ethics Service, reference 14/LO/0346.
Latent fingerprints were also collected from an additional female and a male volunteer who were not patients of the drug and alcohol treatment service. The hands of the volunteers were not washed prior to deposition of the fingerprints. A second female volunteer with no drug use history provided a sample of urine and oral fluid.
To investigate the possibility of detecting exogenous compounds in fingerprints, artificial fingerprints with controlled contamination were prepared. A polymeric dummy finger was rubbed over forehead and cheek and subsequently loaded with explosives by rubbing the dummy over the explosive loaded tissue paper. Standard solutions (1 ml, 100 μg/ml) of TNT and RDX were absorbed on regular tissue paper, which was placed in a petridish. The tissue paper was wetted with approximately 2 ml of ethanol. The loaded dummy finger was subsequently placed on different microscope slides to make contaminated fingerprints.
All experiments were performed in compliance with UK law and the University of Surrey institutional guidelines, and the experiments were approved by both the University of Surrey Ethics Committee as well as the National Research Ethics Service (reference 14/LO/0346). Informed consent was obtained for all sample collections.
LESA
Materials
Methanol, ethanol and acetonitrile used in preparation of electrospray solvents were purchased from Fisher Scientific (Leicestershire, U.K.). Water was purified by an ELGA Option 3 system (Marlow, UK). Formic acid (FA) was purchased from Sigma-Aldrich Company Ltd. (Dorset, U.K.).
Surface Sampling
Automated sample analysis was performed using the LESA Points software (Advion Ithaca, NY), which controls the TriVersa Nanomate. This platform was used to select the sampling location (x and y co-ordinates) and the z position, relative to the plate height, for sampling routines using the Nanomate probe. The LESA sampling routine involved the collection of a conductive tip from the Advion tip rack before moving to solvent well containing the electrospray solvent solution. The Nanomate probe aspirated a pre-set volume into the conductive tip. The probe relocated to the predetermined location on the surface then descended to 0.2 mm above the surface. The tip dispensed between 0.5 and 3 μL onto the sample forming a liquid micro-junction (LMJ) between the tip and the surface. The LMJ was maintained to allow sufficient time for analytes to be dissolved into the solution (1–3 seconds)). The solvent was then reaspirated into the tip (1–3 μL). Finally the tip was rotated and engaged with the back of the ESI chip, and nanospray ionization was initiated. The Triversa Nanomate was coupled with a Thermo Fisher Scientific Orbitrap Velos mass spectrometer.
Mass Spectrometry
Samples were introduced at a flow rate of ~80 nL/min with a gas pressure of 0.3 psi, a tip voltage of 1.4 kV and a capillary temperature of 250 °C. MS data were collected in full scan mode at a resolution of 100 000 at m/z 400. Each scan comprised 3 co-added microscans. For all optimisation experiments the Automatic Gain Control (AGC) was used to accumulate sufficient ions for analysis. The AGC target was 1x106 with fill times as indicated in Table 1.
Table 1.
Optimised LESA methods used for analysis of fingerprints, saliva, explosives and proteins in fingerprints. Solvent A = 70 : 30 Methanol : Water with 0.1% formic acid; Solvent B = 70:30 methanol : water with 2% formic acid;
| Matrix | Analyte | ||||
|---|---|---|---|---|---|
|
| |||||
| Natural fingerprint | saliva | Urine | Proteins | explosives | |
|
| |||||
| solvent composition | A | A | A | B | A |
| solvent volume (μl) | 3 | 0.7 | 0.7 | 2 | 3 |
| dispense (μl) | 2 | 0.5 | 0.5 | 1 | 2 |
| delay (s) | 3 | 2 | 2 | 1 | 2 |
| aspirate (μl) | 3 | 0.8 | 0.8 | 2 | 3 |
| dispensation height (mm) | 0.4 | 0.4 | 0.4 | 0.2 | 0.4 |
| aspiration height (mm) | 0.2 | 0.2 | 0.2 | 0 | 0.2 |
| delivery time (min) | 3 | 3 | 3 | 2.5 | 3 |
| gas pressure (psi) | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| voltage (kV) | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| Fill time | 43 ms | 9 ms | 3 ms | 43 ms | 43 ms |
Collision Induced Dissociation (CID)
CID was performed in the linear ion trap with nitrogen gas at a normalised collision energy of 30%. Ions were detected in the Orbitrap at a resolution of 100,000 at m/z 400. The AGC target was 5x104 with a maximum fill time of 2 s. Each scan comprised 1 microscan. The precursors selected and major product ion m/z values are listed in Table 2.
Table 2.
Compounds detected in a single fingerprint, a sample of urine (3 analyses) and a sample of oral fluid (3 analyses) using LESA; list taken from Girod et al [25]. √=detected with counts >3x the blank; ND = not detected
| Analyte | Monoisotopic Mass (M+H) | Chemical Formula | Fingerprint | Oral Fluid | Urine |
|---|---|---|---|---|---|
|
| |||||
| Amino Acids | |||||
| Glycine | 76.0399 | C2H5NO2 | y | ND | ND |
| Histidine | 156.0773 | C6H9N3O2 | y | √ | √ |
| Serine | 106.0504 | C3H7NO3 | y | √ | √ |
| Alanine | 90.0555 | C3H7NO2 | y | √ | √ |
| Isoleucine / leucine | 132.1025 | C6H13NO2 | y | √ | √ |
| Threonine | 120.0661 | C4H9NO3 | y | √ | √ |
| Arginine | 175.1195 | C6H14N4O2 | y | √ | √ |
| Tyrosine | 182.0817 | C9H11NO3 | y | √ | √ |
| Asparagine | 133.0613 | C4H8N2O3 | ND | √ | √ |
| Lysine | 147.1134 | C6H14N2O2 | y | √ | √ |
| Valine | 118.0868 | C5H11NO2 | y | √ | √ |
| Aspartic Acid | 134.0453 | C4H7NO4 | y | √ | √ |
| Methionine | 150.0589 | C5H11NO2S | y | ND | √ |
| Taurine | 126.0225 | C2H7NO3S | y | ND | √ |
| Citrulline | 176.1035 | C6H13N3O3 | y | √ | √ |
| Ornithine | 133.0977 | C5H12N2O2 | y | √ | √ |
| Glutamic Acid | 148.061 | C5H9NO4 | y | √ | √ |
| Phenylalanine | 166.0868 | C9H11NO2 | y | √ | √ |
|
| |||||
| Fatty acids | |||||
| Octanoic acid (8:0) | 145.123 | C8H16O2 | √ | √ | √ |
| Nonanoic acid (9:0) | 159.1387 | C9H18O2 | √ | √ | √ |
| Decanoic acid (10:0) | 173.1543 | C10H20O2 | √ | √ | √ |
| Dodecanoic acid (12:0) | 201.1856 | C12H24O2 | √ | ND | √ |
| Tridecanoic acid (13:0) | 215.2013 | C13H26O2 | ND | ND | ND |
| Myristoleic acid (14:1) | 227.2013 | C14H26O2 | √ | ND | ND |
| Myristic acid (14:0) | 229.2169 | C14H28O2 | √ | ND | √ |
| Pentadecenoic acid (15:1) | 241.2169 | C15H28O2 | √ | √ | ND |
| Pentadecanoic acid (15:0) | 243.2326 | C15H30O2 | √ | √ | √ |
| Palmitoleic acid (16:1) | 255.2326 | C16H30O2 | √ | √ | √ |
| Palmitic acid (16:0) | 257.2482 | C16H32O2 | √ | √ | √ |
| Margaric acid (17:0) | 271.2639 | C17H34O2 | √ | √ | √ |
| Heptadecenoic acid (17:1) | 269.2482 | C17H32O2 | √ | ND | √ |
| Linoleic acid (18:2) | 281.2482 | C18H32O2 | √ | ND | ND |
| Oleic acid (18:1) | 283.2639 | C18H34O2 | √ | √ | √ |
| Stearic acid (18:0) | 285.2795 | C18H36O2 | √ | √ | |
| Nonadecanoic acid (19:0) | 299.2952 | C19H38O2 | √ | √ | ND |
| Eicosanoic acid (20:0) | 313.3108 | C20H40O2 | √ | ND | ND |
| Heneicosanoic acid (21:0) | 327.3265 | C21H42O2 | ND | ND | ND |
| Docosanoic acid (22:0) | 341.3421 | C22H44O2 | √ | ND | ND |
| Tricosanoic acid (23:0) | 355.3578 | C23H46O2 | ND | ND | ND |
| Tetracosanoic acid (24:0) | 369.3734 | C24H48O2 | ND | ND | ND |
|
| |||||
| Miscellaneous | |||||
| caffeine | 195.0882 | C8H10N4O2 | √ | ND | √ |
| caffeine metabolite, Paraxanthine | 181.0726 | C7H8N4O2 | √ | ND | √ |
| cholesterol | 369.3521 | C27H46O | √ | ND | ND |
| creatinine | 114.0667 | C4H7N3O | √ | √ | √ |
| squalene | 411.3991 | C30H50 | √ | ND | ND |
| phenol | 95.0497 | C6H6O | √ | √ | √ |
| riboflavin | 377.1461 | C17H20N4O6 | y | ND | ND |
| Uric Acid | 169.0362 | C5H4N4O3 | √ | ND | √ |
| Urea | 61.0402 | CH4N2O | √ | ND | √ |
| Lactic Acid | 91.0395 | C3H6O3 | √ | √ | √ |
| alpha-Tocopherol | 431.3891 | C29H50O2 | y | ND | ND |
| delta-Tocopherol | 403.3578 | C27H46O2 | y | ND | ND |
Data processing
Mass spectrometry data were analyzed using Xcalibur 2.10 software (Thermo Fisher Scientific) where the Xtract program was used to calculate monoisotopic masses. For all other data processing, data in the Thermo RAW format were converted to mzML using msconvert as part of ProteoWizard [32] Sequential spectra that corresponded to the same injection (as determined by time from the total ion chromatogram) were summed together and outputted to mzML using a custom MATLAB script. The summed spectra were then converted to imzML using imzMLConverter [33]. All further data processing was performed in MATLAB using in-house software.
Spectra were queried for a compound of interest by extracting the region of the spectra that spanned 20 ppm error either side of the accurate mass. Orthogonal matching pursuit (OMP) was then used to fit peaks to the reduced spectral region [34]. OMP was applied with 7 iterations and a dictionary composed of Gaussian functions (with standard deviation of 1) and an additional constraint that no fitted peaks could be within 1 standard deviation. The fitted peak with the lowest ppm error was assigned as a match.
MALDI MS imaging
Fingerprint samples were coated with CHCA matrix (5 mg/mL in 80% MeOH, 0.1% TFA) using a TM-Sprayer (HTX Technologies, LLC). 8 coatings were deposited. The matrix was sprayed with a flow rate 0.115 mL/min and track speed of 1333 mm/min. The capillary temperature was 90°C and the spacing between tracks was 3mm.
MALDI MSI data were acquired with a QSTAR XL QqToF instrument with an oMALDI 2 ion source using Analyst QS 1.1 with oMALDI server 5.1 (AB Sciex). An Nd:YAG (Elforlight: FQS-200-1-Y-355) diode pumped solid state (DPSS) laser (Elforlight, Daventry, UK). The laser was triggered from the QSTAR XL instrument using the existing TTL trigger signal in conjunction with a function generator (Thurlby Thander Instruments, TG2000 20MHz DDS) triggering the laser at a repetition rate of 1000 Hz. Images were acquired at 100 x 100 μm, using a raster speed of 0.2 mm s−1.
MALDI MS data were converted from proprietary file format to mzML using AB MS Data Converter (AB Sciex version 1.3) and then converted to imzML using imzMLConverter [33] and using in house software.
Results and Discussion
Chemical profiling of fingerprints, oral fluid and urine
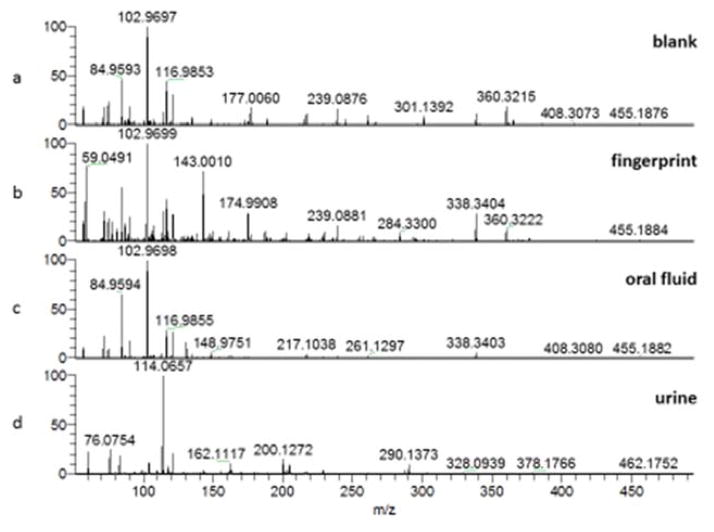
Full scan data for fingerprint, oral fluid and urine samples are shown in Figure 1. The urine spectrum is dominated by the creatinine peak at m/z 114. Table 2 shows the compounds searched for (taken from a list of previously detected substances in the review by Girod et al [26]) and the corresponding peaks detected in fingerprint, urine and oral fluid samples. Other peaks are present at higher abundance in the spectra, but these could not be assigned.
Figure 1.
Full scan mass spectra showing Liquid Extraction Surface Analysis (LESA) of (a) blank, (b) natural fingerprint (c) oral fluid (d) urine
In the fingerprint, 17 amino acids were detected, together with 18 fatty acids and 12 miscellaneous substances. It is remarkable to detect this many substances in a single analysis of a fingerprint. The donor used here was female, and female donors are known to give particularly poor fingerprints [22], showing the great potential for this method for fingerprint chemical profiling. Previous reviews of fingerprint chemical profiling [22], [26] indicate that a range of analytical techniques have previously been required to survey such a wide range of compounds within a fingerprint. Table 2 shows how LESA coupled to mass spectrometry opens up the possibility of doing this within a single analysis. Surveying such a large number of compounds in an individual fingerprint may assist future investigations designed to determine the age of a fingerprint, the identity of a donor from a smudged fingerprint, or indeed may permit medical diagnostics to be carried out from a fingerprint as a convenient sampling matrix.
Similarly, Table 2 demonstrates that an array of compounds including lipids, amino acids and other substances can be detected by LESA in urine and oral fluid. This opens up the possibility of using LESA as a tool to rapidly probe an array of urine and oral fluid samples. In biomarker discovery, the ability to survey a wide array of compounds without targeting particular species is key to a successful outcome. Table 2 demonstrates the ability of LESA to do exactly this, at a comparatively high sample throughput compared with the chromatography methods that are conventionally used in these studies. LESA coupled to high resolution mass spectrometry could therefore add significant value to future investigations in this area.
Drugs in Biological Matrices
Spectra acquired from LESA-MS analysis of fingerprint, oral fluid and urine samples collected from a donor without a drug use history did not reveal any peaks of interest for ions relating to cocaine, methadone, heroin and their respective metabolites.
Analysis of Patient Samples
Oral Fluid
The patient’s oral fluid screened positive for cocaine and opiates using the LGC immunoassay test. LESA MS analysis of corresponding oral fluid sample indicated presence of cocaine, EME and BZE, corroborating results from immunoassay screening. However, heroin and 6-AM were not detected in oral fluid by LESA. The oral fluid was suspended in a buffer solution provided as part of the collection kit, which has the effect of considerably diluting the oral fluid sample. No extraction from the buffer was carried out prior to LESA, and this, or differing solubilities of analytes in LESA solvents, may explain the reason for the apparent lack of sensitivity of the LESA method to heroin in this matrix. The sensitivity of LESA for oral fluid could be improved by repeated spotting and drying procedures to increase the volume of sample. Methadone and its metabolites were not detected by either method in oral fluids. Methadone was selected for analysis because the patient was on a methadone treatment programme.
Fingerprints
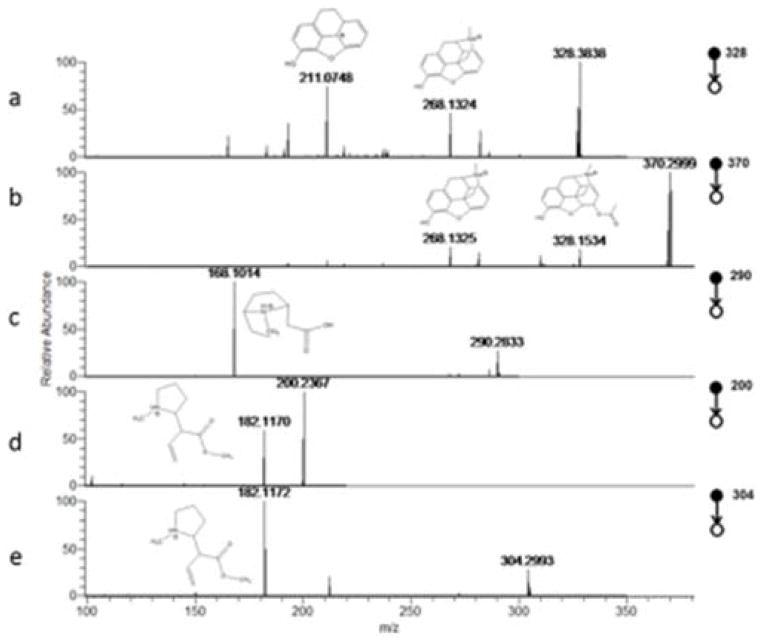
CID spectra revealed detection of expected product ions, consistent with standards of cocaine, BZE, Heroin and 6-AM, in all four regions of the sampled fingerprint, in direct agreement with the LGC immunoassay test. EME was detected in 3 out of the 4 locations analysed. Example CID spectra are given in Figure 2. No ions relating to methadone or its metabolites were detected. Methadone was not detected via immunoassay measurement. There was therefore good agreement between the LESA fingerprint and immunoassay oral fluid screen for all analytes.
Figure 2.
Collision induced Dissociation (CID) spectra produced by liquid extraction surface analysis (LESA) analysis of a fingerprint from a donor attending a drug rehabilitation service, showing fragment ion peaks corresponding to (a) 6-AM (b) heroin (c) cocaine (d) benzolyecgonine (e) methylecgonine
Urine
Cocaine, and associated metabolites, in addition to the 6-AM metabolite were successfully detected in the donor urine sample via LESA-MS and immunoassay. Unsuccessful detection of ions of heroin in the urine of the donor can be explained by a difference in the detection window (varying time of excretion post-administration) for the different matrices studied here. Additionally, it has previously been shown that opiates are detected more readily in sweat than in urine [41].
Once again, methadone and metabolites were not detected using either method in the patient’s urine. A failure to detect these compounds in any sample, by either method indicates that, perhaps, the patient had not in fact consumed methadone within a detectable window. Further work must be conducted to establish limits of detection and to better understand these issues.
Table 3 summarises the data obtained from LESA-MS of the patient’s oral fluid, urine and fingerprints.
Table 3.
Drug and metabolite compounds detected in fingerprint, oral fluid and urine samples from the same donor. Four points on a single fingerprint from a donor were chosen at random and analysed using LESA. Three 0.2 μL spots of oral fluid in a buffer solution and three 0.2 μL spots of urine were analysed; the table shows the samples in which the relevant parent and precursor ions were detected. ND = Not Detected
| Drug and associated metabolites | Parent ion m/z detected (M+H)+ | Δ ppm from accurate mass | CID transitions: precursor → product ion | Oral fluid immune assay screen | Fingerprint (LESA) | Oral Fluid (LESA) | Urine (LESA) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cocaine | 304.1538 | 3.5 | 304→272, 182, 150, 82 | Y | Y | Y | Y |
| EME | 200.1272 | 5.3 | 200→182, 82 | Y | Y | Y | |
| BZE | 290.1377 | 7.3 | 290→272, 168, 150 | Y | Y | Y | |
|
| |||||||
| Methadone | ND | N/A | ND | N | N | N | N |
| EMDP | ND | ND | N | N | N | ||
| EDDP | ND | ND | N | N | N | ||
|
| |||||||
| Heroin | 370.1640 | 3.9 | 370→328, 310, | Y (opiates) | Y | N | N |
| 6-AM | 328.1531 | 5.4 | 211,193 328→310, 286, 211,193,165 |
Y | N | Y | |
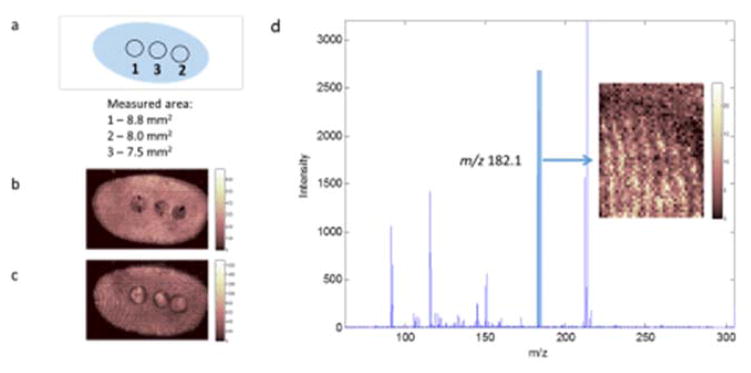
A limitation of the LESA method for fingerprint chemical analysis is the lack of spatial resolution. In drug testing cases it may be necessary to take a sample testing positive for a drug using LESA and to verify the location of a substance within a fingerprint ridge, if a higher level of confirmation is required. To investigate the potential of carrying out this type of analysis, MALDI images of the fingerprint which had previously been analysed using LESA are presented in Figure 3. The images (3b and 3c) of ions detected at m/z 638.6 and 550.6 clearly show the three areas sampled from this fingerprint using LESA, and allow the LESA sampling area to be determined. Figure 3d shows a MALDI MS/MS spectrum acquired during CID of m/z 304 for the area of the fingerprint highlighted in Figure 3a, showing the characteristic fragment ion for cocaine at m/z 182. The image of this fragment ion is presented as an inset, showing some ridge detail. Whilst MALDI has been used before in imaging mode on fingerprints spiked with cocaine [20] and in profiling mode to detect cocaine in the fingerprint of a drug user [19], this appears to be the first report of MALDI imaging of drugs in a fingerprint from a drug user. Whilst quantification of substances in fingerprint residue using MALDI is known to be difficult to achieve [22], a two-step process of (a) quantification of a drug residue in a fingerprint using LESA, and then (b) imaging using MALDI would thereby provide quantification and visualisation of a drug metabolite within a fingerprint ridge.
Figure 3.
Matrix Assisted Laser Desorption Ionisation (MALDI) images of a fingerprint previously analysed using liquid extraction surface analysis (LESA). (a) representation of the areas analysed by LESA; images of (b) m/z 638.6 and {c} m/z 550.6; (d) MS/MS spectrum acquired during CID of m/z 304 for the area of the fingerprint highlighted in the inset, showing the characteristic fragment ion for cocaine at m/z 182.
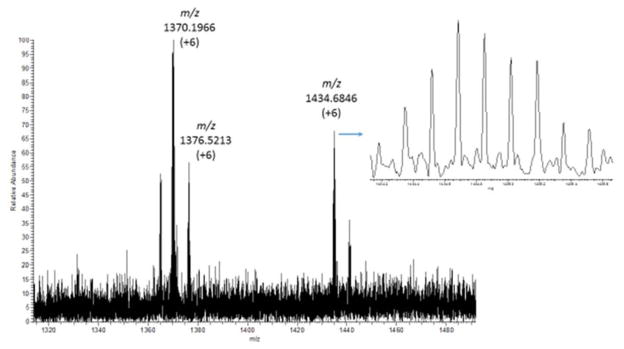
A different LESA method, as described in Table 1 was developed for the detection of peptides and proteins in fingerprints. Application of this method to a fingerprint from a male donor yielded the spectra presented in Figure 4. Several multiply charged ions are detected, indicating the potential of this method for profiling peptides and proteins directly in latent fingerprints. The spectrum presented in figure 4 shows detection of an ion at m/z 1434.68. This +6 charge state ion reveals that molecules of approximately 8600 daltons are detected using this method. Identifications of proteins and peptides in fingerprints have been made previously by others [26], most notably by Francese’s group [24] using MALDI. There are several possible advantages of using LESA compared to techniques such as MALDI. These advantages include improved sensitivity afforded by larger sampling regions; opportunities for using multiple fragmentation techniques within a single analysis (e.g. analysis of multiply charged ions can allow electron transfer dissociation (ETD) as well as CID to be used for improved sequence coverage); and long electrospray survey times achieved by using nano-electrospray flow rates for analysis of microliter volumes, which allows for multiple fragmentation events. Previous research has shown how hundreds of proteins can be detected from digested tissues and blood spots using LESA coupled to LC/MS [35]. Introducing a chromatographic step into LESA-MS analysis of fingerprints, following sample digestion, will likely enable detection and identification of many proteins from a single location of a single fingerprint and will form the topic of further work.
Figure 4.
Full scan mass spectrum produced by the “protein” LESA method developed for fingerprints in the range m/z 1300 to 1500
Detection of Explosives in a Contaminated Fingerprint
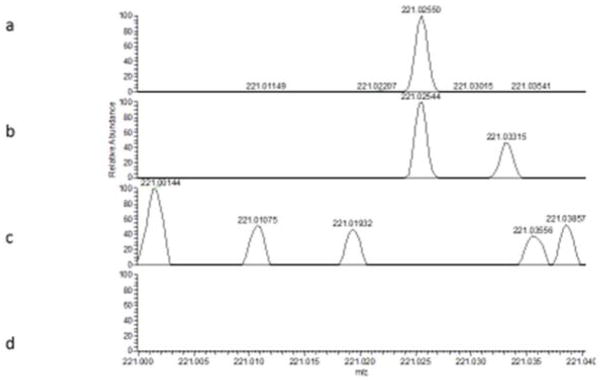
Figures 5a shows full scan spectra collected during analysis of a standard of RDX. The mass spectrum shows the presence of a peak at m/z 221.0255, which can be assigned to [M-H]− [36]. Figure 5b shows that this peak is also present in the fingerprint sample, and Figures 5c and d show that the peak is also not present in either the TNT standard (Figure 5c) or the fingerprints that were blank for RDX (but spiked with TNT) (Figure 5d). In contrast, no peaks corresponding to TNT were found in the contaminated fingerprint sample, implying a lack of sensitivity to this compound, or that the compound was unstable in the fingerprint matrix. However, for RDX, these results do demonstrate the possibility of using LESA to detect explosives in contaminated fingerprints. Other mass spectrometry methods including Direct Analyte Probed nanoextraction (DAPNe) [37], Direct Analysis in Real Time [36], desorption electrospray ionisation (DESI) [6] and MALDI [38] have also been demonstrated for this application. LESA offers complementary features compared with MALDI and DESI as described above for proteins. In the case of explosive detection from fingerprints, the long electrospray time that LESA offers allows multiple CIDs to be carried out from a single spot, leaving the rest of the fingerprint untouched. This is significant if a range of explosive or drug substances are to be scanned for. In contract to DAPNe, LESA probes a larger area (offering enhanced sensitivity) and has a robotic system for high throughput, automated analysis. Future work should explore the limits of detection of LESA for these analytes in a fingerprint matrix, compare with the other techniques that have been previously demonstrated to show promise for this application and explore the integration of LESA into the forensic workflow.
Figure 5.
Full scan spectra for standards of (a) RDX (c) TNT and artificial fingerprints contaminated with (b) RDX and (d) TNT
Conclusions
We have demonstrated the potential for LESA to rapidly gain qualitative information from a range of biofluid samples including urine, oral fluid and fingerprints. The detection of drugs and metabolites in the oral fluid, urine and fingerprints of drug users make LESA an interesting candidate for high throughput drug testing, because the absence of sample preparation and chromatography step decreases the time spent on sample handling and analysis. In contrast to other ambient techniques such as DESI, DART, PADI and paper spray, the process of surface sampling and ionisation is more clearly decoupled. This will likely provide an opportunity for superior quantification, and inclusion of multiple reference or internal standards, and will be considered for these analytes and sample types in further work.
The broad array of compounds detected in a single fingerprint makes LESA a very attractive technique for the study of fingerprint chemistry, an area which is rapidly growing in forensic science, due to the possibility of determining fingerprint age as well as the identity of the donor and the development of superior development reagents. We have shown that because the LESA method only consumes a small area of a fingerprint, it could be used in conjunction with imaging mass spectrometry methods (e.g. MALDI) which have limited quantitative capability, if confirmation is required that a particular substance is located within a fingerprint ridge.
More broadly, this method of sampling a large number of compounds from either oral fluid, urine and fingerprints is highly relevant to medical diagnostics and metabolomics, because an array of samples can be rapidly probed, allowing high throughput analysis to be carried out.
Acknowledgments
The authors would like to acknowledge the National Institute of Health Research for funding through the Clinical Research Network Portfolio ID 17487 and would like to thank Fiona Robinson and Sarah Hamilton at Surrey and Borders NHS Trust as well as Mahado Ismail from the University of Surrey for their help with the collection of fingerprint, oral fluid and urine samples.
References
- 1.Takats Z, Wiseman J, Gologan B, Cooks RG. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionisation. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 2.Ifa DR, Manicke N, Dill A, Cooks RG. Latent Fingerprint Chemical Imaging by Mass Spectrometry. Science. 321(5890):805. doi: 10.1126/science.1157199. [DOI] [PubMed] [Google Scholar]
- 3.Ratcliffe L, Rutten F, Barrett D, Whitmore T, Seymour D, Greenwood C, Arandan-Gonzalvo Y, Robinson S, McCourstra M. Surface Analysis Under Ambient Conditions Using Plasma Assisted Desorption Ionisation Mass Spectrometry. Anal Chem. 2007;49(16):6094–6101. doi: 10.1021/ac070109q. [DOI] [PubMed] [Google Scholar]
- 4.Cody RB, Alaramse J, Dupont Durst H. Versatile New Ion Source for the Analysis of Materials in Open Air Under Ambient Conditions. Anal Chem. 2005;77(8):2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Liu J, Cooks RG, Ouyang Z. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angewandte Chemie. 2010;122(5):889–892. doi: 10.1002/anie.200906314. [DOI] [PubMed] [Google Scholar]
- 6.Ifa D, Jackson A, Paglia G, Cooks RG. Forensic Applications of Ambient Ionsiation Mass Spectrometry. Anal Bioanal Chem. 2009;394:1995–2008. doi: 10.1007/s00216-009-2659-2. [DOI] [PubMed] [Google Scholar]
- 7.Green FM, Salter TL, Stokes P, Gimore S, O’Connor G. Ambient Mass Spectrometry: Advances and Applications in Forensics Surface and Interface Analysis. 2010;42(5):347–357. [Google Scholar]
- 8.Harris GA, Gathena AS, Ferndandez FM. Ambient Sampling / Ionisation Mass Spectrometry : Applications, Current trends. Anal Chem. 2011;83(12):4508–4538. doi: 10.1021/ac200918u. [DOI] [PubMed] [Google Scholar]
- 9.Kertesz V, Van Berkel GJ. Fully automated liquid extraction-based surface sampling and ionization using a chip-based robotic nanoelectrospray platform. J Mass Spectrom. 2010;45:252–260. doi: 10.1002/jms.1709. [DOI] [PubMed] [Google Scholar]
- 10.Marshall P, Toteu-Djomte V, Bareille P, Perry H, Brown G, Baumert M, Biggadike K. Correlation of skin blanching and percutaneous absorption for glucocorticoid receptor agonists by matrix-assisted laser desorption ionization mass spectrometry imaging and liquid extraction surface analysis with nanoelectrospray ionization mass spectrometry. Anal Chem. 2010;82:7787–7794. doi: 10.1021/ac1017524. [DOI] [PubMed] [Google Scholar]
- 11.Parson WB, Koeniger SL, Johnson RW, Erickson J, Tian Y, Stedman C, Schwartz A, Tarcsa E, Cole R, Van Berkel GJ. Analysis of chloroquine and metabolites directly from whole-body animal tissue sections by liquid extraction surface analysis (LESA) and tandem mass spectrometry. J Mass Spectrom. 2012;47:1420–1428. doi: 10.1002/jms.3068. [DOI] [PubMed] [Google Scholar]
- 12.Brown SHJ, Huxtable LH, Willcox MDP, Blanksby SJ, Mitchell TW. Automated surface sampling of lipids from worn contact lenses coupled with tandem mass spectrometry. Analyst. 2013;138:1316–1320. doi: 10.1039/c2an36189b. [DOI] [PubMed] [Google Scholar]
- 13.Edwards RL, Creese AJ, Baumert M, Griffiths P, Bunch J, Cooper HJ. Hemoglobin variant analysis via direct surface sampling of dried blood spots coupled with high-resolution mass spectrometry. Anal Chem. 2011;83:2265–2270. doi: 10.1021/ac1030804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negrusz A, Cooper G. Clarke’s Analytical Forensic Toxicology. London, UK: Pharmaceutical Press; 2013. [Google Scholar]
- 15.Drummer Olaf H. Introduction and Review of Collection Techniques and Applications of Drug Testing of Oral Fluid Therapeutic Drug Monitoring. 2008;30(2):203–206. doi: 10.1097/FTD.0b013e3181679015. [DOI] [PubMed] [Google Scholar]
- 16.Bosker WM, Huestis MA. Oral Fluid Testing for Drugs of Abuse Clinical Chemistry. 2009;55(11):1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legget R, Lee-Smith EE, Jickells SM, Russell DA. Intelligent Fingerprinting: simultaneous identification of drug metabolites and individuals by using antibody-functionalised nanoparticles. Angewandte Chemie. 2007;199:4179–4181. doi: 10.1002/anie.200700217. [DOI] [PubMed] [Google Scholar]
- 18.Kuwayama K, Yamamuro T, Tsjuikawa K, Miyaguchi H, Kanamori T, Iwata Y, Inoue H. Time-course measurements of drugs and metabolites transferred from fingerprints after drug administration: usefulness of fingerprints for drug testing Forensic Toxicology. 2014;32:235–242. [Google Scholar]
- 19.Bailey MJ, Bradshaw R, Francese S, Salter TL, Costa C, Ismail M, Webb PR, Bosman I, Wolff K, de Puit M. Rapid detection of cocaine, benzoylecgonine and methylecgonine in fingerprints using surface mass spectrometry. Analyst 2015. 2015;140:6254–6259. doi: 10.1039/c5an00112a. [DOI] [PubMed] [Google Scholar]
- 20.Groeneveld G, de Puit M, Bleay S, Bradshaw R, Francese S. Detection and mapping of illicit drugs and their metabolites in fingermarks by MALDI MS and compatibility with forensic techniques. Scientific Reports. 2015;5 doi: 10.1038/srep11716. Article number: 11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey MJ, Ismail M, Bright N, Everson D, Costa C, De Puit M, Bleay S, Elad ML, Cohen Y, Geller B, Webb RP, Watts JF. Enhanced imaging of developed fingerprints using mass spectrometry imaging. Analyst. 2013;138(21):6246–6250. doi: 10.1039/c3an01204b. [DOI] [PubMed] [Google Scholar]
- 22.Bailey MJ, Bright NJ, Hinder S, Jones BN, Webb RP, Croxton RS, Francese S, Ferguson LS, Wolstenholme R, Jickells S, Jones BJ, Ojeda JJ, Kazarian SG, Bleay S. Chemical characterization of latent fingerprints by matrix-assisted laser desorption ionization, time-of-flight secondary ion mass spectrometry, mega electron volt secondary mass spectrometry, gas chromatography/mass spectrometry, X-ray photoelectron spectroscopy, and attenuated total reflection Fourier transform infrared spectroscopic imaging: An intercomparison. Analytical Chemistry. 2012;84(20):8514–8523. doi: 10.1021/ac302441y. [DOI] [PubMed] [Google Scholar]
- 23.Bright NJ, Webb R, Hinder SJ, Kirkby KJ, Ward NI, Watts JF, Bleay S, Bailey MJ. Determination of the deposition order of overlapping latent fingerprints and inks using Secondary Ion Mass Spectrometry (SIMS) Anal Chem. 2012;84(9):4083–4087. doi: 10.1021/ac300185j. [DOI] [PubMed] [Google Scholar]
- 24.FERGUSON Leesa Susanne, WULFERT Florian, WOLSTENHOLME Rosalind, FONVILLE Judith Marlou, CLENCH Malcolm, CAROLAN Vikki Amanda, FRANCESE Simona. Direct detection of peptides and small proteins in fingermarks and determination of sex by MALDI mass spectrometry profiling. The Analyst. 2012;137(20):4686–4692. doi: 10.1039/c2an36074h. [DOI] [PubMed] [Google Scholar]
- 25.BRADSHAW Robert, BLEAY Stephen, WOLSTENHOLME Rosalind, CLENCH Malcolm, FRANCESE Simona. Towards the integration of matrix assisted laser desorption ionisation mass spectrometry imaging into the current fingermark examination workflow. Forensic Science International. 2013;232(1–3):111–124. doi: 10.1016/j.forsciint.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Girod A, Ramotowski R, Weyermann C. Composition of fingermark residue: A qualitative and quantitative review. Forensic Science International. 2012;223(1–3):10–24. doi: 10.1016/j.forsciint.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Hazarika P, Russell DA. Advances in Fingerprint Analysis. Angewandte Chemie 2012. 2012;51(15):3524–3531. doi: 10.1002/anie.201104313. [DOI] [PubMed] [Google Scholar]
- 28.Cadd S, Islam M, Manson P, Bleay S. Fingerprint composition and aging: A literature review. Science & Justice. 2014;55(4):219–238. doi: 10.1016/j.scijus.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 29.de Puit M, Ismail M, Xu Xioama. LCMS Analysis of Fingerprints, the Amino Acid Profile of 20 Donors. Journal of Forensic Sciences. 2013;59(2):364–370. doi: 10.1111/1556-4029.12327. [DOI] [PubMed] [Google Scholar]
- 30.Ricci C, Phiriyavityopas P, Curum N, Chan KL, Jickells S, Kazarian SG. Chemical Imaging of Latent Fingerprints. Appl Spectrosc. 2007;61(5):514–22. doi: 10.1366/000370207780807849. [DOI] [PubMed] [Google Scholar]
- 31.Bright NJ, Willson TR, Driscoll D, Reddy SM, Webb RP, Bleay S, Ward NI, Kirkby KJ, Bailey MJ. Chemical changes exhibited by latent fingerprints after exposure to vacuum conditions. Forensic science international. 2013;230(1–3):81–6. doi: 10.1016/j.forsciint.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 32.Chambers MC, et al. Nature Biotechnology. 2012;30:918–920. doi: 10.1038/nbt.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Race AM, Styles IB, Bunch J. Journal of Proteomics. 2012;75(16):5111–5112. doi: 10.1016/j.jprot.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 34.Denis L, et al. Inverse Problems. 2009;25:115017. [Google Scholar]
- 35.Sarbsby J, Martin NJ, Bunch J, Cooper HJ. J Am Soc Mass Spectrom. 2014;25(11):1953–61. doi: 10.1007/s13361-014-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H, Zenobi R. Neutral desorption sampling of biological surfaces for rapid chemical characterization by extractive electrospray ionization mass spectrometry. Nature Protocols. 2008;3:1467–1475. doi: 10.1038/nprot.2008.109. [DOI] [PubMed] [Google Scholar]
- 37.Clemons K, Dake J, Sisco E, Verbek G. Forensic Science International. 2013;231(1–3):98–101. doi: 10.1016/j.forsciint.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan-Sandquist K, LeBeu MA, Miller ML. Forensic Science International. 2014;235:68–77. doi: 10.1016/j.forsciint.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Kintz P, Tracqui A, Mangin P, Edel Y. Sweat Testing in Opiod Users with a Sweat Patch. J Anal Toxicol. 1996;20(6):393–7. doi: 10.1093/jat/20.6.393. [DOI] [PubMed] [Google Scholar]