Abstract
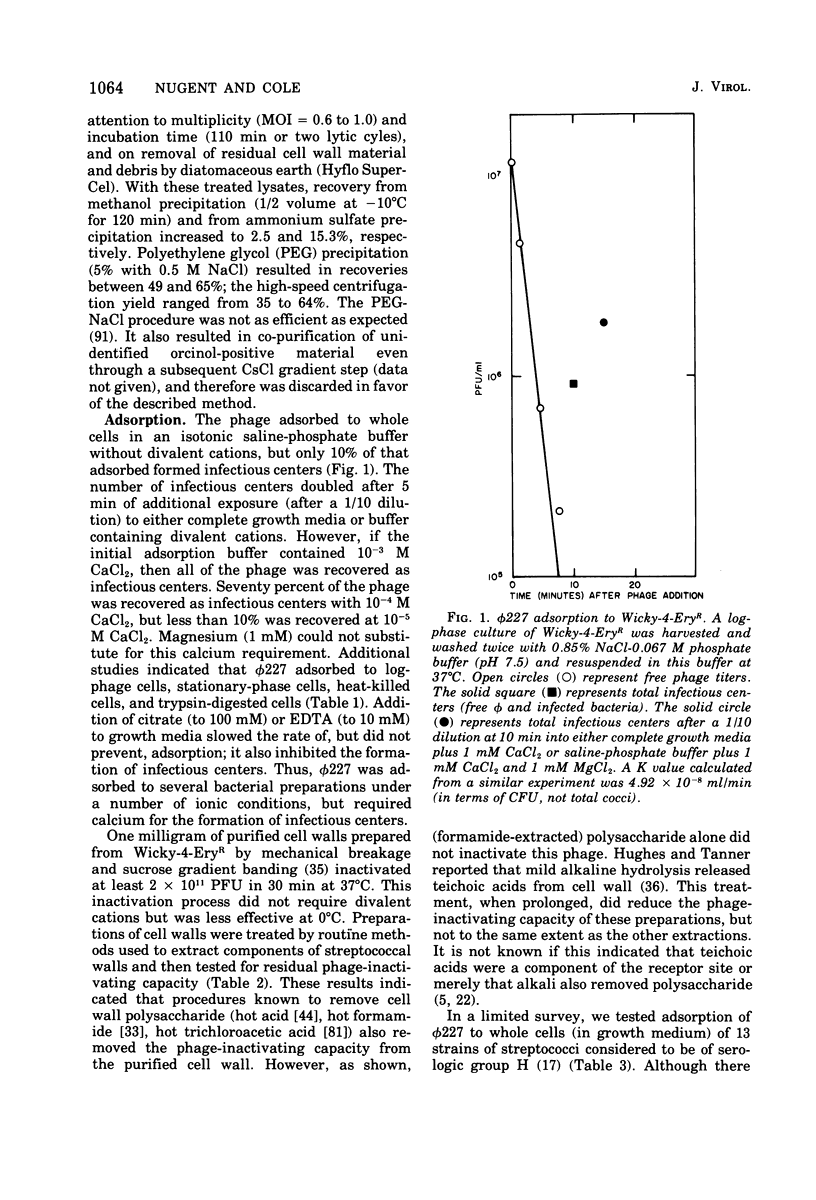
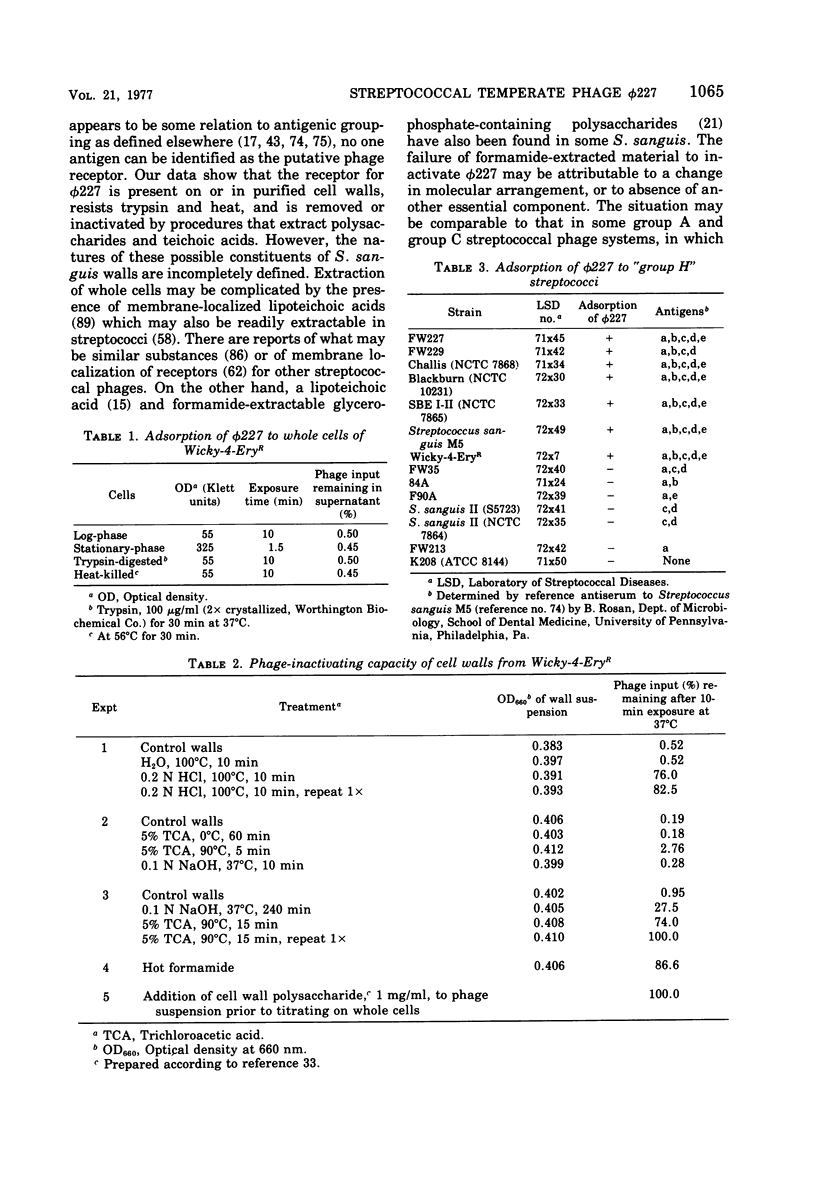
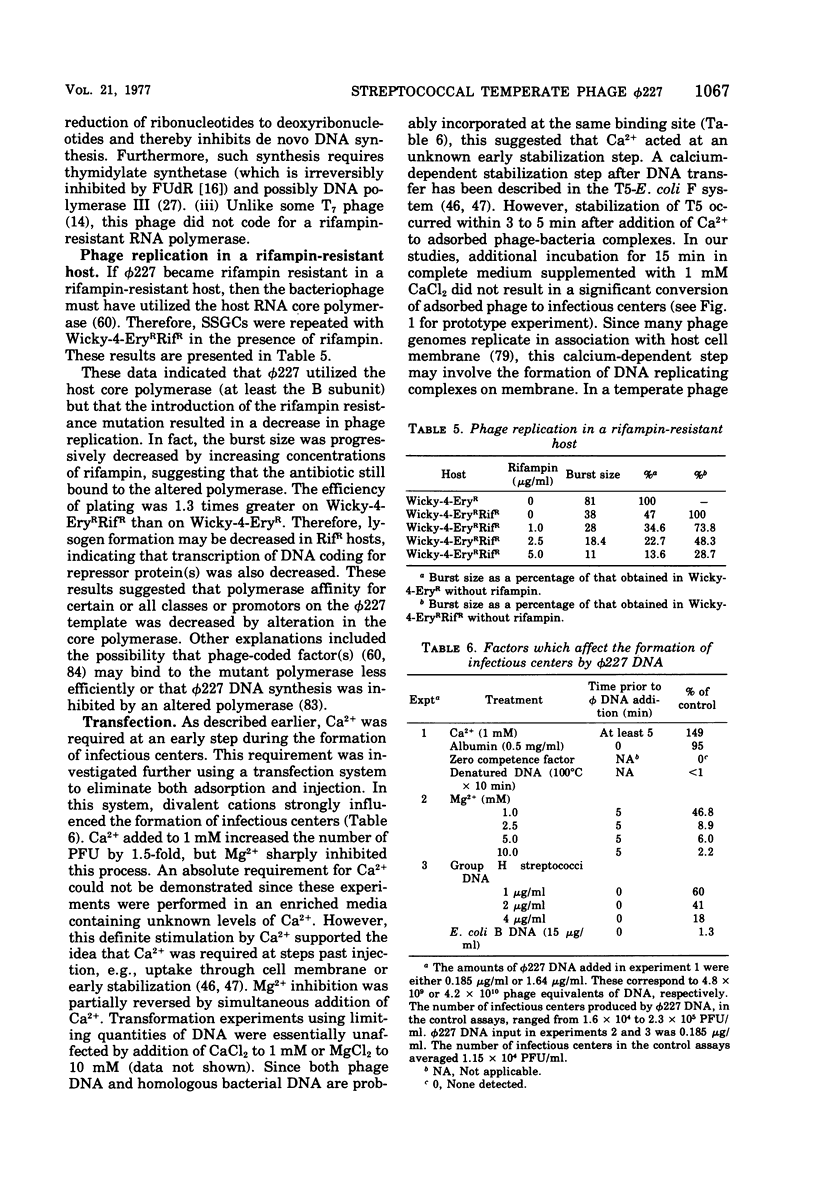
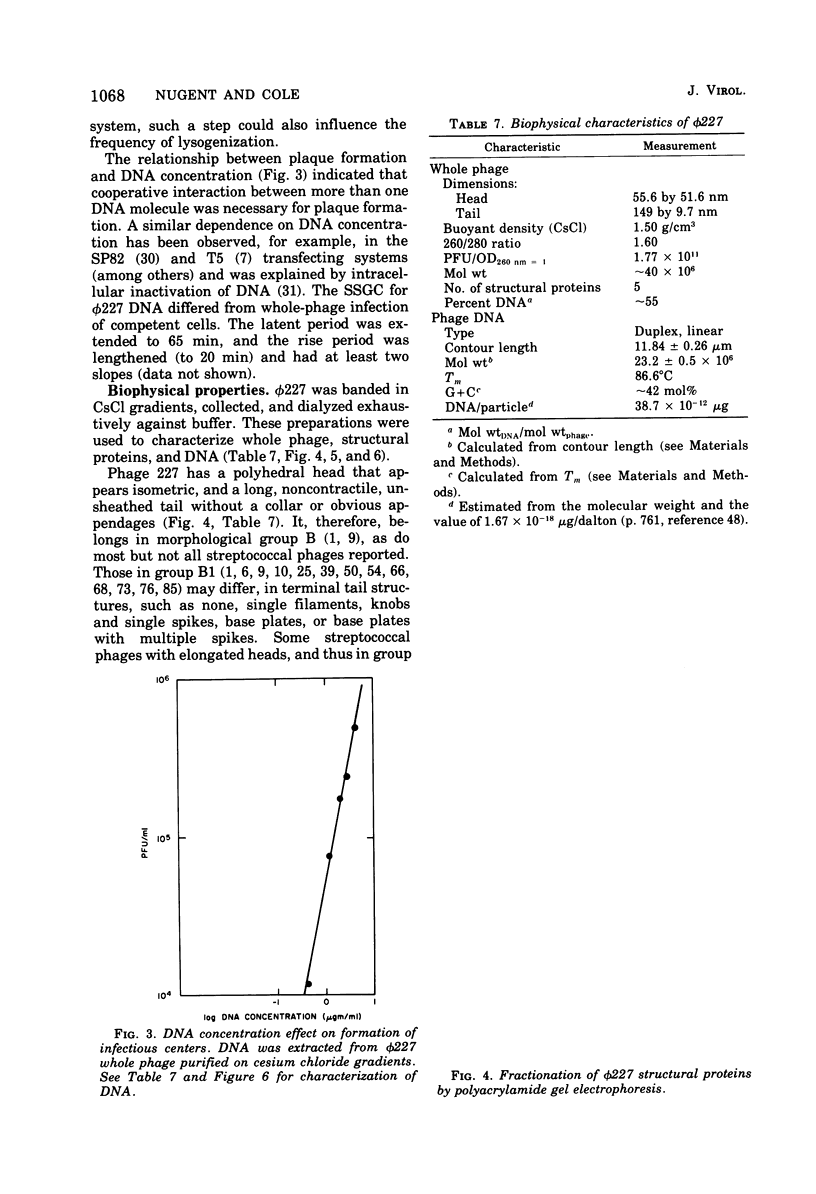
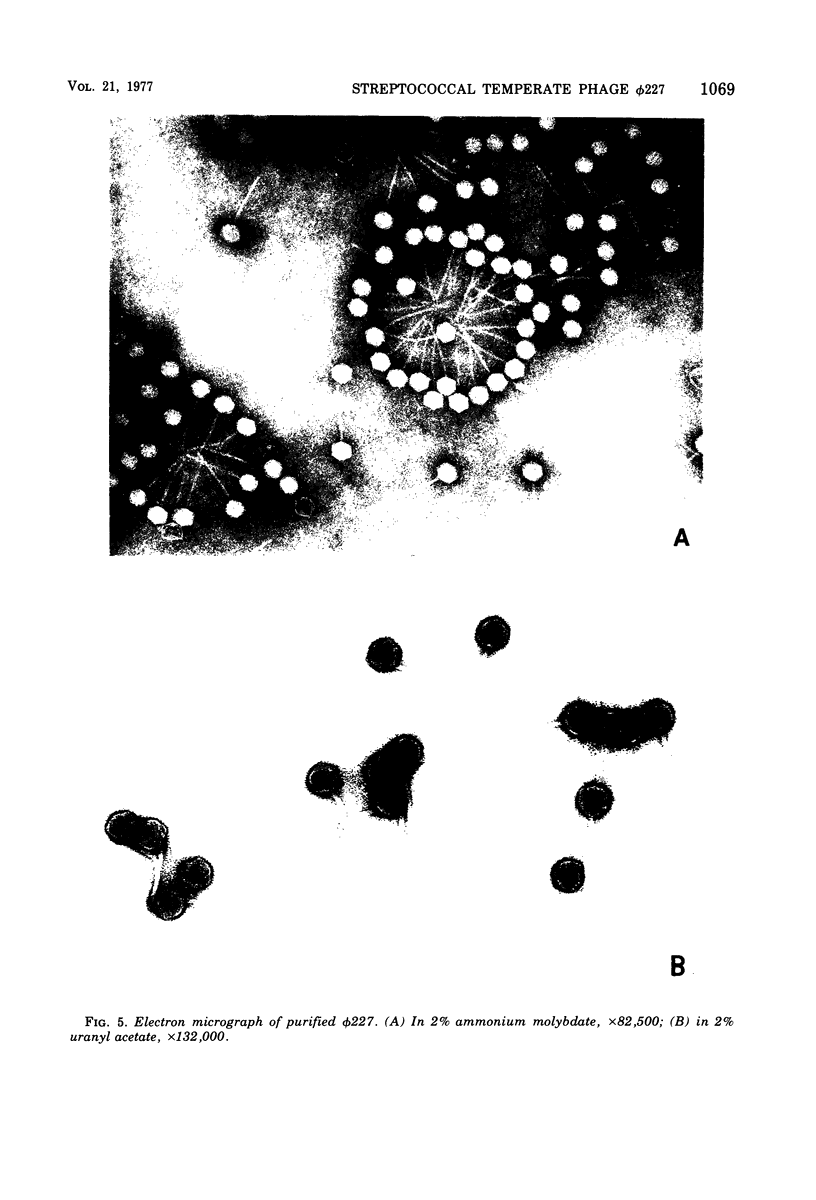
phi 227, a temperate phage from a group H streptococcus (Streptococcus sanguis), was propagated vegetatively in group H strain Wicky 4-EryR, and its characteristics were determined. A procedure dependent on multiplicity of infection, incubation time, and treatment of crude lysates with diatomaceous earth was found to optimize phage yield, resulting in titers of 1 X 10(10) to 2 X 10(10) PFU/ml. Without prior treatment with diatomaceous earth, subsequent purification procedures (methanol, ammonium sulfate, polyethylene glycol) gave recoveries of less than 1% of crude lysate titers. Adsorption of phi227 to host cells was relatively unaffected by the medium, but calcium (not substituted by magnesium) was required for formation of infectious centers. The phage receptor was present on purified cell walls, resisted trypsin and heat, and was removed ty hydrochloric acid, trichloracetic acid, and hot formamide: however, formamide-extracted material failed to inactivate phage, and the nature of the receptor is unknown. Single-step growth experiments showed a latent period of 39 min and a burst size of 100 PFU/infectious center; results were unaffected by omission of supplemental Ca2+, by supplementation with Mg2, addition of glucose, or changes of pH between 6.35 and 8.0; but increased temperature (40 to 43 degrees C) shortened the latent period and decreased the burst size. The latent period was prolonged in genetically competent host cells and in chemically defined medium; and in the latter, the burst size was smaller. Phage replication was sensitive to those metabolic inhibitors which inhibited the host streptococcus: these included rifampin, fluorodeoxyuridine, hydroxyurea, dihydrostreptomycin, and 6-P-hydroxyphenylazouracil. The data suggest that phi227 does not code for a rifampin-resistant RNA polymerase. However, in a rifampin-resistant host strain, phage replication and lysogen formation were both decreased suggesting that altered host core polymerase had less affinity for (some) promotors on the phi227 template. In transfection, a Ca2+-dependent stabilization step that was inhibited by Mg2+ was demonstrated; transformation was not affected by either Ca2+ or Mg2+, and the site and nature of the stabilization are unknown. More than one molecule of DNA was required for plaque formation. Biophysical characterization showed a type B phage of buoyant density (CsCl) 1.50, containing five proteins and 54.8% DNA. The duplex linear DNA had a molecular weight (calculated from contour length) of 23.2 X 10(6) and a guanine plus cytosine content (calculated from melting point) of 42.3 mol%. Similar characterizations of streptococcal phages, including biophysical data, have not been previously available.
Full text
PDF


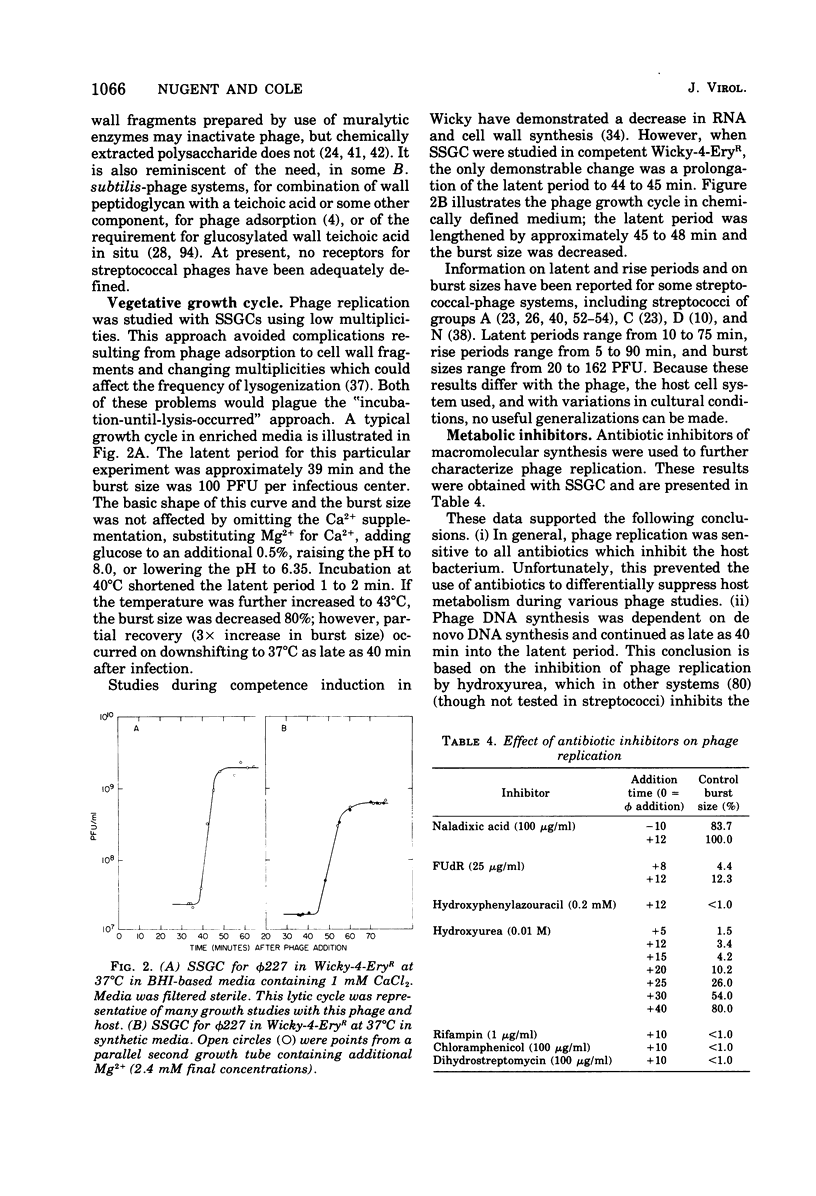




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann H. W., Caprioli T., Kasatiya S. S. A large new Streptococcus bacteriophage. Can J Microbiol. 1975 Apr;21(4):571–574. doi: 10.1139/m75-080. [DOI] [PubMed] [Google Scholar]
- Archibald A. R., Coapes H. E., Stafford G. H. The action of dilute alkali on bacterial cell walls. Biochem J. 1969 Aug;113(5):899–900. doi: 10.1042/bj1130899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Coapes H. E. The influence of growth conditions on the presence of bacteriophage-receptor sites in walls of Bacillus subtilis W23. Biochem J. 1971 Nov;125(2):667–669. doi: 10.1042/bj1250667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D., JOHNSON R. M., DEVILLE W. B. PHYSICAL AND CHEMICAL PROPERTIES OF A BACTERIAL VIRUS AS RELATED TO ITS INHIBITION BY STREPTOMYCIN. Virology. 1965 Mar;25:439–453. doi: 10.1016/0042-6822(65)90065-6. [DOI] [PubMed] [Google Scholar]
- Benzinger R., Scheible P. Transfection of Escherichia coli spheroplasts. IV. Transfection of rec+ and rec minus spheroplasts by native, denatured, and renatured T5 bacteriophage DNA after repair of single-strand breaks by polynucleotide ligase. J Virol. 1974 May;13(5):960–966. doi: 10.1128/jvi.13.5.960-966.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailsford M. D., Hartman P. A. Characterization of Streptococcus durans bacteriophages. Can J Microbiol. 1968 Apr;14(4):397–402. doi: 10.1139/m68-063. [DOI] [PubMed] [Google Scholar]
- Buchwald M., Steed-Glaister P., Siminovitch L. The morphogenesis of bacteriophage lambda. I. Purification and characterization of lambda heads and lambda tails. Virology. 1970 Oct;42(2):375–389. doi: 10.1016/0042-6822(70)90281-3. [DOI] [PubMed] [Google Scholar]
- Bujard H. Electron microscopy of single-stranded DNA. J Mol Biol. 1970 Apr 14;49(1):125–137. doi: 10.1016/0022-2836(70)90381-5. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Chiu T. H., Emdur L. I., Platt D. Lipoteichoic acids from Streptococcus sanguis. J Bacteriol. 1974 May;118(2):471–479. doi: 10.1128/jb.118.2.471-479.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Calandra G. B., Huff E., Nugent K. M. Attributes of potential utility in differentiating among "group H" streptococci or Streptococcus sanguis. J Dent Res. 1976 Jan;55:A142–A153. doi: 10.1177/002203457605500106011. [DOI] [PubMed] [Google Scholar]
- Emdur L. I., Saralkar C., McHugh J. G., Chiu T. H. Glycerolphosphate-containing cell wall polysaccharides from Streptococcus sanguis. J Bacteriol. 1974 Nov;120(2):724–732. doi: 10.1128/jb.120.2.724-732.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELTON L. D., PRESCOTT B., KAUFFMANN G., OTTINGER B. Pneumococcal antigenic polysaccharide substances from animal tissues. J Immunol. 1955 Mar;74(3):205–213. [PubMed] [Google Scholar]
- Fischetti V. A., Barron B., Zabriskie J. B. Studies on streptococcal bacteriophages. I. Burst size and intracellular growth of group A and group C streptococcal bacteriophages. J Exp Med. 1968 Mar 1;127(3):475–488. doi: 10.1084/jem.127.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Zabriskie J. B. Studies on streptococcal bacteriophages. II. Adsorption studies on group A and group C streptococcal bacteriophages. J Exp Med. 1968 Mar 1;127(3):489–505. doi: 10.1084/jem.127.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett E. A. An electron microscope study of a streptococcal bacteriophage. J Gen Virol. 1967 Jul;1(3):281–284. [PubMed] [Google Scholar]
- Friend P. L., Slade M. D. Characteristics of group A streptococcal bacteriophages. J Bacteriol. 1966 Jul;92(1):148–154. doi: 10.1128/jb.92.1.148-154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Gass K. B., Low R. L., Cozzarelli N. R. Inhibition of a DNA polymerase from Bacillus subtilis by hydroxyphenylazopyrimidines. Proc Natl Acad Sci U S A. 1973 Jan;70(1):103–107. doi: 10.1073/pnas.70.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Ionesco H., Schaeffer P. Teichoic acids as components of a specific phage receptor in Bacillus subtilis. Biochim Biophys Acta. 1966 Aug 24;124(2):415–417. doi: 10.1016/0304-4165(66)90211-x. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- HEYMANN H., MANNIELLO J. M., BARKULIS S. S. Structure of streptococcal cell walls. I. Methylation study of C-polysaccharide. J Biol Chem. 1963 Feb;238:502–509. [PubMed] [Google Scholar]
- Hayward G. S., Smith M. G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J Mol Biol. 1972 Feb 14;63(3):383–395. doi: 10.1016/0022-2836(72)90435-4. [DOI] [PubMed] [Google Scholar]
- Horne D. S., Perry D. Effect of competence induction on macromolecular synthesis in a group H streptococcus. J Bacteriol. 1974 Jun;118(3):830–836. doi: 10.1128/jb.118.3.830-836.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff E., Silverman C. S. Lysis of Staphylococcus aureus cell walls by a soluble staphylococcal enzyme. J Bacteriol. 1968 Jan;95(1):99–106. doi: 10.1128/jb.95.1.99-106.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Tanner P. J. The action of dilute alkali on some bacterial cell walls. Biochem Biophys Res Commun. 1968 Oct 10;33(1):22–28. doi: 10.1016/0006-291x(68)90248-9. [DOI] [PubMed] [Google Scholar]
- KJEMS E. Studies on streptococcal bacteriophages. 2. Adsorption, lysogenization, and one-step growth experiments. Acta Pathol Microbiol Scand. 1958;42(1):56–66. [PubMed] [Google Scholar]
- KRAUSE R. M. Studies on bacteriophages of hemolytic streptococci. I. Factors influencing the interaction of phage and susceptible host cell. J Exp Med. 1957 Sep 1;106(3):365–384. doi: 10.1084/jem.106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh B. P., Shimmin P. D. Morphology of the bacteriophages of lactic streptococci. Appl Microbiol. 1974 Feb;27(2):411–415. doi: 10.1128/am.27.2.411-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourilsky P. Lysogenization by bacteriophage lambda. I. Multiple infection and the lysogenic response. Mol Gen Genet. 1973 Apr 12;122(2):183–195. doi: 10.1007/BF00435190. [DOI] [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. I. Some basic kinetic features. Virology. 1960 Apr;10:501–513. doi: 10.1016/0042-6822(60)90132-x. [DOI] [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. II. Dissociation of calcium-independent and calcium-dependent processes. Virology. 1960 Apr;10:514–529. doi: 10.1016/0042-6822(60)90133-1. [DOI] [PubMed] [Google Scholar]
- Lai C., Listgarten M., Rosan B. Serology of Streptococcus sanguis: localization of antigens with unlabeled antisera. Infect Immun. 1973 Sep;8(3):475–481. doi: 10.1128/iai.8.3.475-481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D., Bujard H., Wolff B., Russell D. Electron microscopy of size and shape of viral DNA in solutions of different ionic strengths. J Mol Biol. 1967 Jan 28;23(2):163–181. doi: 10.1016/s0022-2836(67)80024-x. [DOI] [PubMed] [Google Scholar]
- Leonard C. G., Ranhand J. M., Cole R. M. Competence factor production in chemically defined media by noncompetent cells of group H Streptococcus strain Challis. J Bacteriol. 1970 Nov;104(2):674–683. doi: 10.1128/jb.104.2.674-683.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Malke H. Characteristics of transducing group A streptococcal bacteriophages A 5 and A 25. Arch Gesamte Virusforsch. 1970;29(1):44–49. doi: 10.1007/BF01253879. [DOI] [PubMed] [Google Scholar]
- Malke H. Prophage induction by N-methyl-N'-nitro-N-nitrosoguanidine in a group A streptococcus. Nature. 1967 May 20;214(5090):811–812. doi: 10.1038/214811a0. [DOI] [PubMed] [Google Scholar]
- Malke H. Transduction of Streptococcus pyogenes K 56 by temperature-sensitive mutants of the transducing phage A 25. Z Naturforsch B. 1969 Dec;24(12):1556–1561. doi: 10.1515/znb-1969-1214. [DOI] [PubMed] [Google Scholar]
- Matsuno T., Slade H. D. Composition and properties of a group A streptococcal teichoic acid. J Bacteriol. 1970 Jun;102(3):747–752. doi: 10.1128/jb.102.3.747-752.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonnell M., Lain R., Tomasz A. "Diplophage": a bacteriophage of Diplococcus pneumoniae. Virology. 1975 Feb;63(2):577–582. doi: 10.1016/0042-6822(75)90329-3. [DOI] [PubMed] [Google Scholar]
- Mitra S. Inhibition of M13 phage synthesis by rifampicin in some rifampicin-resistant Escherichia coli mutants. Virology. 1972 Nov;50(2):422–430. doi: 10.1016/0042-6822(72)90394-7. [DOI] [PubMed] [Google Scholar]
- Oram J. D. Isolation and properties of a phage receptor substance from the plasma membrane of Streptococcus lactis ML 3. J Gen Virol. 1971 Oct;13(1):59–71. doi: 10.1099/0022-1317-13-1-59. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The adsorption of phage to group N streptococci. The specificity of adsorption and the location of phage receptor substances in cell-wall and plasma-membrane fractions. J Gen Virol. 1968 Jul;3(1):103–119. doi: 10.1099/0022-1317-3-1-103. [DOI] [PubMed] [Google Scholar]
- PAKULA R., HULANICKA E., WALCZAK W. Transformation reactions between different species of streptococci and between streptococci, pneumococci and staphylococci. Schweiz Z Pathol Bakteriol. 1959;22(2):202–214. doi: 10.1159/000160592. [DOI] [PubMed] [Google Scholar]
- PAKULA R., WALCZAK W. On the nature of competence of transformable streptococci. J Gen Microbiol. 1963 Apr;31:125–133. doi: 10.1099/00221287-31-1-125. [DOI] [PubMed] [Google Scholar]
- Pakula R., Spencer L. R., Anderson N., Goldstein P. A. A comparative study of transformable and nontransformable isolates derived from two strains of streptococci. Can J Microbiol. 1973 Feb;19(2):207–216. doi: 10.1139/m73-032. [DOI] [PubMed] [Google Scholar]
- Parmelee C. E., Carr P. H., Nelson F. E. ELECTRON MICROSCOPE STUDIES OF BACTERIOPHAGE ACTIVE AGAINST STREPTOCOCCUS LACTIS. J Bacteriol. 1949 Apr;57(4):391–397. doi: 10.1128/jb.57.4.391-397.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C. L., Cole R. M. Transfection of group H streptococci. J Bacteriol. 1973 Mar;113(3):1505–1506. doi: 10.1128/jb.113.3.1505-1506.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C. L., Colon A. E., Leonard C. G., Cole R. M. Isolation of bacteriophages from group H streptococci. J Virol. 1972 May;9(5):876–878. doi: 10.1128/jvi.9.5.876-878.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L. C., Ranhand J. M., Leonard C. G., Colon A. E., Cole R. M. Inhibition of transformation in group H streptococci by lysogeny. J Bacteriol. 1973 Mar;113(3):1217–1222. doi: 10.1128/jb.113.3.1217-1222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. M., Rutberg L. Linked transformation of bacterial and prophage markers in Bacillus subtilis 168 lysogenic for bacteriophage phi 105. J Bacteriol. 1969 Jun;98(3):874–877. doi: 10.1128/jb.98.3.874-877.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS C. G., SARLES W. B. CHARACTERIZATION OF ENTEROCOCCUS BACTERIOPHAGES FROM THE SMALL INTESTINE OF THE RAT. J Bacteriol. 1963 Jun;85:1378–1385. doi: 10.1128/jb.85.6.1378-1385.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M., Leonard C. G., Cole R. M. Autolytic activity associated with competent group H streptococci. J Bacteriol. 1971 Apr;106(1):257–268. doi: 10.1128/jb.106.1.257-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M., Theodore T. S., Cole R. M. Protein difference between competent and noncompetent cultures of a group H Streptococcus. J Bacteriol. 1970 Oct;104(1):360–362. doi: 10.1128/jb.104.1.360-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B., Lai C. H., Listgarten M. A. Streptococcus sanguis: a model in the application in immunochemical analysis for the in situ localization of bacteria in dental plaque. J Dent Res. 1976 Jan;55:A124–A141. doi: 10.1177/002203457605500105011. [DOI] [PubMed] [Google Scholar]
- Russell H., Norcross N. L., Kahn D. E. Isolation and characterization of Streptococcus agalactiae bacteriophage. J Gen Virol. 1969 Sep;5(2):315–317. doi: 10.1099/0022-1317-5-2-315. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel P. J., Schaechter M. The role of the host cell membrane in the replication and morphogenesis of bacteriophages. Annu Rev Microbiol. 1973;27:261–282. doi: 10.1146/annurev.mi.27.100173.001401. [DOI] [PubMed] [Google Scholar]
- Sinha N. K., Snustad D. P. Mechanism of inhibition of deoxyribonucleic acid synthesis in Escherichia coli by hydroxyurea. J Bacteriol. 1972 Dec;112(3):1321–1324. doi: 10.1128/jb.112.3.1321-1334.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade H. D. Extraction of Cell-Wall Polysaccharide Antigen from Streptococci. J Bacteriol. 1965 Sep;90(3):667–672. doi: 10.1128/jb.90.3.667-672.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Hofschneider P. H. Replication of bacteriophage M 13: inhibition of single-strand DNA synthesis by rifampicin. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1634–1637. doi: 10.1073/pnas.69.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby J. G., Tiraby E., Fox M. S. Pneumococcal bacteriophages. Virology. 1975 Dec;68(2):566–569. doi: 10.1016/0042-6822(75)90300-1. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K., Brock T. D. Purification and properties of a bacteriophage receptor material from Streptococcus faecium. Biochim Biophys Acta. 1966 Jun 29;121(2):298–314. doi: 10.1016/0304-4165(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Villarejo M., Hua S., Evans E. A., Jr Protein components of bacteriophages lambda and lambda virulent. J Virol. 1967 Oct;1(5):928–934. doi: 10.1128/jvi.1.5.928-934.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamaker L. W., Skjold S., Maxted W. R. Characterization of bacteriophages from nephritogenic group A streptococci. J Infect Dis. 1970 Apr;121(4):407–418. doi: 10.1093/infdis/121.4.407. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Young F. E. The influence of temperate bacteriophage phi105 on transformation and transfection in Bacillus subtilis. Biochem Biophys Res Commun. 1972 Apr 28;47(2):365–371. doi: 10.1016/0006-291x(72)90722-x. [DOI] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZABRISKIE J. B. THE ROLE OF TEMPERATE BACTERIOPHAGE IN THE PRODUCTION OF ERYTHROGENIC TOXIN BY GROUP A STREPTOCOCCI. J Exp Med. 1964 May 1;119:761–780. doi: 10.1084/jem.119.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]