Summary
Escalated aggression can have devastating societal consequences, yet underlying neurobiological mechanisms are poorly understood. Here we show significantly increased inter-male mouse aggression when neurotransmission is constitutively blocked from either of two subsets of serotonergic, Pet1+ neurons – one identified by dopamine receptor D1(Drd1a)::cre driven activity perinatally, the other by Drd2::cre from pre-adolescence onward. Blocking neurotransmission from other Pet1+ neuron subsets of similar size and/or overlapping anatomical domains had no effect on aggression compared to controls, suggesting subtype-specific serotonergic neuron influences on aggression. Using established and novel intersectional genetic tools, we further characterized these subtypes across multiple parameters, showing both overlapping and distinct features in axonal projection targets, gene expression, electrophysiological properties, and effects on non-aggressive behaviors. Notably, Drd2::cre-marked 5-HT neurons exhibited D2-dependent inhibitory responses to dopamine in slices, suggesting direct and specific interplay between inhibitory dopaminergic signaling and a serotonergic subpopulation. Thus, we identify specific serotonergic modules that shape aggression.
Graphical Abstract

Introduction
Throughout the animal kingdom, species-typical aggressive behaviors are used to acquire or safeguard food, mating partners, progeny, and territory and are essential for individual and population survival (Marler, 1976). While specific behavior patterns differ between species, the motivation toward aggression has been strongly conserved evolutionarily (Lorenz, 1966). Likewise, aggression is an inherent thread in the fabric of human society, but when escalated and uncontrolled, as can occur in disorders such as schizophrenia, intermittent explosive disorder, autism, or even dementia (Volavka, 2002), the outcome can be detrimental to the individual and society. Across these varied DSM (American Psychiatric Association, 2013) disease categories, the behavioral presentation of aggression is one integral endophenotype, perhaps reflecting a shared underlying component at the level of specific cells, circuits, and/or genes – as conceptually put forth by NIMH’s RDoC (Research Domain Criteria; http://www.nimh.nih.gov/research-priorities/rdoc/constructs/rdoc-matrix.shtml). Here we discriminate specific brain serotonergic neuronal subtypes linked to abnormally high levels of inter-male aggression in mice, and analyze these neuronal modules across multiple levels (RDoC “units of analysis”), from the molecular and cellular to the organismal.
Motivating this work in part are the many pharmacological manipulations and gene association studies establishing serotonin (5-hydroxytryptamine, 5-HT) and the neurons that produce it as shaping aggression levels in animals and humans (Audero et al., 2013, Lesch and Merschdorf, 2000, Zalsman et al., 2011, Takahashi and Miczek, 2014). Increasingly, evidence points to an association between decreased serotonergic tone in the adult and an increased potential for pathological aggression (Hendricks et al., 2003, Takahashi and Miczek, 2014, Saudou et al., 1994, Audero et al., 2013, Angoa-Perez et al., 2012, Mosienko et al., 2012, Alenina et al., 2009). In line, current treatments for patients displaying impulsive aggression include substances that enhance 5-HT levels, e.g. selective serotonin reuptake inhibitors; (Bond, 2005, Coccaro and Kavoussi, 1997, Reist et al., 2003) or monoamine oxidase A inhibitors (Hollander, 1999). Such treatments, though, target the entire serotonergic neuronal system and thus can trigger undesirable and even dangerous side effects due to the multiplicity of behaviors and physiological processes modulated by 5-HT (reviewed in (Hale et al., 2012, Lucki, 1998). Increasing evidence suggests that the many functions modulated by the serotonergic system are, partially, the collective result of distinct serotonergic neuronal modules, each governing a particular set of functions (Brust et al., 2014, Molliver, 1987, Fernandez et al., 2015, Gaspar and Lillesaar, 2012, Hale et al., 2012, Kim et al., 2009, Okaty et al., 2015). A key question, then, is whether there are specialized distinct serotonergic neurons that shape facets of aggression or whether instead, global changes in serotonergic tone are necessary.
Recent work has demonstrated the utility of subdividing the 5-HT neuronal system based on patterns of gene expression therein identifying distinct molecular subtypes of serotonergic neurons that can be targeted for functional studies (Brust et al., 2014, Spaethling et al., 2014, Commons et al., 2003, Fox and Deneris, 2012, Wylie et al., 2010, Fernandez et al., 2015, Jensen et al., 2008, Okaty et al., 2015). To test whether there exist specialized serotonergic neurons involved in establishing normative levels of aggression, we implemented intersectional genetic strategies in mice (Awatramani et al., 2003, Jensen et al., 2008, Kim et al., 2009) to functionally silence molecularly-defined subsets of serotonergic neurons and assess the effects on aggressive behavior. Resultant findings reveal functional modularity within the serotonergic neuronal system at the behavioral, cellular, and hodological level, providing insight into the cellular and molecular substrates that may influence behaviors such as aggression.
Results
Neurotransmission by serotonergic neurons is required for normative levels of adult inter-male rodent aggression
We employed molecular genetic tools to probe whether neurotransmission from serotonergic neurons is required for normative levels of aggressive behavior in adult male mice, and if so, to determine which subtype(s) of serotonergic neurons underlie this function. We “silenced” vesicular neurotransmission from 5-HT neurons constitutively through recombinase-dependent expression of a tetanus toxin light chain-GFP fusion, referred to throughout as “tox” (Kim et al., 2009), which impedes Vamp2-dependent exocytosis of neurotransmitters. We previously established tox as potent and effective in serotonergic neurons (Kim et al., 2009).
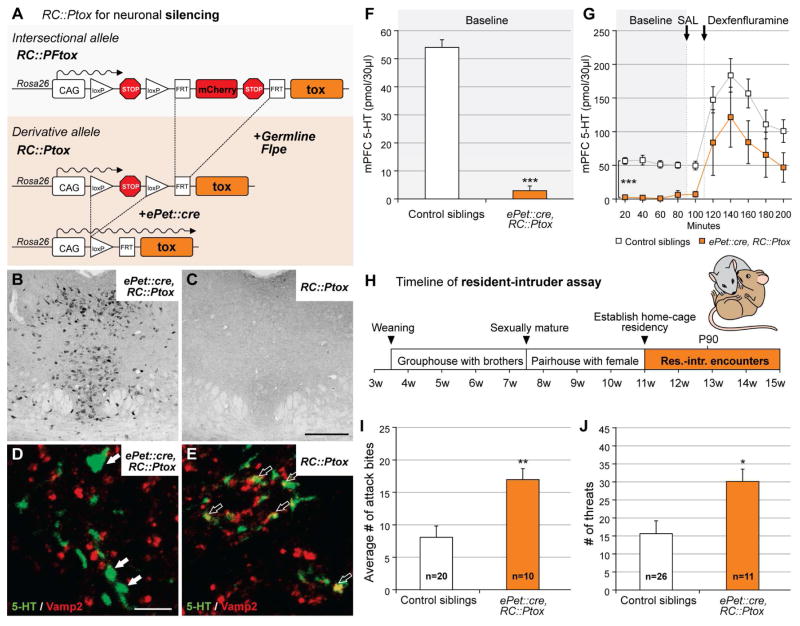
Using a double transgenic strategy pairing the pan-serotonergic ePet::cre driver (Scott et al., 2005) with the Cre-responsive RC::Ptox allele (Kim et al., 2009) Figure 1A), we observed robust and reproducible tox expression restricted to regions containing the serotonergic raphe nuclei in double transgenic ePet::cre, RC::Ptox animals (referred to here as Pet1-silenced animals; Figure 1B), but not single transgenic RC::Ptox control littermates (Figure 1C). Immunohistochemical analyses of brain tissue from double transgenic ePet::cre, RC::Ptox mice further showed that 5-HT-positive axonal varicosities and synaptic boutons were diminished in VAMP2, consistent with VAMP2 cleavage by tox, the documented method of tetanus toxin light chain activity (Link et al., 1992, Kim et al., 2009) Figure 1D, E). Additionally, serotonergic axonal processes in brains of ePet::cre, RC::Ptox mice appeared enlarged qualitatively (Figures 1D) relative to controls (Figure 1E), consistent with a build-up of 5-HT-loaded vesicles due to disrupted release (Kim et al., 2009). Finally, microdialysis experiments (Figure 1F) sampling the medial prefrontal cortex (mPFC; Figure S1), a known projection target of serotonin neurons (Azmitia and Segal, 1978), demonstrated a near absence of extracellular 5-HT in ePet::cre, RC::Ptox transgenic mice at baseline (controls vs. ePet::cre, RC::Ptox, M-W U < 0.0001, p = 0.008). Systemic administration of fenfluramine (3mg/kg, i.p.), which has been shown to induce serotonin release via reversal of the serotonin transporter (Rothman et al., 2003), increased extracellular 5-HT in both Pet1-silenced and control siblings (Figure 1G; main effect of time course F(9, 119) = 17.1, p < 0.001) although serotonin levels remained higher in controls (main effect of genotype F(1, 119) = 10.3, p = 0.009). These data suggest that normal vesicular 5-HT release was blocked, but that viable axons were still present in the mPFC of ePet::cre, RC::Ptox mice.
Figure 1. Silencing serotonin neurons en masse increases aggression.
A) Schematic illustrates strategy for pan-serotonergic tox-mediated silencing. Silencing of Pet1 neurons was accomplished by pairing RC::Ptox, a Cre-only responsive derivative allele of RC::PFtox, with the driver ePet::cre.
B and C) Photomicrographs of the dorsal raphe showing immunostaining for GFP from ePet::cre, RC::Ptox (B) and RC::Ptox control (C) mice illustrate the effectiveness of tox-GFP fusion protein expression when ePet::cre is present. Scale bar = 500 μm.
D and E) Serotonergic axon terminals in the trigeminal nucleus show that Vamp2 staining is absent from serotonergic terminals in ePet::cre, RC::Ptox mice (D, no yellow), but that immuno-labeled 5-HT and Vamp2 colocalize in RC::Ptox controls (E, yellow). Scale bar = 5 μm.
F) As measured by in vivo microdialysis, baseline serotonin levels (mean ± SEM) in the mPFC were significantly lower in ePet::cre, RC::Ptox (orange) than in RC::Ptox (white) controls (M-W U < 0.0001, p = 0.008).
G) 5-HT levels (mean ± SEM) in mPFC dialysate samples from ePet::cre, RC::Ptox (orange) and control (white) mice before and after administration of dexfenfluramine (3mg/kg, i.p), which induces non-exocytotic release of neurotransmitter.
H) Timeline of experimental design for resident-intruder assay illustrating rearing/housing conditions of male test subjects (residents).
I) Number of attack bites (mean ± SEM) demonstrated by ePet::cre, RC::Ptox mice (orange) and control siblings (white) during the resident-intruder assays (M-W U = 38, p = 0.005).
J) Number of lateral threats (mean ± SEM) demonstrated by ePet::cre, RC::Ptox mice (orange) and control siblings (white) during the resident-intruder assays (M-W U = 51.5, p=0.03).
To determine the impact of en masse silencing of serotonergic neurons on aggressive behaviors, we subjected individual Pet1-silenced mice and sibling controls to an ethologically guided version of the resident-intruder assay (Fish et al., 1999, Miczek and O’Donnell, 1978) (Figure 1H). Each test mouse (resident male) was “primed” with a brief non-contact exposure to a male breeder, after which an intruder male was placed into the resident’s home cage. This assay provided high enough baseline levels of aggression to detect increases or decreases (Miczek and O’Donnell, 1978, Fish et al., 1999).
Using this assay, Pet1-silenced mice displayed more aggression than control siblings. Pet1-silenced mice delivered significantly more attack bites (Figure 1I; 17.0 ± 1.7 (Pet1-silenced, n=10) versus 8.1 ± 1.7 (control, non-tox-expressing siblings, n=20) M-W U = 38, p = 0.005; breakdown of sibling controls by single-transgenic genotype is shown in Figure S2A), displayed significantly more lateral threats toward intruders, (Figure 1J; 30.1 ± 3.4 versus 15.6 ± 3.6; M-W U = 51.5, p=0.03) and spent significantly more time tail rattling than controls (Table S1; 3.5s ± 1.0s vs. 1.4s ± 0.4s; M-W U = 45, p=0.01). Although Pet1-silenced mice tended to demonstrate their first attack bite sooner and to pursue intruders more often than controls, these differences did not reach statistical significance (Table S1).
These data are consistent with the model that functional deficiency in serotonergic neurons is associated with increased aggression (Hendricks et al., 2003, Audero et al., 2013, Angoa-Perez et al., 2012, Mosienko et al., 2012, Alenina et al., 2009). The present findings further establish an experimental platform to delineate those serotonergic neurons that are responsible for these effects on aggression.
Specific subtypes of brain serotonergic neurons shape inter-male aggression levels
We used a dual recombinase (Cre and Flpe)-based intersectional approach (Figure 2; (Jensen et al., 2008, Awatramani et al., 2003, Kim et al., 2009, Ray et al., 2011, Dymecki et al., 2010) to test whether there exist specialized aggression-influencing subtypes of serotonergic neurons. By leveraging molecular differences across serotonergic neurons to drive tox expression, we silenced discrete molecular subtypes of serotonergic neurons and assayed the impact on aggression using the resident-intruder test described above.
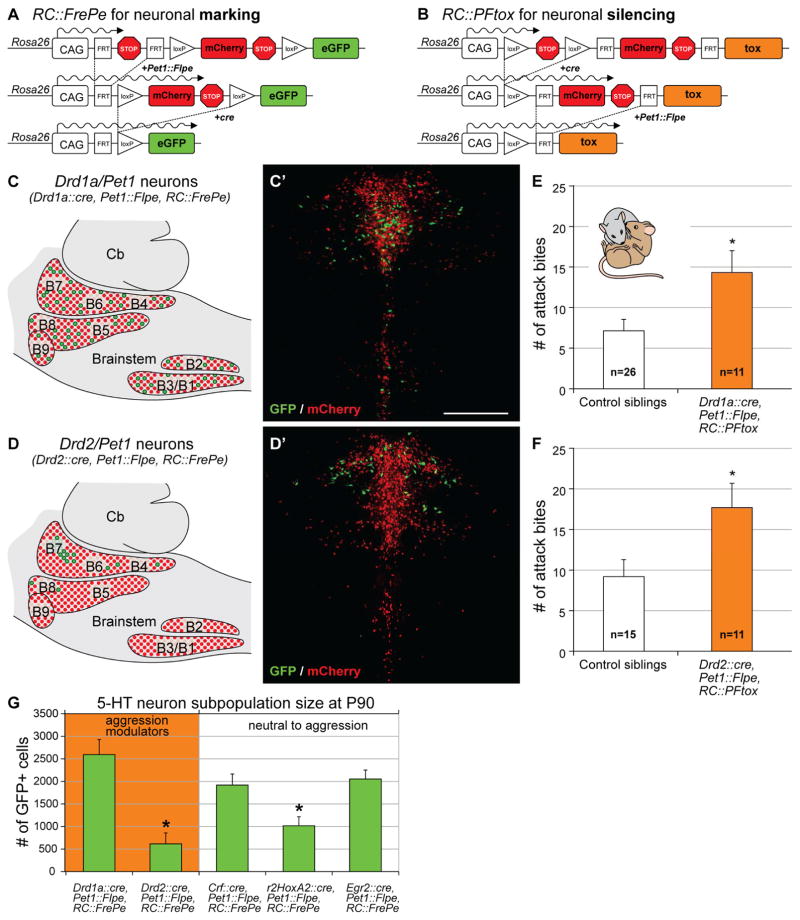
Figure 2. Serotonergic subtypes that modulate aggression.
A and B) Schematics illustrate strategies for labeling (A) or silencing (B) subtypes of serotonergic neurons.
C/C′ and D/D′) Cartoons of a sagittal brain slice illustrate the distributions of the Drd1a/Pet1 (C) and Drd2/Pet1 subtypes (D) within the serotonergic raphe (B1–B9). Photomicrographs show the presence of GFP+ Drd1a/Pet1 (C′) and GFP+ Drd2/Pet1 neurons (D′) within the context of the remaining Pet1+ population labeled by mCherry in coronal brain slices through the DR (B7). Cb, cerebellum Scale bar = 50 μm (C′ and D′).
E and F) Drd1a/Pet1-silenced (E) and Drd2/Pet1-silenced (F) mice exhibited more attack bites than control siblings during the resident-intruder assay (Drd1a/Pet1: M-W U=75, p=0.023 and Drd2/Pet1: M-W U=42, p=0.036).
G) Number of GFP+ neurons (mean ± SEM) for each of the 5 subtypes detected using the RC::FrePe reporter allele in the serotonergic raphe system of male mice (P90; one-way ANOVA, F(4,10)=10.47, p=0.001, Fisher’s LSD, P<0.05).
To gain genetic access to molecular subsets of serotonergic neurons, we partnered the intersectional reporter allele RC::FrePe (Figure 2A; (Bang et al., 2012, Brust et al., 2014) or silencing allele RC::PFtox (Figure 2B; (Kim et al., 2009), respectively, with the pan-serotonergic Pet1::Flpe driver and one of multiple cre driver lines. We chose five cre drivers whose expression delineates subsets distributed throughout the serotonergic raphe nuclei. Three of these drivers (Gong et al., 2007) targeted subtypes with constituent neurons residing in the dorsal raphe nucleus (DR): dopamine receptor type-I (Drd1a::cre), dopamine receptor type-II (Drd2::cre), and corticotropin-releasing factor (Crf::cre). We additionally selected two cre drivers, r2Hoxa2::cre (Awatramani et al., 2003) and Egr2::cre (Voiculescu et al., 2000), which together encapsulate most median raphe (MR; also referred to as the prepontine raphe, PnR (Alonso et al., 2013)) and raphe magnus (RMg) neurons (Jensen et al., 2008), respectively. We refer to the captured intersectional neuron subtypes as Drd1a/Pet1 (Figure 2C, C′), Drd2/Pet1 (Figure 2D, D′), Crf/Pet1 (Figure S3A, A′), r2Hoxa2/Pet1 (Figure S3B, B′), and Egr2/Pet1 (Figure S3C, C′).
We analyzed the effect of tox-mediated silencing of each molecularly defined neuronal subtype. Silencing of either the Drd1a/Pet1 or the Drd2/Pet1 neuron subtypes resulted in an increase in number of attack bites compared to non-tox-expressing littermate controls (Drd1a/Pet1- silenced (n=11): 14.3±2.7 attack bites, control siblings (n=26): 7.3±1.4 attack bites, M-W U=75, p=0.023; Drd2/Pet1-silenced (n=11): 17.7±3.0 attack bites, control siblings (n=15): 9.2±2.2 attack bites, M-W U=42, p=0.036; Figure 2E and F; breakdown of controls by genotype shown in Figure S2B, C). By contrast, silencing of Crf/Pet1 neurons (n=12, 10.6±3.1 attack bites), r2HoxA2/Pet1 neurons (n=11, 7.8±1.7 attack bites), or Egr2/Pet1 neurons (n=12, 7.6±2.1 attack bites), did not increase the number of attack bites relative to control siblings (Crf/Pet1 controls, n=22, 10.9±1.7 attack bites; U=170, p=0.75; r2HoxA2/Pet1 controls, n=24, 4.5±1.1 attack bites, M-W U=94.5, p=0.14; Egr2/Pet1 controls, n=23, 8.6±1.8 attack bites, M-W U=132.5, p=0.85; Figure S3D, E, F; Figure S4A, B, C).
We next queried whether silencing of Drd1a/Pet1 or Drd2/Pet1 neurons affected other aspects of aggressive behavior (Table S2). We observed a significant increase in the number of lateral threats when we silenced either of the two subtypes (Drd1a/Pet1-silenced: 26.6±4.8 vs. control siblings: 13.8±2.9, M-W U=76.5, p=0.025 and Drd2/Pet1-silenced: 34.5±6.3 vs. control siblings: 15.0±3.5, M-W U=36, p=0.017). Drd1a/Pet1-silenced mice (90.8±25.5s) also had a significantly shorter average latency to attack than respective sibling controls (171.9±22.9s, M-W U=82.5, p=0.046). Silencing either subtype failed to affect time spent tail rattling or number of pursuits. These data show that the Drd1a/Pet1 and Drd2/Pet1 subtypes of serotonergic neurons influence some but not all salient elements of rodent aggressive behaviors.
To rule out that the observed aggression phenotypes might result from silencing a sufficiently large number of non-specific serotonergic neurons, we quantified the cell population size of each of the Pet1 neuron subsets tested in the resident-intruder assay. We counted the number of GFP+ cells in the brains of male mice generated by pairing RC::FrePe with Pet1::Flpe and subtype-specific cre drivers. We found that the Drd1a/Pet1, Crf/Pet1 and Egr2/Pet1 subsets were of comparable size with 2594±333, 1918±247, and 2052±200 GFP+ cells per brain, respectively (Figure 2G). The Drd2/Pet1 and r2HoxA2/Pet1 subsets were significantly smaller with 616±241 and 1016±199 GFP+ cells per brain, respectively (Figure 2G; one-way ANOVA, Genotype F(4,10)=10.47, p=0.001, Fisher’s LSD, P<0.05). The aggression-relevant Drd2/Pet1 subset was the smallest serotonergic population tested, indicating that the observed aggression phenotypes were not the result of silencing a threshold number of non-specific serotonergic neurons, but rather resulted from silencing distinct serotonergic neuron subtypes.
Drd1a/Pet1 and Drd2/Pet1 neurons modulate unique sets of non-aggressive behavior
We next investigated the broader role these neuron subtypes play in regulation of motor and emotionally relevant behavior. We quantified non-aggressive behaviors during the resident-intruder assays and found that Drd1a/Pet1-silenced mice spent significantly less time making ‘nose-to-nose’ and ‘nose-to-anogenital region’ contacts with the intruder (Drd1a/Pet1-silenced: 2.5s±0.7s, control siblings: 7.2s ± 1.0s (mean ± SEM); M-W U=48, p=0.002) and significantly more time walking around the cage compared to control siblings (Drd1a/Pet1-silenced: 110.7s ± 6.4s, control siblings: 91.8s ± 3.4s; M-W U=67, p=0.016). Drd1a/Pet1-silenced mice did not differ significantly from controls in rearing, digging, or grooming behaviors (Table S3). No changes in non-aggressive behaviors during the resident-intruder assay were detected in Drd2/Pet1-silenced mice (Table S3).
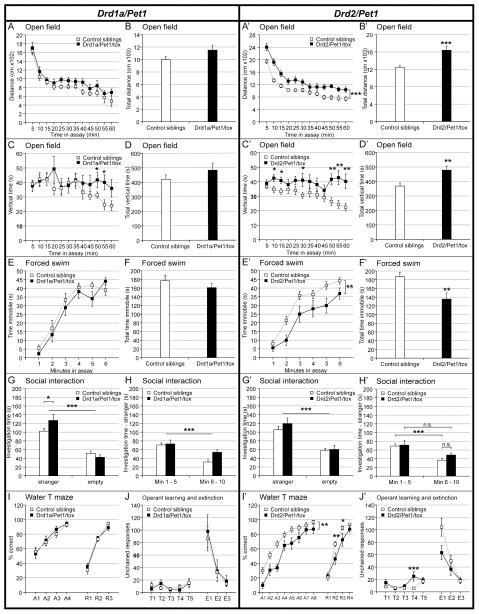
Further, we subjected new cohorts of Drd1a/Pet1- and Drd2/Pet1-silenced mice and their control littermates to behavioral assays assessing neurological functions, emotional responses, social behavior, and cognitive functions (Figures 3 and S5, and Tables S4 and S5). Although the Drd2/Pet1 subset is significantly smaller in neuron number and more circumscribed anatomically than the Drd1a/Pet1 subset, Drd2/Pet1-silencing affected a wider range of behavioral outcomes. Drd1a/Pet1-silenced males demonstrated subtle, yet statistically significant phenotypes in 2 of the 10 tests (open field and social interaction tests; Figure 3A–J), whereas Drd2/Pet1-silenced males showed a relatively robust array of behavioral phenotypes in 4 of the 10 tests (open field, forced swim test, water-T-maze, and operant learning; Figure 3A′–J′). See supplementary Tables S4 and S5 for statistical analysis of behavioral assays. Both Drd1a/Pet1- and Drd2/Pet1-silenced mice exhibited hyperactivity in novel environments, although the phenotype was more evident in the Drd2/Pet1-silenced animals (Figure 3A–D and A′–D′). Drd2/Pet1-silenced mice also demonstrated hyperactivity in the forced swim test and hyperactivity or impulsivity in the water-T-maze and operant learning assays (Figure 3E′, F′, I′, J′). Drd1a/Pet1-silenced mice did not show phenotypes in these assays, but spent significantly more time with the stranger mouse in the social interaction test as compared to sibling controls (Figure 3G, H). These results suggest that Drd2/Pet1-silenced animals exhibit more persistent motor activity in a novel environment but not in the home cage, as well as potentially impaired spatial learning ability. By contrast, Drd1a/Pet1-silenced mice demonstrate only slight hyperactivity and increased social preference.
Figure 3. Behavioral phenotyping of Drd1a/Pet1- and Drd2/Pet1-silenced mice.
All values shown are mean ± SEM. Complete statistical measures are provided in Tables S4 (Drd1a/Pet1) and S5 (Drd2/Pet1).
A/A′ – D/D′) Open field. Horizontal distance traveled during a 60 min. open field assay is shown in 5 min. bins (A/A′) and as total distance traveled (B/B′). Time spent in vertical exploration is shown in 5 min. bins (C/C′) and as total vertical exploration time (D/D′).
E/E′ and F/F′) Forced Swim. Average time spent immobile during each minute of the forced swim test (E/E′) and the total time spent immobile (F/F′).
G/G′ and H/H′) Three chamber social interaction. Average time spent investigating the perforated container holding a stranger mouse (under perforated cup) or investigating an empty perforated container during the three-chamber social interaction assay. (H/H′) shows the average time spent at the container with the stranger mouse binned into first and second halves of the assay.
I/I′) Water T-Maze. The graphs plot the % of correct arm choices during acquisition and reversal learning phases for Drd1a/Pet1-silenced (I), Drd2/Pet1-silenced (I′), and control mice.
J/J′) Operant learning task. The graphs plot the number of unchained responses, lever presses in the absence of stimuli, during training and extinction phases for the Drd1a/Pet1-silenced (J), Drd2/Pet1-silenced (J′), and control mice.
Drd1a/Pet1 and Drd2/Pet1 neuron subtypes differ in anatomical distribution and onset of respective Drd::cre expression
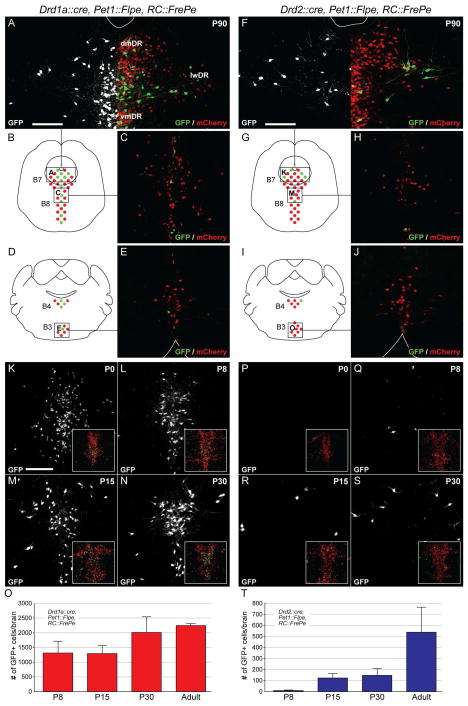
We next examined Drd1a/Pet1 and Drd2/Pet1 neuron distribution across the serotonergic raphe. The DR (comprised of nuclei B7, B6, and B4 (Dahlstroem and Fuxe, 1964, Steinbusch, 1981), with B4 here considered the caudal-most DR domain) can be broken down into the ventromedial (vmDR), dorsomedial (dmDR), and lateral wing regions (Hale and Lowry, 2011, Peyron et al., 1995). In the adult male brain, Drd1a/Pet1 neurons were detected throughout raphe nuclei largely enriched along the midline (Figures 4A–E). By contrast, Drd2/Pet1 neurons localized to the rostral-most DR (B7; Figures 4F–J), especially the lateral wings (Figures 4A and 4F), with few cells in the caudal DR, MR (B8), and medullary raphe (B3, RMg).
Figure 4. Reporter labeling of intersectional and subtractive serotonin neuron populations.
Intersectional Drd1a/Pet1 and Drd2/Pet1 neurons express GFP, whereas the subtractive population of 5-HT neurons (Pet1-only) expresses mCherry, as labeled by the intersectional reporter RC::FrePe.
A–C) Images show Drd1a/Pet1 GFP+ and Pet1-only mCherry+ neurons from regions of the DR (A) and MR (C) as diagrammed in (B). In (A), the left side of the image shows in white Drd1a/Pet1 neurons, and the right half shows both Drd1a/Pet (green) and Pet1-only (red) neurons. dm, dorsomedial; lw, lateral wings; vm, ventromedial. White line (A and F) delineates the cerebral aqueduct. Scale bars = 50 μm.
D and E) Drawing (D) illustrates the brain section from which the RMg was imaged (E). White line delineates ventral brain surface.
F–H) Images show Drd2/Pet1 GFP+ and Pet1-only mCherry+ neurons from regions of the DR (F) and MR (H) as diagrammed in (G). In (F) the left side of the image shows in white only the Drd2/Pet1 neurons, and the right half shows both Drd2/Pet1 and Pet1 neurons. dm, dorsomedial; lw, later wings; vm, ventromedial. White line delineates cerebral aqueduct.
I and J) Drawing (I) illustrates the brain section from which the RMg was imaged (J). White line delineates the ventral brain surface.
K–N) Images show Drd1a/Pet1 GFP+ neurons within the DR of mice at P0 (K), P8 (L), P15 (M), and P30 (N). Insets show same region labeled with GFP and mCherry.
O) Average number (mean ± SEM) of GFP+ Drd1a/Pet1 neurons within the DR at P8, P15, P30, and adult (>P90).
P–S) Images show Drd2/Pet1 GFP+ neurons within the DR of mice at P0 (P), P8 (Q), P15 (R), and P30 (S). Insets show the same region labeled with GFP and mCherry.
T) Average number (mean ± SEM) of GFP+ Drd2/Pet1 neurons within the DR at P8, P15, P30, and adult (>P90).
As intersectional reporter expression served as a proxy for the onset of putative dopamine receptor gene and tox expression, we examined the ontogeny of GFP expression driven by RC::FrePe. Pet1 and Pet1::Flpe expression begins in the newly postmitotic precursor serotonin neurons around E12.5 (Jensen et al., 2008, Scott et al., 2005), with Drd expression subsequently. We detected Drd1a/Pet1 GFP+ cells in late gestation embryos (data not shown), with numerous marked cells by P0 (Figure 4K) and increasing at later ages (Figure 4K–O). By contrast, Drd2/Pet1 GFP+ neurons were observed starting around P8, were consistently detectable by P15, and increased in number between P30 and adulthood (Figures 4P–T). Thus, while a considerable number of Drd1a/Pet1 neurons demonstrated Cre activity embryonically, most Drd2/Pet1 neurons only showed Cre activity (and thus GFP and tox expression) later during post-natal development (i.e., P8 and later).
Drd2/Pet1 neurons express functional D2 receptors
To determine whether dopamine receptor genes are indeed expressed in the adult Drd1a/Pet1 and Drd2/Pet1 cell populations, we manually sorted fluorescently labeled cells (Hempel et al., 2007) from the DR of adult triple transgenic mice. RNA was extracted from single cells and, in the case of Drd1a/Pet1 mice, a pooled cell sample (~30 cells). The presence of endogenous Drd1a and Drd2 transcripts as well as select pan-serotonergic marker genes was assessed using qRT-PCR and/or RNA-seq.
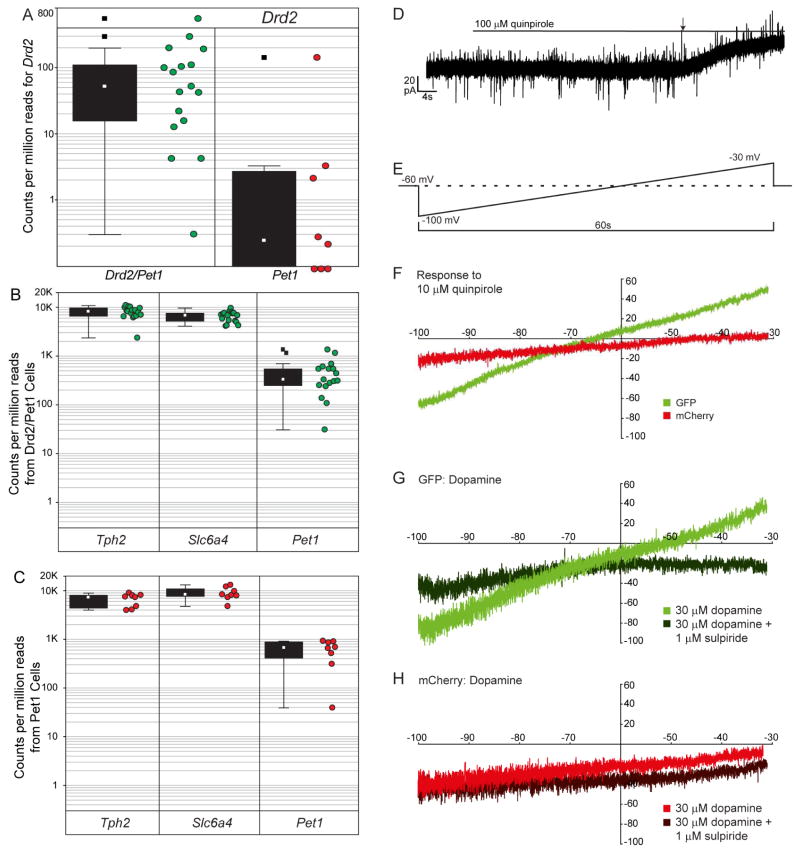
Using RNA-seq, we readily detected Drd2 transcripts in 17 of 17 cells with 16 of 17 having a counts-per-million reads (CPM; Anders, et al. 2013) greater than one, a commonly applied detection threshold (Figure 5A). To determine whether the Drd2::cre driver was capturing near all Drd2-expressing 5-HT neurons, we also measured gene expression in 8 mCherry+ Pet1-only neurons. Three of 8 Pet1-only cells had a Drd2 CPM > 1, only one of which was above the lower quartile range of Drd2 transcript expression levels in Drd2/Pet1 GFP+ cells (Figure 5A). Applying a generalized linear model likelihood ratio test using edgeR (Anders, et al. 2013), we confirmed that transcript counts were significantly greater in the Drd2/Pet1 sample group than in the Pet1-only group (p=.03) by a factor of 6. By contrast, select 5-HT marker transcripts including tryptophan hydroxylase 2 (Tph2), serotonin transporter (Slc6a4 also referred to as Sert), and Pet1 were detected in all GFP+ and mCherry+ neurons by RNA-Seq (Figure 5B, C). Together, these data indicate that the Drd2/Pet1 neurons are bona fide serotonergic neurons that express endogenous Drd2 transcripts. Transcripts for Drd1a, Drd3, Drd4, and Drd5 were not detected in Drd2/Pet1 GFP+ neurons by RNA-seq (data not shown). Our data further show that the few Pet1-only neurons that harbor Drd2 transcripts do so at significantly lower levels (close to the detection limit by RNA-seq) suggesting there may be a critical threshold of Drd2 transcriptional activity to effectively drive the Drd2::cre transgene and perhaps to drive functionally meaningful levels of Drd2 receptor protein.
Figure 5. Validation of dopamine receptor expression and function.
A) Counts per million reads (CPM) for Drd2 as measured using RNA-seq in Drd2/Pet1 and Pet1-only neurons. Box plots show the median (open square), upper and lower quartiles (box), non-outlier min/max (whiskers), and outliers (filled square). The associated raw data (green and red dots) are shown to the right of the box plots.
B) Box plots show summaries and raw data of RNA-seq CPMs for three serotonergic marker genes expressed by the Drd2/Pet1 neurons shown in (A): tryptophan hydroxylase 2 (Tph2), 5-HT transporter (Slc6a4), and Pet1.
C) Box plots show summaries and raw data of RNA-seq CPMs for Tph2, Slc6a4, and Pet1 expressed by the Pet1-only neurons shown in (A).
D) An example trace from a GFP+ Drd2/Pet1 neuron shows outward positive current elicited by bath application of 100 μM quinpirole, a D2-like receptor agonist. The line indicates when quinpirole was added to ACSF reservoir, and the arrow indicates beginning of the response.
E) Schematic illustrates ramp protocol employed before and after drug exposure to further explore the pharmacological responses to quinpirole and dopamine. From the holding potential of −60 mV, the membrane potential was dropped to −100 mV, raised over the course of 1 min. to −30 mV, and then stepped back to −60 mV. To isolate drug induced current, pre-drug ramps were subtracted from post-drug ramps.
F) Shown are average current responses to 10 μM quinpirole for GFP+ Drd2/Pet1 (n=8) and mCherry+ Pet1-only (n=5) neurons.
G) Average current responses (Post minus Pre) are shown for dopamine alone (light) and dopamine (30 μM) in the presence of the D2-like receptor antagonist sulpiride (1 μM, dark) for Drd2/Pet1 neurons (n=4).
H) Average current responses (Post minus Pre) are shown for dopamine (30 μM) alone (light) versus in the presence of sulpiride (1 μM, dark) for mCherry+ Pet1-only neurons (n=4).
We next examined whether Drd2/Pet1 neurons contained functional Drd2 receptors. We obtained whole-cell electrophysiological recordings from Drd2/Pet1 (GFP+) and Pet1-only (mCherry+) neurons. In voltage clamp recordings from GFP+ Drd2/Pet1 neurons, bath application of the D2 receptor agonist quinpirole (100 μM), which targets Drd2, Drd3, and Drd4, induced a small outward positive current (Figure 5D, 5.4 ± 2.3 pA, 1-sample t-test against H0=0, n = 13, t = 2.38, p = 0.034). As recordings were carried out in the presence of glutamate and GABAA receptor antagonists and a blocker of voltage-gated sodium channels, the data suggest that the change in current observed was a direct response.
We next examined current change in response to a voltage ramp (−100 to −30 mV over 1 min, Figure 5E), before and after drug application for both GFP+ Drd2/Pet1 (n=8) and mCherry+ Pet1-only (n=5) neurons. The drug-specific response was calculated by subtracting pre- from post-drug ramp-induced current. GFP+ Drd2/Pet1 neurons, but not mCherry+ Pet1-only neurons, demonstrated a voltage-dependent response to administration of 10 μM quinpirole: at low voltages, GFP+ neurons showed a positive inward (excitatory) current while at voltages greater than ~−65 mV they showed a positive outward (inhibitory) current (Figure 5F). The nature of the quinpirole-induced GFP+ neuron response suggests the activation of a G-protein coupled inwardly rectifying potassium channel (GIRK; (Lesage et al., 1994, Sahlholm et al., 2008)).
Administration of 30 μM dopamine recapitulated the quinpirole-induced response in GFP+ Drd2/Pet1 (Figure 5G), but not mCherry+ Pet1-only neurons (Figure 5H). This current response in GFP+ neurons was blocked by the D2 antagonist sulpiride (1 μM), which abrogated the positive outward current at depolarizing membrane potentials indicating that the GIRK-like response was mediated by D2 receptors. These data indicate that Drd2/Pet1 neurons express functional D2 receptors, which mediate an inhibitory effect on this neuronal subtype. The low levels of Drd2 transcript detected in some mCherry+ Pet1-only neurons is likely insufficient to drive functional protein expression. Interestingly, in both GFP+ and mCherry+ neurons, a positive inward current persisted in the presence of dopamine and sulpiride, suggesting that dopamine also had an excitatory effect (direct or indirect) on serotonergic neurons that was D2-independent.
In contrast to Drd2/Pet1 neurons, Drd1a expression in Drd1a/Pet1 GFP-marked neurons was ambiguous. We analyzed 23 individual GFP+ Drd1a/Pet1 cells derived from 3 adult Drd1a::cre, Pet1::Flpe, RC::FrePe animals (Table S6) as well as a pool of ~30 GFP+ cells from a fourth animal. qRT-PCR and RNA-seq revealed Drd1a transcript in the pooled sample but not the single cells. Most samples also expressed Tph2, Sert, and Pet1. These data suggest that either Drd1a was not expressed by GFP+ Drd1a/Pet1 cells in adult animals (or expressed in only a very small number of cells) or that expression levels were below our detection limit.
Because Drd1a/Pet1 GFP+ neurons are observed perinatally, we examined Drd1a transcript levels in single Drd1a/Pet1 neurons from P4 (n=4) and P10 (n=8) pups (Table S7). Reads for Drd1a were observed in 1 of 4 cells at P4 and 4 of 8 cells at P10, but only 2 of the P10 cells had CPM>1. All 12 cells expressed Tph2, Sert, and Pet1. It is possible that some DR serotonergic neurons marked by the Drd1a::cre driver reflect ectopic transgene expression; however, as GFP serves as a permanent lineage tracer, Drd1a could be expressed largely transiently during development and down regulated by adulthood, as supported by our increased single-cell Drd1 transcript detection frequency at earlier ages.
Low levels of Drd2 transcript were detected in 8 of the 35 Drd1a/Pet1 neurons assayed across all ages (Tables S6 and S7), suggesting that there may be some overlap between the two serotonergic neuronal lineages during adulthood, and that this sub-subtype may contribute to the shared behavioral phenotypes observed in our neurotransmission silencing experiments. We thus further explored and compared other aspects of cell phenotype between the two lineage-marked populations that might indicate shared versus divergent mechanisms of behavioral regulation, specifically axonal projection patterns.
Drd1a/Pet1 and Drd2/Pet1 neuron subtypes exhibit unique axonal bouton distribution profiles
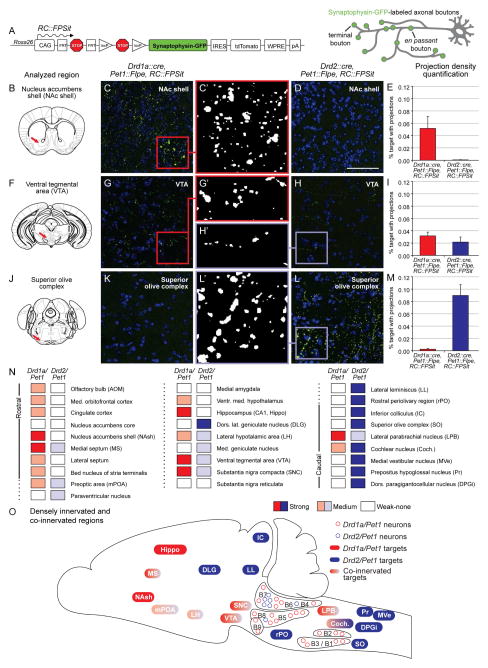
We mapped axonal projections of the Drd1a/Pet1 and Drd2/Pet1 neuron subtypes, using our engineered ROSA26 knock-in intersectional allele, RC::FPSit (Figure 6A), which labels axonal boutons (terminal and en passant) of a given neuron subtype with the presynaptic marker synaptophysin-GFP (Li et al., 2010). Some brain regions were only innervated by the Drd1a/Pet1 subtype (Figure 6B–E), some by both subtypes (Figure 6F–I), and others only by the Drd2/Pet1 subtype (Figure 6J–M).
Figure 6. Axonal projection patterns of Drd1a/Pet1 and Drd2/Pet1 neurons.
A) The ROSA26/CAG knock-in allele, RC::FPSit, allows for visualization of subtype-specific axonal terminals with intersectionally-expressed synaptophysin-GFP.
B–E) Some areas, such as the nucleus accumbens (NAc) shell, were innervated by Drd1a/Pet1 neurons, but not the Drd2/Pet1 population. Schematic (B) illustrates the coronal section from which photomicrographs were taken for Drd1a/Pet1 (C) and Drd2/Pet1 (D) mice. In each image, GFP (green) and DAPI (blue) staining are shown. Center panel (C′) shows thresholded GFP+ axonal terminals from the Drd1a/Pet1 subtype while (E) shows quantification of the area covered by GFP+ staining (average % target area with projections). Scale bar = 50 μM.
F–I) A few regions were innervated by both subtypes as in the ventral tegmental area (VTA). Schematic (F) indicates region analyzed. Representative images from Drd1a/Pet1 (G) and Drd2/Pet1 (H) mice demonstrate GFP+ puncta in the VTA of both genotypes as is depicted in the respective thresholded images (G′ and H′). Quantification of the area covered by GFP+ staining is shown in (I).
J–M) Other regions were innervated only by the Drd2/Pet1 population as in the superior olivary complex (SO). Schematic (J) indicates the region analyzed. Representative images from Drd1a/Pet1 (K) and Drd2/Pet1 (L and L′) mice demonstrate GFP+ puncta in the SO of Drd2/Pet1 but not Drd1a/Pet1 mice. Quantification of the area covered by GFP+ staining is shown in (M).
N) The binned level of innervation for the 28 brain regions analyzed in Drd1a/Pet1 and Drd2/Pet1 brains is indicated with white (no innervation, < 0.005% coverage), light (weak innervation, 0.005% to 0.03% coverage), or dark (strong innervation, > 0.03% coverage) shading. Actual percentages provided in Figure S4.
O) In a sagittal representation, the densest areas of innervation and areas of co-innervation are shown for the Drd1a/Pet1 (red shades) and Drd2/Pet1 (blue shades) neuron subtypes. As in (N), light shades represent modest innervation; dark shades, dense innervation.
Apparent in adult (P90) brains from male triple transgenic Drd1a::cre, Pet1::Flpe, RC::FPSit or Drd2::cre, Pet1::Flpe, RC::FPSit animals was that both subsets innervated numerous brain targets despite their small population size (Figure 6N, O, and 6S). Based on a qualitative examination of innervation patterns, we chose for quantitative analyses 28 brain regions encompassing domains innervated by either the Drd1a/Pet1 and/or Drd2/Pet1 subtypes. We developed a largely automated imaging workflow that allowed for objective quantitative comparison of innervation density across different brains regions. Based on this output (Figure S6), we binned the analyzed target areas into categories of strong, medium, and weak-to-no-innervation (Figure 6N). We found that Drd1a/Pet1 neurons projected strongly to forebrain nuclei, many of which are part of aggression circuits (Figure 6B–I, N, O, and S6). By contrast, Drd2/Pet1 neurons most prominently innervated more caudal brain areas, especially areas known to be involved in sensory (largely auditory) processing (Figure 6F–M–O, and S6). Some areas were co-innervated by Drd1a/Pet1 and Drd2/Pet1 (Figures 6F–I, N, O, and S6).
Discussion
Here we report discovery of two molecularly defined subtypes of serotonergic neurons, each critical for shaping aggressive social interactions in the mouse. One Pet1 serotonergic cell subtype is distinguished by Drd2::cre driver activity from pre-adolescence onward and endogenous Drd2 expression, the other by Drd1a::cre activity perinatally with detectable endogenous Drd1a transcript in some but not all reporter-marked cells. Silencing of other subsets of serotonergic neurons, some of larger population size, did not affect aggressive behavior indicating that constitutive silencing only of specific subtypes of serotonin neurons is sufficient to escalate adult male aggression. The Drd2/Pet1 and Drd1a/Pet1 subtypes are comprised of remarkably few neurons, yet their axonal projections reach a broad range of targets. Differences between these subtypes in anatomical distribution, molecular expression, temporal onset of BAC-driven Cre activity, and projection profile suggest that they may modulate aggression through distinct mechanisms. Collectively, this work reveals the existence of molecularly, hodologically, and developmentally distinct subtypes of serotonergic neurons that are uniquely capable of influencing inter-male aggression.
Functional and anatomical differences between aggression modulating 5-HT neuron subtypes
Silencing either the Drd1a/Pet1 or the Drd2/Pet1 subset of serotonergic neurons resulted in increased displays of aggressive behaviors in adult male mice, suggesting a model in which vesicular neurotransmission from these specific neuron subtypes, either during development and/or adulthood, is required to achieve normative, tempered levels of aggression in the adult. Silencing of the Drd2/Pet1 subtype further resulted in some form of hyper-arousal induced by novel or stressful environments. As overall home cage activity measurement was unchanged, a generic motor phenotype is unlikely. By contrast, the Drd1a/Pet1-silenced animals displayed a phenotype in the social interaction assay, which may implicate the Drd1a/Pet1 subtype in the regulation of social behaviors more generally. These differences in behavioral phenotypes may reflect distinctions observed between the two subtypes in anatomy, dopamine receptor gene expression, and projection targets.
We found that the two subtypes share some anatomical domains (largely the rostral dorsal DR), but largely differ in distribution within the raphe. The developmental onset of the respective Drd::cre drivers also differed between the Drd1a/Pet1 and Drd2/Pet1 subtypes. These findings suggest that although tox-mediated silencing is constitutive, its onset differed between the subtypes. While Drd1a/Pet1 neuron labeling/silencing commenced embryonically, that for Drd2/Pet1 neurons began at adolescence. Adolescence in mice is associated with functional changes in DR serotonin neurons and has been identified as a sensitive period for 5-HT-mediated modulation of emotional behavior in mice (Gross et al., 2002, Yu et al., 2014, Liu et al., 2010, Rood et al., 2014). From our present data, we can deduce that embryonic or early post-natal silencing of the Drd2/Pet1 neurons is not necessary to induce behavioral phenotypes, but rather that subtype-specific neuronal silencing during later post-natal periods is sufficient.
Despite these differences, we observed some overlap between the subtypes, suggesting that the shared aggression phenotype upon neuronal silencing could stem from a neuronal sub-subtype denoted by a history of expression of Pet1/Drd1a/Drd2. However, the extent of these shared features was limited: Drd2 transcript levels in Drd1a/Pet1 cells are lower than those in Drd2/Pet1 cells proper while the shared anatomical and hodological properties are also a limited minority. Probing these Pet1/Drd1a/Drd2low cells for a role in aggression modulation requires new tools with even greater cell subtype resolution.
Projection targets of aggression-relevant Drd1a/Pet1 and Drd2/Pet1 serotonergic neurons
We developed a dual-recombinase allele, RC::FPSit, coding for a synaptophysin-GFP fusion protein enabling bouton visualization and thus enhanced subtype innervation mapping. Several of the innervated brain regions have documented roles in modulating aggressive behavior (Nelson and Trainor, 2007) or contain dopaminergic neuron cell bodies. Drd1a/Pet1 neurons mostly innervated rostral brain structures known to constitute aggression circuits, while Drd2/Pet1 projection targets showed a surprising caudal bias. The majority were centers associated with auditory processing. Notably some human disorders associated with 5-HT dysregulation involve sound hypersensitivity or aberrant auditory processing (Kahkonen et al., 2007, Lucker and Doman, 2012, Wyss et al., 2013). Thus, the behavioral phenotypes in the Drd2/Pet1-silenced animals could be related to altered sensory processing. Importantly, Drd2/Pet1-silenced mice exhibited a normal startle reflex, suggesting that hearing is not overtly impaired. Overall, projection targets of the Drd1a/Pet1 and Drd2/Pet1 neuron subtypes differ considerably, possibly reflecting distinct circuits through which each subset influences behavior.
Both the Drd1a/Pet1 and the Drd2/Pet1 subtypes send dense projections to the dopaminergic midbrain nuclei, namely the ventral tegmental area (VTA) and the substantia nigra pars compacta (SNc). Such a pattern suggests reciprocal circuitry, wherein midbrain dopaminergic neurons send projections to the rostral serotonergic system and vice versa (Niederkofler et al., 2015). Sources of dopaminergic innervation of the Drd/Pet1 subtypes may include the rostral DR in addition to the SNc and midbrain VTA. While the midbrain VTA dopaminergic neurons have been shown to directly impact aggressive behaviors (Yu et al., 2014), the rostral DR dopaminergic neurons have recently been shown to influence the subjective experience of social isolation (Matthews et al., 2016). It remains to be definitively demonstrated, however, whether dopamine-mediated regulation of serotonergic neurons influences serotonin-mediated modulation of aggressive behaviors.
Electrophysiology suggests that dopamine differentially influences Drd2/Pet1 neurons and Pet1-only DR serotonergic neurons
Gene expression analysis and electrophysiology studies provide evidence for a direct effect of dopamine on subsets of serotonergic neurons that we have identified as shaping aggression. Transcriptional profiling of Drd2/Pet1 neurons from adult animals confirmed the presence of Drd2 transcript in nearly all cells analyzed, while electrophysiological studies further revealed that Drd2/Pet1 neurons show a distinct functional response to dopamine as compared to Pet1-only neurons. Future studies will investigate the causal relationship between dopamine-induced inhibition of Drd2/Pet1 neurons and aggression.
Multiscale analysis of inter-male rodent aggression
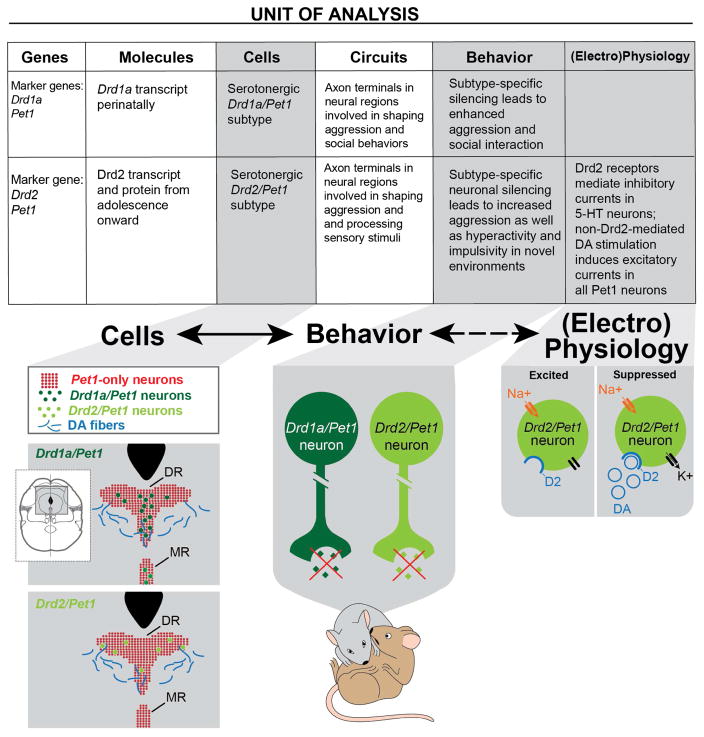
We investigated the neural correlates of aggression across multiple “units of analysis” as summarized in Figure 7 using a framework resembling the NIMH’s RDoC (http://www.nimh.nih.gov/research-priorities/rdoc/constructs/rdoc-matrix.shtml). We identified two subtypes of serotonergic neurons (“cells”) each distinguished by the expression of either the Drd1a or Drd2 marker genes along with serotonergic defining genes such as Tph2, Pet1, Slc6a4 (marker “genes”; Figure 7). We showed that silencing either of these neuronal subtypes enhanced aggressive behavior in adulthood (“behavior”). Further, we demonstrated that these serotonergic subtypes project to specific brain regions and reside in a region of the DR that receives dopaminergic innervation (the foundation for “circuitry” analyses). Our electrophysiological studies in turn demonstrate that while dopamine has an excitatory effect on most serotonergic neurons, Drd2 expressed in the Drd2/Pet1 neurons mediates an inhibitory current, which dampens the excitatory state of the cell (“physiology”). By considering neuronal subtypes underlying aggression at multiple biological levels, we are able to identify several points where further experimental investigation might (1) elucidate substrates for pharmacological treatments to temper impulsive aggression and (2) further our understanding of how neuronal populations in the brain affect the generation and modulation of complex behaviors.
Figure 7. Summary of findings within an RDoC-like matrix.
Chart summarizes findings within context of the NIMH RDoC matrix. Characteristics of the Drd1a/Pet1 and Drd2/Pet1 neuronal subtypes are subdivided amongst six of the RDoC “units of analysis” including genes, molecules, cells, circuits (innervation), behavior, and (electro)physiology).
Schematics illustrate a subset of the key findings. (From left to right) “Cells” illustrates the differential anatomical distribution of the Drd/Pet1 subtypes and the Pet1-only 5-HT neurons in the DR. Dopaminergic fibers innervate the rostral DR (Kalen et al., 1988) where both Drd/Pet1 and Pet1-only 5-HT neurons reside. “Behavior” shows that suppressed release of neurotransmitter (green diamonds) from either of the Drd/Pet1 subtypes enhances aggression.
“Electrophysiology” depicts the dopamine (blue circles)-induced inhibitory current (black arrow) mediated by D2 dopamine receptors (blue half circle) specifically in Drd2/Pet1 neurons. The inhibitory current would counteract other excitatory currents (orange arrow) within the cell. Molecular genetic tools have facilitated relating “Cells” and “Behavior” findings (solid arrow), while it remains to be demonstrated that the Drd2-mediated inhibitory current in Drd2/Pet1 5-HT neurons influences aggressive behavior (dotted arrow).
Experimental procedures
Detailed methods are provided in Supplemental Experimental Procedures (SEP).
Mouse lines
Procedures were in accord with institutional animal care and use committee policies at Harvard Medical School. Transgenics Pet1::Flpe (Jensen et al., 2008) and ePet::cre (Scott et al., 2005) were used to access serotoninergic neurons. Transgenics Drd1a::cre (Gong et al., 2007), Drd2::cre (Gong et al., 2007), Crf::cre (https://www.mmrrc.org/catalog/sds.php?mmrrc_id=30850), and (Rse2)HoxA2::cre (Awatramani et al., 2003), and knock-in transgene Egr2::cre (Egr2Cki)(Voiculescu et al., 2000, Gong et al., 2007) targeted subsets of serotonergic cells when used intersectionally with Pet1::Flpe. Transgenics were backcrossed onto C57BL/6J for over nine generations, with the exception of Crf::cre, which were a mixture of FVB/N, ICR, and C57BL/6 (https://www.mmrrc.org/catalog/sds.php?mmrrc_id=30850).
Recombinase lines were crossed to reporter or effector lines including RC::FrePe (Bang et al., 2012, Engleka et al., 2012) and its derivate RC::rePe (Cre-dependent only)(Ray et al., 2011), which encode a GFP reporter; RC::PFtox (Kim et al., 2009) and its derivate RC::Ptox (Cre-dependent only) which encode a tetanus toxin light chain-GFP fusion; and RC::FPSit encoding a synaptophysin-GFP fusion.
Resident-intruder assay
Species-typical aggression was studied using a resident-intruder (R–I) assay similar to previous reports (Miczek and O’Donnell, 1978, Fish et al., 1999). After weaning, transgenic male mice, “residents”, were group-housed with male siblings until sexual maturity (~P55), when each resident was pair-housed with a female and sired pups. After 3.5 to 4 weeks, resident males were assayed for aggression toward a wild-type (CFW, Charles River) intruder mouse over the course of multiple trials until aggression stabilized –.defined as the trial in which the average number of attack bites demonstrated by a single resident was within 20% variability of the previous 3 trials.
Behavioral phenotyping
Except for R-I assays, all behavioral experiments were performed in the Harvard NeuroDiscovery Center following a two-week acclimation period, conducted in the order described in the SEP and involved mice 2–4.5 months of age at the beginning of the analysis.
Immunohistochemistry
Immunohistochemistry was used to assess: localization and effectiveness of tetanus toxin light chain, anatomical distribution of targeted subsets of serotonin neurons, and projection patterns of targeted cell populations. Primary antibodies employed were chick anti-GFP (Abcam, ab13970–100) or rabbit anti-GFP (gift from Devreotes Lab), rabbit anti-dsRed to detect mCherry (Clontech, 632496), goat anti-serotonin (Abcam, ab66047), and rabbit anti-Vamp2 (Synaptic Systems, 104202).
Electrophysiology
Whole cell patch techniques were applied to fluorescently labeled neurons in mouse brain slices. Voltage clamp recordings were acquired from Drd2/Pet1 (GFP+) and Pet1-only (mCherry+) neurons in the DR, and responses to dopamine (30 μM, Sigma) quinpirole (10–100 μM, D2-selective agonist, Sigma), or sulpiride (1 μM, D2-selective antagonist, Sigma) measured. Current response to a voltage ramp from −100 mV to −30 mV over the course of ~1 min was used to extrapolate information about dopamine induced currents. Details provided in SEP.
Gene Expression
Cell sorting
Gene expression for select transcripts was assessed in a pooled (~30 cells) and single-cell samples for GFP+ Drd1a/Pet1 neurons, and single-cell samples from GFP+ Drd2/Pet1 and mCherry+ (Drd2-)/Pet1 cells. As previously described (Hempel et al., 2007, Okaty et al., 2015), cells were isolated via protease treatment and physical dissociation by pipette trituration of DR brain tissue; individual neurons identified by fluorescent phenotype were collected by micropipette.
RNA preparation
RNA was collected using a PicoPure RNA Isolation Kit (Arcturus), reverse transcribed to cDNA, and linearly amplified using the Ovation RNA-seq System V2 kit (Nugen).
RNA sequencing
cDNA samples were sonicated (Covaris), integrated into RNA-seq libraries using the Ovation Ultralow DR Multiplex System 1–8 Kit (Nugen), quality controlled using TapeStation and qPCR, and sequenced using the Illumina HiSeq 2500 RNA-seq platform at the Harvard Biopolymers Facility.
Statistical analyses
Behavior data were analyzed using statistical packages including Prism Graphpad, StatView 5.0 software (SAS Institute, Cary, NC), Excel 2010, and Statistica (Dell). Statistical tests performed are given with relevant results with the exception of behavioral phenotyping data, which is included in supplemental Tables S4, S5. Electrophysiology data were analyzed with Clampfit 9.2 software (Axon Instruments). Transcript sequence data was analyzed using EdgeR (Anders et al., 2013, Okaty et al., 2015, Robinson et al., 2010).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants: AA021622 (LH), AA013983 (KM), 5R21DA023643-02 (VN, BO, SD), R01 DA034022 (BO, BR, SD, TA, VN), R21MH083613 (SD). Additional support provided by: G.V.R. Khodadad Program for the Study of Genetic, Neurobiological, and Physiochemical Processes of Excessive (Pathological) Selfishness and Aggressive Behavior Fund at Harvard Medical School (VN, BO, TA, SD); the Swiss National Science Foundation PA00P3_131504 and PA00P3_142183 (VN). A portion of the behavioral work was carried out in the NeuroBehavior Laboratory Core subsidized by the Harvard NeuroDiscovery Center; we extend appreciation to Director, Dr. Barbara Caldarone, and Dymecki lab members including Rebecca Senft for discussions and Drs. Rachael Brust and Ryan Dosumu-Johnson for pilot work. We thank Dr. Emily Newman, Ms. Mallory Rice, and Ms. Jia Jia Mai for technical assistance, and the HMS Biopolymers Facility Core for assistance in evaluating sample library quality and next generation sequencing of samples. Authors declare no conflicts of interest.
Footnotes
Author Contributions:
VN, TA, and SD conceived the study. VN performed the behavioural experiments. VN, TA and AN performed the histology and cell counts. VN and AN generated the RC::FPSit mouse. VN and TA quantified the projection targets. TA and BO performed the RT-PCR and RNA-seq experiments. BR performed the electrophysiological recordings. LH performed the microdialysis and HPLC experiments. KM provided expertise in the resident-intruder paradigm. VN, TA, BR, BO and SD wrote the manuscript.
Accession Numbers
Sequencing data are available at GEO Datasets (GSE87758).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALENINA N, KIKIC D, TODIRAS M, MOSIENKO V, QADRI F, PLEHM R, BOYE P, VILIANOVITCH L, SOHR R, TENNER K, HORTNAGL H, BADER M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci U S A. 2009;106:10332–7. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALONSO A, MERCHAN P, SANDOVAL JE, SANCHEZ-ARRONES L, GARCIA-CAZORLA A, ARTUCH R, FERRAN JL, MARTINEZ-DE-LA-TORRE M, PUELLES L. Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains. Brain Struct Funct. 2013;218:1229–77. doi: 10.1007/s00429-012-0456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMERICAN PSYCHIATRIC ASSOCIATION. Diagnostic and statistical manual of mental disorders. Washington D.C: 2013. [Google Scholar]
- ANDERS S, MCCARTHY DJ, CHEN Y, OKONIEWSKI M, SMYTH GK, HUBER W, ROBINSON MD. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat Protoc. 2013;8:1765–86. doi: 10.1038/nprot.2013.099. [DOI] [PubMed] [Google Scholar]
- ANGOA-PEREZ M, KANE MJ, BRIGGS DI, SYKES CE, SHAH MM, FRANCESCUTTI DM, ROSENBERG DR, THOMAS DM, KUHN DM. Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity. J Neurochem. 2012;121:974– 84. doi: 10.1111/j.1471-4159.2012.07739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUDERO E, MLINAR B, BACCINI G, SKACHOKOVA ZK, CORRADETTI R, GROSS C. Suppression of serotonin neuron firing increases aggression in mice. J Neurosci. 2013;33:8678–88. doi: 10.1523/JNEUROSCI.2067-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AWATRAMANI R, SORIANO P, RODRIGUEZ C, MAI JJ, DYMECKI SM. Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat Genet. 2003;35:70–5. doi: 10.1038/ng1228. [DOI] [PubMed] [Google Scholar]
- AZMITIA EC, SEGAL M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–67. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- BANG SJ, JENSEN P, DYMECKI SM, COMMONS KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOND AJ. Antidepressant treatments and human aggression. Eur J Pharmacol. 2005;526:218–25. doi: 10.1016/j.ejphar.2005.09.033. [DOI] [PubMed] [Google Scholar]
- BRUST RD, CORCORAN AE, RICHERSON GB, NATTIE E, DYMECKI SM. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep. 2014;9:2152–65. doi: 10.1016/j.celrep.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCCARO EF, KAVOUSSI RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry. 1997;54:1081–8. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- COMMONS KG, CONNOLLEY KR, VALENTINO RJ. A neurochemically distinct dorsal raphelimbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–15. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- DAHLSTROEM A, FUXE K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta Physiol Scand Suppl, SUPPL. 1964;232:1–55. [PubMed] [Google Scholar]
- DYMECKI SM, RAY RS, KIM JC. Mapping cell fate and function using recombinase-based intersectional strategies. Methods Enzymol. 2010;477:183–213. doi: 10.1016/S0076-6879(10)77011-7. [DOI] [PubMed] [Google Scholar]
- ENGLEKA KA, MANDERFIELD LJ, BRUST RD, LI L, COHEN A, DYMECKI SM, EPSTEIN JA. Islet1 derivatives in the heart are of both neural crest and second heart field origin. Circ Res. 2012;110:922–6. doi: 10.1161/CIRCRESAHA.112.266510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ SP, CAULI B, CABEZAS C, MUZERELLE A, PONCER JC, GASPAR P. Multiscale single-cell analysis reveals unique phenotypes of raphe 5-HT neurons projecting to the forebrain. Brain Struct Funct. 2015 doi: 10.1007/s00429-015-1142-4. [DOI] [PubMed] [Google Scholar]
- FISH EW, FACCIDOMO S, MICZEK KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT(1B) receptor agonist CP-94,253. Psychopharmacology (Berl) 1999;146:391–9. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- FOX SR, DENERIS ES. Engrailed is required in maturing serotonin neurons to regulate the cytoarchitecture and survival of the dorsal raphe nucleus. J Neurosci. 2012;32:7832–42. doi: 10.1523/JNEUROSCI.5829-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASPAR P, LILLESAAR C. Probing the diversity of serotonin neurons. Philos Trans R Soc Lond B Biol Sci. 2012;367:2382–94. doi: 10.1098/rstb.2011.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONG S, DOUGHTY M, HARBAUGH CR, CUMMINS A, HATTEN ME, HEINTZ N, GERFEN CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–23. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS C, ZHUANG X, STARK K, RAMBOZ S, OOSTING R, KIRBY L, SANTARELLI L, BECK S, HEN R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- HALE MW, LOWRY CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–64. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- HALE MW, SHEKHAR A, LOWRY CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol. 2012;32:695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMPEL CM, SUGINO K, NELSON SB. A manual method for the purification of fluorescently labeled neurons from the mammalian brain. Nat Protoc. 2007;2:2924–9. doi: 10.1038/nprot.2007.416. [DOI] [PubMed] [Google Scholar]
- HENDRICKS TJ, FYODOROV DV, WEGMAN LJ, LELUTIU NB, PEHEK EA, YAMAMOTO B, SILVER J, WEEBER EJ, SWEATT JD, DENERIS ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–47. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- HOLLANDER E. Managing aggressive behavior in patients with obsessive-compulsive disorder and borderline personality disorder. J Clin Psychiatry. 1999;60(Suppl 15):38–44. [PubMed] [Google Scholar]
- JENSEN P, FARAGO AF, AWATRAMANI RB, SCOTT MM, DENERIS ES, DYMECKI SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–9. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHKONEN S, YAMASHITA H, RYTSALA H, SUOMINEN K, AHVENINEN J, ISOMETSA E. Dysfunction in early auditory processing in major depressive disorder revealed by combined MEG and EEG. J Psychiatry Neurosci. 2007;32:316–22. [PMC free article] [PubMed] [Google Scholar]
- KALEN P, SKAGERBERG G, LINDVALL O. Projections from the ventral tegmental area and mesencephalic raphe to the dorsal raphe nucleus in the rat. Evidence for a minor dopaminergic component. Exp Brain Res. 1988;73:69–77. doi: 10.1007/BF00279662. [DOI] [PubMed] [Google Scholar]
- KIM JC, COOK MN, CAREY MR, SHEN C, REGEHR WG, DYMECKI SM. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron. 2009;63:305–15. doi: 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESAGE F, DUPRAT F, FINK M, GUILLEMARE E, COPPOLA T, LAZDUNSKI M, HUGNOT JP. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett. 1994;353:37–42. doi: 10.1016/0014-5793(94)01007-2. [DOI] [PubMed] [Google Scholar]
- LESCH KP, MERSCHDORF U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav Sci Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- LI L, TASIC B, MICHEVA KD, IV, ANOV VM, SPLETTER ML, SMITH SJ, LUO L. Visualizing the distribution of synapses from individual neurons in the mouse brain. PLoS One. 2010;5:e11503. doi: 10.1371/journal.pone.0011503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINK E, EDELMANN L, CHOU JH, BINZ T, YAMASAKI S, EISEL U, BAUMERT M, SUDHOF TC, NIEMANN H, JAHN R. Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun. 1992;189:1017–23. doi: 10.1016/0006-291x(92)92305-h. [DOI] [PubMed] [Google Scholar]
- LIU C, MAEJIMA T, WYLER SC, CASADESUS G, HERLITZE S, DENERIS ES. Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci. 2010;13:1190–8. doi: 10.1038/nn.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORENZ K. On aggression. London: Methuen; 1966. [Google Scholar]
- LUCKER JR, DOMAN A. Auditory hypersensitivity and autism spectrum disorders: an emotional response. Autism Science Digest: The Journal of Autismone. 2012:103–108. [Google Scholar]
- LUCKI I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–62. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- MARLER P. On animal aggression. The roles of strangeness and familiarity. Am Psychol. 1976;31:239–46. doi: 10.1037//0003-066x.31.3.239. [DOI] [PubMed] [Google Scholar]
- MATTHEWS GA, NIEH EH, VANDER WEELE CM, HALBERT SA, PRADHAN RV, YOSAFAT AS, GLOBER GF, IZADMEHR EM, THOMAS RE, LACY GD, WILDES CP, UNGLESS MA, TYE KM. Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell. 2016;164:617–31. doi: 10.1016/j.cell.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICZEK KA, O’DONNELL JM. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology (Berl) 1978;57:47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- MOLLIVER ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- MOSIENKO V, BERT B, BEIS D, MATTHES S, FINK H, BADER M, ALENINA N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl Psychiatry. 2012;2:e122. doi: 10.1038/tp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON RJ, TRAINOR BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–46. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- NIEDERKOFLER V, ASHER TE, DYMECKI SM. Functional Interplay between Dopaminergic and Serotonergic Neuronal Systems during Development and Adulthood. ACS Chem Neurosci. 2015;6:1055–70. doi: 10.1021/acschemneuro.5b00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKATY BW, FRERET ME, ROOD BD, BRUST RD, HENNESSY ML, DEBAIROS D, KIM JC, COOK MN, DYMECKI SM. Multi-Scale Molecular Deconstruction of the Serotonin Neuron System. Neuron. 2015;88:774–91. doi: 10.1016/j.neuron.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEYRON C, LUPPI PH, KITAHAMA K, FORT P, HERMANN DM, JOUVET M. Origin of the dopaminergic innervation of the rat dorsal raphe nucleus. Neuroreport. 1995;6:2527–31. doi: 10.1097/00001756-199512150-00019. [DOI] [PubMed] [Google Scholar]
- RAY RS, CORCORAN AE, BRUST RD, KIM JC, RICHERSON GB, NATTIE E, DYMECKI SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–42. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIST C, NAKAMURA K, SAGART E, SOKOLSKI KN, FUJIMOTO KA. Impulsive aggressive behavior: open-label treatment with citalopram. J Clin Psychiatry. 2003;64:81–5. [PubMed] [Google Scholar]
- ROBINSON MD, MCCARTHY DJ, SMYTH GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROOD BD, CALIZO LH, PIEL D, SPANGLER ZP, CAMPBELL K, BECK SG. Dorsal raphe serotonin neurons in mice: immature hyperexcitability transitions to adult state during first three postnatal weeks suggesting sensitive period for environmental perturbation. J Neurosci. 2014;34:4809–21. doi: 10.1523/JNEUROSCI.1498-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHMAN RB, CLARK RD, PARTILLA JS, BAUMANN MH. (+)-Fenfluramine and its major metabolite, (+)-norfenfluramine, are potent substrates for norepinephrine transporters. J Pharmacol Exp Ther. 2003;305:1191–9. doi: 10.1124/jpet.103.049684. [DOI] [PubMed] [Google Scholar]
- SAHLHOLM K, MARCELLINO D, NILSSON J, FUXE K, ARHEM P. Differential voltage-sensitivity of D2-like dopamine receptors. Biochem Biophys Res Commun. 2008;374:496–501. doi: 10.1016/j.bbrc.2008.07.052. [DOI] [PubMed] [Google Scholar]
- SAUDOU F, AMARA DA, DIERICH A, LEMEUR M, RAMBOZ S, SEGU L, BUHOT MC, HEN R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–8. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- SCOTT MM, WYLIE CJ, LERCH JK, MURPHY R, LOBUR K, HERLITZE S, JIANG W, CONLON RA, STROWBRIDGE BW, DENERIS ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472–7. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPAETHLING JM, PIEL D, DUECK H, BUCKLEY PT, MORRIS JF, FISHER SA, LEE J, SUL JY, KIM J, BARTFAI T, BECK SG, EBERWINE JH. Serotonergic neuron regulation informed by in vivo single-cell transcriptomics. FASEB J. 2014;28:771–80. doi: 10.1096/fj.13-240267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBUSCH HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI A, MICZEK KA. Neurogenetics of aggressive behavior: studies in rodents. Curr Top Behav Neurosci. 2014;17:3–44. doi: 10.1007/7854_2013_263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOICULESCU O, CHARNAY P, SCHNEIDER-MAUNOURY S. Expression pattern of a Krox-20/Cre knock-in allele in the developing hindbrain, bones, and peripheral nervous system. Genesis. 2000;26:123–6. doi: 10.1002/(sici)1526-968x(200002)26:2<123::aid-gene7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- VOLAVKA J. Neurobiology of violence. Washington, DC: American Psychiatric Pub; 2002. [Google Scholar]
- WYLIE CJ, HENDRICKS TJ, ZHANG B, WANG L, LU P, LEAHY P, FOX S, MAENO H, DENERIS ES. Distinct transcriptomes define rostral and caudal serotonin neurons. J Neurosci. 2010;30:670–84. doi: 10.1523/JNEUROSCI.4656-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYSS C, HITZ K, HENGARTNER MP, THEODORIDOU A, OBERMANN C, UHL I, ROSER P, GRUNBLATT E, SEIFRITZ E, JUCKEL G, KAWOHL W. The loudness dependence of auditory evoked potentials (LDAEP) as an indicator of serotonergic dysfunction in patients with predominant schizophrenic negative symptoms. PLoS One. 2013;8:e68650. doi: 10.1371/journal.pone.0068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU Q, TEIXEIRA CM, MAHADEVIA D, HUANG Y, BALSAM D, MANN JJ, GINGRICH JA, ANSORGE MS. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZALSMAN G, PATYA M, FRISCH A, OFEK H, SCHAPIR L, BLUM I, HARELL D, APTER A, WEIZMAN A, TYANO S. Association of polymorphisms of the serotonergic pathways with clinical traits of impulsive-aggression and suicidality in adolescents: a multi-center study. World J Biol Psychiatry. 2011;12:33–41. doi: 10.3109/15622975.2010.518628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.