Abstract
Background
The supplement Pycnogenol® (PYC) has been used for the treatment of several chronic diseases including allergic rhinitis (AR). However, the in vivo effects on allergic inflammation have not been identified to date.
Aims
To investigate the treatment results of PYC on allergic inflammation in a rat model of allergic rhinitis.
Study Design
Animal experimentation.
Methods
Allergic rhinitis was stimulated in 42 rats by intraperitoneal sensitization and intranasal challenge with Ovalbumin. The animals were divided into six subgroups: healthy controls, AR group, AR group treated with corticosteroid (dexamethasone 1 mg/kg; CS+AR), healthy rats group that were given only PYC of 10 mg/kg (PYC10), AR group treated with PYC of 3mg/kg (PYC3+AR), and AR group treated with PYC of 10 mg/kg (PYC10+AR). Interferon-γ (IFN-γ), interleukin-4 (IL-4), interleukin-10 (IL-10), and OVA-specific immunoglobulin E (Ig-E) levels of serum were measured. Histopathological changes in nasal mucosa and expression of tumor necrosis factor-α (TNF-α) and IL-1β were evaluated.
Results
The levels of the IL-4 were significantly decreased in the PYC3+AR, PYC10+AR and CS+AR groups compared with the AR group (p=0.002, p<0.001, p=0.006). The production of the IFN-γ was significantly decreased in the PYC3+AR and PYC10+AR groups compared with the AR group (p=0.013, p=0.001). The administration of PYC to allergic rats suppressed the elevated IL-10 production, especially in the PYC3+AR group (p=0.006). Mucosal edema was significantly decreased respectively after treatment at dose 3 mg/kg and 10 mg/kg PYC (both, p<0.001). The mucosal expression of TNF-α has significantly decreased in the PYC3+AR and PYC10+AR groups (p=0.005, p<0.001), while the IL-1β expression significantly decreased in the CS+AR, PYC3+AR, and PYC10+AR groups (p<0.001, p=0.003, p=0.001).
Conclusion
PYC has multiple suppressive effects on allergic response. Thus, PYC may be used as a supplementary agent in allergic response.
Keywords: Pycnogenol, allergic rhinitis, IL-4, IL-10, OVA-specific IgE, TNF-α, IL-1β, eosinophil, edema, goblet cell
Allergic rhinitis (AR) is a common illness which is characterized by Immunoglobulin (Ig) E-mediated inflammation (1). The inflammatory response is associated with an increase in the numbers of eosinophils, mucus secretion and increased production of Th2-type cytokines, such as IL-4, IL-5, and IL-13 (2). Interactions between inflammatory mediators and neural and glandular structures cause symptoms such as sneezing, nasal obstruction and rhinorrhea (3,4). Studies have revealed that this disease decreases the quality of life and labor productivity of affected patients, and negatively affects the economy (5). Many important pharmacological agents have been used to treat AR (6).
Pycnogenol® (PYC) is a natural plant extract achieved from the French maritime pine Pinus pinaster bark (Pinus maritime) (7,8). It has been used as a herbal dietary supplement because of its anti-oxidant effect in several chronic diseases. Besides its anti-oxidant effect, it may have an anti- inflammatory affect (9).
A few studies have investigated the effect of PYC on allergic asthma (9–12). Although these studies have demonstrated the effect of PYC on allergic disease, its mechanism for this effect is not clear. Thus, in this study, we investigated the treatment results of PYC on allergic inflammation in a rat model of allergic rhinitis.
MATERIALS AND METHODS
Animals
This study was conducted on 43, 12–15 month-old female Wistar rats, obtained from the Experimental Animal Center of University. The rats were housed under standardized conditions (12 h dark/light cycle, 20±1°C room temperature) in regular cages and allowed free access to food and water. The study was approved by the Committee on Ethics in Animal Experimentation of University (HADYEK 60583101/2014/029) and obeyed the international ethical standards.
Production of the AR model and treatment protocol
Forty-three rats were randomized into six subgroups (allergic rhinitis treated with PYC (Horpag Research Ltd; Genova, Switzerland) 3mg/kg (PYC3+AR) group n=8 the other five groups n=7/per cage): healthy control, allergic rhinitis (AR), allergic rhinitis treated with corticosteroid (dexamethasone 1 mg/kg; CS+AR), healthy rats given only Pycnogenol® 10 mg/kg (PYC 10), allergic rhinitis treated with Pycnogenol® 3mg/kg (PYC3+AR), allergic rhinitis treated with PYC 10mg/kg (PYC10+AR).
Allergic rhinitis groups (AR, CS+AR, PYC3+AR, PYC10+AR) were sensitized by intraperitoneal injection of 1 mL saline containing 30 mg of aluminum hydroxide and 0.3 mg of ovalbumin (Sigma A5253, Interlab; İstanbul, Turkey) once every other day for 14 days (for a total of seven injections per rat), while control and only PYC treated rat groups were given 30 mg/kg aluminum hydroxide in1 mL saline. Then, allergic rhinitis groups (AR, CS+AR, PYC3+AR, PYC10+AR) were sensitized by intranasal dripping of 2% ovalbumin, two or three drops per administration, once a day for the following 7 days (Figure 1) (13,14), while control and only PYC-treated groups were given saline drops. Meanwhile, during the sensitization period, allergic rhinitis treated groups (CS+AR, PYC3+AR, PYC10+AR) were also given either dexamethasone or PYC in high and low doses intraperitoneally once every day. PYC was dissolved in 2% ethanol (ETOH) and immediately applied to the rats according to bodyweight. Except CS+AR group (because it has own vehicle), all of the remaining groups took 2% ETOH intraperitoneally. CS+AR group served as a comparison of PYC treatment with the standard therapy. Only the PYC group was used to clarify the safety of PYC.
FIG. 1.

Allergic rhinitis rat model
Histopathological examination
All of the animals were sacrificed at post-experiment day 21 under the ketamine (Ketalar, Eczacıbaşı; İstanbul, Turkey) and xylazine (Rompun, Bayer; İstanbul, Turkey) (50 and 5 mg/kg, respectively) anesthesia. Blood samples were taken by cardiac puncture; they were centrifuged and the sera were kept at −80°C for the measurement of interleukin and interferon levels. The nasal respiratory tissues were harvested and fixed in 10% formalin solution overnight. Thereafter, they were decalcified and coronal sections were chosen from the middle segment of the sinonasal cavity (maxillary sinus and olfactory region) as the principal area of the histopathological examination.
Paraffin blocks were formed and anatomically similar sections were collected. Finally, 4 μm-thick sections were stained with hematoxylin and eosin (H&E). The nasal mucosa edema, goblet cell, and eosinophil counts were evaluated as a semi-quantitative parameter. The Goblet cell count was indicated as (0) normal, (1) slight increase or (2) severe increase; eosinophils as (0) <10/high, high power field (HPF) and (1) >10/HPF; and edema as (0) absent, (1) mild, (2) moderate and (3) severe.
Cytokines and OVA- specific immunoglobulin E in serum
To evaluate the allergic reaction, the following were measured by enzyme-linked immunosorbent assay (ELISA; Baoshan District, Shanghai, China): interferon (IFN)-γ for T helper 1 (Th1) immune reaction, interleukin-4 (IL-4) for T helper 2 (Th2) immune reaction, IL-10 for T regulator (Treg) and serum OVA-specific immunoglobulin E (IgE) levels. Procedures were performed according to the manufacturer’s instructions.
Immunohistochemistry
Immunohistochemical staining was done by using DAKO Autostainer Universal Staining System (Autostainer Link 48 DAKO; Glostrup, Denmark). Firstly, 4μm sections were mounted on positive-loaded glass slides. Then the sections were deparaffinized with xylol and dehydrated by passing through an ethyl alcohol series. Subsequently, antigen retrieval was performed in a thermostatic bath (PT Link) at 96°C (10 mm/L citrate buffer, pH 6) for 40 minutes. The sections were incubated with anti- TNF-α (cat. No: NB600-587, Novus Biologicals; CO, USA) and anti-IL-1β (cat. No. SC-7884, Santa Cruz Biotechnology; USA) primary antibodies for 60 minutes. Using the streptavidin-biotin immunoperoxidase technique (K8000 Envision Flex, DAKO; Glostrup, Denmark), an automated system was used. In order to provide a colored image, immunoreactions with Diaminobenzidine tetrachloride (DAB) were displayed. For background staining, the sections were counter-stained with hematoxylin. For dehydration, the sections were passed through an alcohol series with increasing strength and then cleared in xylol, lined with balsam. After the staining stage, the sections were examined under light microscope (Olympus BX51; Tokyo, Japan) with 4, 10, 20, and 40 magnifications by a single pathologist blinded to the patient’s history and CT findings. A minimum of 200 cells was counted in the mostly stained areas (hot spots). They were scored as: (0) <5% staining, (1) 5–10% staining, (2) 11–20% staining and (3) >20% staining.
Statistical analysis
Statiscial Package for the Social Sciences version 19.0 (IBM Corp.; Armonk, NY, USA) was used for calculations and statistical analysis. Comparisons between groups were analyzed using the Kruskal Wallis test. Dunn’s post-hoc test was used to compare between selected groups. Descriptive statistics were showed as median (25–75 percentiles) and interquartile range or frequency. In terms of variables with no normal distribution, non-parametric tests were used for analysis. Hence, confidence intervals were not calculated. P values <0.05 were considered significant.
RESULTS
Effects of PYC on the levels of cytokines and OVA-specific IgE in serum
To demonstrate the effect of PYC on OVA-induced allergic responses in rat, the levels of OVA-specific IgE, IL-4, IL-10 and IFN-γ were measured in OVA-sensitized rats by ELISA. OVA-specific IgE levels were increased by sensitization (p=0.039) (Figure 2a). The administration of a dose of 3mg/kg (median 159.73 pg/ml, IQR: 54.09) and 10mg/kg PYC (173.67 pg/mL, IQR: 43.94) significantly inhibited OVA-specific IgE production in the AR group (median: 203.09 pg/mL, IQR: 23.33) (p=0.007, p=0.001, respectively). This effect was independent of the PYC doses (Figure 2a). The levels of the Th2-type cytokine, IL-4, were significantly decreased in the PYC3+AR (median: 45.83 pg/mL, IQR: 23), PYC10+AR (median: 52.08 pg/mL, IQR: 14.22) and CS+AR (median: 43.95 pg/mL, IQR: 17.73) groups compared with the AR group (median: 67.31 pg/mL, IQR: 16.72) (p=0.002, p<0.001, p=0.006, respectively). The results are summarized in Figure 2b.
FIG. 2.

a, d. The Ovalbumin-specific IgE levels in the serum of rat groups. *Significantly higher than that of control, PYC10, PYC3+AR and PYC10+AR groups (p<0.05) (a). The interleukin-4 (IL-4) levels in the serum of rat groups. *Significantly lower than that of PYC3+AR, PYC10+AR groups. **Significantly higher than that of CS+AR, PYC3+AR and PYC10+AR groups (p<0.05). ***Significantly lower than that of PYC10 group (p<0.05) (b). The interferon (IFN)-γ levels in the serum of rat groups. *Significantly higher than that of PYC10, PYC3+AR and PYC10+AR groups (p<0.05). **Significantly lower than that of control and CS+AR groups (p<0.05). ***Significantly lower than that of control group (p<0.05) (c). The interleukin-10 (IL-10) levels in the serum of the rat groups. *Significantly higher than that of PYC3+AR and PYC10+AR groups (p<0.05) (d).
AR: allergic rhinitis; CS+AR: corticosteroid+AR; PYC: Pycnogenol®10 mg/kg; PYC3+AR: Pycnogenol® 3mg/kg +AR; PYC10+AR: Pycnogenol® 10mg/kg+AR
The production of the Th1 cytokine, IFN-γ, was significantly decreased in the PYC3+AR (median: 26.741 pg/mL, IQR: 7.41 pg/mL), and PYC10+AR (median: 22.65 pg/mL, IQR: 5.99) groups compared with the AR group (median: 40.23 pg/mL, IQR: 18.19) (p=0.013, p=0.001, respectively; Figure 2c).
Treg levels are shown in Figure 2d. IL-10 was increased in the AR group (median: 180.83 pg/mL, IQR: 141.70) compared with the control group (median: 118 pg/mL, IQR: 72.3). The administration of PYC to allergic rats suppressed the elevated IL-10 production, especially in the PYC3+AR group (median: 99.70 pg/mL, 61.37) (p=0.006).
Effects of PYC on the histopathological changes in nasal mucosa
The histopathological examination of the nasal mucosa of the control group was normal while edema, eosinophil infiltration and goblet cell count were increased in the OVA-sensitized group (Figure 3). The eosinophil infiltration and edema were present in the nasal mucosa of the OVA-sensitized rat by sensitization (p=0.012, pg=0.001, respectively). That eosinophilic infiltration was significantly decreased in the PYC3+AR and PYC10+AR groups (p=0.001, p=0.012, respectively, Table 1). Degree of edema was summarized in Table 2. Edema was significantly decreased respectively after treatment at dose 3 mg/kg and 10 mg/kg PYC (both, p<0.001). The edema reducing effect of dose of 3 mg/kg PYC and 10 mg/kg PYC were more effective than corticosteroids (p=0.012, p=0.018, respectively). Interestingly, edema and eosinophilic inflammation decreased slightly in the corticosteroid group. However, goblet cell count did not differ significantly between the AR, control, and treatment groups (Table 3).
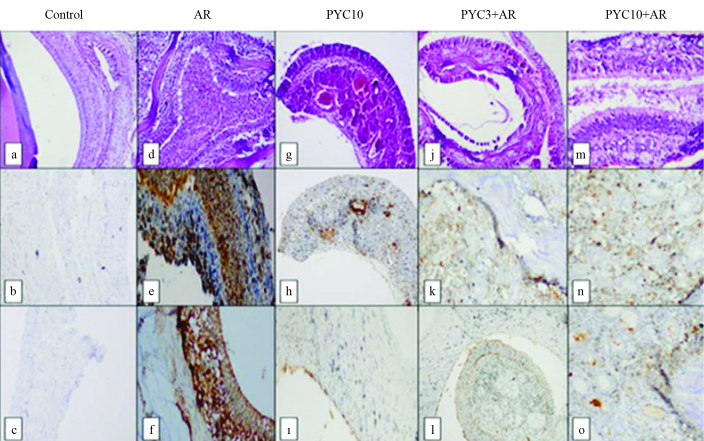
FIG. 3.
a–o. There is a normal appearance in the control group (a) (HE, ×100); there was no immunohistochemical staining with TNF-α (b) and IL-1β (c); There is severe eosinophil infiltration, and edema (d), severe and diffuse immunohistochemical staining with TNF-α (e) and IL-1β (f) in the allergic rhinitis group. There is mild edema (g), mild immunohistochemical staining with TNF-α (h) and IL-1β (i) in the CS+AR group; there is mild edema (j), mild immunohistochemical staining with TNF-α (k) and IL-1β(l) in the PYC3+AR group; there is mild edema (m), mild immunohistochemical staining with TNF-α (n) and IL-1β (o) in the PYC10+AR group.
AR: allergic rhinitis; CS+AR: corticosteroid+AR; PYC: Pycnogenol®10 mg/kg; PYC3+AR: Pycnogenol® 3mg/kg +AR; PYC10+AR: Pycnogenol® 10mg/kg+AR.
TABLE 1.
The eosinophil count in the nasal mucosa of the rat groups.
| Groups | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Eosinophil counts | Control (n) | AR* (n) | CS+AR (n) | PYC10 (n) | PYC3+AR (n) | PYC10+AR (n) | p |
| 0 | 6 | 2 | 5 | 6 | 8 | 6 | 0.031 |
| 1 | 1 | 5 | 2 | 1 | 0 | 1 | |
| Total | 7 | 7 | 7 | 7 | 8 | 7 | |
(0) eosinophil count<10/hpt, (1) eosinophil count>10/hpt.
Significantly higher than that of control, PYC10, PYC3+AR, PYC10+AR groups (p<0.05).
AR: allergic rhinitis; CS+AR: corticosteroid+AR; PYC: Pycnogenol®10 mg/kg; PYC3+AR: Pycnogenol® 3 mg/kg +AR; PYC10+AR: Pycnogenol® 10 mg/kg+AR
TABLE 2.
The edema degree of the nasal mucosa of the rat groups.
| Groups | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Edema | Control (n) | AR* (n) | CS+AR** (n) | PYC10 (n) | PYC3+AR (n) | PYC10+AR (n) | p |
| 0 | 5 | 0 | 1 | 6 | 7 | 6 | <0.001 |
| 1 | 2 | 3 | 6 | 1 | 1 | 1 | |
| 2 | 0 | 4 | 0 | 0 | 0 | 0 | |
| Total | 7 | 7 | 7 | 7 | 8 | 7 | |
(0) absence, (1) mild, (2) moderate, (3) severe.
Significantly higher than that of control, PYC10, PYC3+AR, PYC10+AR groups (p<0.05).
Significantly higher than that of PYC10, PYC3+AR, and PYC10+AR groups (p<0.05).
AR: allergic rhinitis; CS+AR: corticosteroid+AR; PYC: Pycnogenol®10 mg/kg; PYC3+AR: Pycnogenol® 3 mg/kg +A; PYC10+AR: Pycnogenol® 10 mg/kg+AR
TABLE 3.
The goblet cell count in the nasal mucosa of the rat groups.
| Groups | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Goblet cell | Control (n) | AR (n) | CS+AR (n) | PYC10 (n) | PYC3+AR (n) | PYC10+AR (n) | p |
| 0 | 5 | 3 | 5 | 6 | 6 | 5 | >0.05 |
| 1 | 2 | 4 | 2 | 1 | 2 | 2 | |
| Total | 7 | 7 | 7 | 7 | 8 | 7 | |
(0) normal, (1) slight increase, (2) severe increase.
AR: allergic rhinitis; CS+AR: corticosteroid+AR; PYC: Pycnogenol®10 mg/kg; PYC3+AR: Pycnogenol® 3 mg/kg +AR; PYC10+AR: Pycnogenol® 10 mg/kg+AR
Effects of PYC on expression of TNF-α and IL-1β in nasal mucosa
The expression of TNF-α has significantly decreased in the PYC3+AR and PYC10+AR groups (p=0.005, p<0.001, respectively), while sensitization of OVA significantly increased the expression of TNF-α (p<0.001) (Figure 3b–o). The expression level of IL-1β was significantly higher in the AR group than in the control group (p<0.001). The IL-1β expression significantly decreased in the CS+AR, PYC3+AR, and PYC10+AR groups (p<0.001, p=0.003, p=0.001, respectively) (Figure 3b–o).
DISCUSSION
Pycnogenol has been used as a herbal dietary supplement in European countries because of its anti-oxidant effect (8). Recent studies have demonstrated its potential anti-inflammatory activity in several chronic diseases (8,15). It was reported that PYC is a hepatoprotective agent when administered at a dose of 10 mg/kg in a model of nonalcoholic fatty liver disease caused by oxidative stress (16). Choi and Yan (10) reported that 10–100 mg/kg PYC inhibits histamine and pro-inflammatory cytokines and reduces allergic response. Studies on asthma showed that PYC relieves symptoms of asthma and reduces the leukotrienes levels (11,12). In a study which is the first about the effect of PYC on AR, Wilson et al. (7) reported that PYC reduces allergic rhinitis symptoms if supplementation is started 5 weeks before the allergy season begins. Although PYC’s effect on allergic disease is known, its mechanism is not clear. In view of these studies, we have thought that 10 mg/kg of PYC application by intraperitoneally once daily for 7 days is adequate for an allergic rhinitis animal model. We used the OVA-induced (sensitization/challenge) mouse AR model, which was previously tested in many studies (17,18).
The present study clarified the effects of PYC on allergic rhinitis in a rat model. OVA sensitization and challenge in our study led to an increase in OVA-specific IgE levels. Hence, the model exhibits the characteristic pathology of rhinitis. We evaluated systemic cytokine profiles of Th1 and Th2 cells which are known to play important roles in allergic rhinitis. Th2 cytokines, such as IL-4 and IL-13, are produced by mast cells, T cells, macrophage and epithelial cells and they are important for the differentiation of T cells from the Th2 type (19). Both the IL-4 and IL-13 regulate IgE isotype switching in B cells (20). Allergic rhinitis is an inflammation which has Th2 cell predominance. However recent studies have shown IFN-γ increase; IFN-γ does not play an inhibitor role in AR and it may supports Th2 cells cytokine activity in allergic response. It was found that IFN-γ levels are decreased by corticosteroid treatment (21,22). As expected, IFN-γ and IL-4 were increased after induction sensitization in the AR group compared to the control group and corticosteroid therapy reduced the levels of these cytokines in our study (Figure 2b, 2c). PYC reduced the production of IL-4 and IFN-γ in serum of the sensitized rats. In addition, there were no significant differences in IL-4 levels between PYC doses. Therefore, this result showed that PYC alleviates the allergic inflammatory response via Th1 and Th2 cytokines.
Treg lymphocytes have a suppressive effect on allergic inflammation. IL-10, which is one of the Treg cytokines, inhibits the proinflammatory effect of the mast cells and eosinophils (23). Benson et al. (21) found that IL-10 level in nasal fluid is increased patients with AR compared to the control group during pollen season and reduced by corticosteroid therapy. In another study, IL-10 was found to be significantly decreased in AR patients compared to the control group (24). According to our data, IL-10 level was increased in AR group and it significantly decreased after PYC treatment at doses both 3 mg/kg and 10 mg/kg. We demonstrated to PYC had no increasing effect on the production of IL-10 like corticosteroid therapy. However, IL-10 level may decreased due to suppressed allergic response by PYC.
AR has histopathological changes such as an increase in eosinophil infiltration, ciliated cell failure, goblet cell and mucosal edema (4). TNF-α and IL-1β, which are critical in the pathogenesis of AR, are proinflammatory cytokines produced by mast cells and eosinophils (4,25). We demonstrated that PYC is a potent inhibitor of eosinophil infiltration and edema. It was more effective on the edema than corticosteroids. In addition, these effects were independent of dose. Additionally, PYC strongly reduced the levels of TNF-α and IL-1β in the sinonasal mucosa of AR rats. There were no significant differences between PYC doses and corticosteroids in terms of TNF-α and IL-1β concentration. Therefore, PYC may alleviate allergic response by suppressing TNF-α and IL-1β.
The anti-allergic effect of PYC in allergic rhinitis using an OVA allergen in a rat model of allergic rhinitis was evaluated in our study. These findings demonstrated that PYC inhibits allergic rhinitis response by reducing nasal mucosal edema and eosinophilia, Th1 and Th2 cytokines, the expression of TNF-α and IL-1β and allergen-specific antibodies. Additionally, there were no significant differences in effects of PYC doses and the effect of PYC on edema was significantly more potent than corticosteroids. This study may be used as a guide to future investigations which should be conducted with PYC in human models.
Acknowledgement
We wish to thank Horpag Research Ltd. for generously gifting us with Pycnogenol®.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Animal Ethical Committee of the ADU (HADYEK 60583101/2014/029).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - C.G., B.D.; Design - C.G., B.D.; Supervision - C.G., M.Y.; Resource - C.G.,A.E.,Y.B.; Materials - B.D., İ.M., M.Y.; Data Collection and/or Processing - M.Y., İ.M.; Analysis and/or Interpretation - İ.K.Ö.; Literature Search - A.E., Y.B.; Writing - C.G., A.E., Y.B.; Critical Reviews - B.D., C.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. https://doi.org/10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 2.El Gazzar M, El Mezayen R, Marecki JC, Nicolls MR, Canastar A, Dreskin SC. Anti-inflammatory effect of Pycnogenol in a mouse model of allergic lung inflammation. Int Immunopharmacol. 2006;6:1135–42. doi: 10.1016/j.intimp.2006.02.004. https://doi.org/10.1016/j.intimp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Kleinjan A, van Nimwegen M, Leman K, Hoogsteden HC, Lambrecht BN. Topical treatment targeting sphingosine-1-phosphate and sphingosine lyase abrogates experimental allergic rhinitis in a murine model. Allergy. 2013;68:204–12. doi: 10.1111/all.12082. https://doi.org/10.1111/all.12082. [DOI] [PubMed] [Google Scholar]
- 4.Jung HW, Jung JK, Park YK. Comparison of the efficacy of KOB03, ketotifen, and montelukast in an experimental mouse model of allergic rhinitis. Int Immunopharmacol. 2013;16:254–60. doi: 10.1016/j.intimp.2013.04.011. https://doi.org/10.1016/j.intimp.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Valovirta E, Myrseth SE, Palkonen S. The voice of the patients: allergic rhinitis is not a trivial disease. Curr Opin Allergy Clin Immunol. 2008;8:1–9. doi: 10.1097/ACI.0b013e3282f3f42f. https://doi.org/10.1097/ACI.0b013e3282f3f42f. [DOI] [PubMed] [Google Scholar]
- 6.Kim BY, Shin JH, Park HR, Kim SW, Kim SW. Comparison of antiallergic effects of pneumococcal conjugate vaccine and pneumococcal polysaccharide vaccine in a murine model of allergic rhinitis. Laryngoscope. 2013;123:2371–7. doi: 10.1002/lary.24047. https://doi.org/10.1002/lary.24047. [DOI] [PubMed] [Google Scholar]
- 7.Wilson D, Evans M, Guthrie N, Sharma P, Baisley J, Schonlau F, et al. A randomized, double-blind, placebo-controlled exploratory study to evaluate the potential of pycnogenol for improving allergic rhinitis symptoms. Phytother Res. 2010;24:1115–9. doi: 10.1002/ptr.3232. https://doi.org/10.1002/ptr.3232. [DOI] [PubMed] [Google Scholar]
- 8.Taner G, Aydın S, Aytaç Z, Başaran AA, Başaran N. Assessment of the cytotoxic, genotoxic, and antigenotoxic potential of Pycnogenol® in in vitro mammalian cells. Food Chem Toxicol. 2013;61:203–8. doi: 10.1016/j.fct.2013.06.053. https://doi.org/10.1016/j.fct.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Shin IS, Shin NR, Jeon CM, Hong JM, Kwon OK, Kim JC, et al. Inhibitory effects of Pycnogenol® (French maritime pine bark extract) on airway inflammation in ovalbumin-induced allergic asthma. Food and Chem Toxicol. 2013;62:681–6. doi: 10.1016/j.fct.2013.09.032. https://doi.org/10.1016/j.fct.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Choi YH, Yan GH. Pycnogenol inhibits immunoglobulin E-mediated allergic response in mast cells. Phytother Res. 2009;23:1691–5. doi: 10.1002/ptr.2812. https://doi.org/10.1002/ptr.2812. [DOI] [PubMed] [Google Scholar]
- 11.Lau BH, Riesen SK, Truong KP, Lau EW, Rohdewald P, Barreta RA. Pycnogenol as an adjunct in the management of childhood asthma. J Asthma. 2004;41:825–2. doi: 10.1081/jas-200038433. https://doi.org/10.1081/JAS-200038433. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini S, Pishnamazi S, Sadrzadeh SM, Farid F, Farid R, Watson RR. Pycnogenol® in the management of asthma. J Med Food. 2001;4:201–9. doi: 10.1089/10966200152744472. https://doi.org/10.1089/10966200152744472. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Zheng M. Nuclear factor kappa B pathway down-regulates aquaporin 5 in the nasal mucosa of rats with allergic rhinitis. Eur Arch Otorhinolaryngol. 2011;268:73–81. doi: 10.1007/s00405-010-1282-3. https://doi.org/10.1007/s00405-010-1282-3. [DOI] [PubMed] [Google Scholar]
- 14.Xu YY, Liu X, Dai LB, Zhou SH. Effect of Tong Qiao drops on the expression of eotaxin, IL-13 in the nasal mucosa of rats with allergic rhinitis. J Chin Med Assoc. 2012;75:524–9. doi: 10.1016/j.jcma.2012.07.003. https://doi.org/10.1016/j.jcma.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Schoonees A, Visser J, Musekiwa A, Volmink J. Pycnogenol® (extract of French maritime pine bark) for the treatment of chronic disorders. Cochrane Database Syst Rev. 2012;18:CD008294. doi: 10.1002/14651858.CD008294.pub4. https://doi.org/10.1002/14651858.cd008294.pub4. [DOI] [PubMed] [Google Scholar]
- 16.Mei L, Mochizuki M, Hasegawa N. Hepatoprotective effects of pycnogenol in a rat model of non-alcoholic steatohepatitis. Phytother Res. 2012;26:1572–4. doi: 10.1002/ptr.4602. https://doi.org/10.1002/ptr.4602. [DOI] [PubMed] [Google Scholar]
- 17.Tatar A, Parlak SN, Yayla M, Ugan RA, Polat E, Halici Z. Effects of allergic rhinitis and desloratadine on the submandibular gland in a rat allergy model. Int Forum Allergy Rhinol. 2015;5:1164–9. doi: 10.1002/alr.21589. https://doi.org/10.1002/alr.21589. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Zheng M. Nuclear factor kappa B pathway down-regulates aquaporin 5 in the nasal mucosa of rats with allergic rhinitis. Eur Arch Otorhinolaryngol. 2011;268:73–81. doi: 10.1007/s00405-010-1282-3. https://doi.org/10.1007/s00405-010-1282-3. [DOI] [PubMed] [Google Scholar]
- 19.Settipane RA, Schwindt C. Chapter 15: Allergic rhinitis. Am J Rhinol Allergy. 2013;27(Suppl 1):52–5. doi: 10.2500/ajra.2013.27.3928. https://doi.org/10.2500/ajra.2013.27.3928. [DOI] [PubMed] [Google Scholar]
- 20.Ying S, Durham SR, Corrigan CJ, Hamid Q, Kay AB. Phenotype of cells expressing mRNA for Th2-type (interleukin 4 and interleukin 5) and Th1-type (interleukin 2 and interferon gamma) cytokines in bronchoalveolar lavage and bronchial biopsies from atopic asthmatic and normal control subjects. Am J Respir Cell Mol Biol. 1995;12:477–87. doi: 10.1165/ajrcmb.12.5.7742012. https://doi.org/10.1165/ajrcmb.12.5.7742012. [DOI] [PubMed] [Google Scholar]
- 21.Benson M, Strannegård IL, Strannegård O, Wennergren G. Topical steroid treatment of allergic rhinitis decreases nasal fluid TH2 cytokines, eosinophils, eosinophil cationic protein, and IgE but has no significant effect on IFN-gamma, IL-1beta, TNF-alpha, or neutrophils. J Allergy Clin Immunol. 2000;106:307–12. doi: 10.1067/mai.2000.108111. https://doi.org/10.1067/mai.2000.108111. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Barrenäs F, Bruhn S, Mobini R, Benson M. Increased IFN-gamma activity in seasonal allergic rhinitis is decreased by corticosteroid treatment. J Allergy Clin Immunol. 2009;124:1360–2. doi: 10.1016/j.jaci.2009.09.037. https://doi.org/10.1016/j.jaci.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Osguthorpe JD. Pathophysiology of and potential new therapies for allergic rhinitis. Int Forum Allergy Rhinol. 2013;3:384–92. doi: 10.1002/alr.21120. https://doi.org/10.1002/alr.21120. [DOI] [PubMed] [Google Scholar]
- 24.König K, Klemens C, Eder K, San Nicoló M, Becker S, Kramer MF, et al. Cytokine profiles in nasal fluid of patients with seasonal or persistent allergic rhinitis. Allergy Asthma Clin Immunol. 2015;11:26. doi: 10.1186/s13223-015-0093-x. https://doi.org/10.1186/s13223-015-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang HQ, Sun Y, Xu F. Therapeutic effects of interleukin-1 receptor antagonist on allergic rhinitis of guinea pig. Acta Pharmacol Sin. 2003;24:251–5. [PubMed] [Google Scholar]