Abstract
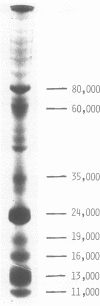
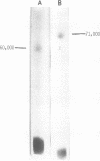
The bovine leukemia virus (BLV) was purified from a chronically infected fetal lamb kidney cell line. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of this virus revealed the presence of eight distinguishable viral components with molecular weights ranging from 80,000 to 11,000. The major component is a non-glycosylated protein having a molecular weight of 24,000 (p24). At least three heavier polypeptides were found, one of them representing a glycoprotein (gp 60). In addition, four minor polypeptides with respective molecular weights of 19,000, 16,000, 13,000, and 11,000 were identified. In a complement fixation assay using naturally occurring antibodies of a leukemic cow, four polypeptides, which included gp 60, p35, p24, and p16, were found to be reactive.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcement L. J., Karshin W. L., Naso R. B., Jamjoom G., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins: presence of p30 and envelope p15 sequences in precursor polypeptides. Virology. 1976 Feb;69(2):763–774. doi: 10.1016/0042-6822(76)90504-3. [DOI] [PubMed] [Google Scholar]
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H. Polypeptides of avian RNA tumor viruses. 1. Isolation and physical and chemical analysis. Virology. 1970 Dec;42(4):1097–1112. doi: 10.1016/0042-6822(70)90357-0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Huper G., Green R. W., Graf T. Biochemical properties of oncornavirus polypeptides. Biochim Biophys Acta. 1974 Dec 31;355(3-4):220–235. doi: 10.1016/0304-419x(74)90011-0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Langlois A. J., Schäfer W. Polypeptides of mammalian oncornaviruses. IV. Structural components of murine leukemia virus released as soluble antigens in cell culture. Virology. 1975 Dec;68(2):550–555. doi: 10.1016/0042-6822(75)90297-4. [DOI] [PubMed] [Google Scholar]
- Boulanger P. Technique of a Modified Direct Complement-Fixation Test for Viral Antibodies in Heat Inactivated Cattle Serum. Can J Comp Med Vet Sci. 1960 Sep;24(9):262–269. [PMC free article] [PubMed] [Google Scholar]
- Calafat J., Hageman P. C., Ressang A. A. Structure of C-type virus particles in lymphocyte cultures of bovine origin. J Natl Cancer Inst. 1974 Apr;52(4):1251–1257. doi: 10.1093/jnci/52.4.1251. [DOI] [PubMed] [Google Scholar]
- Ferrer J. F. Antigenic comparison of bovine type C virus with murine and feline leukemia viruses. Cancer Res. 1972 Sep;32(9):1871–1877. [PubMed] [Google Scholar]
- Ferrer J. F., Avila L., Stock N. D. Serological detection of type C viruses found in bovine cultures. Cancer Res. 1972 Sep;32(9):1864–1870. [PubMed] [Google Scholar]
- Gilden R. V., Long C. W., Hanson M., Toni R., Charman H. P., Oroszlan S., Miller J. M., Van der Maaten M. J. Characteristics of the major internal protein and RNA-dependent DNA polymerase of bovine leukaemia virus. J Gen Virol. 1975 Dec;29(3):305–314. doi: 10.1099/0022-1317-29-3-305. [DOI] [PubMed] [Google Scholar]
- Green R. W., Bolognesi D. P., Schäfer W., Pister L., Hunsmann G., De Noronha F. Polypeptides of mammalian oncornaviruses. I. Isolation and serological analysis polypeptides from murine and feline C-type viruses. Virology. 1973 Dec;56(2):565–579. doi: 10.1016/0042-6822(73)90058-5. [DOI] [PubMed] [Google Scholar]
- Guillemain B., Chevrier L., Boiron M., Parodi A. L., Lévy D. Présence d'anticorps fixant le complément chez des bovins atteints de lymphocytose persistante. Résultats préliminaires concernant le cheptel bovin français. C R Acad Sci Hebd Seances Acad Sci D. 1975 Feb 17;280(7):915–917. [PubMed] [Google Scholar]
- Ishizaki R., Luftig R. B., Bolognesi D. P. Outer membrane of avian myeloblastosis virus. J Virol. 1973 Dec;12(6):1579–1588. doi: 10.1128/jvi.12.6.1579-1588.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Portetelle D., Mammerickx M., Cleuter Y., Dekegel D., Galoux M., Ghysdael J., Burny A., Chantrenne H. Bovine leukemia virus: an exogenous RNA oncogenic virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1014–1018. doi: 10.1073/pnas.73.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald H. C., Graves D. C., Ferrer J. F. Isolation and characterization of an antigen of the bovine C-type virus. Cancer Res. 1976 Apr;36(4):1251–1257. [PubMed] [Google Scholar]
- Miller J. M., Miller L. D., Olson C., Gillette K. G. Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst. 1969 Dec;43(6):1297–1305. [PubMed] [Google Scholar]
- Miller J. M., Olson C. Precipitating antibody to an internal antigen of the C-type virus associated with bovine lymphosarcoma. J Natl Cancer Inst. 1972 Nov;49(5):1459–1462. [PubMed] [Google Scholar]
- Miller J. M., Van der Maaten M. J. A complement-fixation test for the bovine leukemia (C-type) virus. J Natl Cancer Inst. 1974 Dec;53(6):1699–1702. [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Livingston D. M. Radioimmunoassay of mammalian type C viral proteins. I. Species specific reactions of murine and feline viruses. J Immunol. 1972 Sep;109(3):570–577. [PubMed] [Google Scholar]
- Stock N. D., Ferrer J. F. Replicating C-type virus in phytohemagglutinin-treated buffy-coat cultures of bovine origin. J Natl Cancer Inst. 1972 Apr;48(4):985–996. [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian RNA tumor viruses: relatedness of the interspecies antigenic determinants of the major internal protein. J Virol. 1975 Jun;15(6):1332–1341. doi: 10.1128/jvi.15.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto Y. A., Puentes M. J., Young L. J., Cardiff R. D. Structure of the mouse mammary tumor virus: polypeptides and glycoproteins. J Virol. 1974 Feb;13(2):411–418. doi: 10.1128/jvi.13.2.411-418.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Maaten M. J., Miller J. M., Boothe A. D. Replicating type-C virus particles in monolayer cell cultures of tissues from cattle with lymphosarcoma. J Natl Cancer Inst. 1974 Feb;52(2):491–497. doi: 10.1093/jnci/52.2.491. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiland F., Ueberschär S., Straub O. C., Kaaden O. R., Dietzschold B. C-type particles in cultured lymphocytes from highly leukemic cattle. Intervirology. 1974;4(3):140–149. doi: 10.1159/000149853. [DOI] [PubMed] [Google Scholar]