Abstract
Innate lymphoid cells (ILCs) are a family of innate immune cells that have diverse functions during homeostasis and disease. Subsets of ILCs have phenotypes that mirror polarized T helper subsets in their expression of core transcription factors and effector cytokines. Given the similarities between these two classes of lymphocytes, it is important to understand which functions of ILCs are specialized and which are redundant in comparison to T cells. Here, we discuss genetic mouse models that have been used to dissect the contributions of ILCs versus T cells, and review our current understanding of the specialized in vivo functions of ILCs.
In 1986, Mosmann and Coffman identified T helper 1 (TH1) and T helper 2 (TH2) subsets by demonstrating that CD4+ T cell clones can be divided into two groups with distinct patterns of cytokine expression1. These findings, together with the discovery of T helper 17 (TH17) cells almost two decades later, formed the helper T cell paradigm, a concept which functionally defined subsets of polarized CD4+ T cells by the specific cytokines they produce. Remarkably, a similar paradigm has recently emerged from within the innate immune compartment with the discovery of a new group of lymphocytes called innate lymphoid cells (ILCs). ILCs are a functionally diverse but developmentally related family of innate lymphocytes that have phenotypes that resemble those of polarized T cell subsets. Specific cell types within this lymphocyte class have been recognized for decades; the first identified ILC, the conventional natural killer (cNK) cell, was discovered over 40 years ago2, while the lymphoid tissue inducer (LTi) cell was described in neonatal mouse lymph nodes in 19923. In more recent years, additional cell types within this family have been identified4, 5, 6, 7, 8, 9, 10, generating increased interest in ILCs and their functions during homeostasis and inflammation.
With the recognition that innate lymphoid populations have striking similarities to polarized CD4+ T cell subsets, the ILC family was divided into three groups that parallel TH1, TH2, and TH17 cells. Group 1 ILCs (ILC1s) are analogous to TH1 cells, as they express the transcription factor T-bet and produce interferon-γ (IFN-γ). ILC1s include Eomes− IL-7R+ ILC1s as well as Eomes+ IL-7R− cNK cells, although cNK cells arise from a divergent developmental pathway and are perhaps more analogous to CD8+ cytotoxic T cells because they produce high amounts of granzymes and perforin. Eomes− and Eomes+ ILC1s represent two extremes of a broad spectrum of ILC1 phenotypes that were previously attributed to NK populations. These cells, which exhibit varying cytolytic activities and contrasting requirements for Eomes and other transcription factors such as T-bet and Nfil3, include intestinal intraepithelial ILC1s, salivary gland ILC1s, and uterine ILC1s11, 12, 13, 14. Group 2 ILCs (ILC2s) are analogous to TH2 cells in that they express high amounts of the transcription factor GATA-3, and produce interleukin 5 (IL-5), IL-9, and IL-13 during both helminth infection and allergic inflammation4, 5, 6. These cells are subdivided based on responsiveness to the epithelial-derived cytokines IL-33 and IL-2515. Finally, group 3 ILC3s (ILC3s) express the transcription factor RORγt, an isoform of the gene Rorc16. These cells produce IL-22 and/or IL-17A during extracellular fungal and bacterial infection, and are analogous to TH17 cells. ILC3s include LTi cells that are required for secondary lymphoid tissue development in the fetus17, as well as CCR6+ LTi-like cells, NKp46+ ILC3s, and CCR6−NKp46− ILC3s in adult mice. In humans, ILC3s isolated from tonsils express NKp448.
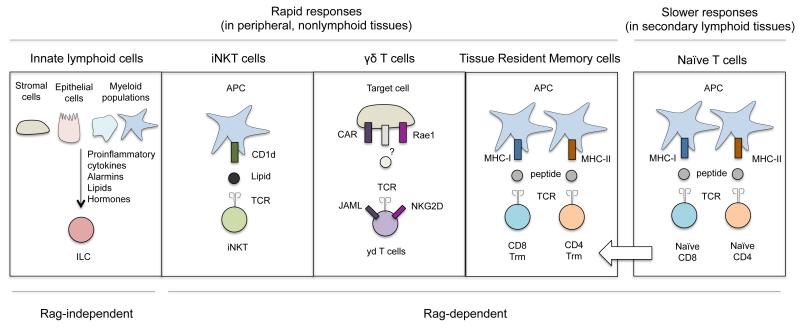
Although ILCs and helper T cells have similar effector programs, these cells are regulated in distinct ways. Naïve CD4+ T cell activation requires priming by major histocompatibility complex (MHC)-peptide-T cell receptor (TCR) interactions and costimulatory signals; both are provided by direct contact with an antigen-presenting cell. In contrast, ILCs do not require specific antigen or costimulation, and are instead activated by an expanding list of soluble mediators that include proinflammatory cytokines, alarmins, lipids, and hormones released by stromal, epithelial, and myeloid populations. ILCs and CD4+ T cells also require different amounts of time to mount effector responses. T cell priming takes place over several days in lymphoid organs as these cells undergo activation and clonal expansion, acquire effector functions, and produce the surface molecules required to migrate to nonlymphoid tissues. By comparison, most ILCs take up permanent residence in nonlymphoid tissues during the steady state18, and are able to produce substantial amounts of cytokines upon stimulation without further differentiation. Thus, ILCs are physically and functionally poised to be immediate sources of effector cytokines in peripheral tissues, placing these cells among the ranks of other rapidly responding tissue resident lymphocytes such as iNKT cells, γδ T cells, and tissue resident memory cells (TRM) (Fig.1). The limited activation requirements of ILCs allow them to serve as first responders during the early stages of a primary immune response, as well as produce cytokines in response to subtle perturbations during homeostasis.
Fig 1. ILCs, innate-like T cells, and conventional T cells are functionally compartmentalized based on tissue residency and activation pathways.
ILCs, iNKT cells, γδ T cells, and TRM cells are tissue resident populations that can rapidly produce cytokines at sites of infection. In contrast, primary conventional T cell responses take place over several days as these cells clonally expand and differentiate in secondary lymphoid organs, and then migrate to nonlymphoid tissues. ILCs are mainly activated by an array of soluble mediators, while the activation of iNKT cells, γδ T cells, and conventional T cells primarily involves direct interactions with TCR ligands presented by other cell types.
Given the similar effector programs of ILCs and T cells, an obvious question is: what are the unique contributions of ILCs in vivo? To distinguish the contributions of ILCs from those of T cells, the functions of ILCs are frequently interrogated in T cell-deficient mice. For example, a common strategy is to compare the phenotypes of Rag-deficient mice, which lack adaptive lymphocytes but have intact ILCs, with mice deficient in both Rag and Il2rg (the common gamma chain, or γc), which lack both adaptive lymphocytes and ILCs. As a result, there remains a major gap in our knowledge surrounding the activities of ILCs under physiological settings. Studying ILCs in the context of an intact T cell compartment will be required to (1) identify functions of ILCs that are distinct from those performed by T cells, and to (2) determine how ILCs communicate with T cells during an immune response. Additionally, the shared and distinct regulatory elements that govern lineage determination and function in ILCs and T cells need to be explored. Expansion within these areas of research will be of particular importance if investigators propose to selectively modulate ILC function in patients to improve disease outcomes.
Mouse models for assessing ILC function: considering adaptive immunity
Early studies characterizing ILCs with Rag-deficient and Rag- and Il2rg-doubly deficient mice demonstrated that these cells were not of the T cell lineage, but were lymphocytes that require γc cytokines to develop. Since then, Rag-deficient mice have been essential for showing that cytokines derived from ILCs can promote microbial killing, granulocyte migration, epithelial barrier integrity, smooth muscle contraction, and mucus production in the context of T cell immunodeficiency. However, there are potential caveats regarding ILCs in Rag-deficient mice. ILC2s and ILC3s in Rag-deficient mice are elevated in number in tissue, and can produce increased amounts of cytokine both at rest and after stimulation in vitro19, 20, 21, 22. These changes likely result from an excess of γc cytokines that arises in the absence of T cells. IL-7, which promotes proliferation, survival, and changes in fate decisions in ILCs, is normally limited by T cell consumption in wild-type mice, but is significantly increased in the serum of Rag-deficient mice23. These observations should be considered when assessing the functional contributions of ILCs with this system.
Moreover, although ILCs do not express rearranged antigen-specific receptors, a proportion of cNK cells, ILC2s, and ILC3s express Rag genes during development and are altered by Rag-deficiency in a cell-intrinsic manner24, 25. Rag-deficient ILCs have increased histone H2AX phosphorylation, a marker for double stranded DNA break events, and Rag2−/− cNK cells exhibit a reduced response to radiation-induced DNA damage. Rag-deficient cNK cells also have reduced cellular fitness, with Rag2−/− cNK cells displaying increased apoptosis during the steady state and diminished survival after murine cytomegalovirus (MCMV) infection. The cell-intrinsic effects of Rag-deficiency on cellular fitness in ILC2s and ILC3s are unknown.
A second commonly employed strategy to study ILC function relies on the depletion of ILCs using antibodies against Thy1. Most ILCs and all T cells express high amounts of cell surface Thy1, and ILCs are thus largely eliminated by administering a depleting Thy1 antibody into Rag-deficient mice26. In some studies, chimeric mice containing T cells that express a Thy1 variant not recognized by depleting antibodies have been used in order to deplete the ILC compartment without affecting T cells27. With the Thy1 depletion system, variable efficiencies of cell depletion have been reported in blood and tissue, potentially due to the method of antibody administration28, 29. An important consideration with these studies is that Thy1 expression is not restricted to lymphocytes, but is also expressed by several other cell types including fibroblasts, endothelial cells, neurons, basophils, and dendritic cells. The effects of Thy1 antibody treatment on these cell types have not been fully explored.
Importantly, the use of these models has provided critical information on ILC biology, and has moved the field forward to a point where researchers can start testing how ILCs function in the presence of adaptive immunity using more advanced tools. So far, the overlapping genetic requirements of T cells and ILCs have made it difficult to manipulate innate lymphoid populations in mice without simultaneously altering T cell populations. As of yet, no single transcription factor has been shown to be uniquely required by ILCs but not other immune cells for their development or function. Genes necessary for normal ILC precursor specification (such as Id2, Tcf7, Gata3, Tox, and Nfil3) and genes required for ILC subset maturation (such as Bcl11b, Tbx21, and Rorc(γt)) are required either during thymocyte development or by polarized T cells for their functions. Similarly, strategies for generating ILC-specific Cre-recombinase mice have been complicated by the shortage of positive, exclusive lineage markers for ILCs. This is a particular limitation for ILC2s and ILC3s, which are identified in part by the absence of cell surface markers for other lineages, including T cells.
In general, two strategies for genetically targeting ILCs have been used to divide the innate and adaptive lymphoid compartments: (1) using Cre-lox systems to target two genes expressed by ILCs that in combination, are minimally expressed or required by T cells, and (2) driving expression of a floxed diphtheria toxin receptor (DTR) allele in both T cells and ILCs, then “rescuing” T cells from DTR expression through T cell-specific expression of Cre recombinase. Some examples of effective Cre recombinase lines that have been used to study ILC subsets, but that are not necessarily specific to ILCs, include transgenic Ncr1-Cre mice30, 31, 32, which drive Cre expression in all subsets of group 1 ILCs as well as NKp46+ ILC3s; knock-in Il5-Cre mice33, which target ILC2s but also an IL-5-expressing subset of TH2 cells; and transgenic Rorc(γt)-Cre mice16, which drive Cre expression in ILC3s, as well as in T cells during thymopoiesis. These mice have been crossed to several floxed alleles of cytokines and transcription factors expressed by ILCs. Additional selected mouse models that have been used to study ILCs are listed (Table 1).
Table 1.
Genetic strategies for targeting ILCs
| Geneticall y engineered allele(s) |
Ref | Category | ILC subset(s) targeted |
T cells targeted (Effects on lymphoid tissue development are also noted) |
|---|---|---|---|---|
| Ncr1-Cre |
30,
31, 32 |
Conditional | ILC1s and NKp46+ ILC3s |
None known |
|
Ncr1-Cre x Mcl1fl/fl |
72, 75 | Conditional deletion of Mcl1 in NKp46+ cells |
NK cells and NKp46+ ILC3s; effects on other ILC1s are not reported |
None known |
|
Ncr1-Cre x Rosa-DTA |
72 | Conditional expression of diphtheria toxin by NKp46+ cells |
ILC1s and NKp46+ ILC3s |
None known |
|
Ncr1-Cre x Tgfbr2fl/fl |
76 | Conditional deletion of TGF-β receptor in NKp46+ cells |
NKp46+ salivary gland ILC1s as well as other Tgfbr2- dependent ILC1s |
None known |
| Il5-Cre | 33 | Conditional, knock-in | IL-5+ ILC2s | Cre is expressed by IL- 5-producing TH2 cells |
|
Cd4-Cre x ICOSfloxD TRflox |
65 | The diptheria toxin receptor (DTR) is expressed by ICOS+ non-T cells |
ILC2s and other CD4− ILCs that express ICOS |
T cells are rescued from DTR expression at the double positive stage in the thymus |
| Rora sg/sg | 77, 78 | Knockout | ILC2s | Unknown whether all TH2 subsets are intact |
|
Rorc(yt)- Cre |
79 | Conditional | ILC3s | Cre is expressed by T cells during thymopoiesis |
| Rorc(yt)−/− | 16 | Knockout | ILC3s | T cells lack RORyt, leading to increased thymocyte apoptosis. Almost all secondary and tertiary lymphoid organs are absent. |
|
Rorc(yt)- Cre x Ahrfl/fl |
71 | Conditional deletion of Ahr in Rorc(yt)- expressing cells |
NKp46+ ILC3s and LTi-like cells are greatly diminished |
Loss of the aryl hydrocarbon receptor (AHR) in all T cells. ILFs and CPs are absent. |
|
Ncr1-Cre x Rorc(yt)fl/fl |
71 | Conditional deletion of Rorc(yt) in NKp46+ cells |
NKp46+ ILC3s | None known |
| Il7r-Cre | 80 | Conditional, knock-in | ILC1s, ILC2s, and ILC3s |
Cre is expressed by T cells |
| Zbtb16-Cre | 81 | Conditional | ILC1s, ILC2s, and ILC3s are targeted, but not LTi cells. Lower proportions of NK cells express Zbtb16. |
Cre is expressed by NK T cells and MAIT cells. Also, some background expression occurs before the hematopoietic stem cell (HSC) stage. |
| Nfil3 −/− |
11,
82, 83, 84, 85, 86 |
Knockout | The development of cNK cells, ILC1s, ILC2s, and ILC3s is defective. Nfil3- independent ILC1 populations persist in the salivary gland and uterus. |
Effects on TH17 cells development reported. |
The discovery of additional ILC-specific genes should allow for more targeted manipulation of ILCs at different stages during development and maturation. Such genes are starting to be uncovered through the Immunological Genome (ImmGen) Project, which has provided gene expression analyses of intestinal ILC subsets34. Additional work directly comparing gene expression and regulation in analogous effector T cell and ILC subsets (for example, IL-5-expressing TH2 cells versus ILC2s), will be important for generating tools to distinguish contributions by these populations35. Gene expression in ILC subsets should also be compared to TRM cells. These subsets of memory T cells, following expansion and differentiation, migrate into nonlymphoid tissues where they reside without recirculating in blood36. ILCs are likely to share more core transcriptional signatures with TRM than with effector T cell subsets based on genes associated with tissue residency and activation.
Unique in vivo activities of ILCs versus T cells in mice
Several unique functions of murine ILCs have been described during fetal and neonatal development, under steady state conditions in adults, and after irradiation. At this point, there is less known about the non-redundant functions of ILCs during an immune response, although ILCs have been shown to regulate epithelial cells, T cells, and myeloid populations during infection. Here, we discuss selected examples of unique activities of ILCs, while additional functions of ILCs are discussed in the companion reviews in this issue.
ILCs in gestation and neonatal life
Secondary lymphoid tissue organogenesis occurs in the fetus and is dependent on a specialized subset of ILC3, the LTi cell17. During fetal development, LTi cells and their precursors develop in the fetal liver and migrate to peripheral tissues, where they undergo further maturation and induce lymph node and Peyer’s patch development via lymphotoxin-α1β2 (LT α1β2)37. Interactions between LTα1β2+ LTi cells and lymphotoxin-β receptor (LTβR)+ stromal cells (also known as stromal organizer cells) initiate development by inducing stromal production of adhesion molecules and chemokines, including VCAM-1, the CXCR5 ligand CXCL13, and the CCR7 ligands CCL19 and CCL21. These factors initiate a positive feedback loop by locally recruiting additional LTi cells and ILC precursor populations to the developing lymphoid organ. The absence of LTi cells (as in Rorc−/− mice), lymphotoxin signaling (as in Lta−/−, Ltb−/− or Ltbr−/− mice), or the chemokine receptors CXCR5 and CCR7 lead to severe deficiencies or complete abrogation of secondary lymphoid tissue development.
The postnatal development of tertiary lymphoid organs in the gut is similarly driven by ILC3s. NKp46− LTi-like ILC3s induce the development of cryptopatches (CPs) and isolated lymphoid follicles (ILFs) though activation of LTβR signaling38, 39. However, unlike secondary lymphoid tissue organogenesis, tertiary lymphoid tissue development in the gut requires additional signals from the environment. Peptidoglycan from intestinal microbes drives the development of ILFs in a NOD1-dependent manner40. In addition, the aryl hydrocarbon receptor (AHR), a sensor of diet-, microbe- and endogenously-derived planar aromatic hydrocarbons, is required for normal expansion of adult ILC3s in the small intestine41, 42, 43. Thus, tertiary lymphoid structures are absent in AHR-deficient mice, while secondary lymphoid tissue development proceeds normally in the absence of AHR.
During pregnancy, ILC1s have been implicated in promoting vascular changes in the uterus. In humans, uterine spiral arteries undergo remodeling during pregnancy to increase blood and nutrient flow to the fetus and placenta. In mice, the equivalent vascular structures are the major decidual arteries, which undergo remodeling during pregnancy in an IFN-γ-dependent manner44. Data strongly suggest that ILC1s are required for this process. Remodeling of murine uterine vasculature requires lymphocytes and IL-15, whereas T cells are not required13, 44, 45, 46. Importantly, mice with Nfil3-deficient lymphocytes exhibit defects in uterine vascular remodeling13, 47. Nfil3-deficient mice lack uterine Eomes+CD49b+CD49a− cNK cells, and have reduced numbers of a second uterine ILC1 subset that expresses CD49a13, suggesting that these populations are important for normal vascular remodeling. Additional studies will be required to determine the specific contributions of ILC1 subsets and the mechanisms behind uterine vascular remodeling.
Constitutive production of cytokine
ILC2s actively secrete type 2 cytokines in the absence of infection. In naïve mice, ILC2s produce the eosinophil growth factor IL-5 in the heart, kidney, lung, muscle, skin, gut, and uterus33. IL-5 secretion follows circadian rhythms regulated by food intake, with serum IL-5 and blood eosinophil levels elevated at the beginning of the light cycle, and reduced at the beginning of the dark cycle, as well as after fasting. Although the effects of circadian IL-5 secretion and blood eosinophil levels on host physiology are unclear, the induction of this pathway following food intake suggests that they might have roles in regulating dietary metabolism. Indeed, increased adiposity has been observed in eosinophil-deficient mice48.
Recently, ILC2s have been shown to regulate the development of a specialized gut epithelial lineage, the tuft (or brush) cell49, 50, 51. Tuft cells, which previously had no known function, have been implicated as sentinel cells that detect parasitic worms. This epithelial population is a unique producer of IL-25, an IL-17 family cytokine that activates ILC2s. During the steady state, intestinal ILC2s produce IL-13, which acts on the epithelial stem cell compartment at the base of the crypt-villus axis to promote a basal level of tuft cell development. In turn, tuft cells produce IL-25 at a level that maintains steady-state ILC2 activation. During infection with worms, this regulatory circuit between tuft cells and ILC2s is enhanced in a manner dependent on the tuft cell chemosensory signaling molecule Trpm550, leading to tuft cell and goblet cell hyperplasia, and thereby promoting worm expulsion49, 50, 51. Thus, ILC2s have unique functions in the pathways that detect the initial presence of parasitic worms in the gut.
ILC3s also actively produce effector cytokines in the steady state. In unchallenged mice, ILC3s are the major source of IL-22, a cytokine that induces the proliferation and survival of intestinal epithelial cells (IECs). ILC3-derived IL-22 also induces the production of the anti-microbial peptide Reg3γ and α(1,2)-fucosylation of IECs, which contribute to the maintenance of homeostatic interactions with gut microbiota10, 52. Intestinal NKp46− ILC3s are located at the bases of intestinal crypts in cryptopatches, while NKp46+ ILC3s are widely distributed throughout the lamina propria. Thus, it is possible that these subsets may provide IL-22 to epithelial cells at different locations along the crypt-villus axis. It is not clear whether basal IL-22 production by ILC3s is induced by microbiota, since there are conflicting reports of whether intestinal flora induces or represses IL-22 production by ILC3s7, 10, 19, 41. Interestingly, gut epithelial IL-25 negatively regulates ILC3s, and IL-25 expression by the epithelium is reported to increase with age19. Future studies should shed light on how aging correlates with changes in ILC populations in the gut.
Finally, it has been proposed that NKp46+ and NKp46− ILC3s in the gut constitutively produce GM-CSF in response to IL-1β secreted by macrophages53. In the absence of GM-CSF, dendritic cell and macrophage populations are reduced in the colon. Additionally, dendritic cells from Csf2−/− mice have blunted TGF-β production, and macrophages from these same mice produce less IL-10 than wild-type mice. Thus, ILC3s may contribute to the maintenance of normal cellularity and myeloid functionality in the gut lamina propria under steady-state conditions through GM-CSF production.
Radioresistance
ILC3s have been observed to persist in mice exposed to lethal doses of gamma-radiation. The radioresistance of these cells is presumably due to their slow cell division rate54, which reduces the fraction of the population in radiosensitive phases of the cell cycle, and may allow more time for DNA repair to take place. In comparison, T cells are extremely radiosensitive, and are greatly reduced three days post-irradiation.
Radioresistant ILC3s have been shown to protect mice from tissue damage induced by irradiation as well as allogeneic responses during graft versus host disease (GVHD). In mice and humans, irradiation induces proliferating epithelial cells in intestinal crypts to undergo apoptosis55. This disrupts crypt-villus organization, and can create breaches in the intestinal epithelial barrier. Regeneration of gut epithelium requires Lgr5+ intestinal stem cells (ISCs), which reside at the bases of crypts and give rise to all lineages of gut epithelial cells55, 56. During GVHD, IL-22 derived from radioresistant ILC3s protects this ISC pool. GVHD can be experimentally induced by transplanting irradiated mice with bone marrow and T cells from MHC mismatched mice, or from mice that have mismatched minor histocompatibility antigens (MHAs). Mice that lack IL-22 have increased gut pathology, more severe loss of ISCs, and reduced survival in this model57.
Similarly, thymic regeneration after irradiation is enhanced by IL-22 secreted by radioresistant ILC3s. In the thymus, irradiation induces loss of thymocytes as well as the loss of cortical and medullary thymic epithelial cells (cTECs and mTECs), which are required for positive and negative T cell selection. IL-22 expression is increased after irradiation, and enhances the regeneration of both cTECs and mTECs58, restoring normal thymopoiesis. These studies have important implications for the roles of ILCs in tissue regeneration and protection against GVHD in patients undergoing bone marrow transplantation.
Regulation of T cells
Both ILC2s and ILC3s have been reported to express MHC class II59. Because TECs, dendritic cells, macrophages, and B cells require MHC class II to present antigen to T cells, it has been hypothesized that ILCs may also directly interact with T cells with this molecule. Multiple groups have shown that, unlike professional antigen presenting cells, MHC class II-expressing ILC3s do not induce the expansion of T cells in culture60, 61. Instead, Rorc(γt)-Cre × H2-Ab1fl/fl mice, which have MHC class II deleted from both ILC3s and T cells, have increased TH17 responses in the gut60, 61. There have been conflicting reports on whether this phenotype is linked with intestinal inflammation. Two groups found no gut inflammation in Rorc(γt)-Cre × H2-Ab1fl/fl mice60, 62, while another group found that these mice develop spontaneous intestinal inflammation and rectal prolapse61. These differences may be due to the variances in microbial communities across mouse facilities. Although the mechanisms behind the increased TH17 responses in Rorc(γt)-Cre × H2-Ab1fl/fl mice are not well understood, it has been proposed that ILC3s directly induce cell death of microbe-specific CD4+ T cells via mechanisms similar to those that promote negative selection of T cells in the thymus63. ILC3s migrate from the intestine to interfollicular regions of the draining mesenteric lymph nodes in a CCR7-dependent manner, and the impact of this trafficking on T cells is unclear64. Outside of the gut, loss of MHC class II expression by ILC3s was reported to lead to reduced T cell responses in the spleen in an OT-II adoptive transfer model43, suggesting that the functions of MHC class II in ILC3s may be different across tissues. In ILC2s, MHC class II expression is partly dependent on trogocytosis65. Similar to ILC3s, ILC2s do not induce T cell proliferation in vitro, although ILC2s may enhance TH2 polarization in T cells stimulated with plate-bound anti-CD3 and anti-CD2865, 66. Instead, MHC class II-expressing ILC2s have been proposed to induce T cells to produce IL-2, which feeds back on ILC2s to promote activation65.
ILCs also regulate T cells indirectly by modulating macrophage and dendritic cell function. During a primary response, IL-13 produced by ILC2s promote dendritic cell migration from the lung to the draining lymph node, where these dendritic cells prime naïve T cells67. During a secondary response, IL-13 secreted by ILC2s induce IRF4+CD11b+CD103− dendritic cells in the lung and skin to produce CCL17, a ligand to the TH2-associated chemokine receptor CCR468. In the absence of IL-13 or ILC2s during a recall response, CCL17 is not expressed in the lung, and the accumulation of memory TH2 cells in the lung is reduced. Likewise, ILC3-derived GM-CSF production in the gut induces macrophages and dendritic cells to produce TGF-β and IL-10, which promote regulatory T (Treg) cell expansion53.
Overlapping roles of murine T cells and ILCs during infection
The pathogen Citrobacter rodentium (C. rodentium), a model for enteropathic Escherichia coli (E. coli), has been used extensively to demonstrate that IL-22 has protective roles during intestinal inflammation. Both T cells and ILC3s produce IL-22 in this model. In studies using Rag2−/− mice, ILC3s have been shown to be important for controlling infection. Infected Rag2−/− mice have a survival advantage over Rag2−/−Il2rg−/− mice7, and depletion of IL-22 in Rag2−/− mice decreases survival69. Additionally, mice that lack IL-23, a cytokine that induces ILC3s to secrete IL-22, rapidly succumb to infection after inoculation with standard experimental doses of bacteria69. However, studies using mice with intact T cells have indicated that ILC3s may have redundant roles in this model. Mice infected with low doses of C. rodentium exhibit survival that is independent of IL-23, and thus, independent of ILC3s70. Instead, IL-22-expressing CD4+ T cells are crucial for survival at low inoculums. Notably, mice lacking functional NKp46+ ILC3s exhibit no differences in survival or weight loss compared to wild-type mice after infection with standard inoculums71, 72, indicating that NKp46+ ILC3s are not required for host protection against C. rodentium. However, mice deficient in NKp46+ ILC3s develop ulceration and bleeding in cecums during infection, indicating that these cells have other specialized functions in this model that still need to be characterized72.
Comparing ILCs and T cells in human
Recently, genome-wide comparisons between transcriptomes and epigenomes in human ILC and helper T cell subsets have been performed73. In cells isolated from inflamed pediatric tonsils, active enhancers in T cells are enriched for AP1 and IRF binding motifs, while active enhancers in ILCs are enriched for Runx and ETS binding motifs. The different transcription factors that bind these motifs likely reflect differences in T cell and ILC activation pathways (i.e. by TCR signaling versus cytokine receptor signaling). ILC3s and TH17 cells also exhibit super-enhancers within IL-22 and IL-17 loci, respectively, reflecting the different capacities of ILC3s and TH17 cells to produce these cytokines. Transcriptional profiling of human ILCs conducted at the single cell level using RNA-seq recently demonstrated that there is further heterogeneity in Lin−CD127+ cells defined as ILC3s based on transcriptional clustering and expression of RORC74. Thus, single cell comparisons between human T cells and ILCs may provide additional ontogenic and functional insights.
Concluding remarks
The overlapping and unique functions of ILCs and T cells are still being defined in mouse and human. The development of more specific genetic tools to study ILCs in mice will be crucial to identify specialized activities of these cells during disease states. Expansion within this area, and in research characterizing regulatory pathways in these cells, will be essential to identify therapeutic interventions for human diseases that act either selectively on ILCs or collectively on lymphocytes.
Acknowledgements
The authors would like to thank M. Robinette, M. Patnode, M. Cella, and G. Oltz for critical reading of the manuscript. We also thank J. O’Shea for helpful discussion. This work was supported by the NIH grants AI095542, DE021255, and DK103039, and by the Kenneth Rainin Foundation. J.K.B received support from the grant T32 HL731737 and is a Cancer Research Institute Irvington Postdoctoral Fellow.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 3.Kelly KA, Scollay R. Seeding of neonatal lymph nodes by T cells and identification of a novel population of CD3-CD4+ cells. Eur J Immunol. 1992;22:329–334. doi: 10.1002/eji.1830220207. [DOI] [PubMed] [Google Scholar]
- 4.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 7.Satoh-Takayama N, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luci C, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 10.Sanos SL, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs A, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-γ-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortez VS, Fuchs A, Cella M, Gilfillan S, Colonna M. Cutting edge: Salivary gland NK cells develop independently of Nfil3 in steady-state. J Immunol. 2014;192:4487–4491. doi: 10.4049/jimmunol.1303469. [DOI] [PubMed] [Google Scholar]
- 13.Boulenouar S, et al. The Residual Innate Lymphoid Cells in NFIL3-Deficient Mice Support Suboptimal Maternal Adaptations to Pregnancy. Front Immunol. 2016;7:43. doi: 10.3389/fimmu.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klose CS, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential 'inflammatory' type 2 innate lymphoid cells. Nat Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 17.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3− LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 18.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350:981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawa S, et al. RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 20.Van Gool F, et al. Interleukin-5-producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood. 2014;124:3572–3576. doi: 10.1182/blood-2014-07-587493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korn LL, et al. Conventional CD4+ T cells regulate IL-22-producing intestinal innate lymphoid cells. Mucosal Immunol. 2014;7:1045–1057. doi: 10.1038/mi.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roediger B, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guimond M, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–157. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karo JM, Schatz DG, Sun JC. The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells. Cell. 2014;159:94–107. doi: 10.1016/j.cell.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. J Immunol. 2011;187:5505–5509. doi: 10.4049/jimmunol.1102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buonocore S, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner JE, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckelhart E, et al. A novel Ncr1-Cre mouse reveals the essential role of STAT5 for NK-cell survival and development. Blood. 2011;117:1565–1573. doi: 10.1182/blood-2010-06-291633. [DOI] [PubMed] [Google Scholar]
- 31.Merzoug LB, et al. Conditional ablation of NKp46+ cells using a novel Ncr1(greenCre) mouse strain: NK cells are essential for protection against pulmonary B16 metastases. Eur J Immunol. 2014;44:3380–3391. doi: 10.1002/eji.201444643. [DOI] [PubMed] [Google Scholar]
- 32.Narni-Mancinelli E, et al. Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc Natl Acad Sci U S A. 2011;108:18324–18329. doi: 10.1073/pnas.1112064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nussbaum JC, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinette ML, et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shih H-Y, et al. Regulomes Reveal Mechanisms for Acquisition of Innate Lymphoid Cell Functionalities. Cell. 2016 In Press. [Google Scholar]
- 36.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 37.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 38.Taylor RT, Lügering A, Newell KA, Williams IR. Intestinal cryptopatch formation in mice requires lymphotoxin alpha and the lymphotoxin beta receptor. J Immunol. 2004;173:7183–7189. doi: 10.4049/jimmunol.173.12.7183. [DOI] [PubMed] [Google Scholar]
- 39.Hamada H, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 40.Bouskra D, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 41.Lee JS, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu J, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiss EA, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 44.Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ashkar AA, et al. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol. 2003;171:2937–2944. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- 46.Barber EM, Pollard JW. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol. 2003;171:37–46. doi: 10.4049/jimmunol.171.1.37. [DOI] [PubMed] [Google Scholar]
- 47.Redhead ML, et al. The Transcription Factor NFIL3 Is Essential for Normal Placental and Embryonic Development but Not for Uterine Natural Killer (UNK) Cell Differentiation in Mice. Biol Reprod. 2016 doi: 10.1095/biolreprod.116.138495. [DOI] [PubMed] [Google Scholar]
- 48.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerbe F, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529:226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howitt MR, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–1333. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pickard JM, et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 2014;514:638–641. doi: 10.1038/nature13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mortha A, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawa S, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 55.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 56.Hua G, et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology. 2012;143:1266–1276. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanash AM, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dudakov JA, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinette ML, Colonna M. Innate lymphoid cells and the MHC. HLA. 2016;87:5–11. doi: 10.1111/tan.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goto Y, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hepworth MR, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Burg N, et al. Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc Natl Acad Sci U S A. 2014;111:12835–12840. doi: 10.1073/pnas.1406908111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hepworth MR, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science. 2015;348:1031–1035. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mackley EC, et al. CCR7-dependent trafficking of RORγ+ ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun. 2015;6:5862. doi: 10.1038/ncomms6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliphant CJ, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirchandani AS, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 67.Halim TY, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halim TY, et al. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17:57–64. doi: 10.1038/ni.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 70.Basu R, et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song C, et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med. 2015;212:1869–1882. doi: 10.1084/jem.20151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rankin LC, et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat Immunol. 2016;17:179–186. doi: 10.1038/ni.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koues OI, et al. Direct gene regulatory pathways for human innate versus adaptive lymphoid cells. Cell. 2016 doi: 10.1016/j.cell.2016.04.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Björklund Å, et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17:451–460. doi: 10.1038/ni.3368. [DOI] [PubMed] [Google Scholar]
- 75.Sathe P, et al. Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat Commun. 2014;5:4539. doi: 10.1038/ncomms5539. [DOI] [PubMed] [Google Scholar]
- 76.Cortez VS, et al. Transforming growth factor-B signaling guides the differentiation of innate lymphoid cells in salivary glands. Immunity. 2016 doi: 10.1016/j.immuni.2016.03.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Halim TY, et al. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 78.Wong SH, et al. Transcription factor RORα is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 80.Schlenner SM, et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 81.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geiger TL, et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med. 2014;211:1723–1731. doi: 10.1084/jem.20140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seillet C, et al. Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med. 2014;211:1733–1740. doi: 10.1084/jem.20140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu X, et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. Elife. 2014;3 doi: 10.7554/eLife.04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu W, et al. NFIL3 orchestrates the emergence of common helper innate lymphoid cell precursors. Cell Rep. 2015;10:2043–2054. doi: 10.1016/j.celrep.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 86.Yu X, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342:727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]