Abstract
Objectives.
The disablement process can be viewed conceptually as a progression from disease to impairment to functional limitation and finally disability (frequently operationalized as activity limitation). This article assesses the extent to which early phases of the process are associated with individual-level disability trajectories by age.
Method.
We use data from seven waves of the Health and Retirement Study, 1998 to 2010, to investigate for individuals aged 65–84 years how baseline sociodemographic characteristics and self-reported disease, pain, and functional limitation (physical, cognitive, or sensory) are related to the dynamics of limitations in activities of daily living (ADLs). Our modeling approach jointly estimates multiperiod trajectories of ADL limitation and mortality and yields estimates of the number of, shapes of, and factors associated with the most common trajectories.
Results.
Individual probability of ADL limitation can best be described by three common trajectories. In comparison with disease, pain, and functional limitation, sociodemographic characteristics have weak associations with trajectory group membership. Notably, neither sex nor education is strongly associated with group membership in multivariate models.
Discussion.
The analysis confirms the importance of the early phases of the disablement process and their relationships with subsequent trajectories of activity limitation.
Key Words: Disability, Functional health status, Health disparities, Mortality, Transition models
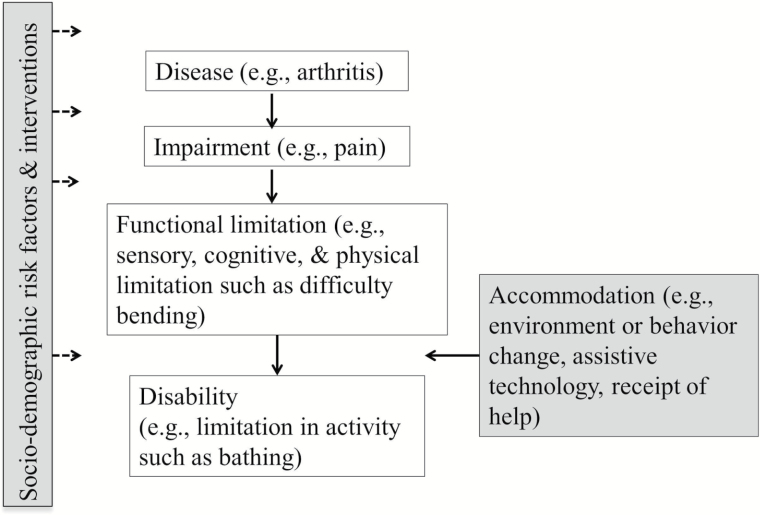
The conceptualization of the disablement process as proposed by Nagi (1965), Pope and Tarlov (1991), and Verbrugge and Jette (1994) is depicted in Figure 1. A disease, such as arthritis, may lead to impairment, such as joint stiffness and pain. Impairment may in turn result in functional limitation (cognitive, sensory, or physical), such as difficulty bending, which may ultimately result in a disability such as a limitation in an activity of daily living (ADL), one of which is bathing. This framework is a stylized representation of an inherently dynamic developmental course (Gill & Kurland, 2003). Such risk factors as sociodemographic attributes as well as interventions may affect onset and progression of each phase, including recovery. Especially in the transition from functional to activity limitation, dynamics may be altered by changes in environment and behavior, assistive technology use, and personal help. Understanding how the phases of the disablement process relate to each other is valuable for both research and clinical purposes, and understanding how activity limitation develops is important for delaying or even preventing it (Guralnik & Ferrucci, 2003; Jette, Assmann, Rooks, Harris, & Crawford, 1998). Our goal is to assess how characteristics measured early in the disablement process are associated with subsequent trajectories of ADL disability.
Figure 1.
Disablement process.
Most national-level analyses of the multiperiod dynamics of the disablement process focus on changes over time at the individual level in indicators of the last two phases, namely, functional and activity limitations (e.g., Liang et al., 2008, 2010; Taylor, 2011; Yang & Lee, 2010; Zimmer, Martin, Jones, & Nagin, 2014; Zimmer, Martin, Nagin, & Jones, 2012). Analyses typically assess how sociodemographic risk factors such as sex (Liang et al., 2008) and education (Taylor, 2011), among others, are related to trajectories of functional and activity limitations. Many studies also look at the association of disease, the first phase of the disablement process, with limitation trajectories (Gill, Gahbauer, Han, & Allore, 2010; Liang et al., 2008). Data on impairment, the second phase, are typically not collected through population-level surveys, and analyses of the relation of impairment to subsequent trajectories of functional and activity limitation are rare if not nonexistent. Also fairly uncommon are analyses that attempt to model how the third phase, functional limitations, is associated with multiperiod patterns or trajectories of activity limitations, thus explicitly probing the last transition posited in the disablement process. Guralnik and colleagues (1995, 2000) show that lower-extremity function, as indicated by performance measures, is predictive of ADL disability at a later period, but do not examine multiperiod trajectories. Dodge, Du, Saxton, & Ganguli (2006), a study of older nondemented Pennsylvanians, finds that relatively poor baseline cognitive function is predictive of poorer trajectories of instrumental activities of daily living (IADL), but physical and sensory functional limitations are not included in their models. In an article focusing primarily on the influence of individual diseases on disability trajectories, Taylor and Lynch (2011) find that sensory limitations play only minor roles in trajectories of a combined indicator of instrumental and basic activity limitations, but they do not examine the roles of cognitive and physical functional limitations.
Thus, there is an abundance of excellent research probing various links in the disablement process, but to our knowledge, no analysis that specifically focuses on assessing at one time the predictive value of all three of the early stages of the process on the evolution of late-life disability. Ideally, one would make such an assessment in a fully dynamic model with changes in the first three phases being related to changes in the last phase. Such a model might also allow for feedback loops, for example, the development of secondary conditions as a result of disability (Field & Jette, 2007). Unfortunately, currently available data sources do not allow modeling the disablement process in all its complexity. Nevertheless, given the extent to which this conceptual framework is invoked in disability research and given the interest in understanding the individual experiences underlying population-level trends in late-life disability, we believe that it is of value to model the relation of the early phases of the process measured in the cross-section to the dynamics of the last phase. The idea is to see whether baseline indicators of one of the three early phases of the disablement process give us relatively more information than the others about how disability develops over time.
This article uses seven waves of longitudinal data from the Health and Retirement Study (HRS) for 1998 to 2010 to examine how sociodemographic risk factors, self-reported diseases, a global pain index, and sensory, cognitive, and physical functional limitations at baseline are associated with multiperiod trajectories of limitations in ADLs. Activity limitation and mortality trajectories are jointly estimated (Haviland, Jones, & Nagin, 2011). Such an approach is particularly important given that attrition from panel surveys of older adults is typically from mortality, which is not random but is associated with activity limitation. We identify the number and shapes of the most common trajectories of activity limitation and estimate the association of the baseline indicators of the earlier phases of the disablement process, as well as various sociodemographic risk factors, with the probability of membership in a particular trajectory group.
Method
Data
The HRS, a nationally representative biennial survey of Americans aged 50 and older, was launched in 1992 (University of Michigan, 2014). For purposes of our analysis, we limit the sample to ages 65–84 in 1998, the year when many aspects of the survey as currently conducted were established. We begin with age 65, because data on some of the cognitive function measures of interest were not collected for those who were younger. We use data from seven biennial waves from 1998 to 2010, when surviving members of our sample would typically be aged 77–96 years. Our initial sample of 9,471 includes 5,327 women and 4,144 men with responses regarding activity limitation for at least one survey wave (only 6 cases excluded from the original 9,477). Because our focus is on identifying the most common ADL limitation trajectories of this baseline sample and on assessing how baseline characteristics are associated with subsequent trajectories, we use 1998 cross-sectional weights in all models.
Except for outcome measures, missing item responses of which there were few were recoded as 0 for categorical variables and as sex-specific means for continuous variables. We assessed sensitivity of results to this recoding of missing item response by re-estimating models including flags indicating missing values. In only one instance, as will be mentioned, were results materially different, but the difference is not of importance for our conclusions. Because of the very small number of missing responses for most variables, including the missing flags in models occasionally resulted in singular or asymmetric variance matrices, which prevented calculation of standard errors. Accordingly, we opted not to include missing flags in models whose results are presented here.
Outcome Measures
At each survey wave respondents were asked whether, because of a health, physical, or emotional problem, they have difficulty expected to last at least 3 months with six ADLs (dressing, walking across a room, bathing, eating, getting in or out of bed, and using the toilet). HRS respondents who reported no difficulty dressing and only one difficulty with physical functions (described in Functional Limitation Measures) were not asked the rest of the ADL questions and were assumed to have no difficulty with any ADL. For each wave and individual, we constructed a summary indicator of any ADL difficulty, which is equal to 1 if the respondent reported one or more difficulties, 0 if all six questions were answered and no difficulties reported, and missing otherwise. Other specifications, such as a continuous measure involving counts of ADL limitations, were considered. However, our purpose is to examine how earlier phases of the disablement process relate to reaching the last phase of the disablement process, disability. Therefore, we have used a binary specification indicating difficulty with at least one ADL, rather than number or severity of ADL limitations. Table 1 shows that in 1998, 18.3% of the sample reported one or more ADL difficulty.
Table 1.
Weighted Frequencies of Variables at Baseline in 1998 for Analysis Sample (n = 9,471)
| % or mean | % missing | |
|---|---|---|
| Any ADL difficulty | 18.31 | — |
| Age (years; mean) | 73.06 | — |
| Female | 57.17 | — |
| Black | 8.30 | 0.05 |
| Hispanic | 4.99 | 0.06 |
| Poor child health | 1.72 | 0.24 |
| Childhood SES score (0–7 adversities; mean)a | 2.03 | 28.38 |
| Education (years; mean) | 11.78 | 0.31 |
| Married | 57.97 | 0.08 |
| Urban residence | 42.65 | — |
| Residence in the South | 35.01 | 0.08 |
| Arthritis ever | 54.10 | 0.08 |
| Cancer ever | 13.59 | 0.11 |
| Diabetes ever | 14.45 | 0.07 |
| Heart problem ever | 26.91 | 0.06 |
| Lung problem ever | 8.77 | 0.06 |
| Psychological problem ever | 9.06 | 0.04 |
| Stroke ever | 9.40 | 0.06 |
| BMI < 19.5 | 5.26 | 1.08 |
| 19.5 ≤ BMI < 25.0 | 37.66 | 1.08 |
| 25.0 ≤ BMI < 30.0 | 39.39 | 1.08 |
| 30.0 ≤ BMI < 35.0 | 13.39 | 1.08 |
| 35.0 ≤ BMI < 40.0 | 3.15 | 1.08 |
| BMI ≥ 40.0 | 1.16 | 1.08 |
| Pain level (0–3; mean) | 0.51 | 0.15 |
| Any physical functional difficulty | 69.92 | 5.80 |
| Cognitive summary score (0–35; mean) | 22.17 | 7.72 |
| Poor eyesight | 6.42 | 0.03 |
| Poor hearing | 6.14 | 0.06 |
| Proportion of responses per individual by proxy (mean) | 11.72 | — |
| Proportion of interviews per individual face-to-face (mean) | 48.33 | 0.00 |
Notes. ADL = activity of daily living; BMI = body mass index; SES = socioeconomic status.
aSee Montez and Hayward (2014) for details.
Information about death was ascertained at follow-up. By the 2010 wave, 4,832 or 51% had died. An additional 757 or 8% were otherwise missing.
Sociodemographic Risk Factors
We focused on variables that have been found to be associated with ADLs in the cross-section and that are unlikely to change as the onset of a limitation is recognized. So, for example, we included whether someone was married, but we did not include living arrangements. The former typically occurs earlier in life, whereas the latter may change in concert with changes in limitations. Nor did we include a measure of income because activity limitation may affect one’s financial well-being. Baseline distributions of the measures we use are shown in Table 1.
We considered including time-varying indicators of some of these variables, but for several reasons decided not to. Most importantly, as will be discussed in the Statistical Analysis subsection, we were not able to use a time-varying specification for all indicators of the first three phases of the disablement process, our primary interest. Moreover, supplementary analysis (results not shown) that included as a time-varying covariate marital status, the most likely sociodemographic candidate for this alternative specification, indicated that being married had similar effects on the shapes of each of the common ADL trajectories that best fit the data. We found the marital status variable much more informative as a baseline indicator of risk of membership in the most common trajectory groups that we otherwise identified.
Based on previous findings, we expected being older, a woman, Black, Hispanic, or a resident of the South to be associated with a higher probability of ADL limitation (Liang et al., 2008; Murray et al., 2006), but education, marriage, and urban residence to be protective (Schoeni, Freedman, & Martin, 2008). There is a growing literature documenting the influence of early-life factors on late-life health (Freedman, Martin, Schoeni, & Cornman, 2008). We explored two indicators of childhood well-being, namely, childhood health and a childhood socioeconomic status (SES) score based on several potential adversities: low education of father, low education of mother, family poverty, family move for financial reasons, family received financial help, father had a blue collar occupation, and never lived with father (Montez & Hayward, 2014).
Disease and Impairment Measures
HRS asked about diseases, generally in the form of “Has a doctor ever told you that you have . . . .” Among the disease groups that we use in our models (arthritis, cancer, diabetes, heart problem, lung problem, psychological problem, stroke, and obesity), the one exception is arthritis, the question for which was in the form of “Have you ever had or has a doctor ever told you that you have arthritis or rheumatism.” Heart problem included heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems. Lung problem included chronic conditions such as bronchitis and emphysema, and psychological problem was defined as an emotional, nervous, or other psychiatric problem. Body mass index (BMI), an indicator of obesity, which the American Medical Association (AMA) has recognized as a disease (AMA, 2013), was calculated using self-reported weight and height.
Pain, our only indicator of the second phase of the disablement process, was ascertained via two questions: (i) Are you often troubled by pain and (ii) how bad is the pain most of the time (mild, moderate, severe)? We generated a pain-level indicator that ranged from 0 for none to 3 for severe.
Functional Limitation Measures
For physical functional limitation, questions similar in format to the ADL questions asked about sitting 2 hours, getting up from a chair, climbing several flights of stairs, lifting and carrying 10 pounds, stooping, picking up a dime, reaching arms above the shoulders, moving a large object, and walking several blocks. We constructed a summary indicator of any physical function difficulty, which is 1 if the respondent reported one or more difficulties, 0 if all questions were answered and no difficulties reported, and missing otherwise. As shown in Table 1, more than two thirds of respondents reported difficulty with at least one physical function at baseline.
Cognitive function was ascertained using a modified Telephone Interview Cognitive Screen (maximum score of 35). Twenty points related to immediate and delayed recall, 4 points to date orientation, 4 points to word recognition and naming the president and vice president, 2 points to backwards counting, and 5 points to serial-7 subtraction.
Respondents were asked in 1998 to rate on a 5-point scale their eyesight, using glasses or corrective lenses. We created a variable indicating poor eyesight, including the lowest category on the scale and reports of being legally blind. Responses to a question regarding hearing (with a hearing aid if used) were handled similarly.
Statistical Analysis
There were two stages in the modeling process that were ultimately carried out simultaneously. The first identified common trajectories of ADL difficulty and mortality, and the second estimated associations of baseline risk factors with these trajectories. We used a group-based trajectory (GBT) model developed by Nagin and colleagues (Haviland et al., 2011; Jones & Nagin, 2007; Jones, Nagin, & Roeder, 2001; Nagin, 1999, 2005). The approach is based on finite mixture modeling and uses maximum likelihood estimation to identify groups of individuals following a discrete number of common patterns. Early applications of this technique and other approaches used to investigate multiperiod trajectories of late-life limitation (e.g., hierarchical linear models) have assumed that all attrition (including both loss to follow-up and mortality) is random (clearly not a good assumption in late life), have examined decedents and survivors separately, or have included a control variable for having died (which is conceptually awkward because disability precedes mortality). Exceptions include Taylor and Lynch’s modeling of mortality as a distal outcome in a latent class analysis of disability trajectories (2011) and Wolf and colleagues’ (2015) incorporation of imputed time-to-death in disability trajectory modeling (2015).
More straightforward is the recent enhancement of the GBT approach to allow for the joint modeling of the outcome of interest and nonrandom missingness (Haviland et al., 2011). Using this methodology, we were able to model mortality probabilities by age jointly with the estimation of ADL disability trajectories. Because mortality represents the vast majority of attrition in our data set (4,832 of 5,589 drop-out cases), this approach substantially reduces the number of cases for which attrition is assumed to be random (n = 757). The joint modeling of ADL trajectories with mortality treats death as more than a separate outcome; it permits estimation of disability trajectories by age that account for whether individuals within each trajectory group are more or less likely to die by a given age. Each individual’s unconditional likelihood of ADL limitation, which is maximized in the estimation procedure, and the estimated size of each trajectory group are functions of the probability of dying (Haviland et al., 2011). This approach has been applied by Zimmer and colleagues in analysis of ADL and mortality trajectories in China (2012) and physical functional limitation and mortality trajectories in Taiwan (2014). The details of this enhanced technique and the related TRAJ plug-in for STATA are provided elsewhere (Haviland et al., 2011; Jones, 2014; Jones & Nagin, 2012, 2013; Zimmer et al., 2012).
Outputs of the GBT model include (i) the number of trajectory groups that most efficiently characterize the course of limitation, (ii) coefficients that describe the shape of the limitation trajectory by age for each group, and (iii) the estimated proportion of the population at baseline most likely to follow each trajectory. Given the dichotomous nature of our primary outcome, any ADL difficulty, trajectories were modeled as logit functions of age. Age is measured at each survey wave and is linked to the outcome at that age. We investigated first-, second-, and third-order polynomials of age. By exploring higher order polynomials of age, we allowed for the possibility that the recovery from ADL limitation that regularly occurs at the individual level could be represented by the shape of one of the common trajectory groups we identified. Mortality trajectories were modeled as logit functions of linear age at last survey wave. To facilitate model convergence (Jones, 2014), age was scaled by subtracting 77, the average age of our sample’s respondents across all seven waves, and dividing by 10.
Different numbers of groups and possible specifications of age for each group were investigated. The best base model was chosen on the basis of the largest Bayesian Information Criterion (BIC) and two diagnostic tests suggested by Nagin (2005). The BIC [calculated by the TRAJ software plug-in as equal to log-likelihood – 0.5 * (number of parameters) * ln (sample size)] takes into consideration both explanation of variance (log-likelihood) and a penalty for adding variables and possibly overfitting. The BIC as calculated here is negative, so the largest BIC is the least negative one.
The two diagnostic tests proposed by Nagin (2005) are based on posterior probabilities, which are calculated postestimation and indicate the probability that an individual belongs to each of the groups in the model. For each individual, the posterior probabilities sum to one, and the largest posterior probability for each individual is an indication of the most likely group to which the individual belongs. One test of model fit is that the average posterior probability across individuals who are most likely to belong to a particular group is .70 or higher, a threshold proposed by Nagin (2005). A second looks for similarity in proportions of the sample associated with the groups on the basis of highest posterior probabilities and proportions generated by the maximum likelihood assignments.
After ascertaining the best base trajectory model and the associated number of groups and specification of age for each group, we re-fit the model simultaneously estimating the association of baseline sociodemographic characteristics, disease, pain, and functional limitation to membership in specific ADL trajectory groups using a multinomial logistic regression. Our aim was to determine using BIC scores which variables when added to the best base trajectory model provided the best fit. All the variables added in this second stage of the modeling were assessed at baseline. It would have been possible to include time-varying specifications of pain and functional limitations in the initial investigation of the best base trajectory model, but given the nature of the disease questions (has a doctor ever told you that you have . . . ), such a specification would not have been possible for diseases. Although we would have been able to measure onset of a disease, we would not have been able to measure recovery. We thought it would be important to treat the first three phases of the disablement process equally, so opted for the baseline specification of all risk factors included in the second stage of the analysis (multinomial logistic regression). In any case, interpretation of results of base trajectory models using additional time-varying variables beyond age would have been complicated by the 2-year survey interval and the possibility of feedbacks such as the development of secondary conditions as a result of disability.
In all our multinomial regression models of the various risk factors for membership in different trajectory groups, we also controlled for the proportion of survey waves for which the respondent’s information was obtained from a proxy and for the proportion of survey waves for which the questionnaire was administered to the respondent face-to-face as opposed to by phone. Several studies have found that proxy respondents are more likely to report limitation than are respondents themselves (Rodgers & Miller, 1997; Santos-Eggimann, Zobel, & Berod, 1999; Todorov & Kirchner, 2000), and Rodgers and Miller (1997) have documented an association between face-to-face interview and reports of greater ADL limitation.
Results
Table 2 shows the results from the best-fitting base trajectory model, which has three groups. Age is modeled with a linear specification for the ADL limitation trajectories of Groups 1 and 3, and a quadratic for Group 2. Maximum likelihood estimation assigned about 40% of the baseline sample each to Groups 1 and 2 and less than 20% to Group 3. Among those most likely to belong to each group on the basis of posterior probabilities, average posterior probabilities for each group are .83, .79, and .83, respectively, well above the .70 criterion suggested by Nagin (2005). Group assignments based on posterior probabilities are also similar to maximum likelihood group assignments, 40.8 versus 39.5% for Group 1, 41.9 versus 42.8% for Group 2, and 17.4 versus 17.7% for Group 3. We also fit the base model separately by sex, but results for each sex (not shown) were substantially the same as those with sexes combined that are presented here.
Table 2.
Maximum Likelihood Logit Results for ADL Limitation and Mortality Trajectories from Best Base Model (n = 9,471)
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Parameters for any ADL limitation trajectory | |||
| Intercept | −4.849 (0.255)*** | −1.302 (0.139)*** | 1.685 (0.141)*** |
| Linear scaled age | 3.045 (0.160)*** | 1.930 (0.066)*** | 1.464 (0.118)*** |
| Quadratic scaled age | — | 0.521 (0.088)*** | — |
| Parameters for mortality trajectory | |||
| Intercept | −3.331 (0.211)*** | −1.851 (0.049)*** | −1.222 (0.049)*** |
| Linear scaled age at previous wave | 1.800 (0.189)*** | 1.135 (0.061)*** | 0.804 (0.076)*** |
| Group size (%) | 39.5 | 42.8 | 17.7 |
| BIC = −33481.99 | |||
Notes. Standard errors are in parentheses. ADL = activity of daily living; BIC = Bayesian Information Criterion.
***p < .001.
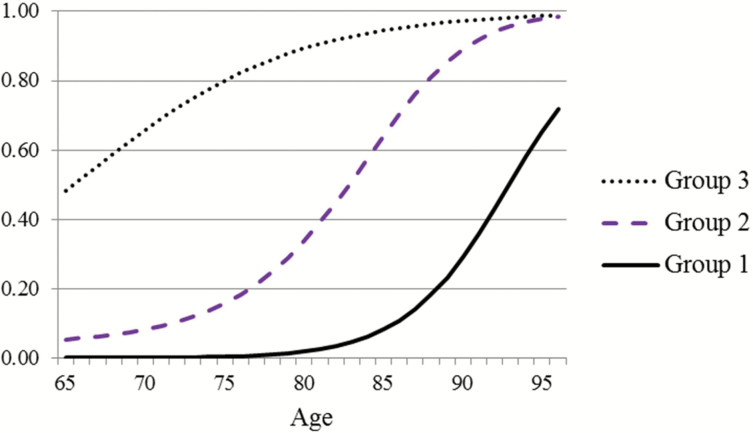
Figure 2 shows for each group the predicted probabilities by age of ADL limitation. For more than 80% of the sample who are members of Groups 1 and 2, probability of any ADL limitation remains relatively low until age 80, but for Group 3, the probability is already .48 at age 65. For all three groups, probabilities are high by the mid-90s.
Figure 2.
Predicted probability of any activity of daily living limitation by age and group.
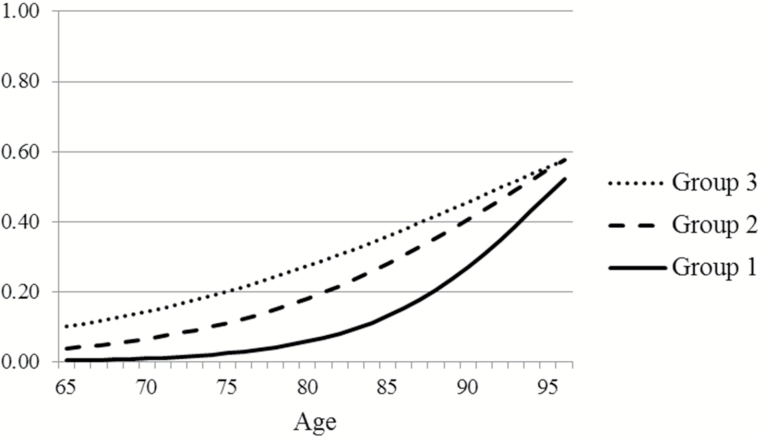
Predicted probabilities of mortality by age in Figure 3 follow a hierarchy by group similar to that of ADL limitation, with Group 1 lowest and Group 3 highest. However, as indicated by the greater vertical distance among the ADL trajectories than among the mortality trajectories, at most ages, differentials across groups in the probability of having an ADL disability are greater than differentials in the probability of dying. For example, mortality probabilities for Groups 2 and 3 are similar to each other, which suggests that members of Group 3 may spend a longer period with ADL limitation at the end of their lives than members of Group 2. Indeed, when the age at which the probability of having an ADL disability reaches .50 is compared with the age at which the probability of dying reaches .50, for Group 1, the ages are 93 and 96 years, respectively, yielding a difference of 3 years; for Group 2, the ages are 83 and 94 years with a difference of 11 years; and for Group 3, the ages are 66 and 93 years with a difference of 27 years. Thus, for people most likely to be members of Group 1, the chances are good that they will survive well into old age without an ADL disability. In contrast, for people most likely to be members of Group 3, the results suggest that a substantial proportion will live a number of years with an ADL disability.
Figure 3.
Predicted probability of mortality by age at previous survey wave and group.
To assess how the disability results might be affected by not jointly modeling attrition due to mortality, we re-fit the basic model without jointly modeling mortality. The results (not shown) of such a model with age similarly specified (linear in Groups 1 and 3 and quadratic in Group 2; which was the best specification in this case also) indicate that not modeling mortality results in an overestimation (47.2% vs 39.5%) of membership in Group 1, the group with the lowest probabilities of any ADL disability, and underestimation of membership in Groups 2 (37.0% vs 42.8%) and 3 (15.8% vs 17.7%). The shapes of the three trajectories also are affected. The general impression is that ignoring mortality and assuming that it is random results in an underestimation of the probability of disability, which is what one would expect if there is a greater likelihood of disability among those about to die.
Table 3 shows the association of sociodemographic factors, disease, pain, and functional limitations with trajectory group membership. Bivariate results are for models with base trajectory specification as reported in Table 2 plus a multinomial logit regression with the specific variable, controls for proportion of waves answered by proxy and proportion of waves with face-to-face interviews, and a constant. For each variable, the table presents logit coefficients for risk of membership in Groups 2 and 3 versus Group 1, the reference category. Also shown for each variable is the BIC from its bivariate model. Sensitivity analyses that included missing flags in the bivariate models resulted in smaller (worse) BICs than for comparable bivariate models without flags in all cases except for that of childhood SES score. However, in either specification, coefficients on childhood SES were very small, and sensitivity analysis in multivariate models indicated that inclusion of the missing flag did not alter results substantively.
Table 3.
Results From Multinomial Logit Risk-Factor Estimation of Group Membership (n = 9,471)a
| Bivariate models | Sociodemographic model | Disease model | Functional limitation model | Full model | Parsimonious model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficients | Coefficients | Coefficients | Coefficients | Coefficients | Coefficients | |||||||||
| Group 2 | Group 3 | Group 2 | Group 3 | Group 2 | Group 3 | Group 2 | Group 3 | Group 2 | Group 3 | Group 2 | Group 3 | |||
| vs | vs | BIC | vs | vs | vs | vs | vs | vs | vs | vs | vs | vs | ||
| Group 1 | Group 1 | BIC | order | Group 1 | Group 1 | Group 1 | Group 1 | Group 1 | Group 1 | Group 1 | Group 1 | Group 1 | Group 1 | |
| Female | −0.016 | 0.471*** | −33,171.81 | 16 | −0.129 | 0.289** | −0.186† | 0.007 | ||||||
| Black | 0.707*** | 1.025*** | −33,162.95 | 15 | 0.482*** | 0.674*** | 0.424** | 0.773*** | 0.486** | 0.798*** | ||||
| Hispanic | 0.334* | 0.739*** | −33,184.49 | 20 | −0.028 | 0.230 | 0.215 | 0.671** | ||||||
| Poor child health | 0.358 | 1.285*** | −33,180.89 | 18 | 0.253 | 1.100** | −0.245 | 0.004 | ||||||
| Childhood SES score | 0.080** | 0.181*** | −33,176.82 | 17 | 0.001 | 0.063† | −0.051 | −0.059 | ||||||
| Years of education | −0.091*** | −0.144*** | −33,107.67 | 12 | −0.080*** | −0.107*** | −0.023 | 0.010 | ||||||
| Married | −0.336*** | −0.756*** | −33,141.42 | 13 | −0.295*** | −0.521*** | −0.278** | −0.506*** | −0.230** | −0.492*** | ||||
| Urban residence | −0.028 | −0.100 | −33,194.66 | 22 | 0.029 | 0.002 | 0.051 | 0.049 | ||||||
| Residence in South | 0.138† | 0.329*** | −33,185.81 | 21 | 0.073 | 0.153† | 0.061 | 0.133 | ||||||
| Arthritis ever | 0.906*** | 1.797*** | −32,912.58 | 3 | 0.869*** | 1.623*** | 0.483*** | 0.695*** | 0.486*** | 0.743*** | ||||
| Cancer ever | 0.315** | 0.525*** | −33,181.92 | 19 | 0.325** | 0.479** | 0.297* | 0.584*** | 0.277* | 0.545*** | ||||
| Diabetes ever | 1.238*** | 1.843*** | −33,044.47 | 7 | 1.163*** | 1.556*** | 1.145*** | 1.441*** | 1.181*** | 1.545*** | ||||
| Heart problem ever | 0.558*** | 1.197*** | −33,082.32 | 11 | 0.338** | 0.735*** | 0.088 | 0.443*** | 0.121 | 0.431*** | ||||
| Lung problem ever | 1.451*** | 2.227*** | −33,064.20 | 10 | 1.532*** | 2.125*** | 1.397*** | 1.922*** | 1.416*** | 1.884*** | ||||
| Psychological problem ever | 1.223*** | 2.300*** | −33,031.61 | 4 | 1.138*** | 2.049*** | 0.849*** | 1.443*** | 0.811*** | 1.416*** | ||||
| Stroke ever | 0.576*** | 1.884*** | −33,049.07 | 8 | 0.706*** | 1.879*** | 0.490* | 1.576*** | 0.462* | 1.521*** | ||||
| 19.5 ≤ BMI<25.0 | −0.071 | −0.916*** | −33,039.06 | 6 | −0.068 | −0.852*** | 0.067 | −0.445† | ||||||
| 25.0 ≤ BMI<30.0 | 0.075 | −0.476** | 0.049 | −0.447* | 0.152 | −0.053 | ||||||||
| 30.0 ≤ BMI<35.0 | 0.603* | 0.421* | 0.457† | 0.318 | 0.426 | 0.597* | ||||||||
| 35.0 ≤ BMI<40.0 | 1.417*** | 1.805*** | 1.231** | 1.661*** | 1.240* | 1.785*** | 1.062** | 1.809*** | ||||||
| BMI ≥ 40.0 | 1.834* | 3.017*** | 1.175† | 2.492*** | 1.106 | 2.586*** | 0.923 | 2.620*** | ||||||
| Pain level | 0.700*** | 1.406*** | −32,626.00 | 2 | 0.460*** | 1.021*** | 0.449*** | 1.017*** | ||||||
| Physical functional limitation | 1.591*** | 4.599*** | −32,473.95 | 1 | 1.324** | 2.706*** | 1.120*** | 3.455*** | 1.118*** | 3.520*** | ||||
| Cognitive summary score | −0.086*** | −0.133*** | −33,036.10 | 5 | −0.114** | −0.075*** | −0.062*** | −0.108*** | −0.070*** | −0.110*** | ||||
| Poor eyesight | 0.909*** | 2.246*** | −33,061.92 | 9 | 0.149 | 1.275*** | 0.397† | 1.321*** | 0.429† | 1.335*** | ||||
| Poor hearing | 0.729*** | 1.283*** | −33,158.79 | 14 | 0.917† | 0.430** | 0.361 | 0.552* | ||||||
| BIC | −33,100.29 | −32,392.20 | −32,419.81 | −31,818.35 | −31,758.34 | |||||||||
Notes. aAll models (bivariate and multivariate) have the same base trajectory specification as reported in Table 2 and include controls for proportion of responses per individual by proxy, proportion of interviews per individual that are face-to-face, and a constant. BIC = Bayesian Information Criterion; BMI = body mass index.
† p < .10. *p < .05. **p < .01. ***p < .001.
For sake of comparison, we ranked bivariate models by size of BIC. The bivariate model with physical functional limitation has the largest BIC as calculated by TRAJ of −32,473.95, followed by pain level (−32,626.00), arthritis (−32,912.58), psychological problem (−33,031.61), and cognition (−33,036.10). Based on Jones et al., 2001, we highlight only differences between BICs of 5 or more, thus, the difference between the last two is marginal. BICs for the sociodemographic risk factors tend to be smallest, suggesting that those factors are least successful in predicting probability of group membership. All coefficients on the variables are statistically significant except for women, poor child health, and the two lower BMI categories for the Group 2 versus Group 1 comparison and urban for both comparisons.
Next in Table 3, we show a model with all the sociodemographic risk factors, a second that includes all diseases, and a third that includes the four functional limitations. Because we had only one variable representing the impairment stage of the disablement process, namely, pain, the results for the impairment (pain) model are reported in the first column, which shows bivariate results. Diseases as a group appear to be comparatively important (BIC = −32,392.20), followed by functional limitations (−32,419.81), pain (−32,626.00), and finally, sociodemographic risk factors (−33,100.29).
In the disease model, having a lung problem or a BMI in the two categories of 35 and above generally resulted in the greatest risks (largest coefficients) of higher versus lower group membership. However, having a BMI less than 35 is much less associated with differential group membership. Other relatively important disease variables are arthritis, diabetes, psychological problems, and stroke. Coefficients on the cancer and heart disease variables are significantly positive, but small. In moving from bivariate models to the disease model, the sizes of coefficients on heart problem appear to be substantially reduced.
The pain coefficients are not comparable to the disease coefficients given the continuous nature of the pain variable. Notably, in a model with just pain and arthritis (results not shown), the sizes of the pain coefficients are only slightly attenuated in comparison to those from the pain bivariate model, whereas that for the Group 3 versus Group 1 comparison for arthritis is substantially reduced from the arthritis bivariate and disease group models (1.241; down from 1.797 and 1.623, respectively).
In the functional limitation model, coefficients on physical functional limitation remain large, although smaller than in the bivariate model. Coefficients on poor eyesight and poor hearing are also substantially reduced, and poor hearing is no longer significantly associated with Group 2 versus Group 1 membership. The coefficients for cognition are relatively stable, but because of the continuous nature of the variable are not comparable to coefficients on the other three functional limitation variables.
Next in Table 3 is the full model in which all variables are included. The BIC is −31,818.35, the largest thus far. In this model, among the sociodemographic risk factors, significant coefficients remain only for women (marginally for G2 vs G1), Black (both comparisons), Hispanic (G3 vs G2), and being married (both comparisons). All the disease variables and the pain variable remain significant except for the first comparison (G2 vs G1) for heart problem and some of the lower BMI categories, as well as BMI greater than or equal to 40 for the first comparison. In the last case, the result may be a function of the very small proportion of the sample in this category (1.16%). The size of the coefficients for arthritis and psychological problem are substantially smaller than in the disease model.
In the final phase of modeling we sequentially dropped variables from the full model and compared BICs. First to be dropped were variables for which the p values on both their coefficients were .10 or greater, namely, poor child health, childhood SES, education, urban residence, and Southern residence. The resulting model (not shown) had a BIC of −31,777.98, larger than the BIC on the full model, indicating a good tradeoff between explanatory power and number of variables included. Subsequent models (not shown) dropped women and categories of BMI less than 35, then hearing, and finally Hispanic. In the final model, which we label the parsimonious model in Table 3, at least one coefficient for each variable has a p value of less than .05, and the BIC is −31,758.34. Dropping additional variables did not substantially raise the BIC. Of sociodemographic variables, only being Black and being married remain in the parsimonious model. Included are all disease variables, pain, and three of the four functional limitation variables—physical, cognitive, and vision.
Discussion
The probability of ADL limitation by age for older Americans from 1998 to 2010 can best be described by three common trajectories. Members of the group with lowest overall probabilities of ADL limitation at all ages (39.5% of the baseline sample) experienced little chance of ADL difficulty until their mid-80s. In contrast, members of the group with highest probabilities (17.7% of baseline sample) already had an almost 50–50 chance of limitation by age 65, and the probability increased to almost 1.0 by age 90. The mortality probability trajectories of the three groups followed the same hierarchy as ADL limitation trajectories with those in the highest ADL difficulty probability group experiencing the highest probabilities of dying. However, at most ages, the differentials in probability by group were considerably larger for ADL limitation than for mortality. As a result, those in the highest probability group were likely to spend years with limitation before dying, even though their probabilities of dying were relatively high.
In comparison with disease, pain, and functional limitation, sociodemographic characteristics had weak associations with trajectory group membership. This result confirms the importance of the early phases of the disablement process and their relationships with subsequent trajectories of activity limitation.
Neither sex nor education was influential in multivariate models including the other types of factors. Previous analysis of HRS data for the 50-and-older population from 1995 to 2006 (Liang et al., 2008) found using a growth-curve model that SES and prior health status could at least partially explain gender differences in changes in ADL and IADL limitations. Here, we analyze HRS data for an older population and a later period and use a different statistical approach. In additional analysis (results not shown), we added women to the disease, pain, and functional limitation models. The addition to the pain model resulted in virtually no change in the BIC and a substantial reduction in the coefficient for women on the Group 3 versus Group 1 comparison relative to the bivariate female model result (0.245 vs 0.471). These results suggest that besides operating though other sociodemographic factors (as indicated by comparison of the bivariate and sociodemographic models), the association of being women with higher ADL limitation trajectory group membership may be operating indirectly through reported pain.
For education, when we engage in a similar exercise (results not shown), the greatest reductions in coefficient sizes occur when education is added to the functional limitations model. The two coefficients were reduced from −0.091 and −0.144 in the bivariate education model to −0.035 and −0.047, respectively, in the functional limitations plus education model. Thus, the beneficial influence of education may operate indirectly on group membership via functional limitations. It could be that those with less education are more likely to have a functional limitation, given that they have a disease. Other studies focusing on the role of education have found that education may be important for onset of functional and activity limitations but not for progression (Taylor, 2011; Zimmer & House, 2003; Zimmer, Liu, Hermalin, & Chuang, 1998).
Being Black is persistently associated with worse ADL trajectories across models, whereas being married is associated with better trajectories. The former may reflect the cumulative disadvantage that Blacks experience throughout their lives, differential severity and management of disease, differential accommodation of limitations, and differential self-reporting of activity limitation. The latter may reflect the benefits of social support through marriage, differential management of disease, and differential accommodation of limitations. The marriage result also may partially be a measurement artifact, because the HRS question does not specify whether difficulty with activities is to be assessed with or without assistance. It is only after difficulty is acknowledged that a question about receipt of help is asked. Thus, some married people who receive help from spouses may indicate no difficulty, whereas unmarried people may answer the question in terms of difficulty without assistance.
Diseases, which represent the first phase of the disablement process, as a group appear to be the most important in explaining ADL trajectory group membership. The bivariate model with the pain variable, representing the second phase, yields a larger BIC from its bivariate model than those of any of the individual disease bivariate models, but does not do as well as the diseases taken together. Among the disease variables, the largest coefficients in the multivariate models are associated with obesity, lung problem, psychological problem, stroke, arthritis, and diabetes.
Physical and cognitive limitations are significantly associated with membership in groups with higher probability of ADL limitation, as indicated by their bivariate models having the first and fifth largest BICs, respectively. Poor eyesight is also important. Poor hearing, although associated with trajectory group membership bivariately, is not included in the parsimonious model. That hearing is the least associated of the functions with ADL trajectory group membership makes sense given the nature of the specific ADLs (e.g., bathing and dressing). Such may not have been the case had this study focused on trajectories of IADLs, such as shopping and using the telephone.
Looking to the future, the more complex marital histories of the Baby Boom generation may not augur well for future ADL trajectories of the older population, although other forms of social support may be substituted for marriage. It is difficult to predict changes in prevalence of specific diseases, because prevalence is a function of incidence and survival, which in turn are based on prevention and management. Using self-reports of diseases was not ideal for our modeling purposes because reporting may be influenced by access to health care, diagnosis criteria, and health literacy, among other factors. Temporal improvements among older Americans in vision (Martin & Schoeni, 2014) and cognitive function (Sheffield & Peek, 2011) bode well for future ADL trajectories. At the same time, there is evidence of recent increases in physical functional limitations among those aged 40 to 64 and those 65 and older(Martin & Schoeni, 2014), which suggests that, given how physical functional and ADL limitations are linked, all things equal, more older Americans would be members of worse ADL trajectory groups in the future.
An important weakness in this effort to understand better the disablement process and in particular to understand the influence of functional limitations on activity limitation trajectories is the failure to include information on the environment in which activities are carried out, the use of assistive technology, and modification of how activities are performed. Disability or activity limitation is not simply a function of underlying capacity of the individual (as represented by physical, cognitive, and sensory function measures) but rather reflects a gap between that capacity and the demands of the particular activity in the circumstances in which it is being conducted. Although HRS had an experimental module focused on environment and home modification in 2006, accommodations that may influence the course of the disablement process have not been a focus of regular HRS data collection. The new National Health and Aging Trends Study will be an important resource for investigating the influence of these domains in the future (NHATS, 2014).
Also, we would have ideally used performance tests as well as self-reports of physical function, but only in 2006 did HRS begin to conduct these tests on alternate halves of the sample by wave. Thus, multiple waves of ADL data subsequent to these tests are not yet available for the entire sample.
Beyond the global pain measure that we use, additional indicators of impairment such as pain in specific areas of the body and weakness of upper and lower body could also have proven informative. The formulation of the HRS questions regarding disease in terms of ever having experienced it precluded our considering how the evolution of disease prevalence is related to the development of ADL limitation. Because age at baseline varied across individuals and because disease incidence could have occurred at any time before that age, the meaning of disease at baseline differs across individuals. For example, in our analysis, we were not able to distinguish the effect on disability trajectory group membership of the onset of a condition prior to age 65 from the effect of its onset at, say, age 80. Moreover, the inclusion of time-varying indicators of all three of the early stages of the disablement process (disease, pain, and functional limitations) might well have altered our findings regarding the relative importance of these and the sociodemographic factors in modeling trajectory group membership
The possibilities for additional research are many. For example, as the data allow, stratification of the analysis by age may be informative. Future analysis might also profitably explore the association of individual physical functional limitations and more detailed measures of cognition with ADL trajectories and even with trajectories of individual activities, such as bathing. Other research might usefully explore the link between specific diseases and ADL trajectories while jointly modeling mortality. Finally, given the different nature of IADLs and ADLs, modeling trajectories of difficulty with IADLs might provide further insight into how the ability of older people to care for themselves evolves as they age.
In sum, by linking longitudinal trajectories of ADL probability with baseline indicators of disease, impairment, and functional limitations, our analysis provides new empirical support for the conceptual framework of the disablement process presented at the outset. In addition, jointly modeling attrition due to mortality is an important strength of this analysis. The design of this study otherwise has been relatively simple, relying on baseline indicators of the first three phases of the disablement process. Much more research is necessary to assess in its full dynamic complexity the usefulness of the disablement process framework and to estimate the predictive power from phase to phase in the theoretical causal chain.
Funding
This work was supported by the National Institute on Aging of the National Institutes of Health (R21 AG036938 and R01 AG030153). L. G. Martin, Z. Zimmer, and J. Lee planned the study; J. Lee supervised the data extraction; L. G. Martin and Z. Zimmer performed all statistical analyses; L. G. Martin wrote the article; and Z. Zimmer and J. Lee revised the article.
Acknowledgments
The authors thank Drystan Phillips for assistance with the data files.
References
- American Medical Association. (2013). AMA adopts new policies on second day of voting at annual meeting. Retrieved from http://www.ama-assn.org/ama/pub/news/news/2013/2013-06-18-new-ama-policies-annual-meeting.page [Google Scholar]
- Dodge H. H. Du Y. Saxton J., & Ganguli M (2006). Cognitive domains and trajectories of functional independence in nondemented elderly persons. Journal of Gerontology: Medical Sciences, 61A, 1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. J., & Jette A. M (Eds.). (2007). The future of disability in America. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Freedman V. A. Martin L. G. Schoeni R. F., & Cornman J (2008). Declines in late-life disability: The role of early- and mid-life factors. Social Science & Medicine, 66, 1588–1602. doi:10.1016/j.socscimed.2007.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T. M. Gahbauer E. A. Han L., & Allore H. G (2010). Trajectories of disability in the last year of life. New England Journal of Medicine, 362, 1173–1180. doi:10.1056/NEJMoa0909087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T. M., & Kurland B (2003). The burden and patterns of disability in activities of daily living among community-living older persons. Journal of Gerontology: Medical Sciences, 58A, 70–75. [DOI] [PubMed] [Google Scholar]
- Guralnik J. M., & Ferrucci L (2003). Assessing the building blocks of function: Utilizing measures of functional limitation. American Journal of Preventive Medicine, 25(Suppl. 2), 112–121. [DOI] [PubMed] [Google Scholar]
- Guralnik J. M. Ferrucci L. Pieper C. F. Leveille S. G. Markides K. S. Ostir G. V., … Wallace R. B (2000). Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. Journal of Gerontology: Medical Sciences, 55A, M221–M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik J. M. Ferrucci L. Simonsick E. M. Salive M. E., & Wallace R. B (1995). Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. New England Journal of Medicine, 332, 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviland A. B. Jones B. L., & Nagin D. S (2011). Group-based trajectory modeling extended to account for nonrandom participant attrition. Sociological Methods and Research, 40, 367–390. doi:10.1177/0049124111400041 [Google Scholar]
- Jette A. M. Assmann S. F. Rooks D. Harris B. A., & Crawford S (1998). Interrelationships among disablement concepts. Journal of Gerontology: Medical Sciences, 53A, M395–M404. [DOI] [PubMed] [Google Scholar]
- Jones B. L. (2014). TRAJ, group-based modeling of longitudinal data. Retrieved on September 16, 2014 from http://www.andrew.cmu.edu/user/bjones/index.htm [Google Scholar]
- Jones B. L., & Nagin D. S (2007). Advances in group-based trajectory modeling and a SAS procedure for estimating them. Sociological Methods and Research, 35, 542–572. doi:10.1177/0049124106292364 [Google Scholar]
- Jones B. L., & Nagin D. S (2012). A Stata plugin for estimating group-based trajectory models. Research Showcase@CMU. Carnegie Mellon University; Retrieved on July 10, 2015 from http://repository.cmu.edu/heinzworks/406/ [Google Scholar]
- Jones B. L., & Nagin D. S (2013). A note on a Stata plugin for estimating group-based trajectory models. Sociological Methods and Research, 42, 608–613. doi:10.1177/0049124113503141 [Google Scholar]
- Jones B. L. Nagin D. S., & Roeder K (2001). A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research, 29, 374–393. [Google Scholar]
- Liang J. Bennett J. M. Shaw B. A. Quiñones A. R. Ye W. Xu X., & Ofstedal M. B (2008). Gender differences in functional status in middle and older age: Are there any age variations? Journal of Gerontology: Social Sciences, 63B, S282–S292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J. Wang C. Hsu H. Lin H. Lin Y., & Xu X (2010). Trajectory of functional status among older Taiwanese: Gender and age variations. Social Science and Medicine, 71,1208–1217. doi:10.1016/j.socscimed.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. G., & Schoeni R. F (2014). Trends in disability and related chronic conditions among the forty-and-over population: 1997–2010. Disability and Health Journal, 7, S4–S14. doi:10.1016/j.dhjo.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez J. K., & Hayward M. D (2014). Cumulative childhood adversity and active life expectancy among U.S. adults. Demography, 51, 413–435. doi:10.1007/S13524-013-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. J. L. Kulkarni S. Michaud C. Tomijima N. Bulzacchelli M. T. Iandiorio T. J., & Ezzati M (2006). Eight Americas: Investigating causes of mortality disparities across races, counties and race-counties. PLoS Medicine, 3, e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi S. Z. (1965). Some conceptual issues in disability and rehabilitation. In M. B., Sussman (Ed.), Sociology and rehabilitation (pp. 100–113). Washington, DC: American Sociological Association. [Google Scholar]
- Nagin D. S. (1999). Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological Methods, 4, 139–157. [DOI] [PubMed] [Google Scholar]
- Nagin D. S. (2005). Group-based modeling of development. Cambridge, MA: Harvard University Press. [Google Scholar]
- NHATS. (2014). NHATS Round 1 Data Collection Instruments. Retrieved September 22, 2014, from http://www.nhats.org/scripts/instruments/SS.pdf [Google Scholar]
- Pope A. M., & Tarlov A. R (Eds.). (1991). Disability in America: Toward a national agenda for prevention. Washington, DC: National Academy Press. [Google Scholar]
- Rodgers W., & Miller B (1997). A comparative analysis of ADL questions in surveys of older people. Journal of Gerontology: Social Sciences, 52B, 21–36. [DOI] [PubMed] [Google Scholar]
- Santos-Eggimann B. Zobel F., & Berod A. C (1999). Functional status of elderly home care users: Do subjects, informal and professional caregivers agree? Journal of Clinical Epidemiology, 52, 181–186. [DOI] [PubMed] [Google Scholar]
- Schoeni R. F. Freedman V. A., & Martin L. G (2008). Why is late-life disability declining? Milbank Quarterly, 86, 47–87. doi:10.1111/j.1468-0009.2007.00513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield K. M., & Peek M. K (2011). Changes in the prevalence of cognitive impairment among older Americans, 1993–2004: Overall trends and differences by race/ethnicity. American Journal of Epidemiology, 174, 274–283. doi:10.1093/aje/kwr074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. G. (2011). The causal pathway from socioeconomic status to disability trajectories in later life: The importance of mediating mechanisms for onset and accumulation. Research on Aging, 33, 84–108. doi:10.1177/0164027510385011 [Google Scholar]
- Taylor M. G., & Lynch S. M (2011). Cohort differences and chronic disease profiles of differential disability trajectories. Journal of Gerontology: Social Sciences, 66, 729–738. doi:10.1093/geronb/gbr104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A., & Kirchner C (2000). Bias in proxies’ reports of disability: Data from the National Health Interview Survey on Disability. Amerian Journal of Public Health, 90, 1248–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Michigan. (2014). Health and Retirement Study. Retrieved September 16, 2011, from http://hrsonline.isr.umich.edu/index.php [Google Scholar]
- Verbrugge L. M., & Jette A. M (1994). The disablement process. Social Science and Medicine, 38, 1–14. [DOI] [PubMed] [Google Scholar]
- Wolf D. A. Freedman V. A. Ondrich J. A. Seplaki C. L., & Spillman B. C (2015). Disability trajectories at the end of life: A “countdown” model. Journal of Gerontology: Social Sciences, 70, 745–752. doi:10.1093/geronb/gbu182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., & Lee L. C (2010). Dynamics and heterogeneity in the process of human frailty and aging: Evidence from the U.S. older adult population. Journal of Gerontology: Social Sciences, 65B, 246–255. doi:10.1093/geronb/gbp102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer Z., & House J. S (2003). Education, income, and functional limitation transitions among American adults: Contrasting onset and progression. International Journal of Epidemiology, 2, 1089–1097. [DOI] [PubMed] [Google Scholar]
- Zimmer Z. Liu X. Hermalin A., & Chuang Y. C (1998). Educational attainment and transitions in functional status among older Taiwanese. Demography, 35, 361–375. [PubMed] [Google Scholar]
- Zimmer Z. Martin L. G. Jones B. L., & Nagin D. S (2014). Examining late-life functional limitation trajectories and their associations with underlying onset, recovery, and mortality. Journal of Gerontology: Social Sciences, 69, 275–286. doi:10.1093/geronb/gbt099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer Z. Martin L. G. Nagin D. S., & Jones B. L (2012). Modeling disability trajectories and mortality of the oldest old in China. Demography, 49, 291–314. doi:10.1007/S13524-011-0075-7 [DOI] [PubMed] [Google Scholar]