Abstract
Purpose
To evaluate the safety, tolerability, and preliminary efficacy of suprachoroidal injection of triamcinolone acetonide (TA) in patients with noninfectious uveitis.
Methods
In this Phase 1/2 open-label clinical study, a single suprachoroidal injection of 4-mg TA in 100 μL was performed in the study eye of patients with noninfectious intermediate, posterior, or pan-uveitis, and follow-up obtained for 26 weeks.
Results
Nine individuals with chronic uveitis were enrolled. There were 38 reported adverse events (AEs); most were mild or moderate in severity. Approximately half the AEs were ocular. The most common AE was reported by four subjects who experienced ocular pain at or near the time of the injection. All systemic AEs were unrelated to study drug. No steroid-related increases in intraocular pressure (IOP) were observed and no subject required IOP-lowering medication. All eight efficacy-evaluable subjects had improvements in visual acuity. Four subjects, who did not need additional therapy, had on average a greater than 2-line improvement in visual acuity through week 26. Three of four had macular edema at baseline, and two of three had at least a 20% reduction in macular edema at week 26.
Conclusions
The safety and preliminary efficacy data support further investigations of suprachoroidally administered TA as a therapeutic option for the treatment of noninfectious uveitis.
Translational Relevance
Targeted suprachoroidal administration of corticosteroid is a potential local route for the treatment of ocular inflammatory disease, which merits further investigation. (www.ClinicalTrials.gov, NCT01789320)
Keywords: uveitis, triamcinolone acetonide, suprachoroidal space, drug delivery
Introduction
Uveitis is the fifth most common cause of visual loss in the developed world.1 Significant vision loss occurs in up to 35% of children and adults, and uveitis accounts for 10% to 15% of all cases of total blindness in the United States.2,3
Uveitis is classified anatomically as anterior, intermediate, posterior, or pan-uveitis, according to the primary site of inflammation.4 Each of these categories, however, encompasses a number of conditions that can be characterized further along other dimensions including: onset, duration, course, and etiology.
One of the most common causes of vision loss in uveitis is macular edema (ME), which causes visual impairment or blindness in up to one-third of all uveitis patients.1,5 Approximately 60% of patients with intermediate and pan-uveitis suffer from ME, while posterior and anterior uveitis patients have lower incidences of ME (40% and 20%, respectively) as well as associated visual impairment.6
Uveitis is commonly treated with corticosteroids and other immunomodulatory agents; such treatments are either systemic or local. Local treatments are either topical drops, or intraocular or periocular injections or implants. Commonly used corticosteroids include prednisone, triamcinolone, dexamethasone, and fluocinolone.
Triamcinolone acetonide (TA) is a synthetic corticosteroid that has been used for the treatment of various inflammatory conditions for over 50 years.7 TA also has a long history as a treatment for a variety of ocular inflammatory diseases.8 In addition to quieting other complications of inflammation in the eye, TA reduces ME and improves visual outcomes.9 TA is, therefore, a good candidate to evaluate in a new route of ocular administration.
All currently used routes of drug delivery indirectly access the retina and choroid; by diffusion from the outer layers of the eye following topical administration of eye drops or following periocular injection, via diffusion from the vitreous following intravitreal injection or implant, or by distribution to these tissues through the vascular system following systemic treatment.
Topical corticosteroids have historically been ineffective for the treatment of ME, with the exception of difluprednate drops, which have been demonstrated to be able to reduce ME in children and adults with uveitis.10,22 Difluprednate, however, may be more likely than other topical steroids to have the adverse effect of very high intraocular pressure (IOP), particularly in children.10,11
Local therapy with periocular or intravitreal corticosteroid administrations can be used to manage ME associated with uveitis. Intravitreal and periocular injections of TA or the implantation of intraocular steroid-releasing sustained delivery devices in the vitreous have the potential to administer therapeutically effective levels of drug to the posterior segment.1 All local steroid therapy, including periocular and intravitreal corticosteroid injections and corticosteroid releasing implants are associated with the development of cataracts, increased IOP, and exacerbation of pre-existing glaucoma, due primarily to exposure of the anterior segment and lens to these agents.1,12–15
Pharmacokinetic studies following administration of TA into the suprachoroidal space in rabbits, demonstrate 12-fold higher amounts of drug in the retina and sclera-choroid and relatively minimal amounts (ranging from trace to 3%) of drug in the lens and anterior segment compared with exposure of TA following intravitreal injection.16 This rather unique distribution led to the speculation that it might be possible to obtain therapeutic levels of corticosteroid in the retina and choroid, with lower drug levels in the anterior segment, and therefore fewer ocular side effects following suprachoroidal corticosteroid dosing compared with intravitreal corticosteroid administration. In contrast to exposures seen in the ocular tissues, systemic exposure following suprachoroidal administration of TA remains at very low levels. Maximum concentration (Cmax), 12 ng/mL, was achieved on day 1 following injection and TA was detectable out to 60-days postinjection, although at very low levels; systemic levels of TA were not detectable subsequently.
In a pig model of acute uveitis, a 2-mg dose of the drug was as effective when given suprachoroidally as compared with when dosed intravitreally. Further, a suprachoroidal injection of a 0.2-mg dose of TA was significantly (P < 0.03) more effective than a 0.2-mg dose given by intravitreal injection, demonstrating that a 10-fold lower dose administered suprachoroidally was effective in treating inflammation in this model and better than the 0.2-mg dose following intravitreal dosing.17
Tolerability and safety evaluations from single and repeat dosing in animals showed that TA was well tolerated and displayed no specific safety concerns following suprachoroidal injections. This paper reports the 26-week posttreatment observations in a Phase 1/2 open-label study in patients with noninfectious uveitis following a single unilateral suprachoroidal injection of TA.
Methods
This first human clinical study with suprachoroidal injection of TA to patients with noninfectious uveitis was conducted at three sites in the United States (Cole Eye Institute, Cleveland, OH; Northwestern University, Chicago, IL; Truhlsen Eye Institute at the University of Nebraska Medical Center, Omaha, NE) and in accordance with the International Conference on Harmonization E6 Guideline for Good Clinical Practice and the Declaration of Helsinki. The study is registered at www.ClinicalTrials.gov (Identifier NCT01789320) and was approved by each study site's institutional review board. Written informed consent was obtained from all patients prior to any study-specific procedures. The study was conducted from July 2013 through January 2015.
Subject Eligibility
Males and nonpregnant females 18 years or older were eligible. Only one eye of each subject could be enrolled. Each study eye was required to have a diagnosis of noninfectious intermediate, posterior, or pan-uveitis. Further, each study eye was required to have either ME with a central subfield thickness (CST) of greater than or equal to 310 μm as measured by spectral-domain optical coherence tomography (SD-OCT) or a vitreous haze score of greater than or equal to 1.5 based on the Standardization of Uveitis Nomenclature Working Group criteria,3 along with a best corrected visual acuity (BCVA) of +1.0 logMAR (20/200 Snellen equivalent) or better by Early Treatment of Diabetic Retinopathy Study (ETDRS).18 If both eyes were eligible, the eye with worse inflammation or ME was designated as the study eye.
Exclusion criteria included uncontrolled systemic disease that could preclude study participation; immunodeficiency disease for which corticosteroid therapy would be contraindicated; known hypersensitivity to the TA formulation, to fluorescein, or to topical anesthetics; and systemic infection for which an antibiotic was indicated. Subjects could not have used acetazolamide or an investigational drug or device within 1 month of study treatment.
Patients were ineligible if they were monocular or if they had an ocular condition (e.g., active ocular infection, history of suprachoroidal hemorrhage) that might put them at risk. Subjects also could not have active ocular disease or infection, uveitis previously unresponsive to corticosteroid treatment, or newly prescribed use of IOP-lowering medications or difluprednate within 1 month prior to study treatment in either eye.
Exclusion criteria specific to the study eye included IOP greater than 22 mm Hg; history of filtration surgery; glaucoma implant surgery or evidence of glaucomatous optic nerve damage; a history of clinically significant IOP elevation in response to corticosteroid treatment; history of intraocular surgery; presence of anterior (corneal) staphyloma; eye diseases other than uveitis that could compromise central visual acuity; high myopia, defined as a spherical equivalent refraction of −6.00 diopters or greater; conditions that could predispose to scleral thinning; significant media opacity precluding evaluation of the vitreous cavity or visualization of the posterior segment; ocular trauma within 6 months; photocoagulation within 6 months; intravitreal injection of antivascular endothelial growth factor within 2 months; injection of periocular corticosteroids within 3 months; or injection of intraocular corticosteroid or an Ozurdex® (Allergan, Irvine, CA) implant within 6 months; or a Retisert® (Bausch&Lomb, Rochester, NY) implant within 3 years.
Study Design
After determining eligibility and providing consent, patients were administered a single suprachoroidal injection of TA in the study eye on day 1 and observed for 26 weeks. Observations included ocular tolerability, ocular and systemic safety, and changes from baseline at 8 and 26 weeks for IOP, BCVA, clinical examination, and CST.
Eligible subjects received a single injection of 4-mg TA (Triesence®, Alcon, Fort Worth, TX) in 100 μL administered approximately 4-mm posterior to the limbus, into the suprachoroidal space of the study eye via a proprietary microinjector (Clearside Biomedical, Inc., Alpharetta, GA): a 1-mL syringe attached via a Luer lock to a commercially purchased cannula that was cut down to an approximately 1-mm length, 30-G needle (Fig. 1). Scleral thickness near the site of injection was measured by ultrasound biomicroscopy. Prior to injection, the study eye was dilated, topical anesthetic was applied, the eye was sterilized, and a speculum was placed following standard practice at the site, for intraocular injections.
Figure 1.
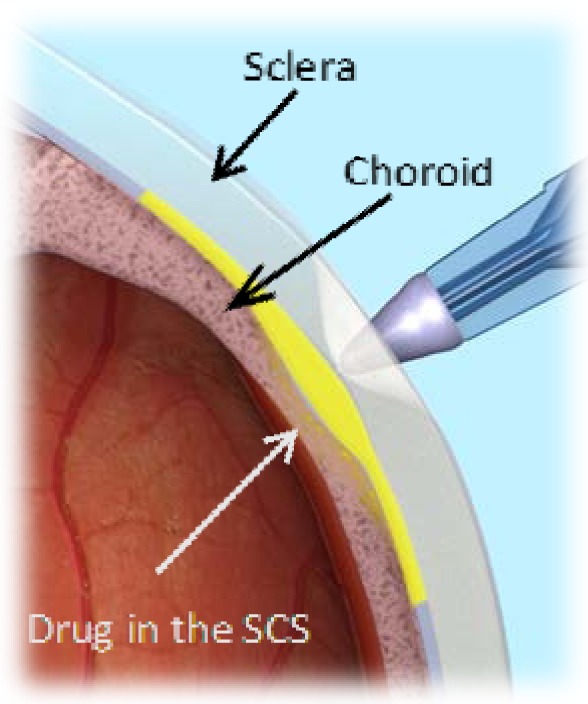
Injection into the suprachoroidal space.
After injection, subjects remained in the clinic for approximately 3 hours for observation: the study eye was assessed immediately after injection and 1 and 3 hours post dose via indirect ophthalmoscopy. Follow-up examinations were conducted 24 hours after the suprachoroidal injection with TA and at weeks 1, 2, 4, 8, 12, 16, 20, and 26. Subjects were allowed to receive additional therapy at the investigator's discretion for lack of efficacy at any point during the study.
Study Assessments
Safety assessments encompassed evaluation of systemic and ocular adverse events (AEs), including frequency, severity, and relationship to study drug. AEs were summarized using the Medical Dictionary for Regulatory Activities (MedDRA) v.17.1. Changes from baseline in vital signs also were monitored.
Additional safety evaluations in both eyes included changes from baseline in IOP, slit-lamp, and fundus examinations. IOP was measured using Goldmann applanation.
Exploratory efficacy assessments evaluated changes from baseline in BCVA and in CST. BCVA was measured using ETDRS charts18 and recorded as base logMAR. CST was evaluated using SD-OCT; images were evaluated for retinal thickness measurements at each clinical site.
With few exceptions, ocular assessments were performed on both eyes at every visit. Data were summarized descriptively for each study visit, and missing values were not imputed. Vitreous haze was graded using a standardized photographic scale ranging from 0 to 4.20
Results
Subjects
Nine individuals were enrolled in the study, five subjects with pan-uveitis, three subjects with anterior and intermediate uveitis, and one subject with intermediate uveitis.
Due to a new procedure being evaluated in this study and to the potential for any amount of study drug, no matter how small, to have been delivered in any subject where a needle was inserted into the eye, safety data was obtained after all attempted injection procedures, for a denominator of 11 attempted injections in 9 individuals. One subject was reported to have not received study drug after two attempts during two separate enrollments. Another subject received the drug when the injection procedure was carried out during the second enrollment while the injection procedure during the first enrollment was reported by the injecting physician as not having provided any drug to the subject. Seven other subjects received their drug treatment when they were enrolled. Thus, among the 11 total attempted injection procedures, eight procedures in eight subjects were reported as receiving an injection of study drug while three procedures in two subjects were reported as resulting in the subject receiving no study drug as described.
One of the subjects who was enrolled twice (9%) discontinued participation under the first study number after 4 weeks, then re-enrolled with a new subject number; this same individual was lost to follow-up 16 weeks following re-enrollment. All other subjects completed the 26-week follow-up.
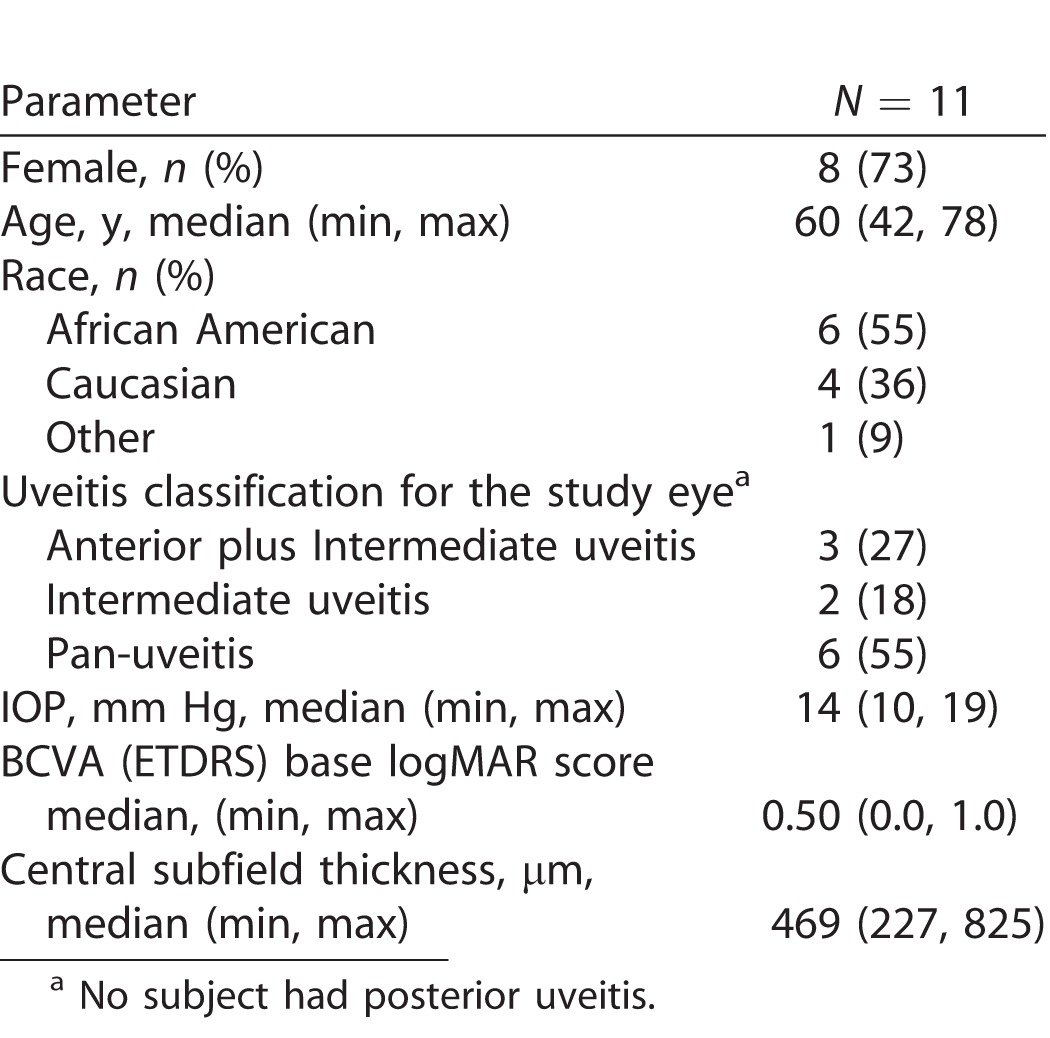
Baseline demographics and disease characteristics are provided in Table 1. The median age was 60 years (range, 42–78), and eight subjects (73%) were female.
Table 1.
Baseline Demographics and Disease Characteristics
Safety
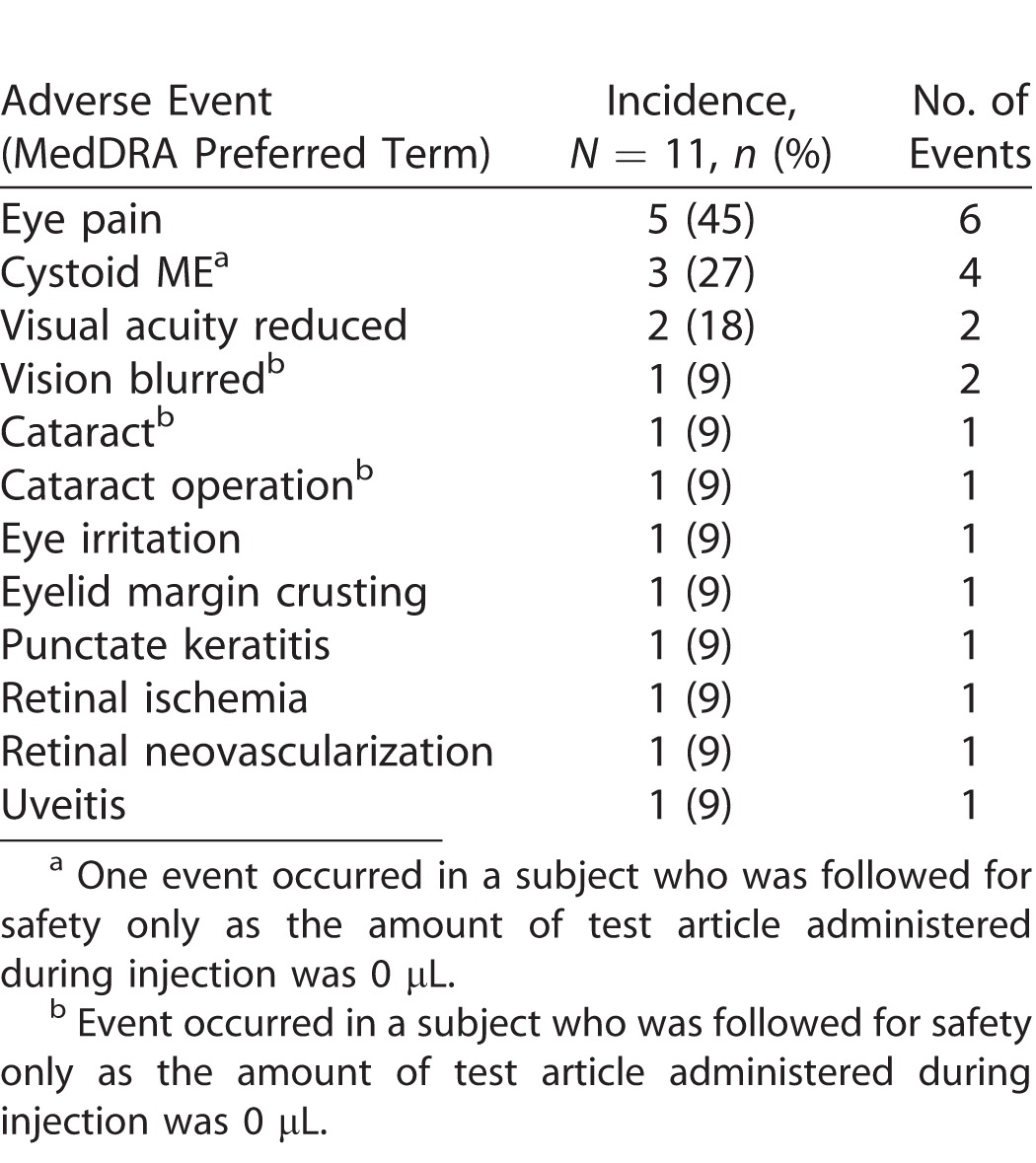
All subjects had at least one AE, and a total of 38 events were reported. Most AEs (89%) were mild or moderate in severity. There were two reports of severe eye pain at the time of injection (related to procedure rather than drug) and one report each of retinal neovascularization and pulmonary embolism (serious AE). None of the events were considered to be related to the study drug. The retinal neovascularisation was felt to be secondary to the underlying disease process rather than treatment. The pulmonary embolism was considered unrelated to the therapy (see below).
Fifty eight percent (22 of 38) of the reported AEs were ocular events (Table 2). Nine ocular AEs in four subjects were considered possibly related to TA by the investigator: seven of the events were complications due to progression of uveitis, including ME (3 events), blurry vision (2 events), and vision decrease (2 events); and one event of progression of cataract leading extraction (1 event). Eye pain, six events in five subjects, was the most commonly reported AE, and was generally reported at or near the time of injection.
Table 2.
Ocular Adverse Events
Systemic AEs were reported in eight subjects, and all were considered to be unrelated to study drug. All systemic AEs were isolated events in a single subject, with the exception of mild headache (3 events in 2 subjects) and localized nonocular infection (2 events in 2 subjects). Mild events reported in one subject each were alopecia, ligament sprain, pyrexia, and sinusitis. Moderate events reported in one subject each were psychological trauma (“patient felt traumatized post injection”), chronic obstructive pulmonary disease, ligament injury, neck pain, tendon injury, and urinary tract infection. One severe event, pulmonary embolism, was reported in a subject with a prior history of pulmonary emboli. This serious AE occurred approximately 10 weeks after treatment and was reported by the Investigator as unrelated.
No trends over time were observed in mean resting systolic blood pressure (BP), diastolic BP, or pulse rate, and no clinically significant changes were reported for any subject.
Baseline IOP ranged from 10 through 19 mm Hg. Mean changes from baseline ranged from −0.1 to 1.3 mm Hg at various time-points. The mean change from baseline was −0.1 mm Hg (range, −5 to 5) at week 8 and 0.9 mm Hg (range, −4 to 6) at week 26. No increases in IOP (defined as 10 mm Hg change from baseline or an absolute increase to >30 mm Hg)3 were observed for any subject during the study (Fig. 2), and no subject required IOP-lowering medication.
Figure 2.
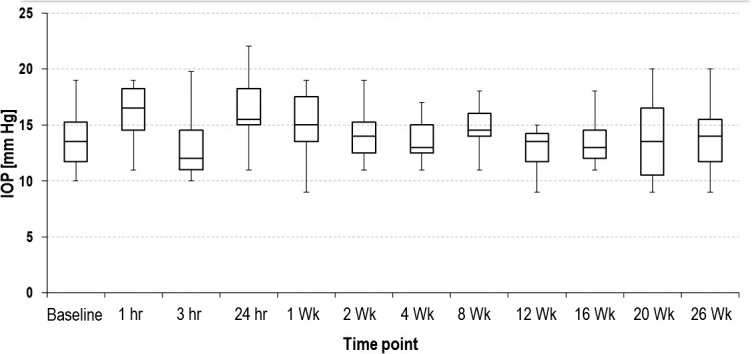
IOP at each time-point: safety population.
Dilated indirect ophthalmoscopy and slit-lamp biomicroscopy parameters were evaluated for changes at each study visit. There were no reported significant changes in the optic nerve, macula, vessels, or periphery of the fundus at any time-points during the course of the study.
Efficacy
Efficacy was evaluated for the per-protocol analysis set, defined as those subjects who received any non-zero amount of study drug (N = 8). Data for subjects who received additional, nonstudy therapy are included in the analyses up to and including the visit when additional therapy was administered. Four of eight efficacy-evaluable subjects in this study were given additional treatment for uveitis: one of the four subjects received additional treatment at week 8. Of the remaining three subjects who received additional treatment, at the investigator's discretion, two were at week 16, and the third was at week 20. Four subjects needed no additional treatment during the entire 26 weeks of the study.
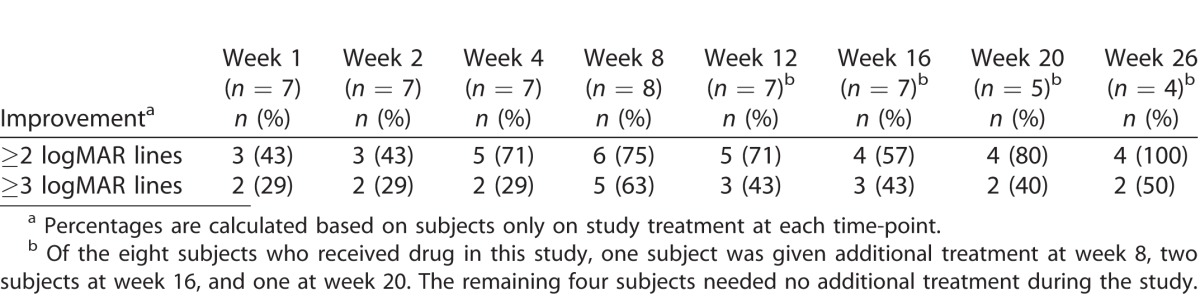
After a single suprachoroidal injection of TA, all eight efficacy-evaluable subjects showed improvements in BCVA. Mean improvements ranged between 0.17 and 0.28 logMar (approximately 8 and 14 letters) sustained through the 26-week study period (Fig. 3). Improvements in BCVA (a gain of ≥ 2 lines)20 were shown by six of eight subjects (75%) at week 8, and by all four subjects (100%) who needed no additional uveitis treatment through the 26-week posttreatment observation period of the study (Table 3).
Figure 3.
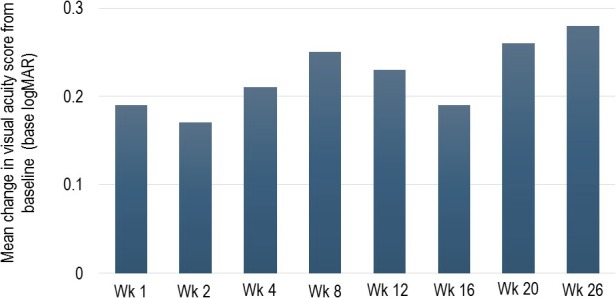
Mean changes in visual acuity. No imputations were performed on the data.
Table 3.
Subjects with Improvements in BCVA
Seven of eight subjects in the per protocol population qualified for the study based on meeting the requirement for ME associated with their uveitis, that is, a measurement of CST greater than or equal to 310 μm by SD-OCT. One subject qualified for the study based upon haze alone with no ME at enrollment. At baseline, retinal thickness ranged from 364 to 825 μm (mean 547 μm) in the study eye for the seven subjects with ME. Mean reductions in retinal thickness ranged from 76 to 154 μm during the 26-week study (Fig. 4).
Figure 4.
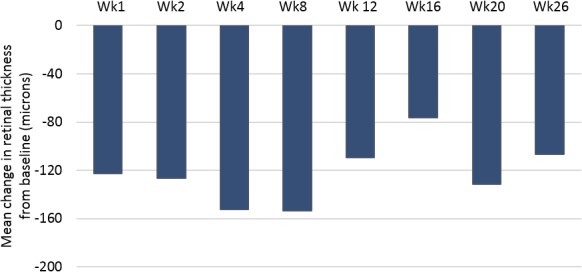
Mean changes in ME. No imputations were performed on the data.
At week 8, the mean decrease in CST was 154 μm (range, 61–375 μm) with all seven subjects showing reductions in retinal thickness. Of these seven, four subjects (57%) had at least a 20% reduction from baseline in CST at week 8. At week 26, three of seven subjects with ME (43%) did not receive additional therapy. The mean decrease in CST at week 26 was 107 μm; all three subjects had reductions (43, 72, and 206 μm) in retinal thickness, and two of the three subjects had at least a 20% reduction in CST from baseline.
All eyes in the study had at least trace (+0.5) haze at baseline. The mean vitreous haze score at baseline was 1.25 (range 0.5–2.0). At week 8, 63% of subjects (5/8) had a vitreous haze score of 0. At Week 26, 50% of those subjects who had not received additional therapy (2/4) had a vitreous haze score of 0. The mean haze score was 0. 5 at week 8 (range 0–2) and 0.63 at week 26 (range 0–1.5).
Discussion
The data from our pilot study support the concept that targeted suprachoroidal administration of corticosteroid merits further exploration as a local route for the treatment of noninfectious uveitis and uveitic ME.
Overall, a single suprachoroidal injection of TA in this small study was well tolerated. Most AEs were ocular and no systemic AEs were considered by investigators to be related to the study drug. All AEs, including eye pain, the most commonly reported AE in this study, will be evaluated in larger numbers of subjects in future studies of suprachoroidal administration of TA. The injection experiences gathered on this study of both full and partial injections will be used to optimize the injector and the injection procedure in future studies.
No increases in IOP were observed, and no subject required IOP-lowering medication over the 26-week study period, after a single 4-mg suprachoroidal injection of TA. Although this was not a head-to-head comparison, in contrast to published literature, 25% of subjects in posterior segment uveitis and retinal vein occlusion studies of dexamethasone implant (Ozurdex) experienced an adverse event of increased IOP, which peaked at approximately 8-weeks post implant and were seen as early as week 3.20 Similarly, 43% of subjects with ME associated with uveitis experienced a rise in IOP of greater than 10 mm Hg in a study of intravitreally administered TA; the mean rise of IOP in this cited study was 10 mm Hg.12 It was reasonable to expect that suprachoroidally injected TA could provide a favorable modulation of adverse effects such as IOP increases due to the potential for a differential distribution of drug, similar to the data observed in preclinical studies of rabbits where the anterior segment tissues were largely spared relative to the distribution following injection of TA into the vitreous.16 Because there were only eight-dosed subjects in this study, data from larger studies, currently underway, are required to substantiate the negligible effect on IOP of suprachoroidal injection of steroid.
Four of eight efficacy-evaluable subjects needed no additional treatment during the 26-week study. All eight subjects showed improvements in BCVA, and the four subjects who needed no additional uveitis treatment during the study had meaningful improvements of greater than or equal to 2 lines in BCVA at week 26. By week 8, 63% of efficacy-evaluable subjects in this study achieved a 3-line improvement from baseline BCVA. This is comparable to improvements reported for 43% of subjects with posterior segment uveitis treated with Ozurdex19,20 and 51% of subjects with ME associated with uveitis treated with intravitreal TA.12 In the current study, this trend for improvement in BCVA was maintained in two of four subjects who remained on study drug alone, during the entire 26-week study.
Among the seven subjects who entered the study with ME, all had at least a 60-μm reduction in CST at week 8 (mean reduction of 154 μm); the three subjects with ME and with evaluable data at week 26, each showed reductions in retinal thickness. In a prospective study of 128 eyes (101 individuals) with ME associated with uveitis, Sugar et al 21 showed that a 20% change in retinal thickness, as measured at the central subfield with time-domain OCT, was above the level of random variation and was associated with improved visual acuity of greater than or equal to 10 letters. In this study, four of seven subjects had at least a 20% reduction in CST at week 8, as did two of three subjects remaining on study drug alone at week 26.
There are several limitations in this pilot study of suprachoroidal TA for uveitis. It was an open-label, single administration study and there were only eight subjects who were treated with a suprachoroidal injection of TA. The suprachoroidal injection procedure is novel, requires physicians to learn the technique and relies on the investigators' experience to ensure accuracy of delivery. Also, all measurements, including visual acuity and ME OCT readings, were performed by the individual sites using standard methods used at the specific site, with no involvement from a centralized, masked reading center. Nevertheless, these clinical data, both safety and efficacy, support the continued development of TA administered suprachoroidally for the treatment of noninfectious uveitis and its associated ME. Ongoing and future controlled studies will include masked centralized evaluations, studies with larger numbers of subjects and repeated injections. A phase 2 study in patients with ME associated with noninfectious uveitis is complete and a phase 3 study in patients with ME associated with noninfectious uveitis has been initiated.
The results from this open-label study are encouraging, providing initial feedback from human subjects following suprachoroidal dosing, and supporting the continued development of suprachoroidal injection of TA as a local pharmacological approach to treat ocular inflammatory diseases.
Acknowledgments
This research was supported by grants from Clearside Biomedical, Inc.
Disclosure: D.A. Goldstein, C; D. Do, C; G. Noronha, E; J.M. Kissner, E; S.K. Srivastava, C; Q.D. Nyugen, C
Dr Goldstein has served as a scientific advisor for Clearside, Santen, XOMA and Bausch and Lomb. She was on the data safety monitoring board for Abbvie. Dr Do has served as scientific advisor for Clearside. Drs Noronha and Kissner are employees of Clearside Biomedical, Inc. Dr Srivastava has served as a scientific advisor for Clearside, Santen, Bausch and Lomb, Sanofi, Regeneron, Allergan, Optos and Carl Zeiss Meditec. Dr. Nguyen has served as scientific advisor for Clearside, is on the Scientific Advisory Board for AbbVie, Santen, XOMA, and Genentech, and chairs the Study Steering Committee for the VISUAL, SAKURA, EYEGUARD, and STOP-UVEITIS studies.
References
- 1. Karim R,, Sykakis E,, Lightman S,, Fraser-Bell S. Interventions for the treatment of uveitic macular edema: a systematic review and meta-analysis. Clin Ophthalmol. 2013. ; 7: 1109–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rothova A,, Suttorp-van Schulten MSA,, Treffers WF, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996. ; 80: 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nussenblatt RB. The history of uveitis. Int Ophthalmol. 1990; 14: 303–308. [DOI] [PubMed] [Google Scholar]
- 4. Jabs DA. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005. ; 140: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dick AD. The treatment of chronic uveitic macular oedema. Br J Ophthalmol. 1994. ; 78: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lardenoye CWTA,, van Kooij B,, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006. ; 113: 1446–1449. [DOI] [PubMed] [Google Scholar]
- 7. KENALOG®-40 Injection (triamcinolone acetonide injectable suspension), Prescribing Information 2016, Bristol Myers Squibb, Princeton, NJ, USA. [Google Scholar]
- 8. Nozik RA. Periocular injection of steroids. Trans Am Acad Ophthalmol Otolaryngol. 1972; 76: 695–705. [PubMed] [Google Scholar]
- 9. Munk MR,, Bolz M,, Huf W,, et al. Morphologic and functional evaluations during development, resolution, and relapse of uveitis-associated cystoid macular edema. Retina. 2013. ; 33: 1673–1683. [DOI] [PubMed] [Google Scholar]
- 10. Slabaugh MA,, Herlihy E,, Ongchin S,, Van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am J Ophthalmol. 2012. ; 153: 932–938. [DOI] [PubMed] [Google Scholar]
- 11. Birnbaum AD,, Jiang Y,, Tessler HH,, Goldstein DA. Elevation of intraocular pressure in patients with uveitis treated with topical difluprednate. Arch Ophthalmol. 2011. ; 129: 667–668. [DOI] [PubMed] [Google Scholar]
- 12. Kok H,, Lau C,, Maycock N,, McCluskey P,, Lightman S. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology. 2005. ; 112: 1–7. [DOI] [PubMed] [Google Scholar]
- 13. Kersey JP,, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006. ; 20: 407–416. [DOI] [PubMed] [Google Scholar]
- 14. Rhee DJ,, Peck RE,, Belmont J,, et al. Intraocular pressure alterations following intravitreal triamcinolone acetonide. Br J Ophthalmol. 2006. ; 90: 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bollinger KE,, Smith SD. Prevalence and management of elevated intraocular pressure after placement of an intravitreal sustained-release steroid implant. Curr Opin Ophthalmol. 2009. ; 20: 99–103. [DOI] [PubMed] [Google Scholar]
- 16. Edelhauser HF,, Verhoeven RS,, Burke B,, Struble CB,, Patel SR. Intraocular distribution and targeting of triamcinolone acetonide suspension administered into the suprachoroidal space. Invest Ophthalmol Vis Sci. 2014. ; 55: 5259. [Google Scholar]
- 17. Gilger BC,, Abarca EM,, Salmon JH,, Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophthalmol Vis Sci. 2013. ; 54: 2483–2492. [DOI] [PubMed] [Google Scholar]
- 18. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985. ; 103: 1796–1806. [PubMed] [Google Scholar]
- 19. Lowder C,, Belfort R,, Lightman S,, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011. ; 129: 545–553. [DOI] [PubMed] [Google Scholar]
- 20. Ozurdex® (dexamethasone intravitreal implant), Prescribing Information 2014, Allergan, Irvine, CA, USA. [Google Scholar]
- 21. Sugar EA,, Jabs DA,, Altaweel MM,, et al. Identifying a clinically meaningful threshold for change in uveitic macular edema evaluated by optical coherence tomography. Am J Ophthalmol. 2011. ; 152: 1044–1052, e5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feller DL,, Srivastava SK,, Pichi F,, Bena J,, Lowder CY. Resolution of noninfectious uveitic cystoid macular edema with topical difluprednate. Retina. 2016. ; Aug 12. [Epub ahead of print]. [DOI] [PubMed]


