Abstract
Recently, we demonstrated that fermentation conditions have a strong impact on subsequent survival of Lactococcus lactis strain MG1363 during heat and oxidative stress, two important parameters during spray drying. Moreover, employment of a transcriptome-phenotype matching approach revealed groups of genes associated with robustness towards heat and/or oxidative stress. To investigate if other strains have similar or distinct transcriptome signatures for robustness, we applied an identical transcriptome-robustness phenotype matching approach on the L. lactis strains IL1403, KF147 and SK11, which have previously been demonstrated to display highly diverse robustness phenotypes. These strains were subjected to an identical fermentation regime as was performed earlier for strain MG1363 and consisted of twelve conditions, varying in the level of salt and/or oxygen, as well as fermentation temperature and pH. In the exponential phase of growth, cells were harvested for transcriptome analysis and assessment of heat and oxidative stress survival phenotypes. The variation in fermentation conditions resulted in differences in heat and oxidative stress survival of up to five 10-log units. Effects of the fermentation conditions on stress survival of the L. lactis strains were typically strain-dependent, although the fermentation conditions had mainly similar effects on the growth characteristics of the different strains. By association of the transcriptomes and robustness phenotypes highly strain-specific transcriptome signatures for robustness towards heat and oxidative stress were identified, indicating that multiple mechanisms exist to increase robustness and, as a consequence, robustness of each strain requires individual optimization. However, a relatively small overlap in the transcriptome responses of the strains was also identified and this generic transcriptome signature included genes previously associated with stress (ctsR and lplL) and novel genes, including nanE and genes encoding transport proteins. The transcript levels of these genes can function as indicators of robustness and could aid in selection of fermentation parameters, potentially resulting in more optimal robustness during spray drying.
Introduction
Owing to their spoilage-preventing, texture-improving and flavor-enhancing properties, lactic acid bacteria have a long history of application in food fermentations [1, 2]. One of the most widely used lactic acid bacteria in the food industry is Lactococcus lactis, notably for the production of cheese and butter(milk) [2]. These milk fermentation processes are typically initiated with the addition of starter cultures containing high concentrations of one or multiple L. lactis strains. During the production of these starter cultures prior to application in the food industry, L. lactis strains encounter severe stresses, for example heat and oxidative stress during spray drying [3–5]. Although spray drying is a cost-effective and energy-efficient method for the preservation of starter cultures, it generally results in a relatively large decrease in viability as compared with other preservation methods such as freezing and freeze drying [6]. Viability of starter cultures is essential for an adequate contribution to the fermentation end-product, justifying the industrial interest to better understand and improve robustness [1].
Genes involved in stress responses appear highly conserved among bacteria, nevertheless regulation of these stress genes can differ between organisms [7, 8]. Recently, we demonstrated a large diversity in heat and oxidative stress survival among L. lactis strains, suggesting differential regulation of stress responses [5]. Furthermore, strains with an L. lactis subsp. cremoris phenotype appeared to have a less efficient response as compared with strains with an L. lactis subsp. lactis phenotype when these strains were pre-adapted to a minor dose of acid, bile or freezing stress, prior to exposure to a lethal dose of the same stress [9].
Previously, we demonstrated that for the L. lactis subsp. cremoris strain MG1363 [10], oxygen level and fermentation temperature strongly affect subsequent survival during heat and oxidative stress assays, respectively [11]. Furthermore, by applying a transcriptome-phenotype matching approach, we revealed transcriptome signatures associated with robustness towards heat and oxidative stress, which could function as indicators for robustness. These transcriptome signatures included the metC-cysK operon, of which the transcript levels positively correlated with robustness. The role of this operon was confirmed by demonstrating an increase in robustness towards oxidative stress of MG1363 after growth in medium lacking cysteine, which has been demonstrated to induce the metC-cysK operon [11, 12].
L. lactis strains that are applied in food industry are diverse in subspecies and isolation source. It remains unclear if the correlation of gene expression levels and robustness as found in strain MG1363 are generic and, therefore, can also be employed for other L. lactis strains to predict their robustness. Specific individual gene transcripts that associated with robustness in MG1363 [11] were previously established to be important during heat, acid and osmotic stress in L. lactis subsp. lactis strain IL1403 [13], suggesting at least partially overlapping stress responses in these two L. lactis strains.
To investigate if other strains have transcriptome signatures for robustness towards heat and oxidative stress similar to or distinct from those of strain MG1363, we applied an identical transcriptome-phenotype matching strategy [11] on three other strains. These three strains were the dairy L. lactis subsp. lactis strain IL1403 [14], the non-dairy L. lactis subsp. lactis strain KF147 [15] and the dairy L. lactis subsp. cremoris strain SK11 [16]. Besides the differences in subspecies and origin, we previously revealed highly diverse robustness phenotypes of these strains[5]. The strains were individually grown under the twelve conditions that were previously applied to MG1363 [11] and the effect of these conditions on heat and oxidative stress survival was assessed. Moreover, we determined full genome transcriptome profiles, allowing association of gene expression and stress survival to identify transcriptome signatures for robustness towards heat and oxidative stress in the individual strains.
Materials and Methods
Strains and fermentations
L. lactis strains IL1403 [14], KF147 [15] and SK11 [16] were cultivated in chemically defined medium (CDM) as described previously [11]. Briefly, the strains were fermented under twelve different conditions varying in sodium chloride concentration (0 or 100 mM), initial pH (6.0 or 6.5), temperature (27, 30 or 35°C) and level of oxygen (static in 50 ml Falcon tube or shaken at 100 rpm in 500 ml shake flask with a cotton plug) (Table 1). Fermentations were performed on two separate days (fermentation number 1–6 on day 1, 7–12 on day 2) and, therefore, a replicate of fermentation 6 was added on day 2 (fermentation 13). Biomass formation was determined by measurement of the optical density (OD) at 600 nm. In the exponential phase of growth (OD600 between 0.5 and 0.7), cells were harvested for heat and oxidative stress survival assays and RNA isolation.
Table 1. Fermentation conditions, growth characteristics and stress survival.
| μ (h-1) | ODfinal | heat stress survival(%) | oxidative stress survival (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fermentation number | salt (mM) | initial pH | temperature (°C) | level of oxygen | IL1403 | KF147 | SK11 | IL1403 | KF147 | SK11 | IL1403 | KF147 | SK11 | IL1403 | KF147 | SK11 |
| 1 | 0 | 6.0 | 27 | high | 0.37 | 0.87 | 0.52 | 1.59 | 2.12 | 1.34 | 0.23 | 0.38 | 1.2 | 6.5 | 0.045 | 0.051 |
| 2 | 100 | 6.5 | 27 | high | 0.43 | 0.77 | 0.57 | 2.43 | 2.56 | 1.81 | 0.75 | 1.1 | 0.36 | 12 | 0.10 | 0.55 |
| 3 | 0 | 6.5 | 27 | low | 0.59 | 0.79 | 0.67 | 2.42 | 2.37 | 2.43 | 0.000092 | 0.012 | 0.63 | 0.0011 | 0.10 | 0.13 |
| 4 | 100 | 6.0 | 27 | low | 0.30 | 0.87 | 0.49 | 1.48 | 1.50 | 1.30 | 4.5 | 0.021 | 0.35 | 0.75 | 0.12 | 0.11 |
| 5 | 0 | 6.0 | 30 | low | 0.59 | 1.16 | 0.66 | 1.56 | 1.83 | 1.46 | 0.000044 | 0.0077 | 2.5 | 0.00046 | 0.27 | 0.074 |
| 6 | 100 | 6.5 | 30 | low | 0.73 | 1.09 | 0.69 | 2.27 | 2.23 | 2.08 | 0.0075 | 0.12 | 4.0 | 0.032 | 0.042 | 0.047 |
| 7 | 0 | 6.5 | 30 | high | 0.74 | 0.94 | 0.63 | 2.62 | 2.73 | 1.98 | 0.0052 | 1.7 | 6.8 | 0.0065 | 0.071 | 0.037 |
| 8 | 100 | 6.0 | 30 | high | 0.39 | 0.85 | 0.57 | 1.53 | 1.80 | 1.50 | 3.2 | 18 | 9.4 | 53 | 0.46 | 0.038 |
| 9 | 0 | 6.0 | 35 | high | 0.80 | 1.12 | 0.26 | 2.18 | 1.99 | 1.16 | 5.3 | 25 | 13 | 0.0029 | 1.7 | 58 |
| 10 | 100 | 6.5 | 35 | high | 0.77 | 1.09 | 0.25 | 2.42 | 2.75 | 1.32 | 2.7 | 53 | 6.4 | 1.2 | 0.18 | 20 |
| 11 | 0 | 6.5 | 35 | low | 1.00 | 1.22 | 0.61 | 2.90 | 2.58 | 1.89 | 0.095 | 0.79 | 34 | 0.040 | 0.012 | 0.0084 |
| 12 | 100 | 6.0 | 35 | low | 0.92 | 1.18 | 0.50 | 1.77 | 1.59 | 1.26 | 0.45 | 0.93 | 7.4 | 0.0042 | 0.00070 | 0.010 |
| 13 | 100 | 6.5 | 30 | low | 0.76 | 1.06 | 0.74 | 2.34 | 2.21 | 2.04 | 0.011 | 0.033 | 3.1 | 0.0066 | 0.0023 | 0.036 |
Fermentation parameters of the various fermentations and resulting maximum growth rates (μ) and optical densities at the end of fermentation (ODfinal) and survival after 60 minutes (IL1403) or 10 minutes (KF147 and SK11) of heat stress and after 30 minutes of oxidative stress of strains IL1403, KF147 and SK11. Survival at the other time point of the stress assays can be found in S1 Table. Survival data represent averages of technical duplicates. Shaken and static fermentations are indicated as a relatively high level of oxygen (“high”) and a relatively low level of oxygen (“low”), respectively.
Heat and oxidative stress survival assays
Stress survival was determined as described previously [11]. Cells were harvested from 5 ml of culture by centrifugation at 1865 × g for 10 minutes and resuspended in 2.5 ml sterile 50 mM sodium phosphate (Merck) buffer pH 7.2. For assessment of heat stress survival, 0.5 ml of the cell suspensions was diluted by adding 0.5 ml of phosphate buffer and were incubated in duplicate in a volume of 0.1 ml at 50°C for 10 and 30 minutes (KF147, SK11) or 30 and 60 minutes (IL1403) in 0.2 ml PCR tubes (Bioplastics BV, Landgraaf, The Netherlands) in a Gene-Amp PCR system 9600 (Applied BioSystems, Foster City, California, USA). For assessment of oxidative stress survival, hydrogen peroxide (Merck) in phosphate buffer was added to 0.25 ml of the cell suspensions in duplicate to a final concentration of 5 mM and an end volume of 0.5 ml, followed by incubation for 30 and 60 minutes at 30°C in a water bath. After incubation, samples were centrifuged at 15,000 × g for 3 minutes and cell pellets were resuspended in 0.5 ml of phosphate buffer. Survival was assessed by spotting serial dilutions in triplicate on M17 agar plates supplemented with 0.5% glucose[17]. Colony forming units (CFU) were determined after incubation of the plates for 72 hours at 30°C.
RNA isolation and DNA microarrays
RNA isolation, subsequent cDNA synthesis and labeling, as well as DNA microarray hybridizations were performed using routine procedures, as described previously for MG1363 [11]. Briefly, aliquots of 5 ml of culture were centrifuged at 4000 × g for 3 minutes at 2°C and cells were resuspended in 0.5 ml cold TE buffer. To this suspension, 500 μl 1:1 phenol/chloroform, 30 μl 10% SDS, 30 μl 3M sodium acetate pH 5.2 and 500 mg 0.1 mm zirconia beads (Biospec Products, Inc., Bartlesville, USA) was added in a 2 ml screw-cap tube and samples were frozen in liquid nitrogen and stored at -80°C. The DNA microarray hybridization scheme contained two connected loops, both containing samples derived on a single day (S1 Fig). A two-dye microarray-based gene expression analysis was performed on a custom-made 60-mer oligonucleotide array (Agilent Technologies, Santa Clara, California, USA, submitted in Gene Expression Omnibus under GEO Series accession number GSE72045) to determine genome-wide gene transcription levels. Co-hybridization of Cy5- and Cy3-labeled cDNA probes was performed on these oligonucleotide arrays at 65°C and 10 rpm for 17 h using GEX HI-RPM buffer (Agilent Technologies). After hybridization, slides were washed and scanned.
Data analysis
Data analysis was performed as previously described for strain MG1363 [11]. The raw expression data were Lowess normalized and scaled to normalized probe expression levels using MicroPreP [18]. Multiple probes were designed for each ORF and the ORF expression level was calculated from the median of its probe signals. Normalized gene expression levels were further analyzed using the R BioConductor packages Biobase and limma (www.bioconductor.org). After 2-log transformation, gene expression levels were plotted against robustness levels and significance of the correlation was assessed by a linear model. We selected the genes with a significant correlation (P < 0.05) at both time points of the stress assay and further analyzed the genes with the most significant correlation by calculating the product of both P-values. To identify a generic transcriptome signature, we used survival at the time point at which the dynamic range of robustness was the largest. As a consequence, the selected time points for heat stress were 60, 10 and 10 minutes for IL1403, KF147 and SK11, respectively, whereas for oxidative stress the selected time point was 30 minutes for all strains. These data were compared with the survival of MG1363 after 30 minutes of heat stress or oxidative stress [11]. Correlation of survival and growth rate or optical density was determined by calculating the Pearson correlation coefficient. Differences in the effect of individual fermentation parameters on growth characteristics and robustness were assessed with a t-test in R (version 3.0.1, www.R-project.org) and differences were considered significant if the P-value was smaller than 0.05.
Results and Discussion
Variations in fermentation conditions impose largely similar effects on the growth characteristics of different L. lactis strains
To compare the effect of fermentation conditions on the growth characteristics, L. lactis strains IL1403, KF147 and SK11 were grown under the twelve different conditions that were previously applied to strain MG1363 [11]. These conditions varied in the level of salt and/or oxygen, as well as fermentation pH and temperature and resulted in variation of growth characteristics (Table 1, S2 Fig). Strain KF147 displayed maximum growth rates (μmax) in the same range (0.7 h-1 to 1.2 h-1) as we previously established for strain MG1363 [11], whereas SK11 had lower growth rates (0.5 h-1 to 0.7 h-1 [Table 1]). Strain IL1403 displayed the largest variation in maximum growth rate, ranging from 0.3 h-1 to 1.0 h-1 (Table 1).
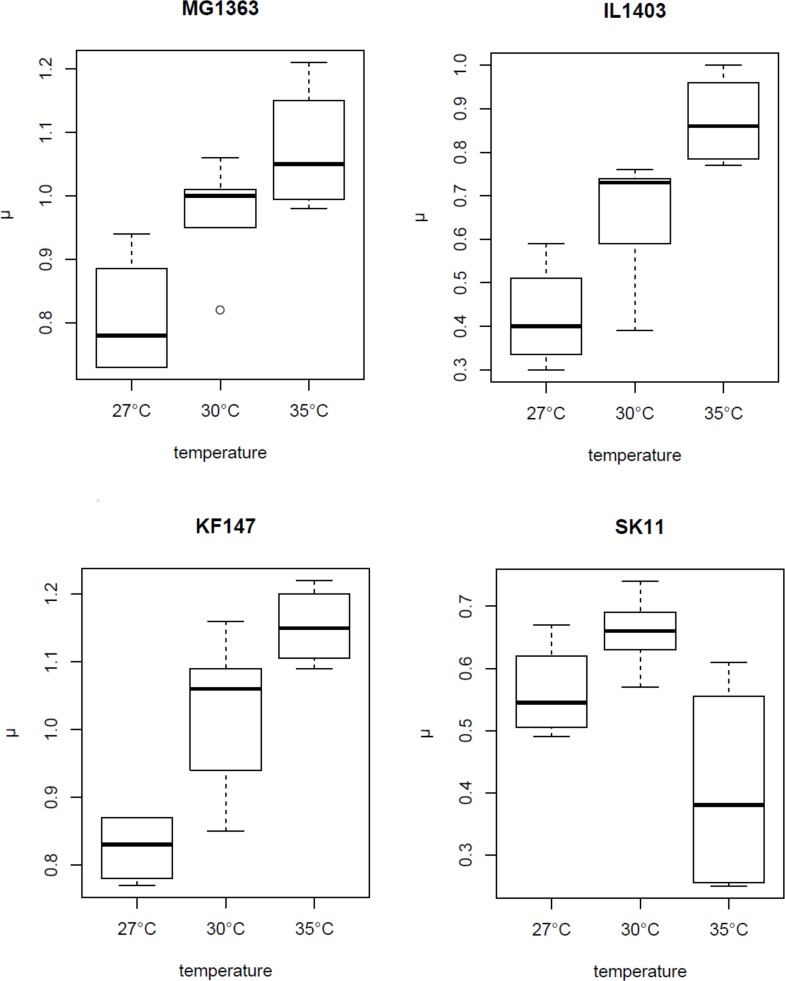
The effect of fermentation temperature on maximum growth rate of the strains KF147 and IL1403 was similar to what we previously observed for MG1363 [11] (Fig 1). In contrast to the other strains, the maximum growth rate of SK11 was significant lower in fermentations at 35°C as compared with 30°C (Fig 1), which is in line with the fact that SK11 has an L. lactis subsp. cremoris phenotype in contrast to MG1363, IL1403 and KF147, which have an L. lactis subsp. lactis phenotype [19]. One of the characteristics that discriminates these phenotypes is that strains with an L. lactis subsp. cremoris phenotype are incapable of growing at high temperature in contrast to strains with an L. lactis subsp. lactis phenotype [20].
Fig 1. Effect of temperature on growth rate.
Boxplots of maximum growth rate (μmax in h-1) of strains MG1363, IL1403, KF147 and SK11 in fermentations at 27, 30 and 35°C.
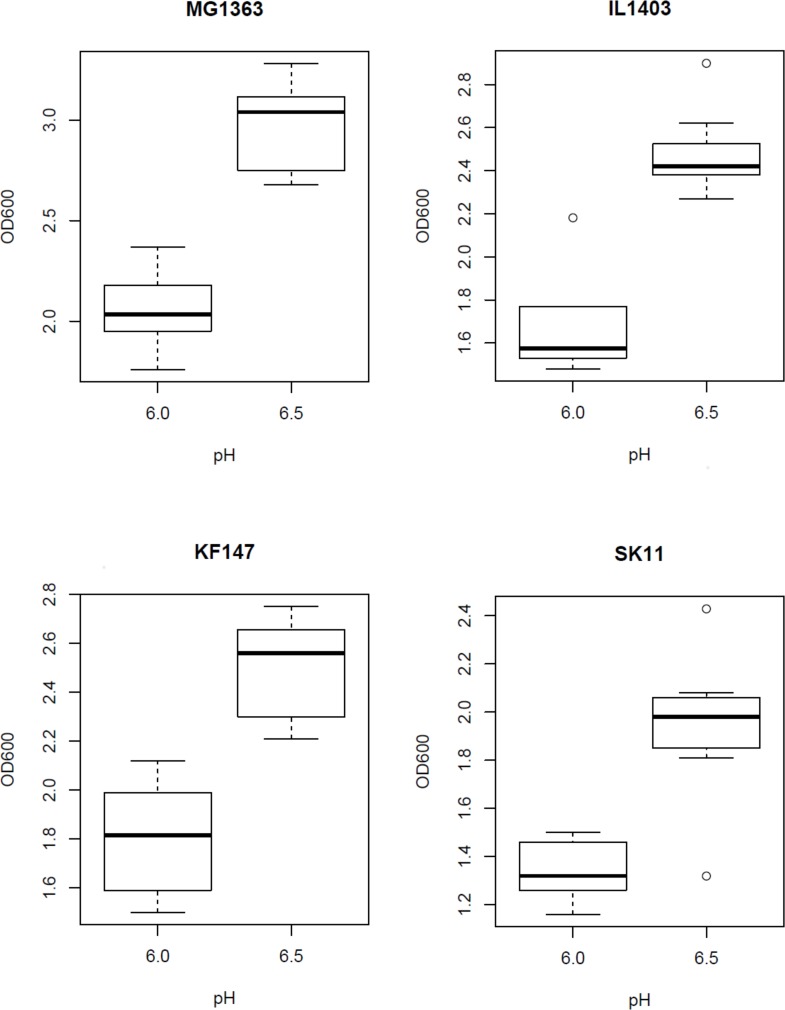
Both biomass formation (ODfinal) and final pH at the end of fermentation were strongly affected by the fermentation conditions and the observed effects were similar for all strains (Table 1). The initial pH of fermentation had the most significant effect on biomass formation. In fermentations with an initial pH of 6.5 a significantly higher biomass formation was reached for all strains as compared to fermentations with an initial pH of 6.0 (Fig 2). The final pH at the end of fermentation was mostly affected by the oxygen level and was significantly lower in fermentations with a relatively low level of oxygen as compared with fermentations with a relatively high level (data not shown). This is in line with an earlier study, which demonstrated that the acidifying ability of L. lactis strain CNRZ 483 decreased as initial oxygen concentration increased [21].
Fig 2. Effect of pH on final OD.
Boxplots of final optical density (ODfinal) of strains MG1363, IL1403, KF147 and SK11 in fermentations with an initial pH of 6.0 or 6.5.
With the notable exception of the effect of fermentation temperature on growth rate of SK11, all other applied fermentation parameters had similar effects on the growth characteristics of the L. lactis strains, revealing an overlap in responses towards the applied fermentation conditions.
The effect of fermentation conditions on robustness is strain-dependent
To study the effect of the fermentation conditions on robustness phenotypes, cells were harvested in exponential phase of growth for assessment of heat and oxidative stress survival phenotypes, representing robustness during spray drying [5]. During the stress assays, survival was determined at two time points, similar as for MG1363 [11]. For KF147 and SK11 the time points for heat stress survival measurement were adjusted because these strains displayed a higher sensitivity towards heat stress as compared with MG1363 and IL1403 (see Materials and Methods). Variation in fermentation conditions resulted in differences in both heat and oxidative stress survival of up to five log units (Table 1, S1 Table). Moreover, the various fermentation conditions had a different impact on the stress survival of the various strains. Strain IL1403 displayed the largest variation in robustness towards both heat and oxidative stress, which is in line with our observation that differences in fermentation conditions imposed the largest variation on growth characteristics of this strain as well. The observed differences in robustness towards both heat and oxidative stress of strain SK11 in the various fermentations demonstrate that contrary to earlier observations by Kim et al. [9] also strains with an L. lactis subsp. cremoris phenotype can have an adaptive response to stress.
As was observed before for strain MG1363 [11], no correlation of growth rate and survival towards heat stress was observed for the three strains. Only strain SK11 displayed a correlation of growth rate and oxidative stress survival (Pearson correlation coefficient = 0.79). Overall, this appears to support the study of Dressaire et al., which demonstrated that downregulation of stress genes at increasing growth rates, as observed in yeast [22], does not occur in L. lactis [23]. This implies that fermentation conditions resulting in improved robustness are not necessarily more time-consuming. Moreover, neither for heat stress nor oxidative stress, correlation of final biomass formation and survival was found, indicating that increased robustness can be achieved without the necessity to reduce yield.
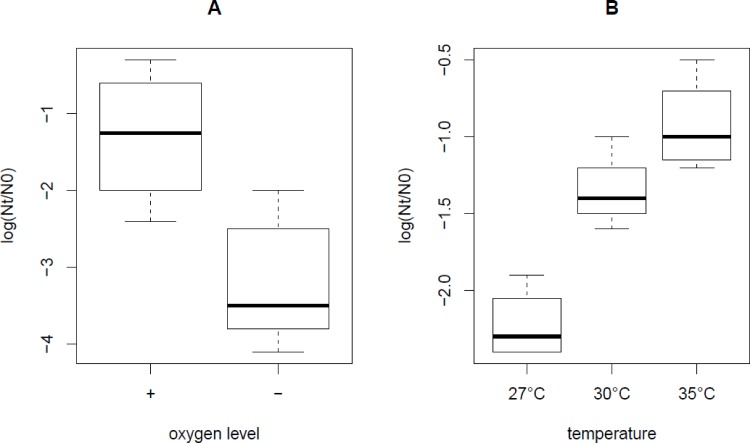
To identify the individual fermentation parameters with the most pronounced effect on heat or oxidative stress survival, we compared survival phenotypes in fermentations with one variant of this parameter with survival phenotypes in fermentations with the other variant of this parameter. Similar to what was previously observed for MG1363 [11], survival of KF147 during heat stress significantly increased during fermentation with a high level of oxygen (Fig 3A), whereas for SK11 robustness towards heat stress significantly increased with increasing fermentation temperature (Fig 3B). Contrasting our earlier observations in MG1363 [11], oxidative stress survival of strains IL1403, KF147 and SK11 was not significantly higher in fermentation at 35°C as compared with fermentations at 27°C. Survival of IL1403, which displayed a large variation in robustness phenotypes in the various fermentations, was not significantly altered by any of the specific individual fermentation parameters (S2 Table).
Fig 3. Heat stress survival of KF147 and SK11.
Boxplots of robustness phenotypes towards 10 minutes of heat stress at relatively low and high oxygen levels for strain KF147 (A) and at various fermentation temperatures for strain SK11 (B). Robustness is expressed as the difference of log CFU/ml after stress (Nt) and before stress (N0).
These experiments demonstrate that fermentation parameters have a substantial impact on subsequent stress survival of L. lactis strains. Irrespective of the strain’s general robustness level [5], survival can be dramatically altered by varying fermentation conditions. Although the fermentation parameters had similar effects on growth characteristics, the effect of specific fermentation parameters on survival is strain-dependent. This indicates that a general fermentation strategy to optimize robustness is difficult to achieve and to accomplish optimal robustness, fermentation conditions should be individually optimized for each L. lactis strain.
Transcriptome-phenotype matching reveals strain-specific associations of gene expression with robustness
We determined the effect of the fermentation parameters on gene expression. As previously demonstrated for strain MG1363 [11], the oxygen level and the fermentation temperature also had the most pronounced effect on gene expression in IL1403, KF147 and SK11 (S3 Fig), which appears to be in line with the observed effect of oxygen level and fermentation temperature on robustness phenotypes of several strains.
Subsequently, we calculated the correlation (according to a linear model) of gene expression levels in the various fermentations with the corresponding robustness phenotypes (S1–S6 Files). Similarly as for MG1363 [11], we selected the genes displaying a significant correlation (P < 0.05) with robustness at both time points of the stress assay. The genes with the most significant correlation at both time points of the stress assay (product of P-values < 5×10−5) were further analyzed (Table 2). For IL1403, 54 and 32 genes met these criteria for heat and oxidative stress survival, respectively. Only two genes displayed a significant correlation with oxidative stress survival in KF147, whereas 174 genes correlated with heat stress survival in this strain. In SK11, 124 and 63 genes displayed a significant correlation with heat and oxidative stress survival, respectively.
Table 2. Individual correlating gene expressions with robustness towards heat stress (A) or oxidative stress (B).
| A | |||||
| Strain | Locus tag | Gene | Function | Correlation | Slope |
| IL1403 | L133770 | rpmH | 50S_ribosomal_protein_L34 | negative | 3.1 |
| L127611 | yveD | hypothetical protein | negative | 0.6 | |
| L36850 | ps104 | prophage_ps1_protein_04 | negative | 0.1 | |
| L52686 | ycfD | hypothetical_protein | negative | 1.1 | |
| L52019 | gntK | gluconate_kinase | positive | 0.4 | |
| L18206 | ysdB | ABC transporter ATP binding protein | negative | 1.8 | |
| L167426 | zitS | zinc ABC transporter substrate binding protein | negative | 1.9 | |
| L94973 | ycjG | hypothetical protein | negative | 3.4 | |
| L14408 | nagB | glucosamine-6-P isomerase | negative | 3.9 | |
| L72115 | yohD | hypothetical protein | negative | 2.9 | |
| L154225 | ylfD | hypothetical protein | negative | 2.4 | |
| L0163 | ribG | riboflavin-specific deaminase | positive | 0.1 | |
| L145739 | floL | flotillin-like protein | negative | 5.2 | |
| L39365 | yqdA | hypothetical protein | negative | 2.8 | |
| L11493 | arsC | arsenate reductase | negative | 1.7 | |
| L175712 | ynhD | hypothetical protein | negative | 3.2 | |
| L196779 | yfjD | tRNA/rRNA methyltransferase | negative | 1.5 | |
| L113377 | ps221 | prophage ps2 protein 21 | negative | 0.4 | |
| L77017 | ykhJ | hypothetical protein | negative | 0.3 | |
| L0397 | rpsT | 30S ribosomal protein S20 | negative | 17.7 | |
| L0275 | dnaN | DNA polymerase III subunit beta | positive | 6.1 | |
| L0063 | aroF | phospho-2-dehydro-3-deoxyheptonate aldolase | negative | 5.2 | |
| L193734 | pdc | phenolic acid decarboxylase | negative | 0.3 | |
| L156445 | ylfH | N-acetylglucosamine catabolic protein | positive | 1.8 | |
| L126998 | yveC | hypothetical protein | negative | 2.4 | |
| L158972 | yjfJ | hypothetical protein | negative | 5.7 | |
| L189881 | rluC | pseudouridine synthase | negative | 1.5 | |
| L109379 | yjaJ | transcription regulator | negative | 4.8 | |
| L198904 | ps304 | prophage ps3 protein 04 | negative | 0.3 | |
| L16848 | ysdA | ABC transporter permease protein | negative | 2.7 | |
| L193031 | yhjA | hypothetical protein | negative | 11.4 | |
| L0064 | aroH | phospho-2-dehydro-3-deoxyheptonate aldolase | negative | 17.4 | |
| L30663 | ycdA | hypothetical protein | negative | 0.8 | |
| L102317 | hslA | HU like DNA-binding protein | negative | 11.1 | |
| L0285 | dnaD | hypothetical protein | positive | 2.7 | |
| L0151 | rgrB | GntR family transcription regulator | negative | 4.1 | |
| L188392 | ybiH | hypothetical protein | positive | 0.2 | |
| L192589 | pydA | dihydroorotate dehydrogenase 1A | negative | 4.8 | |
| L19745 | bar | acyltransferase | negative | 2.4 | |
| L117821 | yxdC | cation-transporting ATPase | negative | 0.5 | |
| L67463 | yuiB | hypothetical protein | negative | 7.9 | |
| L199277 | ps305 | prophage ps3 protein 05 | negative | 0.7 | |
| L71486 | yohC | transcription regulator | negative | 2.0 | |
| L140714 | adk | adenylate kinase | negative | 2.7 | |
| L43222 | recX | recombination regulator RecX | negative | 5.3 | |
| L72684 | ykhE | hypothetical protein | negative | 0.3 | |
| L00096 | rpmF | 50S ribosomal protein L32 | negative | 13.5 | |
| L155044 | dcdA | dCMP deaminase | negative | 1.5 | |
| L122849 | ybcG | hypothetical protein | negative | 7.5 | |
| L3272 | yiaD | putative NADH-flavin reductase | negative | 3.9 | |
| L0416 | rplT | 50S ribosomal protein L20 | negative | 10.5 | |
| L0217 | rlrD | LysR family transcription regulator | negative | 1.7 | |
| L148007 | ybeM | hypothetical protein | negative | 0.9 | |
| L162840 | yhgC | transcription regulator | negative | 0.1 | |
| KF147 | LLKF_1804 | trxB | thioredoxin reductase | positive | 12.6 |
| LLKF_1758 | rarA | ArsR family transcriptional regulator | positive | 0.7 | |
| LLKF_0447 | yeaA | beta-lactamase superfamily Zn-dependent hydrolase | positive | 6.0 | |
| LLKF_2085 | ytgB | hypothetical protein | positive | 17.7 | |
| LLKF_1563 | bglH | beta-glucosidase/ 6-phospho-beta-glucosidase | positive | 0.4 | |
| LLKF_1820 | yrbB | transglycosylase | positive | 26.3 | |
| LLKF_2083 | hypothetical protein | positive | 15.2 | ||
| LLKF_2084 | ytgA | hypothetical protein | positive | 14.1 | |
| LLKF_1723 | excisionase | positive | 0.1 | ||
| LLKF_2082 | ytgH | Gls24 family general stress protein | positive | 16.5 | |
| LLKF_0716 | glgD | glucose-1-phosphate adenylyltransferase regulatory subunit | negative | 2.9 | |
| LLKF_0747 | menC | O-succinylbenzoate synthase | positive | 5.1 | |
| LLKF_0746 | yhdA | 1,4-dihydroxy-2-naphthoyl-CoA thioesterase | positive | 2.2 | |
| LLKF_0965 | yjgC | amino acid ABC transporter substrate-binding protein | positive | 7.0 | |
| LLKF_0036 | pdhC | pyruvate dehydrogenase complex dihydrolipoamide acetyltransferase | positive | 23.9 | |
| LLKF_1210 | hypothetical protein | positive | 1.5 | ||
| LLKF_0039 | lplL | lipoate-protein ligase | positive | 17.5 | |
| LLKF_1293 | AMP-dependent synthetase and ligase family protein | negative | 0.6 | ||
| LLKF_0381 | ydcG | Cro/CI family transcriptional regulator | positive | 5.6 | |
| LLKF_1201 | nanE | N-acetylmannosamine-6-phosphate 2-epimerase | positive | 0.8 | |
| LLKF_0715 | glgC | glucose-1-phosphate adenylyltransferase catalytic subunit | negative | 1.6 | |
| LLKF_0967 | yjgE | amino acid transport, ATP-binding protein | positive | 4.9 | |
| LLKF_1852 | yrfB | NADH-dependent oxidoreductase | positive | 5.8 | |
| LLKF_0684 | CHW repeat-/cell adhesion domain-containing transglutaminase-like protease | negative | 21.7 | ||
| LLKF_1259 | ymdE | hypothetical protein | positive | 16.9 | |
| LLKF_0384 | fhuG | ferrichrome ABC transporter permease FhuG | positive | 2.9 | |
| LLKF_1275 | trmFO | tRNA (uracil-5-)-methyltransferase Gid | positive | 11.6 | |
| LLKF_0110 | pmrB | MF superfamily multidrug resistance efflux pump protein | positive | 0.9 | |
| LLKF_1417 | yngB | fibronectin-binding protein A | positive | 1.9 | |
| LLKF_1270 | ilvA | threonine dehydratase | negative | 2.9 | |
| LLKF_1118 | ykjI | hypothetical protein | positive | 0.7 | |
| LLKF_1265 | ymeB | ABC transporter ATP-binding protein | negative | 0.3 | |
| LLKF_0493 | pyrG | CTP synthase | positive | 7.4 | |
| LLKF_0849 | trmU | tRNA (5-methylaminomethyl-2-thiouridylate)-methyltransferase | positive | 10.2 | |
| LLKF_0664 | scrK | fructokinase | positive | 0.7 | |
| LLKF_0555 | yfhA | GNAT family acetyltransferase | positive | 0.5 | |
| LLKF_1344 | xerD | site-specific tyrosine recombinase XerD | positive | 1.5 | |
| LLKF_2234 | hypothetical protein | negative | 1.5 | ||
| LLKF_2318 | family 2 glycosyltransferase | negative | 0.3 | ||
| LLKF_0959 | yjfG | hypothetical protein | positive | 3.5 | |
| LLKF_0901 | hslB | DNA-binding protein HU | positive | 1.1 | |
| LLKF_0500 | dnaE | DNA polymerase III subunit alpha | positive | 2.0 | |
| LLKF_2242 | hypothetical protein | negative | 1.9 | ||
| LLKF_1294 | acyl carrier protein | negative | 0.3 | ||
| LLKF_1209 | hypothetical protein | positive | 0.8 | ||
| LLKF_0382 | fhuC | ferrichrome ABC transporter ATP-binding protein FhuC | positive | 5.8 | |
| LLKF_0518 | cysK | cysteine synthase | positive | 1.0 | |
| LLKF_2139 | yudI | tRNA-dihydrouridine synthase | positive | 8.4 | |
| LLKF_1001 | ftsE | cell division ATP-binding protein FtsE | positive | 10.8 | |
| LLKF_2231 | ardA | conjugative transposon antirestriction protein | negative | 0.5 | |
| LLKF_1299 | nisK | nisin biosynthesis two-component system, sensor histidine kinase NisK | positive | 0.9 | |
| LLKF_0094 | ABC transporter ATPase protein | negative | 8.1 | ||
| LLKF_0853 | uvrC | excinuclease ABC subunit C | positive | 4.1 | |
| LLKF_0037 | pdhB | pyruvate dehydrogenase E1 component subunit beta | positive | 16.0 | |
| LLKF_1962 | nifU | SUF system FeS assembly protein | positive | 11.1 | |
| LLKF_0964 | yjgB | gamma-D-glutamyl-meso-diaminopimelate peptidase I, NlpC/P60 family | positive | 6.7 | |
| LLKF_2244 | FtsK/SpoIIIE family DNA segregation ATPase | negative | 2.2 | ||
| LLKF_0038 | pdhA | pyruvate dehydrogenase E1 component subunit alpha | positive | 13.4 | |
| LLKF_1851 | yrfA | ArsR family transcriptional regulator | positive | 3.1 | |
| LLKF_0577 | yfiL | GNAT family acetyltransferase | positive | 2.0 | |
| LLKF_1710 | uxaC | uronate isomerase | negative | 0.1 | |
| LLKF_0098 | hypothetical protein | negative | 2.1 | ||
| LLKF_0441 | trxH | thioredoxin | positive | 3.0 | |
| LLKF_0047 | yahA | HAD superfamily hydrolase | positive | 5.6 | |
| LLKF_1812 | yraD | hypothetical protein | positive | 1.1 | |
| LLKF_1857 | ABC transporter ATP-binding protein | negative | 0.3 | ||
| LLKF_0540 | uvrB | excinuclease ABC subunit B | positive | 2.4 | |
| LLKF_1295 | hypothetical protein | negative | 0.5 | ||
| LLKF_1966 | sufC | SUF system FeS cluster assembly protein ATP-dependent transporter SufC | positive | 11.5 | |
| LLKF_0052 | cysD | O-acetyl-L-homoserine sulfhydrolase/O-acetyl-L-serine sulfhydrolase | positive | 1.8 | |
| LLKF_0904 | yjaF | hypothetical protein | positive | 5.6 | |
| LLKF_0162 | ybhA | 5-formyltetrahydrofolate cyclo-ligase | negative | 0.7 | |
| LLKF_0035 | pdhD | pyruvate dehydrogenase complex dihydrolipoamide acetyltransferase | positive | 21.6 | |
| LLKF_1720 | hypothetical protein | negative | 0.1 | ||
| LLKF_1579 | ypaE | hypothetical protein | negative | 4.6 | |
| LLKF_2241 | hypothetical protein | negative | 1.9 | ||
| LLKF_2238 | hypothetical protein | negative | 1.7 | ||
| LLKF_1856 | transcriptional regulator | negative | 0.7 | ||
| LLKF_2243 | replication initiation factor | negative | 1.5 | ||
| LLKF_2233 | CHAP domain family N-acetylmuramoyl-L-alanine amidase | negative | 0.5 | ||
| LLKF_2236 | hypothetical protein | negative | 1.1 | ||
| LLKF_2246 | hypothetical protein | negative | 2.7 | ||
| LLKF_1948 | ysdC | hypothetical protein | negative | 0.3 | |
| LLKF_1167 | ylfFG | acyl-[acyl-carrier-protein] hydrolase | positive | 2.5 | |
| LLKF_1550 | coaA | pantothenate kinase | positive | 2.4 | |
| LLKF_0668 | GFO/IDH/MOCA family oxidoreductase | negative | 0.2 | ||
| LLKF_0861 | choS | glycine betaine ABC transporter permease/substrate-binding protein | positive | 2.7 | |
| LLKF_0999 | yjjH | calcineurin-like phosphoesterase | positive | 1.0 | |
| LLKF_1961 | sufB | cysteine desulfurase activator complex subunit SufB | positive | 13.9 | |
| LLKF_0443 | noxE | NADH oxidase | positive | 29.5 | |
| LLKF_0020 | tilS | tRNA(Ile)-lysidine synthetase | positive | 2.3 | |
| LLKF_0802 | cysK | cysteine synthase | positive | 2.2 | |
| LLKF_0898 | pnuC | nicotinamide mononucleotide transporter/n-ribosylnicotinamide transporter | positive | 4.0 | |
| LLKF_1536 | pp270 | phage protein | positive | 0.6 | |
| LLKF_0661 | scrR | LacI family sucrose operon repressor | positive | 0.8 | |
| LLKF_1521 | pp255 | phage protein | negative | 0.3 | |
| LLKF_0284 | transcriptional regulator | positive | 2.5 | ||
| LLKF_0982 | grpE | molecular chaperone GrpE | negative | 6.4 | |
| LLKF_1261 | leuB | 3-isopropylmalate dehydrogenase | negative | 0.5 | |
| LLKF_2093 | ytgF | 2,3-cyclic-nucleotide 2-phosphodiesterase | positive | 10.6 | |
| LLKF_0100 | short chain dehydrogenase | negative | 5.8 | ||
| LLKF_1331 | ymjE | family 2 glycosyltransferase | positive | 2.3 | |
| LLKF_0093 | ABC transporter permease | negative | 8.2 | ||
| LLKF_1359 | rnhB | ribonuclease HII | positive | 0.9 | |
| LLKF_0165 | ybhD | GNAT family acetyltransferase | positive | 0.5 | |
| LLKF_1075 | pp146 | phage protein | positive | 2.7 | |
| LLKF_0310 | hypothetical protein | negative | 0.6 | ||
| LLKF_0981 | hrcA | Heat-inducible transcription repressor HrcA | negative | 5.7 | |
| LLKF_0695 | hypothetical protein | positive | 7.5 | ||
| LLKF_1578 | ypaD | hypothetical protein | negative | 4.5 | |
| LLKF_1799 | aroD | 3-dehydroquinate dehydratase | negative | 1.9 | |
| LLKF_2229 | conjugative transposon Tn5276 integrase | negative | 0.8 | ||
| LLKF_1872 | yrgF | hypothetical protein | negative | 0.5 | |
| LLKF_1527 | pp261 | phage protein | negative | 0.1 | |
| LLKF_0029 | yafF | hypothetical protein | positive | 0.8 | |
| LLKF_2431 | gntR | RpiR family transcriptional regulator | negative | 2.1 | |
| LLKF_0983 | dnaK | chaperone protein DnaK | negative | 12.8 | |
| LLKF_1695 | thiL | acetyl-CoA acetyltransferase | positive | 4.6 | |
| LLKF_0551 | dfpA | phosphopantothenoylcysteine decarboxylase | positive | 1.5 | |
| LLKF_0663 | scrA | PTS system sucrose-specific transporter subunit IIABC | positive | 0.5 | |
| LLKF_2232 | hypothetical protein | negative | 0.9 | ||
| LLKF_1965 | sufD | SUF system FeS cluster assembly protein SufD | positive | 11.3 | |
| LLKF_0510 | adaA | methylphosphotriester-DNA alkyltransferase | positive | 0.1 | |
| LLKF_1352 | gltB | glutamate synthase large subunit | negative | 5.1 | |
| LLKF_1018 | ribH | riboflavin synthase subunit beta | positive | 0.2 | |
| LLKF_0570 | yfiE | organic hydroperoxide resistance family protein | positive | 24.3 | |
| LLKF_0647 | citB | aconitate hydratase | negative | 0.3 | |
| LLKF_0471 | ligA | NAD-dependent DNA ligase | positive | 2.9 | |
| LLKF_0215 | yqeL | GTP-binding protein | positive | 2.0 | |
| LLKF_0151 | ybgA | hypothetical protein | negative | 0.3 | |
| LLKF_2444 | pp401 | phage integrase | positive | 2.4 | |
| LLKF_1853 | hypothetical protein | positive | 7.3 | ||
| LLKF_1066 | pp137 | phage HNH endonuclease | positive | 0.7 | |
| LLKF_2398 | adhE | alcohol dehydrogenase/ acetaldehyde dehydrogenase | negative | 6.5 | |
| LLKF_1858 | ABC transporter permease | negative | 0.4 | ||
| LLKF_1656 | yphI | hypothetical protein | positive | 0.3 | |
| LLKF_1324 | dltC | D-alanine—poly(phosphoribitol) ligase subunit 2 | negative | 3.2 | |
| LLKF_1284 | recA | recombinase recA, C-terminal fragement | negative | 0.2 | |
| LLKF_1644 | clpB | ATP-dependent Clp protease chaperonin ATPase ClpB | negative | 3.1 | |
| LLKF_0873 | xseA | exodeoxyribonuclease VII large subunit | positive | 2.5 | |
| LLKF_0520 | yfcI | metallo-beta-lactamase family protein | positive | 0.9 | |
| LLKF_1071 | pp142 | phage major head protein | positive | 1.6 | |
| LLKF_1566 | trpA | tryptophan synthase subunit alpha | positive | 0.8 | |
| LLKF_1269 | ilvC | ketol-acid reductoisomerase | negative | 1.8 | |
| LLKF_0822 | rnc | ribonuclease III | positive | 1.5 | |
| LLKF_1132 | cobQ | cobB/cobQ-like glutamine amidotransferase | positive | 3.0 | |
| LLKF_1501 | pp235 | phage terminase large subunit | negative | 0.1 | |
| LLKF_1887 | pstA | phosphate ABC transporter ATP-binding protein | positive | 4.0 | |
| LLKF_1424 | pfkA | 6-phosphofructokinase | negative | 14.2 | |
| LLKF_0854 | mutY | A/G-specific adenine DNA glycosylase | positive | 1.5 | |
| LLKF_0889 | yijB | hypothetical protein | negative | 0.2 | |
| LLKF_0505 | yfaA | hypothetical protein | positive | 0.8 | |
| LLKF_0918 | tcsR | Two-component response regulator | positive | 1.7 | |
| LLKF_0390 | yddD | glyoxalase family protein | positive | 0.2 | |
| LLKF_1805 | ccpA | catabolite control protein A | positive | 4.6 | |
| LLKF_2245 | hypothetical protein | negative | 2.2 | ||
| LLKF_1546 | deoC | deoxyribose-phosphate aldolase | positive | 3.2 | |
| LLKF_1589 | putrescine/ornithine aminotransferase | negative | 0.1 | ||
| LLKF_0270 | nrdD | anaerobic ribonucleoside-triphosphate reductase | negative | 11.7 | |
| LLKF_0313 | hypothetical protein | negative | 0.1 | ||
| LLKF_1486 | pp220 | phage protein | positive | 0.5 | |
| LLKF_0104 | hypothetical protein | negative | 0.2 | ||
| LLKF_2239 | hypothetical protein | negative | 3.0 | ||
| LLKF_1351 | gltD | glutamate synthase small subunit | negative | 3.3 | |
| LLKF_1728 | csc2A | c-terminal membrane anchored cell surface protein | negative | 0.1 | |
| LLKF_2066 | yteB | glycine/D-amino acid oxidase family protein | positive | 0.2 | |
| LLKF_0915 | rpsN | 50S ribosomal protein S14P | negative | 0.1 | |
| LLKF_0385 | fhuD | ferrichrome ABC transporter substrate-binding protein FhuD | positive | 8.9 | |
| LLKF_0640 | pfl | formate acetyltransferase | negative | 11.3 | |
| LLKF_1348 | murI | glutamate racemase | positive | 2.2 | |
| LLKF_2368 | comGE | competence protein ComGE | negative | 0.1 | |
| LLKF_0222 | yccJ | hypothetical protein | positive | 4.8 | |
| SK11 | LACR_2496 | gluconate kinase | positive | ||
| LACR_2183 | manganese transporter NRAMP | positive | 1.5 | ||
| LACR_2273 | hypothetical protein | positive | 25.8 | ||
| LACR_2219 | hypothetical protein | positive | 1.1 | ||
| LACR_1490 | hypothetical protein | positive | 0.2 | ||
| LACR_C29 | hypothetical protein | positive | 15.9 | ||
| LACR_1011 | ABC-type polar amino acid transport system, ATPase component | positive | 12.9 | ||
| LACR_1370 | cation-transporting P-ATPase | positive | 20.0 | ||
| LACR_1188 | hypothetical protein | positive | 3.8 | ||
| LACR_2217 | hypothetical protein | positive | 1.0 | ||
| LACR_1428 | hypothetical protein | positive | 14.1 | ||
| LACR_1467 | hypothetical protein | positive | 8.6 | ||
| LACR_0359 | hypothetical protein | positive | 5.3 | ||
| LACR_2213 | hypothetical protein | positive | 3.3 | ||
| LACR_2358 | integral membrane protein | negative | 6.2 | ||
| LACR_1427 | DeoR family transcriptional regulator | positive | 9.4 | ||
| LACR_1389 | hypothetical protein | positive | 7.1 | ||
| LACR_0544 | hypothetical protein | positive | 0.7 | ||
| LACR_1168 | hypothetical protein | positive | 0.5 | ||
| LACR_1369 | Mn-dependent transcriptional regulator | positive | 6.5 | ||
| LACR_0743 | flavodoxin | positive | 2.0 | ||
| LACR_1502 | hypothetical protein | positive | 1.9 | ||
| LACR_A11 | relaxase/mobilization nuclease domain-containing protein | positive | 39.9 | ||
| LACR_0543 | recU | Holliday junction-specific endonuclease | positive | 3.2 | |
| LACR_0274 | hypothetical protein | positive | 2.4 | ||
| LACR_2272 | hypothetical protein | positive | 5.3 | ||
| LACR_1231 | hypothetical protein | negative | 1.2 | ||
| LACR_0805 | hypothetical protein | positive | 1.3 | ||
| LACR_2216 | hypothetical protein | positive | 2.0 | ||
| LACR_1390 | transcriptional regulator | positive | 19.2 | ||
| LACR_2499 | hypothetical protein | positive | 2.3 | ||
| LACR_0927 | acetyltransferase | positive | 5.2 | ||
| LACR_1715 | cation transport protein | positive | 3.4 | ||
| LACR_0774 | menaquinone-specific isochorismate synthase | positive | 4.0 | ||
| LACR_1524 | Signal transduction histidine kinase | positive | 7.3 | ||
| LACR_2012 | gamma-aminobutyrate permease related permease | negative | 4.4 | ||
| LACR_1302 | xerS | site-specific tyrosine recombinase XerS | positive | 16.5 | |
| LACR_C54 | hypothetical protein | positive | 4.8 | ||
| LACR_0329 | acetyltransferase | positive | 3.0 | ||
| LACR_0302 | transcriptional regulator | positive | 2.0 | ||
| LACR_0398 | asnB | asparagine synthetase B | negative | 17.5 | |
| LACR_A05 | hypothetical protein | positive | 3.0 | ||
| LACR_2026 | ABC-type oligopeptide transport system, periplasmic component | negative | 4.4 | ||
| LACR_2220 | hypothetical protein | positive | 1.4 | ||
| LACR_2522 | hypothetical protein | positive | 4.4 | ||
| LACR_1437 | transposase | positive | 9.2 | ||
| LACR_1714 | ArsR family transcriptional regulator | positive | 3.1 | ||
| LACR_0904 | transcriptional regulator | positive | 0.6 | ||
| LACR_2151 | hypothetical protein | positive | 3.1 | ||
| LACR_1052 | putative exporter of polyketide antibiotics | positive | 3.3 | ||
| LACR_2126 | hypothetical protein | negative | 6.1 | ||
| LACR_1379 | hypothetical protein | positive | 1.2 | ||
| LACR_1525 | hypothetical protein | positive | 2.3 | ||
| LACR_0781 | hypothetical protein | positive | 2.5 | ||
| LACR_1237 | truB | tRNA pseudouridine synthase B | positive | 2.0 | |
| LACR_1261 | hypothetical protein | positive | 1.5 | ||
| LACR_C27 | pyrrolidone-carboxylate peptidase | positive | 6.5 | ||
| LACR_1505 | transposase | positive | 9.0 | ||
| LACR_0803 | hypothetical protein | positive | 1.3 | ||
| LACR_2218 | hypothetical protein | positive | 2.1 | ||
| LACR_2270 | hypothetical protein | positive | 18.7 | ||
| LACR_1987 | murE | UDP-N-acetylmuramoylalanyl-D-glutamate—2,6-diaminopimelate ligase | positive | 3.6 | |
| LACR_1104 | hypothetical protein | negative | 5.0 | ||
| LACR_0812 | putative effector of murein hydrolase LrgA | positive | 4.1 | ||
| LACR_1019 | hypothetical protein | negative | 4.4 | ||
| LACR_1523 | DNA-binding response regulator | positive | 5.0 | ||
| LACR_0804 | hypothetical protein | positive | 2.2 | ||
| LACR_0140 | hypothetical protein | positive | 0.1 | ||
| LACR_0505 | hypothetical protein | negative | 0.4 | ||
| LACR_1362 | transcriptional regulator | positive | 1.8 | ||
| LACR_C28 | dienelactone hydrolase family protein | positive | 14.6 | ||
| LACR_2274 | hypothetical protein | positive | 12.9 | ||
| LACR_1031 | lactose transport regulator | positive | 2.6 | ||
| LACR_1067 | amidase | positive | 0.5 | ||
| LACR_2592 | hypothetical protein | positive | 0.2 | ||
| LACR_1032 | tagatose-6-phosphate kinase | positive | 4.9 | ||
| LACR_0422 | transcriptional regulator | positive | 0.4 | ||
| LACR_0450 | hypothetical protein | positive | 0.4 | ||
| LACR_1982 | pleiotropic transcriptional repressor | positive | 0.1 | ||
| LACR_0809 | hypothetical protein | positive | 2.3 | ||
| LACR_2381 | secY | preprotein translocase subunit SecY | negative | 21.0 | |
| LACR_2340 | hypothetical protein | positive | 1.7 | ||
| LACR_D08 | site-specific recombinase, DNA invertase Pin related protein | negative | 11.5 | ||
| LACR_1260 | hypothetical protein | positive | 1.4 | ||
| LACR_1122 | deoxyuridine 5'-triphosphate nucleotidohydrolase | negative | 6.4 | ||
| LACR_1079 | hypothetical protein | positive | 1.5 | ||
| LACR_2118 | deoxyuridine 5'-triphosphate nucleotidohydrolase | negative | 4.6 | ||
| LACR_0432 | membrane carboxypeptidase (penicillin-binding protein) | positive | 2.6 | ||
| LACR_0807 | sortase (surface protein transpeptidase) | positive | 1.2 | ||
| LACR_1020 | hypothetical protein | negative | 4.4 | ||
| LACR_1164 | hypothetical protein | positive | 0.3 | ||
| LACR_0301 | integrase | positive | 2.2 | ||
| LACR_2515 | ruvB | Holliday junction DNA helicase RuvB | positive | 2.8 | |
| LACR_2119 | hypothetical protein | negative | 1.8 | ||
| LACR_0582 | dinucleoside polyphosphate hydrolase | positive | 1.9 | ||
| LACR_0511 | hypothetical protein | positive | 4.8 | ||
| LACR_0775 | SSU ribosomal protein S5P alanine acetyltransferase | positive | 1.0 | ||
| LACR_2134 | hypothetical protein | negative | 1.9 | ||
| LACR_2116 | hypothetical protein | negative | 1.5 | ||
| LACR_2357 | hypothetical protein | negative | 1.3 | ||
| LACR_2558 | transcriptional regulator | positive | 0.5 | ||
| LACR_0956 | transcriptional regulator | positive | 1.7 | ||
| LACR_1891 | competence protein | negative | 0.2 | ||
| LACR_0094 | D-tyrosyl-tRNA(Tyr) deacylase | positive | 0.7 | ||
| LACR_0201 | hypothetical protein | negative | 5.9 | ||
| LACR_2462 | transposase | positive | 12.1 | ||
| LACR_1458 | N-acetylglucosamine 6-phosphate deacetylase | positive | 3.5 | ||
| LACR_C08 | acetyltransferase | negative | 0.6 | ||
| LACR_1266 | xanthine/uracil permease | negative | 0.9 | ||
| LACR_0870 | HAD superfamily hydrolase | positive | 2.3 | ||
| LACR_D23 | replication initiator protein | positive | 2.5 | ||
| LACR_1635 | transposase | positive | 9.3 | ||
| LACR_0715 | Mg-dependent DNase | positive | 1.4 | ||
| LACR_1856 | hypothetical protein | positive | 1.4 | ||
| LACR_0652 | XRE family transcriptional regulator | positive | 1.5 | ||
| LACR_1631 | thyA | thymidylate synthase | positive | 2.0 | |
| LACR_0249 | HAD superfamily hydrolase | negative | 1.1 | ||
| LACR_0680 | transposase | positive | 12.4 | ||
| LACR_1099 | XRE family transcriptional regulator | positive | 7.6 | ||
| LACR_2061 | TIM-barrel fold family protein | negative | 11.5 | ||
| LACR_1423 | hypothetical protein | positive | 4.0 | ||
| LACR_1063 | ribonucleoside-diphosphate reductase class Ib glutaredoxin subunit | positive | 10.8 | ||
| LACR_0066 | transcriptional regulator | positive | 2.2 | ||
| LACR_C32 | transposase | negative | 20.6 | ||
| B | |||||
| Strain | Locus tag | Gene | Function | Correlation | Slope |
| IL1403 | L162840 | yhgC | transcription regulator | negative | 0.1 |
| L79507 | yahD | hypothetical protein | positive | 2.8 | |
| L0275 | dnaN | DNA polymerase III subunit beta | positive | 7.1 | |
| L104969 | napC | multidrug-efflux transporter | positive | 0.2 | |
| L189822 | ybiK | hypothetical protein | positive | 8.4 | |
| L109527 | rsuA | ribosomal small subunit pseudouridine synthase A | negative | 0.8 | |
| L84992 | ytaB | YtaB | positive | 2.9 | |
| L0165 | ribA | 3,4-dihydroxy-2-butanone 4-phosphate synthase | positive | 0.2 | |
| L4822 | ptsK | HPr kinase/phosphorylase | positive | 5.9 | |
| L196779 | yfjD | tRNA/rRNA methyltransferase | negative | 1.7 | |
| L180241 | mycA | myosin-cross-reactive antigen | positive | 5.4 | |
| L7798 | ps316 | integrase | negative | 1.6 | |
| L30663 | ycdA | hypothetical protein | negative | 0.9 | |
| L20937 | ywdF | hypothetical protein | negative | 3.3 | |
| L190009 | feoB | ferrous ion transport protein B | positive | 8.1 | |
| L0016 | gpsA | NAD(P)H-dependent glycerol-3-phosphate dehydrogenase | positive | 4.8 | |
| L193030 | yjjD | ABC transporter permease protein | positive | 0.9 | |
| L136552 | ybdJ | hypothetical protein | positive | 0.2 | |
| L179531 | ispB | heptaprenyl diphosphate synthase component II | positive | 4.3 | |
| L0241 | uxuB | fructuronate reductase | positive | 0.1 | |
| L177590 | hasC | UTP-glucose-1-phosphate uridylyltransferase | positive | 5.7 | |
| L0274 | dnaA | chromosomal replication initiation protein | positive | 7.2 | |
| L114325 | ybbE | hypothetical protein | negative | 0.8 | |
| L0298 | topA | DNA topoisomerase I | negative | 4.3 | |
| L32731 | ykdB | hypothetical protein | positive | 1.0 | |
| L17893 | yebF | transcription regulator | positive | 1.2 | |
| L180104 | umuC | UmuC | positive | 0.3 | |
| L0101 | metA | homoserine O-succinyltransferase | positive | 1.7 | |
| L197697 | yfjE | flavodoxin | negative | 1.5 | |
| L200024 | hypothetical protein | positive | 0.4 | ||
| L5776 | lgt | prolipoprotein diacylglyceryl transferase | positive | 2.0 | |
| L135900 | ybdI | hypothetical protein | positive | 0.2 | |
| KF147 | LLKF_2311 | family 2 glycosyltransferase | negative | 0.3 | |
| LLKF_0448 | tcsK | Two-component sensor histidine kinase | negative | 5.5 | |
| SK11 | LACR_0741 | hypothetical protein | positive | 0.8 | |
| LACR_0891 | copper/potassium-transporting ATPase | positive | 4.4 | ||
| LACR_E7 | hypothetical protein | positive | 4.1 | ||
| LACR_1450 | fibronectin-binding protein | positive | 1.1 | ||
| LACR_0073 | esterase | positive | 10.0 | ||
| LACR_0714 | hypothetical protein | positive | 3.7 | ||
| LACR_C16 | replication initiator protein | positive | 3.3 | ||
| LACR_0074 | lactoylglutathione lyase related lyase | positive | 7.0 | ||
| LACR_1221 | hypothetical protein | positive | 2.1 | ||
| LACR_0072 | hypothetical protein | positive | 8.5 | ||
| LACR_0920 | copper-potassium transporting ATPase B | positive | 5.0 | ||
| LACR_0959 | hypothetical protein | positive | 1.3 | ||
| LACR_0242 | saccharopine dehydrogenase related protein | positive | 10.1 | ||
| LACR_0451 | ABC-type multidrug transport system, permease component | positive | 3.0 | ||
| LACR_0713 | acetyltransferase | positive | 2.9 | ||
| LACR_0452 | ABC-type multidrug transport system, ATPase component | positive | 5.2 | ||
| LACR_0381 | hypothetical protein | positive | 0.5 | ||
| LACR_1506 | hypothetical protein | positive | 0.3 | ||
| LACR_0744 | lysophospholipase L1 related esterase | positive | 1.5 | ||
| LACR_2167 | N-acetylmuramoyl-L-alanine amidase | positive | 4.5 | ||
| LACR_0347 | ABC-type multidrug transport system, ATPase and permease component | positive | 4.4 | ||
| LACR_1291 | Beta-xylosidase | positive | 0.5 | ||
| LACR_1468 | orotidine 5'-phosphate decarboxylase | positive | 5.4 | ||
| LACR_0240 | NADPH:quinone reductase related Zn-dependent oxidoreductase | positive | 11.5 | ||
| LACR_1051 | ABC-type multidrug transport system, ATPase component | positive | 3.0 | ||
| LACR_0075 | hypothetical protein | positive | 6.7 | ||
| LACR_0241 | nucleoside-diphosphate sugar epimerase | positive | 11.2 | ||
| LACR_0105 | hypothetical protein | positive | 3.3 | ||
| LACR_0629 | major facilitator superfamily permease | positive | 0.3 | ||
| LACR_0164 | hypothetical protein | positive | 3.8 | ||
| LACR_2411 | hypothetical protein | negative | 0.9 | ||
| LACR_1362 | transcriptional regulator | positive | 1.1 | ||
| LACR_0982 | ring-cleavage extradiol dioxygenase | positive | 3.6 | ||
| LACR_0742 | transcriptional regulator | positive | 2.0 | ||
| LACR_0537 | cysteine synthase | positive | 0.4 | ||
| LACR_0743 | flavodoxin | positive | 1.2 | ||
| LACR_D16 | oligopeptidase O1 | negative | 11.3 | ||
| LACR_2476 | transcriptional regulator | positive | 5.7 | ||
| LACR_0839 | sugar metabolism transcriptional regulator | positive | 1.7 | ||
| LACR_1302 | xerS | site-specific tyrosine recombinase XerS | positive | 9.7 | |
| LACR_1290 | endoglucanase | positive | 0.2 | ||
| LACR_2355 | hypothetical protein | positive | 0.8 | ||
| LACR_1976 | negative regulator of genetic competence, sporulation and motility | positive | 2.4 | ||
| LACR_1629 | transcriptional regulator | positive | 2.1 | ||
| LACR_1395 | hypothetical protein | positive | 3.7 | ||
| LACR_1922 | hypothetical protein | negative | 1.1 | ||
| LACR_1267 | hypothetical protein | positive | 0.4 | ||
| LACR_2497 | 6-phosphogluconate dehydrogenase-like protein | positive | 0.9 | ||
| LACR_0431 | tyrosyl-tRNA synthetase | negative | 10.0 | ||
| LACR_0570 | dnaG | DNA primase | positive | 2.4 | |
| LACR_0657 | adenine phosphoribosyltransferase | negative | 5.6 | ||
| LACR_2490 | recX | recombination regulator RecX | positive | 14.2 | |
| LACR_1728 | Mg2+ transporter | positive | 1.6 | ||
| LACR_1751 | transposase | positive | 3.3 | ||
| LACR_0206 | glycosyltransferase | negative | 1.0 | ||
| LACR_1052 | putative exporter of polyketide antibiotics | positive | 2.0 | ||
| LACR_0642 | 6-phosphogluconate dehydrogenase | negative | 5.1 | ||
| LACR_0800 | XRE family transcriptional regulator | positive | 1.2 | ||
| LACR_1078 | transcriptional regulator | negative | 0.1 | ||
| LACR_2545 | ribosomal small subunit pseudouridine synthase A | negative | 1.6 | ||
| LACR_2184 | oxidoreductase | positive | 9.7 | ||
| LACR_0212 | lipopolysaccharide biosynthesis protein | negative | 1.8 | ||
| LACR_1105 | hypothetical protein | positive | 4.3 | ||
Correlating gene expressions with robustness towards heat stress (A) or oxidative stress (B) as assessed by a linear model of the strains IL1403, KF147 and SK11. Genes of which expression correlated with survival in more than one strain (including MG1363 [11]) are indicated in bold. Genes are ranked based on the significance of correlation (lowest P-value on top). Slope represents the average slope of the linear models fitting the data of both time points of the stress assay.
In KF147, the operon encoding the pyruvate dehydrogenase complex (pdhABCD) and a lipoate-protein ligase (lplL) as well as an operon encoding a ferrichrome ABC transporter (fhuCDG) and an operon encoding hypothetical proteins and a Gls24 family general stress protein (ytgH) displayed a positive correlation with heat stress survival. Surprisingly, the heat shock genes grpE and dnaK anti-correlated with robustness towards heat stress of KF147 and also their repressor hrcA displayed anti-correlation [24]. The gene fhuC was previously associated with heat stress survival in MG1363 [11], as well as four other genes: uvrC, cysD, cysK and trpA. In contrast to KF147 and MG1363, these transcripts did not show a significant correlation with heat stress survival in IL1403 nor in SK11, although a previous study by Xie et al. did suggest a role of cysK in heat stress survival of IL1403 [13]. Two other genes were found to associate with heat stress survival in both KF147 and SK11 (rarA and yjgE/ LACR_1011) and one in both IL1403 and SK11 (gntK). However, the majority of the correlating genes were shown to associate with stress survival in only one of the strains. In IL1403 the genes aroF and aroH encoding a phospho-2-dehydro-3-deoxyheptonate aldolase anti-correlated with heat stress survival. The gene aroF was previously shown to be upregulated in this strain during osmotic stress [13], suggesting this gene could be involved in a general stress mechanism. In SK11, multiple genes encoding hypothetical proteins were found to correlate with heat stress survival and also a gene encoding a manganese transporter (LACR_2183). Manganese transport was also associated with heat stress survival in an earlier study, where mtsC, encoding part of a manganese ABC transporter was shown to be present in robust strains and absent in sensitive strains within an L. lactis strain collection [5]. Metal ions have several functions in the cell and can be involved in stabilizing proteins, ribosomes and the cell membrane [25, 26]. Because these cellular components are affected during heat stress [8], manganese might have a role in the prevention of damage caused by heat stress.
Similar as for heat stress, the transcriptome signature associated with oxidative stress survival was highly strain-specific, which is exemplified by the fact that only three genes associated with oxidative stress survival in more than one strain. In both IL1403 and SK11 the gene expressions yahD/ LACR_0073, yjjD/ LACR_1052 and rsuA/ LACR_2545 were found to correlate with oxidative stress survival. In IL1403, 32 genes displayed correlation of expression with survival, among which was the gene feoB, which is involved in iron transport and was previously associated with heat stress survival in MG1363 [11]. In SK11, a gene encoding cysteine synthase positively correlated with oxidative stress survival. In MG1363 we previously demonstrated a link between cysteine metabolism and oxidative stress survival [11]. Sulfur-containing amino acids are readily oxidized and, therefore, cysteine metabolism could be involved in oxidative stress survival by affecting the redox balance in the cell. Furthermore, genes associated with oxidative stress survival in SK11 included genes encoding membrane proteins and regulators. For application as indicators for robustness, the genes with a high variation in gene expression (indicated by the slope in Table 2) appear to be most suitable, because they can be detected with methods such as quantitative PCR. For both heat and oxidative stress, none of the genes were associated with survival in more than two strains, although the majority of the genes that displayed correlation with survival are present in all four strains. This lack in overlap demonstrates that the transcriptome signature associated with stress survival is largely strain-dependent, and the complete transcriptome signature associated with robustness in one strain cannot be extrapolated fully to other strains. This indicates that the mechanisms aiming to improve robustness vary among the strains and, therefore, strategies resulting in improved robustness of one strain do not necessarily increase robustness of other strains. To acquire optimal robustness, the fermentation conditions of each strain require individual optimization.
Generic L. lactis genes associated with robustness towards heat or oxidative stress
To establish whether a generic transcriptome signature for L. lactis exists, we searched for single genes with the most significant correlation with robustness towards heat and oxidative stress in all strains. For this, we chose one time point of the stress assay, in which the range between the extreme values of survival in all fermentations was the largest (see Materials and methods). We selected the orthologous groups (OGs) in which the genes of all four strains displayed either a positive or a negative correlation (P < 0.2, assessed with a linear model) of expression level with robustness phenotype and ranked these on average P-value per OG (Table 3). Notably, the top 10 genes included ctsR, encoding a class three stress genes transcriptional repressor, which displayed negative correlation of expression with oxidative stress survival in all four strains. This gene was previously demonstrated to be a key regulator of heat-shock induced gene expression in MG1363 [27]. The observation that the transcript level of this gene appeared in the top 10 list of most significant correlating genes with oxidative stress survival suggests that CtsR is also involved in oxidative stress regulation in L. lactis. Involvement of CtsR in other stress responses besides heat stress response was already suggested by Frees et al., who demonstrated that the CtsR regulon was induced at low pH [28]. Furthermore, in Bacillus subtilis involvement of CtsR in oxidative stress survival has been previously suggested as transcription of the CtsR regulon was increased during oxidative stress [29]. Besides the significant correlation with heat stress survival of KF147, as mentioned in the previous paragraph, the gene lplL also displayed a positive correlation of expression and heat stress survival in the other three strains. This gene was previously demonstrated to be involved in heat shock response in strain IL1403 [13], which further supports the role of this gene in heat stress survival in L. lactis strains in general. Furthermore, the list contained multiple genes encoding for proteins involved in iron(complex) transport (feoA, fhuD, fhuG and fhuB). The fhu operon may be involved in haem uptake, enabling respiration metabolism in L. lactis [30, 31] and was recently demonstrated to be induced in strain MG1363 during the early phase of growth at high oxygen levels [32]. Furthermore, it has been demonstrated that free intracellular iron increases oxidative stress through generation of ROS from hydrogen peroxide by the Fenton reaction, which causes cellular damage and mortality in stationary phase cells of L. lactis [33]. A link between iron metabolism and heat stress survival has been demonstrated in Bacillus licheniformis, where an overlap in response to heat shock and iron limitation was revealed [34]. Taken together, a link between iron metabolism and stress survival in L. lactis appears likely.
Table 3. Generic correlating gene expressions with robustness towards heat stress (A) or oxidative stress (B).
| A | ||||||||
| locustag IL1403 | locustag SK11 | locustag KF147 | locustag MG1363 | gene | function | correlation | average P-value | maximum P-value |
| L191486 | LACR_1356 | LLKF_1201 | llmg_1317 | yljB/nanE | N-acetylmannosamine-6-phosphate 2-epimerase | positive | 0.025 | 0.039 |
| L101688 | LACR_1561 | LLKF_1575 | llmg_1029 | ypaA | hypothetical protein | negative | 0.026 | 0.062 |
| L195318 | LACR_1054 | LLKF_0997 | llmg_1551 | yjjF/fdhC | formate/nitrite transporter | negative | 0.031 | 0.059 |
| L89001 | LACR_1179 | LLKF_1106 | llmg_1494 | ykiI | ABC transporter permease | positive | 0.035 | 0.095 |
| L64373 | LACR_0052 | LLKF_0039 | llmg_0075 | lplL | lipoate-protein ligase | positive | 0.044 | 0.116 |
| L143312 | LACR_0389 | LLKF_0398 | llmg_0362 | dppA/optS | oligopeptide ABC transporter substrate binding protein | negative | 0.046 | 0.138 |
| L72684 | LACR_1157 | LLKF_1090 | llmg_1513 | ykhE | arsenate reductase | negative | 0.048 | 0.103 |
| L18206 | LACR_1946 | LLKF_1947 | llmg_1957 | ysdB | sodium ABC transporter ATP-binding protein | negative | 0.053 | 0.106 |
| L192240 | LACR_0194 | LLKF_0183 | llmg_0200 | feoA | ferrous iron transport protein A | positive | 0.054 | 0.113 |
| L148945 | LACR_1868 | LLKF_1872 | llmg_0725 | yrgF | hypothetical protein | negative | 0.057 | 0.184 |
| B | ||||||||
| locustag IL1403 | locustag SK11 | locustag KF147 | locustag MG1363 | gene | function | correlation | average P-value | maximum P-value |
| L0223 | LACR_0665 | LLKF_0631 | llmg_0614 | ctsR | class III stress genes transcriptional repressor | negative | 0.025 | 0.055 |
| L128386 | LACR_0373 | LLKF_0384 | llmg_0348 | fhuG | ferrichrome ABC transporter permease FhuG | positive | 0.030 | 0.051 |
| L100027 | LACR_2040 | LLKF_2041 | llmg_2036 | ytbC | hypothetical protein | negative | 0.039 | 0.127 |
| L0046 | LACR_0642 | LLKF_0600 | llmg_0586 | gnd | 6-phosphogluconate dehydrogenase | negative | 0.044 | 0.115 |
| L127476 | LACR_0372 | LLKF_0383 | llmg_0347 | fhuB | ferrichrome ABC transporter permease protein | positive | 0.059 | 0.109 |
| L117074 | LACR_2341 | LLKF_2294 | llmg_2327 | yvdD | glycerol uptake facilitator protein | negative | 0.066 | 0.134 |
| L103246 | LACR_1565 | LLKF_1577 | llmg_1026 | ypaC | methylase for ubiquinone/menaquinone biosynthesis | negative | 0.077 | 0.102 |
| L104745 | LACR_1567 | LLKF_1579 | llmg_1024 | ypaE | hypothetical protein | negative | 0.081 | 0.118 |
| L162870 | LACR_1609 | LLKF_1641 | llmg_0989 | ypgD | ABC transporter ATP binding and permease protein | positive | 0.098 | 0.183 |
| L129403 | LACR_0374 | LLKF_0385 | llmg_0349 | fhuD | ferrichrome ABC transporter substrate binding protein | positive | 0.099 | 0.157 |
Top 10 highest correlating transcript levels with robustness towards heat stress (A) or oxidative stress (B). Average P-value is the average of the P-values of the correlation as assessed by a linear model of the strains MG1363, IL1403, KF147 and SK11 and was used to rank the genes. Maximum P-value indicates the largest P-value of the correlation among the four strains.
Besides the genes that have previously been demonstrated to be involved in stress, the top 10 lists also included genes which to the best of our knowledge have not been associated with stress before. The transcript levels of yljB/nanE, encoding an N-acetylmannosamine-6-phosphate 2-epimerase involved in amino sugar metabolism, displayed the highest correlation in all four strains with robustness towards heat stress. Furthermore, genes encoding transport proteins or hypothetical proteins were among the genes with the most significant correlation of expression and heat or oxidative stress survival in all strains. Revealing the exact mechanism via which the functions encoded by these genes impact on robustness requires additional work.
The strains included in this study varied in type of subspecies, isolation source and general robustness [5] and therefore appear to represent a major part of the L. lactis species. Therefore, it is tempting to suggest that the generic gene expressions associated with robustness in this study can be applied as indicators of robustness for L. lactis strains in general, although individual transcriptome signatures are expected to predict robustness of specific strains more accurately.
Conclusions
In this study we demonstrated that fermentation conditions (e.g. temperature and level of oxygen) have a large impact on heat and oxidative stress survival of L. lactis strains. Therefore, fermentation conditions prior to industrial processing of starter cultures should be carefully selected, and this is true for both intrinsically robust and sensitive strains [5]. The development of a general fermentation strategy for improved robustness of L. lactis starter cultures appears complicated as the effect of fermentation conditions on robustness towards heat and oxidative stress is strain-dependent, even though fermentation conditions have largely similar effects on growth characteristics. The larger part of the transcriptome signatures associated with robustness also appeared strain-specific, indicating that different mechanisms exist to improve robustness. Hence, to obtain optimal robustness in each individual strain tailor-made optimization of fermentation parameters is required. Furthermore, we explored the most significant associations of transcript levels and robustness that overlapped in all four strains, resulting in a generic transcriptome signature associated with robustness in these L. lactis strains, which included both known genes encoding stress related functions and novel genes. This generic transcriptome signature could function as an indicator for robustness and aid the selection of optimal fermentation conditions for optimal robustness during spray drying.
Supporting Information
Numbers indicate fermentations as presented in Table 1. Samples connected with arrows were hybridized together, the arrow head represents Cy5-labeling, the back end Cy3-labeling.
(TIFF)
Growth curves of strains IL1403, KF147 and SK11 in fermentations as presented in Table 1. The data points between the dotted lines indicate the moment of harvesting cells for RNA isolation and stress survival assays.
(TIF)
Numbers indicate the amount of genes that are differently expressed (P < 0.05) by both the individual fermentation parameter (salt, oxygen, pH and temperature) specified in the top row and in the left column. Bars indicate percentages of overlap of differently expressed genes by both fermentation parameters (full bar = 100%).
(TIF)
Survival after 30 minutes of heat stress and after 60 minutes of oxidative stress in the various fermentations of strains IL1403, KF147 and SK11. Survival data represent averages of technical duplicates.
(DOCX)
T-test-based correlation of individual fermentation parameters and robustness. Significant differences (P < 0.05) are underlined.
(DOCX)
Expression levels of genes L0001 –L75633 plotted against survival after 60 minutes heat and 30 min oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Expression levels of genes L75676 –L1889726 plotted against survival after 60 minutes heat and 30 min oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Expression levels of genes LLKF_0001 –LLKF_1273 plotted against survival after 10 minutes heat and 30 minutes oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Expression levels of genes LLKF_1274 –LLKF_2533 and LLKF_p0001 –LLKF_p0036 plotted against survival after 10 minutes heat and 30 minutes oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Expression levels of genes LACR_0001 –LACR_1382 plotted against survival after 10 minutes heat and 30 minutes oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Expression levels of genes LACR_1383 –LACR_2610 and LACR_A01 –LACR_E8 plotted against survival after 10 minutes heat and 30 minutes oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Acknowledgments
This project was carried out within the research programme of the Kluyver Centre for Genomics of Industrial Fermentation, which is part of the Netherlands Genomics Initiative / Netherlands Organization for Scientific Research.
Data Availability
All relevant data are available in the paper and its Supporting Information files. Microarray data are available from GEO (GSE72045).
Funding Statement
AD was funded by the Kluyver centre for Genomics of Industrial Fermentation which is part of the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research (NWO). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bron PA, Kleerebezem M. Engineering lactic acid bacteria for increased industrial functionality. Bioeng Bugs. 2011;2(2):80–7. Epub 2011/06/04. 10.4161/bbug.2.2.13910 [DOI] [PubMed] [Google Scholar]
- 2.Leroy F, and De Vuyst L.. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci Technol. 2004;15:67–78. [Google Scholar]
- 3.Santivarangkna C, Kulozik U, Foerst P. Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J Appl Microbiol. 2008;105(1):1–13. Epub 2008/02/13. 10.1111/j.1365-2672.2008.03744.x [DOI] [PubMed] [Google Scholar]
- 4.Ghandi A, Powell IB, Howes T, Chen XD, Adhikari B. Effect of shear rate and oxygen stresses on the survival of Lactococcus lactis during the atomization and drying stages of spray drying: A laboratory and pilot scale study. J Food Eng. 2012;113(2):194–200. [Google Scholar]
- 5.Dijkstra AR, Setyawati MC, Bayjanov JR, Alkema W, van Hijum SAFT, Bron PA, et al. Diversity in Robustness of Lactococcus lactis Strains during Heat Stress, Oxidative Stress, and Spray Drying Stress. Appl Environ Microbiol. 2014;80(2):603–11. 10.1128/AEM.03434-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peighambardoust SH, Golshan Tafti A, Hesari J. Application of spray drying for preservation of lactic acid starter cultures: a review. Trends Food Sci Technol. 2011;22(5):215–24. [Google Scholar]
- 7.Rallu F, Gruss A, Maguin E. Lactococcus lactis and stress. Antonie Van Leeuwenhoek. 1996;70(2–4):243–51. Epub 1996/10/01. [DOI] [PubMed] [Google Scholar]
- 8.van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek. 2002;82(1–4):187–216. Epub 2002/10/09. [PubMed] [Google Scholar]
- 9.Kim WS, Ren J, Dunn NW. Differentiation of Lactococcus lactis subspecies lactis and subspecies cremoris strains by their adaptive response to stresses. FEMS Microbiol Lett. 1999;171(1):57–65. Epub 1999/02/13. [DOI] [PubMed] [Google Scholar]
- 10.Gasson MJ. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154(1):1–9. Epub 1983/04/01. PubMed Central PMCID: PMC217423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijkstra AR, Alkema W, Starrenburg M, Hugenholtz J, van Hijum S, Bron PA. Fermentation-induced variation in heat and oxidative stress phenotypes of Lactococcus lactis MG1363 reveals transcriptome signatures for robustness. Microb Cell Fact. 2014;13(1):148. Epub 2014/11/05. PubMed Central PMCID: PMC4229599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez M, Kleerebezem M, Kuipers OP, Siezen RJ, van Kranenburg R. Regulation of the metC-cysK operon, involved in sulfur metabolism in Lactococcus lactis. J Bacteriol. 2002;184(1):82–90. Epub 2001/12/14. PubMed Central PMCID: PMC134770. 10.1128/JB.184.1.82-90.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Y, Chou LS, Cutler A, Weimer B. DNA Macroarray profiling of Lactococcus lactis subsp. lactis IL1403 gene expression during environmental stresses. Appl Environ Microbiol. 2004;70(11):6738–47. Epub 2004/11/06. PubMed Central PMCID: PMC525116. 10.1128/AEM.70.11.6738-6747.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chopin A, Chopin MC, Moillo-Batt A, Langella P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984;11(3):260–3. Epub 1984/05/01. [DOI] [PubMed] [Google Scholar]
- 15.Kelly WJ, Davey GP, Ward LJ. Characterization of lactococci isolated from minimally processed fresh fruit and vegetables. Int J Food Microbiol. 1998;45(2):85–92. Epub 1999/01/30. [DOI] [PubMed] [Google Scholar]
- 16.Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, et al. Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci USA. 2006;103(42):15611–6. Epub 2006/10/13. PubMed Central PMCID: PMC1622870. 10.1073/pnas.0607117103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieuwerts S, de Bok FA, Mols E, de Vos WM, van Hylckama Vlieg JE. A simple and fast method for determining colony forming units. Lett Appl Microbiol. 2008;47(4):275–8. Epub 2008/09/10. 10.1111/j.1472-765X.2008.02417.x [DOI] [PubMed] [Google Scholar]
- 18.van Hijum SA, Garcia de la Nava J, Trelles O, Kok J, Kuipers OP. MicroPreP: a cDNA microarray data pre-processing framework. Appl Bioinformatics. 2003;2(4):241–4. Epub 2004/05/08. [PubMed] [Google Scholar]
- 19.Siezen RJ, Bayjanov JR, Felis GE, van der Sijde MR, Starrenburg M, Molenaar D, et al. Genome-scale diversity and niche adaptation analysis of Lactococcus lactis by comparative genome hybridization using multi-strain arrays. Microb Biotechnol. 2011;4(3):383–402. Epub 2011/02/23. 10.1111/j.1751-7915.2011.00247.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teuber M, Geis A. The Genus Lactococcus In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The Prokaryotes: Springer US; 2006. p. 205–28. [Google Scholar]
- 21.Bassit N, Boquien CY, Picque D, Corrieu G. Effect of Initial Oxygen Concentration on Diacetyl and Acetoin Production by Lactococcus lactis subsp. lactis biovar diacetylactis. Appl Environ Microbiol. 1993;59(6):1893–7. Epub 1993/06/01. PubMed Central PMCID: PMC182177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zakrzewska A, van Eikenhorst G, Burggraaff JE, Vis DJ, Hoefsloot H, Delneri D, et al. Genome-wide analysis of yeast stress survival and tolerance acquisition to analyze the central trade-off between growth rate and cellular robustness. Mol Biol Cell. 2011;22(22):4435–46. Epub 2011/10/04. PubMed Central PMCID: PMC3216668. 10.1091/mbc.E10-08-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dressaire C, Redon E, Milhem H, Besse P, Loubiere P, Cocaign-Bousquet M. Growth rate regulated genes and their wide involvement in the Lactococcus lactis stress responses. BMC Genomics. 2008;9:343 Epub 2008/07/23. PubMed Central PMCID: PMC2526093. 10.1186/1471-2164-9-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch B, Kilstrup M, Vogensen FK, Hammer K. Induced levels of heat shock proteins in a dnaK mutant of Lactococcus lactis. J Bacteriol. 1998;180(15):3873–81. Epub 1998/07/31. PubMed Central PMCID: PMC107371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Connor K, Fletcher SA, Csonka LN. Increased expression of Mg(2+) transport proteins enhances the survival of Salmonella enterica at high temperature. Proc Natl Acad Sci U S A. 2009;106(41):17522–7. Epub 2009/10/07. PubMed Central PMCID: PMC2765158. 10.1073/pnas.0906160106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyaval P. Lactic acid bacteria and metal ions. Lait. 1989;69(2):87–113. [Google Scholar]
- 27.Varmanen P, Ingmer H, Vogensen FK. ctsR of Lactococcus lactis encodes a negative regulator of clp gene expression. Microbiology. 2000;146 (Pt 6):1447–55. Epub 2000/06/10. [DOI] [PubMed] [Google Scholar]
- 28.Frees D, Vogensen FK, Ingmer H. Identification of proteins induced at low pH in Lactococcus lactis. Int J Food Microbiol. 2003;87(3):293–300. Epub 2003/10/07. [DOI] [PubMed] [Google Scholar]
- 29.Mostertz J, Scharf C, Hecker M, Homuth G. Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology. 2004;150(Pt 2):497–512. Epub 2004/02/10. 10.1099/mic.0.26665-0 [DOI] [PubMed] [Google Scholar]
- 30.Gaudu P, Lamberet G, Poncet S, Gruss A. CcpA regulation of aerobic and respiration growth in Lactococcus lactis. Mol Microbiol. 2003;50(1):183–92. Epub 2003/09/26. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen MB, Gaudu P, Lechardeur D, Petit MA, Gruss A. Aerobic respiration metabolism in lactic acid bacteria and uses in biotechnology. Annu Rev Food Sci Technol. 2012;3:37–58. Epub 2012/03/06. 10.1146/annurev-food-022811-101255 [DOI] [PubMed] [Google Scholar]
- 32.Cretenet M, Le Gall G, Wegmann U, Even S, Shearman C, Stentz R, et al. Early adaptation to oxygen is key to the industrially important traits of Lactococcus lactis ssp. cremoris during milk fermentation. BMC Genomics. 2014;15(1):1054. Epub 2014/12/04. PubMed Central PMCID: PMC4289295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rezaiki L, Cesselin B, Yamamoto Y, Vido K, van West E, Gaudu P, et al. Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Mol Microbiol. 2004;53(5):1331–42. Epub 2004/09/25. 10.1111/j.1365-2958.2004.04217.x [DOI] [PubMed] [Google Scholar]
- 34.Nielsen AK, Breuner A, Krzystanek M, Andersen JT, Poulsen TA, Olsen PB, et al. Global transcriptional analysis of Bacillus licheniformis reveals an overlap between heat shock and iron limitation stimulon. J Mol Microbiol Biotechnol. 2010;18(3):162–73. Epub 2010/06/10. 10.1159/000315457 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Numbers indicate fermentations as presented in Table 1. Samples connected with arrows were hybridized together, the arrow head represents Cy5-labeling, the back end Cy3-labeling.
(TIFF)
Growth curves of strains IL1403, KF147 and SK11 in fermentations as presented in Table 1. The data points between the dotted lines indicate the moment of harvesting cells for RNA isolation and stress survival assays.
(TIF)
Numbers indicate the amount of genes that are differently expressed (P < 0.05) by both the individual fermentation parameter (salt, oxygen, pH and temperature) specified in the top row and in the left column. Bars indicate percentages of overlap of differently expressed genes by both fermentation parameters (full bar = 100%).
(TIF)
Survival after 30 minutes of heat stress and after 60 minutes of oxidative stress in the various fermentations of strains IL1403, KF147 and SK11. Survival data represent averages of technical duplicates.
(DOCX)
T-test-based correlation of individual fermentation parameters and robustness. Significant differences (P < 0.05) are underlined.
(DOCX)
Expression levels of genes L0001 –L75633 plotted against survival after 60 minutes heat and 30 min oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Expression levels of genes L75676 –L1889726 plotted against survival after 60 minutes heat and 30 min oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Expression levels of genes LLKF_0001 –LLKF_1273 plotted against survival after 10 minutes heat and 30 minutes oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Expression levels of genes LLKF_1274 –LLKF_2533 and LLKF_p0001 –LLKF_p0036 plotted against survival after 10 minutes heat and 30 minutes oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Expression levels of genes LACR_0001 –LACR_1382 plotted against survival after 10 minutes heat and 30 minutes oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Expression levels of genes LACR_1383 –LACR_2610 and LACR_A01 –LACR_E8 plotted against survival after 10 minutes heat and 30 minutes oxidative stress. Survival is expressed as the difference of log CFU/ml after stress and before stress. Numbers indicate fermentations as presented in Table 1. P-values above the plots indicate significance of correlation (assessed by a linear model).
(ZIP)
Data Availability Statement
All relevant data are available in the paper and its Supporting Information files. Microarray data are available from GEO (GSE72045).