INTRODUCTION
On average, only 50% of adults adhere to chronic disease medications1,2; and in the case of high blood pressure (BP), lower levels of adherence are associated with worse BP control and adverse outcomes, including stroke, myocardial infarction, heart failure, and death.3–5 Although effective medications that control BP and reduce the risk of stroke, renal, and cardiovascular disease are available, uncontrolled BP and low adherence to antihypertensive drugs persist as major public health and clinical challenges.6,7 Research in the past decade has identified determinants of poor adherence and explored the impact of interventions to address barriers, improve adherence, and ultimately achieve BP control. Several approaches have proven successful, although no single intervention has emerged as superior in improving adherence and lowering BP across all groups. As the lower systolic BP (SBP) treatment target (<120 mm Hg) suggested by the recent Systolic Blood Pressure Intervention Trial (SPRINT) results8,9 is integrated into clinical practice guidelines,10 new performance standards for BP control will likely emerge and greater attention will be given to improving patient adherence to prescribed therapies in an effort to achieve BP control using the lower target.
Modest changes in adherence can lead to clinically significant reductions in BP.11 In turn, relatively small reductions in BP are associated with improvements in mortality12–14: a reduction in SBP of 3 mm Hg is associated with an 8% reduction in stroke mortality and a 5% reduction in mortality from coronary heart disease.14 Thus, efforts that lead to even modest improvements on adherence can have an appreciable effect on health outcomes at the population level. The purpose of this article is to provide an overview of the current status and recent developments regarding interventions to improve adherence to antihypertensive medications for primary prevention of cardiovascular events.
TYPES OF INTERVENTIONS TO IMPROVE MEDICATION ADHERENCE
Interventions to promote medication adherence may target a number of identified patient-specific barriers: asymptomatic nature of hypertension15,16; depression17–21; comorbidities20; low health literacy22–24; medication complexity, cost, and concerns25–28; use of alternative medicine29–31; poor health care system perceptions32; perceived discrimination26,33; poor communication or provider-patient interaction33–35; medication side effects34,36; forgetfulness37,38; inadequate social support or coping39,40; caring for dependents41; and lack of motivation for self-care.42 Interventions that target these factors can be classified as informational, behavioral, social, or combined.43 Informational interventions use didactic or interactive approaches to educate and motivate patients and to increase understanding of their condition and its treatment.43 Behavioral interventions move beyond the cognitive approaches of informational interventions to influence patient behaviors by shaping, reminding, or rewarding desired behaviors, whereas social interventions enlist family members or others in supporting medication adherence.43 Finally, combined interventions, which are becoming increasingly common, include elements of more than one informational, behavioral, or social strategy. Strategies may vary in intensity, setting (eg, individual, group), mechanism of delivery (eg, face-to-face, technology-mediated), and required personnel (eg, physician, allied health professional, or lay individual) (Table 1).
Table 1.
Strategies for promoting medication adherence
| Strategy | Description | Examples |
|---|---|---|
| Patient education | Didactic or interactive approaches to provide information and educate patients |
• Face-to-face education session • Written or audiovisual education • Mailed instructional material |
| Social support | Enlistment of family members, friends, or other individuals to support patients in taking their medications as prescribed |
• Lay health mentoring • Group support meetings • Family education |
| Patient motivation | Motivation of patients to take their medication as prescribed and removal of barriers that work against their motivation |
• Motivational interviewing • Case management • Problem-solving • Decisional balance activities • Self-monitoring and feedback (see the next two rows) |
| Self-monitoring | Enlistment of patients to monitor their own BP or adherence |
• Home or ambulatory BP monitoring • Home titration90 |
| Feedback | Feedback to patients about their adherence or BP |
• Telemonitoring of BP data • Rewards for meeting BP goals |
| Reminders | Reminders to patients to take their medications |
• Calendars • Alarms • Pillboxes |
| Drug packaging | Changes in packaging of medications, intended to remind patients and/or give feedback about medication taking behavior |
• Pillboxes • Blister packaging • Adherence packets |
| Regimen simplification |
Prescription changes or changes in dosage schedule to simplify the regimen |
• Combination pills • Once-daily dosing |
| Reduction of out- of-pocket costs |
Reduction of patient out-of pocket drug costs |
• Reduced medication copayments • Improved drug prescription coverage |
| Communication or interactions with provider |
Improvements in patient- provider communication or interactions |
• Communication skills training for patients and/or clinicians |
| Allied health providers and collaborative care |
Enlistment of allied health care providers, individually or working as collaborative teams, to implement the intervention |
• Pharmacist-delivered interventions • Nurse-delivered interventions • Team-based care |
Abbreviation: BP, blood pressure
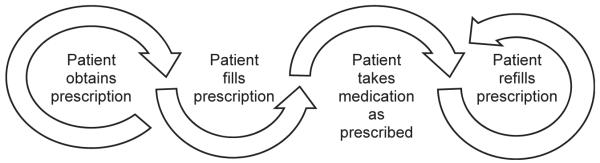
When evaluating the effectiveness of interventions to improve adherence, consideration should be given to the adherence measure used. Validated objective (eg, pharmacy fill,44,45 electronic monitoring46) and subjective (eg, self-report37,38,47–50) measures for assessing medication-taking behavior are available (Table 2). An effect size of d = 0.2 is considered small, d = 0.5 medium, and d = 0.8 large.51 In a recent meta-analysis assessing the impact of adherence interventions, the largest effect sizes were found among studies using objective measures of adherence, including electronic event monitoring (d = 0.621), followed by pharmacy fill measures (d = 0.299) and pill counts (d = 0.299), whereas studies using subjective, self-report measures produced smaller effect sizes (d = 0.232).11 This may be due, in part, to objective measures being less prone to the measurement noise associated with self-report measures, thereby rendering more precise estimates of adherence and easier differentiation between low and high adherence.52 Given that different tools assess different aspects of behavior along the adherence cascade (Fig. 1), use of both objective and self-report measures to identify at-risk patients and target patient-specific needs to improve adherence may facilitate our ability to promote adherence and increase BP control.5
Table 2.
Key medication adherence measures
| Objective | Subjective |
|---|---|
| Pharmacy fill6,17 • Medication Possession Ratio (MPR) • Proportion of Days Covered (PDC)44,45 Electronic monitoring46 Pill counts46 Direct measurement of drug concentration in blood46 |
Morisky Medication Adherence Scale (MMAS), 4- item and 8-item versions37,38,47 • MMAS-4 • MMAS-8 Krousel-Wood 4-item adherence tool (K-Wood-4)48 Hill-Bone Compliance Scale49 Medication Adherence Estimator50 |
Fig. 1.
Adherence behavior cascade.
PROMISING STRATEGIES FOR IMPROVING ADHERENCE
Meta-analyses have linked several intervention characteristics to modest improvements in antihypertensive medication adherence (Table 3). Interventions that provide behavioral rather than informational support,53 are delivered over a longer time frame,11,53 and include more intervention components11 have larger effects on adherence. With respect to health outcomes, larger intervention doses (measured by minutes per session and number of sessions) are more effective at improving BP.55 Moreover, face-to-face versus mediated delivery of adherence interventions (eg, via mail) is associated with a larger decrease in diastolic BP (DBP), but not with a larger decrease in SBP55. Taken together, these findings indicate that effective interventions to promote adherence and improve BP control are likely to require ongoing, sustained focus with repeated contacts and a combination of strategies that can be tailored to patients’ needs. These intervention characteristics are well-aligned with current movement toward patient-centered approaches to the management of chronic diseases. When implementing and evaluating any intervention, and particularly those that are complex and multicomponent, it is critical to attend to feasibility and implementation fidelity, as the lack of effect found in some studies of multicomponent interventions may be due to poor adherence to the intervention.56 Challenges associated with poor adherence to multiple components of complex interventions may be addressed by providing incentives for participation, automating aspects of the intervention (eg, wireless home BP monitoring devices that communicate automatically with clinicians and automated feedback provided to patients), or changing defaults to make it easier to make healthy choices (eg, 90-day instead of 30-day prescription refills).56
Table 3.
Comparing the effects of intervention characteristics on adherence and blood pressure
| Effect on Adherencea | Effect on Blood Pressureb |
|---|---|
| Larger effect • Longer vs shorter time frame (P<.001) • More vs fewer intervention components (P<.001) • Behavioral vs informational53 • Delivered to patients (d = 0.316) vs health care providers (d = 0.107) (P = .030) No difference in effect • Target adherence exclusively (d = 0.318) vs address additional health behaviors (d = 0.292) (P = .768) • Delivered in ambulatory care settings (d = 0.272) vs other setting (d = 0.282) (P = .938) • Delivered in pharmacies (d = 0.432) vs other locations (d = 0.290) (P = .405) • Face-to-face (d = 0.319) vs mediated de- livery (d = 0.259) (P = .400) • Theory-based (d = 0.335) vs not theory- based (d = 0.284) (P = .554) • Larger intervention dose (i.e., no relation- ship between total minutes of intervention and effect size) (P = .534) |
Larger effect Systolic blood pressure • Larger intervention dose (P = .021) • Delivered in pharmacies (d = 0.360) vs other locations (d = 0.226) (P = .031) • Delivered to groups (d = 0.399) vs indi- viduals/families (d = 0.228) (P = .029) Diastolic blood pressure • Larger intervention dose (P = .027) • Face-to-face (d = 0.221) vs mediated delivery (d = 0.060) (P<.05) • Delivered in pharmacies (d = 0.356) vs other locations (d = 0.177) (P = .009) • Delivered to groups (d = 0.376) vs indi- viduals/families (d = 0.179) (P = .018) No difference in effect Systolic blood pressure • Presence vs absence of behavior change theory • Face-to-face (d = 0.256) vs mediated delivery (d = 0.179) (P>.05) • Target patients/families vs health care providers • Delivered in ambulatory care settings vs home/community centers Diastolic blood pressure • Presence vs absence of behavior change theory • Target patients/families vs health care providers • Delivered in ambulatory care settings vs home/community centers |
There are specific strategies that should be considered for inclusion in an antihypertensive medication adherence intervention. Regimen simplification, through once-daily dosing, has long been known to be effective at improving medication adherence.28 Similarly, the use of combination pills may promote medication adherence: in a single study, multivariate odds ratio (OR) for achieving proportion of days covered (PDC) ≥80% at 6-month follow-up using single amlodipine/atorvastatin combination pill versus various combinations of separate pills ranged from 1.95 to 3.10 (all P<.0001).57 There is low strength evidence supporting case management as an effective strategy for promoting adherence and improving BP outcomes.54 Finally, home BP telemonitoring58 and habit-based interventions55 have larger effects on health outcomes compared to other interventions, but evidence for larger effects on adherence is limited.11,58
Three additional promising interventions for improving medication adherence include reduction of out-of-pocket costs (Table 4), use of allied health professionals in promoting medication adherence (Table 5), and self-monitoring of BP (Table 6). Across several individual studies, reductions in patients’ out-of-pocket drug costs (through reduced copayment rates59,60 and introduction of drug insurance coverage)61,62 were associated with significant improvements in adherence. Only 1 study, involving financial incentives equal to copayment expenditures, found no effect on adherence.63 Evidence is limited regarding effects of reduced out-of-pocket costs on health outcomes.
Table 4.
Effectiveness of interventions to reduce out-of-pocket costs: evidence from individual studies
| Study | Description of Intervention | Effect on Adherencea |
|---|---|---|
| Chernew et al,59 2008 |
Reduction of copayment rates for ACE inhibitors, ARBs, and beta- blockers |
• MPR: ○ +2.6% points for ACE inhibi- tors/ARBs (P<.001) ○ +3.0% points for beta-blockers (P<.001) |
| Maciejewski et al,60 2010 |
Reduction of copayment rates | • MPR: ○ +3.4% for diuretics (P<.001) ○ 13.1% for ACE inhibitors (P<.001) ○ +2.7% for beta-blockers (P<.001) ○ +1.3% for calcium-channel blockers (P<.05) |
| Zhang et al,61 2010 |
Introduction of Medicare Part D coverage ($8/$20 copayments for generic/brand medications) to 3 intervention groups with following baseline conditions:
[Comparison group had benefits similar to Part D coverage at baseline.] |
• MPR: ○ +13.5% points for group with no coverage at baseline (95% CI 11.5–15.5) ○ +2.6% points for group with low cap at baseline (95% CI 1.2–4.1) ○ +2.5% points for group with high cap at baseline (95% CI 1.7–3.2) |
| Li et al,62 2012 | Medicare Part D coverage gap, 3 intervention groups with following baseline conditions:
[Comparison group eligible for low- income subsidies during the gap.] |
• PDC <0.8 (low adherence) ○ OR = 1.60 (95% CI 1.50–1.71) for group with no coverage at baseline ○ OR = 1.50 (95% CI 1.30–1.73) for group with generic-only coverage at baseline ○ OR = 1.00 (95% CI 0.88–1.15) for group with brand and generic coverage at baseline |
| Volpp et al,63 2014 |
Financial incentive equal to copayments for all antihypertensive medications, which effectively eliminated copayments |
• MPR: ○ No effect (P = .74) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; CI, confidence interval; MPR, medication possession ratio; OR, odds ratio; PDC, proportion of days covered.
Only Volpp et al assessed the effect of the intervention on BP outcomes; no effect was detected.
Table 5.
Effectiveness of interventions involving allied health professionals (including collaborative care): summary of evidence from reviews and meta-analyses
| Type of Intervention [Review/Meta-analysis] |
Effects on Adherence | Effects on Blood Pressure |
|---|---|---|
| Nurse- and pharmacist-led care [Glynn et al,65 2010] |
• Not assessed | • Mean difference SBP, range: −13 mm Hg to 0 mm Hg • Mean difference DBP, range: −8 mm Hg to 0 mm Hg |
| Pharmacist interventions [Morgado et al,66 2011] |
• 44% of interventions increased adherence |
• WMD SBP: −4.9 mm Hg, 95% CI −5.8 to −4.0 • WMD DBP: −2.6 mm Hg, 95% CI −3.5 to −1.7 |
| Pharmacist-delivered face-to- face education; collaborative care [Viswanathan et al,54 2012]a |
• Pharmacist-delivered edu- cation: low strength evi- dence of benefit • Collaborative care: low strength evidence of no benefit |
• Pharmacist-delivered edu- cation: decreased SBP and DBP (mean decrease not specified) |
| Multiprofessional informational, behavioral, and combined strategies [Mansoor et al,69 2013]a |
• Few informational or com- bined interventions improved adherence • All behavioral interven- tions improved adherence |
• Most informational or combined interventions improved health out- comes, including BP • All behavioral interven- tions improved health out- comes, including BP |
| Team-based care interventions [Proia et al,68 2014] |
• Insufficient information provided |
• Median effect estimate SBP: −5.4 mm Hg, 95% CI −7.2 to −2.0 • Median effect estimate DBP: −1.8 mm Hg, 95% CI −3.2 to 0.7 |
| Pharmacist-led interventions [Cheema et al,64 2014] |
• OR (improved adherence) = 12.1, 95% CI 4.2–34.6 |
• WMD SBP: −6.1 mm Hg, 95% CI −8.4 to −3.8 • WMD DBP: −2.5 mm Hg, 95% CI −3.5 to −1.6 |
| Pharmacist interventions [Santschi et al,67 2014] |
• Not assessed | • WMD SBP: −7.6 mm Hg, 95% CI −9.0 to −6.3 • WMD DBP: −3.9 mm Hg, 95% CI −5.1 to −2.8 |
| Pharmacist-delivered interventions; increased integration of care [Conn et al,11 2015] |
• Pharmacist-delivered inter- vention (d = 0.356) vs not delivered by pharmacist (d = 0.277) (P = .327) • Studies that increased integration across pro- viders (d = 0.185) vs studies without integration (d = 0.344) (P = .021) |
Not assessed |
| Pharmacist, nurse, and physician interventionist [Conn et al,55 2016] |
• Not assessed | • SBP ○ Pharmacist d = 0.317 (P<.001) ○ Nurse, advanced practice d = 0.298 (P = .001) ○ Physician d = 0.218 (P<.001) ○ Nurse, not advanced practice d = 0.142 (P = .089) • DBP ○ Pharmacist d = 0.235 (P<.001) ○ Nurse, advanced practice d = 0.224 (P = .030) ○ Physician d = 0.199 (P<.001) ○ Nurse, not advanced practice d = 0.149 (P = .101) |
Abbreviations: BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure; WMD, weighted mean difference.
Specific data not reported in source
Table 6.
Effectiveness of self-monitoring of blood pressure: summary of evidence from reviews and meta-analyses
| Review/Meta-analysis | Effects on Adherence | Effects on Blood Pressure |
|---|---|---|
| Glynn et al,65 2010 | • Not assessed | • WMD SBP: −2.5 mm Hg, 95% CI −3.7 to −1.3 • WMD DBP: −1.8 mm Hg, 95% CI −2.4 to −1.2 |
| Bray et al,70 2010 | • Not assessed | • WMD SBP: −3.8 mm Hg, 95% CI −5.6 to −2.0 • WMD DBP: −1.5 mm Hg, 95% CI −2.0 to −0.9 |
| van Dalem et al,53 2012 |
• Contradictory results (2 studies) |
• Mean DBP: “significant decrease” (2 studies; data not reported) |
| Uhlig et al,71 2013 | • “A few studies” found effect (data not reported) |
Self-monitoring only • WMD SBP, 6 mo: −3.9 mm Hg, P<.001 • WMD DBP, 6 mo: −2.4 mm Hg, P<.001 • WMD SBP, 12 mo: −1.5 mm Hg, P>.05 • WMD DBP, 12 mo: −0.8 mm Hg, P>.05 Self-monitoring + additional support • Net difference SBP, range, 12 mo: −8.9 to −2.1 mm Hg • Net difference DBP, range, 12 mo: −4.4 to 0.0 mm Hg |
| Fletcher et al,52 2015 | • d = 0.21 (95% CI 0.08–0.34) | • WMD SBP: −4.1 mm Hg, 95% CI −6.7 to −1.4 • WMD DBP: −2.0 mm Hg, 95% CI −2.9 to −1.1 |
| Conn et al,11 2015 | • Self-monitoring (d = 0.381) vs no self-monitoring (d = 0.261) (P = .160) |
• Not assessed |
| Conn et al,55 2016 | • Not assessed | • No difference in effect (effect sizes not reported) |
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure; WMD, weighted mean difference.
Use of allied health professionals, particularly pharmacists, in interventions to improve medication adherence has proliferated in recent years. In interventions to improve BP outcomes, pharmacists deliver education about hypertension and its treatment, identify prescribing and safety issues, and/or dispense lifestyle advice.64 Several reviews and meta-analyses demonstrate associations between pharmacist-delivered interventions and improved BP outcomes.54,55,64–67 In fact, a recent meta-analysis found that pharmacist-delivered interventions had the largest effect on BP outcomes, followed by those delivered by advanced practice nurses, and then physicians.55 Although it is clear that interventions delivered by pharmacists improve BP outcomes, there is a great deal of unexplained heterogeneity in effects67 and it is uncertain if the effect on BP outcomes is mediated by improved adherence or by some other mechanism, as effects on adherence reported across reviews and meta-analyses are inconsistent.11,54,64,66
Team-based collaborative care is a specific type of intervention involving allied health professionals. Multiple reviews and meta-analyses have identified improved BP outcomes associated with collaborative care models68,69; there is, however, limited evidence supporting adherence as the mechanism leading to improved outcomes.11,54,68 A recent meta-analysis found that studies focusing on increasing integration of patient care across providers had a significantly smaller effect on adherence than interventions that did not have a focus on integration.11 These negative findings may be due to a failure to account for differences in effectiveness between multiprofessional behavioral and informational or combined interventions. Mansoor and colleagues69 reported that multiprofessional behavioral interventions improved adherence while multiprofessional informational or combined interventions did not. Further research is needed to identify the best model and determine if multiprofessional interventions are superior to those delivered by a single professional.69
Across a number of systematic reviews and meta-analyses, self-monitoring of BP leads to improvements in BP outcomes,52,53,65,70,71 although the mechanism for this effect remains unclear. Potential mechanisms include pharmacologic (increased medication and better adherence) and nonpharmacologic (healthier lifestyle) factors.52 The evidence for an effect of self-monitoring of BP on adherence is inconsistent,11,53,71 but a recent meta-analysis of the effect of self-monitoring of BP found a “small but significant” effect on medication adherence.52
STRATEGIES WITH UNCERTAIN EFFICACY FOR IMPROVING ADHERENCE
The efficacy of other strategies to improve antihypertensive medication adherence remains uncertain. There is little evidence that, compared to other interventions, informational interventions, particularly those relying on written materials, or social support interventions are more effective at promoting antihypertensive medication adherence or improving health outcomes.11,55 Data supporting the efficacy of particular behavioral interventions are also limited. Interventions using adherence problem-solving, decisional balance activities, and medication administration calendars are not associated with larger improvements in adherence.11 Furthermore, adherence barrier management, rewards for adherence, and adherence goal setting are not associated with larger improvements in BP outcomes.55 According to recent meta-analyses, when compared with other interventions, motivational interviewing, self-monitoring of medication administration/adherence, feedback about adherence, drug packaging, and efforts to improve communication between patients and providers are not associated with larger improvements in adherence and BP control.11,55
Although the research to date does not provide evidence that these interventions are more effective at improving adherence compared to other interventions, it is important to note that much of the research on intervention efficacy lacks methodological rigor, leading to low-quality evidence about which interventions are most effective. More high-quality studies on the effectiveness of various approaches for improving adherence and health outcomes are needed.
NEW FRONTIERS IN THE PROMOTION OF MEDICATION ADHERENCE
Technology-mediated interventions include both medical devices (eg, electronic drug monitors, pillboxes with alarms, home BP monitors, telehealth devices) and information and communication technologies (eg, computers, telephones, cell phones, e-mails, text messages), which may be used to support adherence through education and counseling, self-monitoring and feedback, or provision of reminders.72 Research on the effectiveness of these interventions is under way. A recent review found inconsistent evidence for the effectiveness of technology-mediated interventions to promote medication adherence and improve health outcomes.72 In contrast, a meta-analysis of Internet-based counseling demonstrated a mean reduction in SBP of 3.8 mm Hg (P = .002) and a mean reduction in diastolic BP (DBP) of 2.1 mm Hg (P = .03).73 Another meta-analysis reported that mobile phone text messaging for adherence to medications for chronic disease led to a doubling of the odds of adherence in intervention compared with control participants (OR = 2.11, P<.001).74 Notably, technology-mediated interventions are often just one component of complex, multicomponent interventions and it is difficult to isolate the effects of the technology piece.72,73 High-quality studies with longer follow-up and objective adherence measures are needed to adequately explore the potential of technology-mediated interventions for improving adherence.
Interactive digital interventions, which include interventions accessed through a computer, smartphone, or other handheld device (eg, Web-based or computer programs, or apps for online or offline use) deserve further mention. Several characteristics of interactive digital interventions make them especially promising for the promotion of antihypertensive medication adherence. First, they are interactive, requiring input from users, which can be used to produce tailored content. Second, they can function without the need for input from a health professional, making them potentially cost-effective tools for delivering long-term, multiple-contact adherence support. Finally, once developed, these interventions are highly scalable, able to reach innumerable users for only marginal additional cost. A recent meta-analysis of interactive digital interventions demonstrated that interactive digital interventions are effective in lowering both SBP (weighted mean difference [WMD] −3.74 mm Hg, 95% confidence interval [CI] −5.28 to −2.19) and DBP (WMD −2.37 mm Hg, 95% CI −4.35 to −0.40) compared with usual care.75 Few studies in the review included medication adherence as an outcome measure. Despite the promising results, little is known about the sustainability, long-term effectiveness, and active components of these interventions; thus, the evidence is not robust enough to warrant a policy or practice change at this time.75 A recent content analysis of 166 medication adherence apps found that the extent to which established behavior change techniques are used in adherence apps is limited.76 Future research incorporating advances in behavior change theory and practice will likely guide development in this emerging area.
GAPS IN OUR KNOWLEDGE ABOUT PROMOTING MEDICATION ADHERENCE
Despite evidence supporting the efficacy of a range of promising interventions, gaps in knowledge remain. A 2014 Cochrane review of interventions to promote medication adherence suggests, “It is possible that interventions to date are not very effective because we do not understand in sufficient detail exactly what the adherence problems are.”77 For example, unconscious, self-protective “hidden motives”78 that render patients “immune to change” their medication-taking behavior have recently been identified and may be contributing to nonadherence to antihypertensive medications. Work is under way to fill the gaps in our understanding of these novel barriers: tools to identify individuals with hidden motives for nonadherence are being developed, and interventions to overturn nonadherence mindsets are being designed and tested. These efforts will yield insights into psychological processes underlying nonadherence and may provide a novel approach for improving adherence, BP control, and quality of life in people with hypertension.
Although it is certain that more work is needed to understand the barriers to and underlying mechanisms for adherence, there is also a need to implement and evaluate interventions that address well-established barriers to adherence. For example, a number of studies and a meta-analysis have demonstrated that low adherence to medications is associated with depression and stressful life events.17–19,39 In one study, adjustment by depressive symptoms attenuated the association between social support and antihypertensive medication adherence.18 Yet, with the exception of a trial that found that integrated management of hypertension and depression led to improvements in medication adherence and health outcomes,79 few intervention trials have focused on addressing these barriers. In addition, although some work suggests that depression leads to low adherence through the mechanism of low self-efficacy,80,81 additional research is needed to understand the mechanisms linking depression to low adherence so that targeted interventions can be developed.
In addition, work is needed to uncover sex and race differences in determinants of low adherence and effects of interventions to improve adherence. Racial and ethnic disparities in adherence rates are well-documented82–84; however, little is known about the root causes. Sex differences in determinants of adherence have been identified.34 These efforts at achieving a nuanced understanding of how the relationships between adherence and its determinants are moderated by demographic and other factors will help us to tailor interventions to meet the varied needs of diverse patients. A consideration of demographic differences should be applied to intervention trials as well: a recent meta-analysis found that effect sizes of antihypertensive adherence interventions were larger for older, female, and moderate-to high-income participants, signaling the need to explore alternative interventions for younger, male, and low-income participants.11 In general, there are major gaps in our understanding about how best to tailor interventions to meet the needs of patients with adherence problems, different types of nonadherence (eg, intentional vs unintentional), and different preferences for delivery (eg, technology-mediated vs face-to-face).11,85,86
Finally, further work is needed to fully understand the link between antihypertensive medication adherence and cardiovascular outcomes. Although several studies to date have identified a significant association between adherence and cardiovascular outcomes, including myocardial infarction, heart failure, stroke, and death,5 further work is needed to explore an association between adherence and other outcomes, such as diastolic dysfunction, a condition in which abnormalities in mechanical function of the heart are present during diastole. Hypertension may lead to diastolic dysfunction even in the absence of systolic dysfunction.87 Diastolic heart failure accounts for approximately 40% to 60% of patients with chronic heart failure; the prognosis for these patients may be similar to that of patients with systolic heart failure.88 Appropriate treatment of hypertension together with high patient medication adherence may be key to preventing onset of diastolic dysfunction and other cardiovascular diseases.
SUMMARY
Adherence to antihypertensive medication remains a key modifiable factor in the management of hypertension, an important, preventable risk factor for cardiovascular disease and death.89 Timely attention in clinical and research settings to identifying and addressing barriers to low medication adherence and uncontrolled BP for the general population may interrupt the costly cycle of this chronic disease and prevent the declines in quality of life associated with the consequences of uncontrolled hypertension.
KEY POINTS.
Relatively modest changes in adherence can lead to clinically significant improvements in BP control and reductions in cardiovascular events.
Interventions associated with improved adherence tend to use ongoing, sustained focus, repeated contacts, and multiple strategies for addressing medication-taking behaviors.
Promising strategies to improve antihypertensive medication adherence include regimen simplification, reduction of out-of-pocket costs, use of allied health professionals in delivering interventions (including team-based collaborative care), and self-monitoring of BP.
Research to understand the effects of emerging technology-mediated interventions, mechanisms underlying adherence behavior, and sex-race differences in determinants of low adherence and intervention effectiveness may enhance patient-specific approaches to improve adherence and disease control.
Acknowledgments
This work was supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health (NIH), which funds the Louisiana Clinical and Translational Science Center. Dr. Krousel-Wood also received funding from the NIH for the following grants: 5K12HD043451; U54TR001368-01; 1P20GM109036-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES
The authors have no commercial or financial conflicts of interest to disclose.
REFERENCES
- 1.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002;288(22):2880–3. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 2.Kronish IM, Ye S. Adherence to cardiovascular medications: lessons learned and future directions. Prog Cardiovasc Dis. 2013;55(6):590–600. doi: 10.1016/j.pcad.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiMatteo MR, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–35. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 5.Krousel-Wood M, Holt E, Joyce C, et al. Differences in cardiovascular disease risk when antihypertensive medication adherence is assessed by pharmacy fill versus self-report: the Cohort Study of Medication Adherence among Older Adults (CoSMO) J Hypertens. 2015;33(2):412–20. doi: 10.1097/HJH.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krousel-Wood M, Thomas S, Muntner P, et al. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol. 2004;19(4):357–62. doi: 10.1097/01.hco.0000126978.03828.9e. [DOI] [PubMed] [Google Scholar]
- 7.Krousel-Wood MA, Muntner P, Islam T, et al. Barriers to and determinants of medication adherence in hypertension management: perspective of the cohort study of medication adherence among older adults. Med Clin North Am. 2009;93(3):753–69. doi: 10.1016/j.mcna.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright JT, Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. JAMA. 2016;315(24):2673–82. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569–88. doi: 10.1016/j.cjca.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 11.Conn VS, Ruppar TM, Chase JA, et al. Interventions to improve medication adherence in hypertensive patients: systematic review and meta-analysis. Curr Hypertens Rep. 2015;17(12):94. doi: 10.1007/s11906-015-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook NR, Cohen J, Hebert PR, et al. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155(7):701–9. [PubMed] [Google Scholar]
- 13.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153(5):598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 14.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335(8693):827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 15.Ogedegbe G, Harrison M, Robbins L, et al. Reasons patients do or do not take their blood pressure medications. Ethn Dis. 2004;14(1):158. [PubMed] [Google Scholar]
- 16.Ogedegbe G, Harrison M, Robbins L, et al. Barriers and facilitators of medication adherence in hypertensive African Americans: a qualitative study. Ethn Dis. 2004;14(1):3–12. [PubMed] [Google Scholar]
- 17.Krousel-Wood MA, Frohlich ED. Hypertension and depression: coexisting barriers to medication adherence. J Clin Hypertens (Greenwich) 2010;12(7):481–6. doi: 10.1111/j.1751-7176.2010.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krousel-Wood M, Islam T, Muntner P, et al. Association of depression with antihypertensive medication adherence in older adults: cross-sectional and longitudinal findings from CoSMO. Ann Behav Med. 2010;40(3):248–57. doi: 10.1007/s12160-010-9217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175–82. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang PS, Avorn J, Brookhart MA, et al. Effects of noncardiovascular comorbidities on antihypertensive use in elderly hypertensives. Hypertension. 2005;46(2):273–9. doi: 10.1161/01.HYP.0000172753.96583.e1. [DOI] [PubMed] [Google Scholar]
- 21.Wang PS, Bohn RL, Knight E, et al. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17(7):504–11. doi: 10.1046/j.1525-1497.2002.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egan BM, Lackland DT, Cutler NE. Awareness, knowledge, and attitudes of older Americans about high blood pressure: implications for health care policy, education, and research. Arch Intern Med. 2003;163(6):681–7. doi: 10.1001/archinte.163.6.681. [DOI] [PubMed] [Google Scholar]
- 23.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 24.Ogedegbe G, Mancuso CA, Allegrante JP. Expectations of blood pressure management in hypertensive African-American patients: a qualitative study. J Natl Med Assoc. 2004;96(4):442–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Briesacher BA, Gurwitz JH, Soumerai SB. Patients at-risk for cost-related medication nonadherence: a review of the literature. J Gen Intern Med. 2007;22(6):864–71. doi: 10.1007/s11606-007-0180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kronish IM, Diefenbach MA, Edmondson DE, et al. Key barriers to medication adherence in survivors of strokes and transient ischemic attacks. J Gen Intern Med. 2013;28(5):675–82. doi: 10.1007/s11606-012-2308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edmondson D, Horowitz CR, Goldfinger JZ, et al. Concerns about medications mediate the association of posttraumatic stress disorder with adherence to medication in stroke survivors. Br J Health Psychol. 2013;18(4):799–813. doi: 10.1111/bjhp.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iskedjian M, Einarson TR, MacKeigan LD, et al. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta-analysis. Clin Ther. 2002;24(2):302–16. doi: 10.1016/s0149-2918(02)85026-3. [DOI] [PubMed] [Google Scholar]
- 29.Brown CM, Barner JC, Richards KM, et al. Patterns of complementary and alternative medicine use in African Americans. J Altern Complement Med. 2007;13(7):751–8. doi: 10.1089/acm.2006.6392. [DOI] [PubMed] [Google Scholar]
- 30.Gohar F, Greenfield SM, Beevers DG, et al. Self-care and adherence to medication: a survey in the hypertension outpatient clinic. BMC Complement Altern Med. 2008;8:4. doi: 10.1186/1472-6882-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krousel-Wood MA, Muntner P, Joyce CJ, et al. Adverse effects of complementary and alternative medicine on antihypertensive medication adherence: findings from the cohort study of medication adherence among older adults. J Am Geriatr Soc. 2010;58(1):54–61. doi: 10.1111/j.1532-5415.2009.02639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization . Adherence to long-term therapies: Evidence for action. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 33.Hagiwara N, Penner LA, Gonzalez R, et al. Racial attitudes, physician-patient talk time ratio, and adherence in racially discordant medical interactions. Soc Sci Med. 2013;87:123–31. doi: 10.1016/j.socscimed.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holt E, Joyce C, Dornelles A, et al. Sex differences in barriers to antihypertensive medication adherence: findings from the cohort study of medication adherence among older adults. J Am Geriatr Soc. 2013;61(4):558–64. doi: 10.1111/jgs.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutfey K. On practices of ’good doctoring’: reconsidering the relationship between provider roles and patient adherence. Sociol Health Illn. 2005;27(4):421–47. doi: 10.1111/j.1467-9566.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 36.Gregoire JP, Moisan J, Guibert R, et al. Tolerability of antihypertensive drugs in a community-based setting. Clin Ther. 2001;23(5):715–26. doi: 10.1016/s0149-2918(01)80021-7. [DOI] [PubMed] [Google Scholar]
- 37.Morisky DE, Ang A, Krousel-Wood MA, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Holt EW, Muntner P, Joyce C, et al. Life events, coping, and antihypertensive medication adherence among older adults: the cohort study of medication adherence among older adults. Am J Epidemiol. 2012;176(Suppl 7):S64–71. doi: 10.1093/aje/kws233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fongwa MN, Evangelista LS, Hays RD, et al. Adherence treatment factors in hypertensive African American women. Vasc Health Risk Manag. 2008;4(1):157–66. doi: 10.2147/vhrm.2008.04.01.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyre AD, Krousel-Wood MA, Muntner P, et al. Prevalence and predictors of poor antihypertensive medication adherence in an urban health clinic setting. J Clin Hypertens (Greenwich) 2007;9(3):179–86. doi: 10.1111/j.1524-6175.2007.06372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rollnick S, Miller WR, Butler C. Motivational interviewing in health care: helping patients change behavior. Guilford Press; New York: 2008. [Google Scholar]
- 43.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167(6):540–50. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 44.Nau DP. Proportion of days covered (PDC) as a preferred method of measuring medication adherence. Pharmacy Quality Alliance; Springfield (VA): 2012. [Google Scholar]
- 45.Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care. 2009;15(7):457–64. [PMC free article] [PubMed] [Google Scholar]
- 46.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):1074–90. doi: 10.1016/S0149-2918(99)80026-5. [discussion: 1073] [DOI] [PubMed] [Google Scholar]
- 47.Krousel-Wood M, Islam T, Webber LS, et al. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009;15(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- 48.Krousel-Wood M, Joyce C, Holt EW, et al. Development and evaluation of a self-report tool to predict low pharmacy refill adherence in elderly patients with uncontrolled hypertension. Pharmacotherapy. 2013;33(8):798–811. doi: 10.1002/phar.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MT, Hill MN, Bone LR, et al. Development and testing of the Hill-Bone Compliance to High Blood Pressure Therapy Scale. Prog Cardiovasc Nurs. 2000;15(3):90–6. doi: 10.1111/j.1751-7117.2000.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 50.McHorney CA. The Adherence Estimator: a brief, proximal screener for patient propensity to adhere to prescription medications for chronic disease. Curr Med Res Opin. 2009;25(1):215–38. doi: 10.1185/03007990802619425. [DOI] [PubMed] [Google Scholar]
- 51.Cohen J. Statistical power analysis for the behavioral sciences. revised edition Academic Press; New York: 1977. [Google Scholar]
- 52.Fletcher BR, Hartmann-Boyce J, Hinton L, et al. The effect of self-monitoring of blood pressure on medication adherence and lifestyle factors: a systematic review and meta-analysis. Am J Hypertens. 2015;28(10):1209–21. doi: 10.1093/ajh/hpv008. [DOI] [PubMed] [Google Scholar]
- 53.van Dalem J, Krass I, Aslani P. Interventions promoting adherence to cardiovascular medicines. Int J Clin Pharm. 2012;34(2):295–311. doi: 10.1007/s11096-012-9607-5. [DOI] [PubMed] [Google Scholar]
- 54.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–95. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 55.Conn VS, Ruppar TM, Chase JD. Blood pressure outcomes of medication adherence interventions: systematic review and meta-analysis. J Behav Med. 2016 doi: 10.1007/s10865-016-9730-1. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volpp KG. The counseling African Americans to control hypertension study and ways to enhance the next wave of behavioral interventions. Circulation. 2014;129(20):2002–4. doi: 10.1161/CIRCULATIONAHA.114.009409. [DOI] [PubMed] [Google Scholar]
- 57.Patel BV, Leslie RS, Thiebaud P, et al. Adherence with single-pill amlodipine/atorvastatin vs a two-pill regimen. Vasc Health Risk Manag. 2008;4(3):673–81. [PMC free article] [PubMed] [Google Scholar]
- 58.Omboni S, Gazzola T, Carabelli G, et al. Clinical usefulness and cost effectiveness of home blood pressure telemonitoring: meta-analysis of randomized controlled studies. J Hypertens. 2013;31(3):455–67. doi: 10.1097/HJH.0b013e32835ca8dd. [discussion: 467–58] [DOI] [PubMed] [Google Scholar]
- 59.Chernew ME, Shah MR, Wegh A, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Aff (Millwood) 2008;27(1):103–12. doi: 10.1377/hlthaff.27.1.103. [DOI] [PubMed] [Google Scholar]
- 60.Maciejewski ML, Farley JF, Parker J, et al. Copayment reductions generate greater medication adherence in targeted patients. Health Aff (Millwood) 2010;29(11):2002–8. doi: 10.1377/hlthaff.2010.0571. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Lave JR, Donohue JM, et al. The impact of Medicare Part D on medication adherence among older adults enrolled in Medicare-Advantage products. Med Care. 2010;48(5):409–17. doi: 10.1097/MLR.0b013e3181d68978. [DOI] [PubMed] [Google Scholar]
- 62.Li P, McElligott S, Bergquist H, et al. Effect of the Medicare Part D coverage gap on medication use among patients with hypertension and hyperlipidemia. Ann Intern Med. 2012;156(11):776–84. doi: 10.7326/0003-4819-156-11-201206050-00004. w-263, w-264, w-265, w-266, w-267, w-268, w-269. [DOI] [PubMed] [Google Scholar]
- 63.Volpp K, Troxel A, Long J, et al. A randomized controlled trial of co-payment elimination: the CHORD trial. Am J Manag Care. 2014;21(8):e455–64. [PMC free article] [PubMed] [Google Scholar]
- 64.Cheema E, Sutcliffe P, Singer DR. The impact of interventions by pharmacists in community pharmacies on control of hypertension: a systematic review and meta-analysis of randomized controlled trials. Br J Clin Pharmacol. 2014;78(6):1238–47. doi: 10.1111/bcp.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glynn LG, Murphy AW, Smith SM, et al. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;(3):CD005182. doi: 10.1002/14651858.CD005182.pub4. [DOI] [PubMed] [Google Scholar]
- 66.Morgado MP, Morgado SR, Mendes LC, et al. Pharmacist interventions to enhance blood pressure control and adherence to antihypertensive therapy: review and meta-analysis. Am J Health Syst Pharm. 2011;68(3):241–53. doi: 10.2146/ajhp090656. [DOI] [PubMed] [Google Scholar]
- 67.Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3(2):e000718. doi: 10.1161/JAHA.113.000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47(1):86–99. doi: 10.1016/j.amepre.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mansoor SM, Krass I, Aslani P. Multiprofessional interventions to improve patient adherence to cardiovascular medications. J Cardiovasc Pharmacol Ther. 2013;18(1):19–30. doi: 10.1177/1074248412442001. [DOI] [PubMed] [Google Scholar]
- 70.Bray EP, Holder R, Mant J, et al. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42(5):371–86. doi: 10.3109/07853890.2010.489567. [DOI] [PubMed] [Google Scholar]
- 71.Uhlig K, Patel K, Ip S, et al. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Ann Intern Med. 2013;159(3):185–94. doi: 10.7326/0003-4819-159-3-201308060-00008. [DOI] [PubMed] [Google Scholar]
- 72.Mistry N, Keepanasseril A, Wilczynski NL, et al. Technology-mediated interventions for enhancing medication adherence. J Am Med Inform Assoc. 2015;22(e1):e177–93. doi: 10.1093/jamia/ocu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu S, Dunford SD, Leung YW, et al. Reducing blood pressure with Internet-based interventions: a meta-analysis. Can J Cardiol. 2013;29(5):613–21. doi: 10.1016/j.cjca.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 74.Thakkar J, Kurup R, Laba TL, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. 2016;176(3):340–9. doi: 10.1001/jamainternmed.2015.7667. [DOI] [PubMed] [Google Scholar]
- 75.McLean G, Band R, Saunderson K, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34(4):600–12. doi: 10.1097/HJH.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morrissey EC, Corbett TK, Walsh JC, et al. Behavior change techniques in apps for medication adherence: a content analysis. Am J Prev Med. 2016;50(5):e143–6. doi: 10.1016/j.amepre.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 77.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;(11):CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krousel-Wood M, Kegan R, Whelton PK, et al. Immunity-to-change: are hidden motives underlying patient nonadherence to chronic disease medications? Am J Med Sci. 2014;348(2):121–8. doi: 10.1097/MAJ.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 79.Bogner HR, de Vries HF. Integration of depression and hypertension treatment: a pilot, randomized controlled trial. Ann Fam Med. 2008;6(4):295–301. doi: 10.1370/afm.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chao J, Nau DP, Aikens JE, et al. The mediating role of health beliefs in the relationship between depressive symptoms and medication adherence in persons with diabetes. Res Social Adm Pharm. 2005;1(4):508–25. doi: 10.1016/j.sapharm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 81.Schoenthaler A, Ogedegbe G, Allegrante JP. Self-efficacy mediates the relationship between depressive symptoms and medication adherence among hypertensive African Americans. Health Educ Behav. 2009;36(1):127–37. doi: 10.1177/1090198107309459. [DOI] [PubMed] [Google Scholar]
- 82.Gellad WF, Haas JS, Safran DG. Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study. J Gen Intern Med. 2007;22(11):1572–8. doi: 10.1007/s11606-007-0385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holmes HM, Luo R, Hanlon JT, et al. Ethnic disparities in adherence to antihypertensive medications of medicare part D beneficiaries. J Am Geriatr Soc. 2012;60(7):1298–303. doi: 10.1111/j.1532-5415.2012.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ishisaka DY, Jukes T, Romanelli RJ, et al. Disparities in adherence to and persistence with antihypertensive regimens: an exploratory analysis from a community-based provider network. J Am Soc Hypertens. 2012;6(3):201–9. doi: 10.1016/j.jash.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 85.Conn VS, Ruppar TM, Enriquez M, et al. Medication adherence interventions that target subjects with adherence problems: systematic review and meta-analysis. Res Social Adm Pharm. 2016;12(2):218–46. doi: 10.1016/j.sapharm.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hugtenburg JG, Timmers L, Elders PJ, et al. Definitions, variants, and causes of nonadherence with medication: a challenge for tailored interventions. Patient Prefer Adherence. 2013;7:675–82. doi: 10.2147/PPA.S29549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasan RS, Larson MG, Benjamin EJ, et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33(7):1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 88.Senni M, Redfield MM. Heart failure with preserved systolic function. A different natural history? J Am Coll Cardiol. 2001;38(5):1277–82. doi: 10.1016/s0735-1097(01)01567-4. [DOI] [PubMed] [Google Scholar]
- 89.Farley TA, Dalal MA, Mostashari F, et al. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med. 2010;38(6):600–9. doi: 10.1016/j.amepre.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 90.Margolius D, Bodenheimer T, Bennett H, et al. Health coaching to improve hypertension treatment in a low-income, minority population. Ann Fam Med. 2012;10(3):199–205. doi: 10.1370/afm.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]