Abstract
Case series
Patient: Male, 42 • Female, 30
Final Diagnosis: Human embryonic stem cells showed good therapeutic potential for treatment of multiple sclerosis with lyme disease
Symptoms: Fatigue • weakness in limbs
Medication: —
Clinical Procedure: Human embryonic stem cells transplantation
Specialty: Transplantology
Objective:
Rare disease
Background:
Multiple sclerosis (MS) is an inflammatory and neurodegenerative disease in which the myelin sheath of nerve cells is damaged. It can cause delayed neurologic symptoms similar to those seen in Lyme disease (LD) patients. Thymus derived T-cells (myelin reactive) migrate to the blood brain barrier and stimulate an inflammatory cascade in the central nervous system. Cell based therapies play an important role in treating neurological diseases such as MS and LD.
Case Report:
Human embryonic stem cell (hESC) therapy was used to treat two patients with both MS and LD. The hESCs were administered via different routes including intramuscular, intravenous, and supplemental routes (e.g., deep spinal, caudal, intercostal through eye drops) to regenerate the injured cells. Both the patients showed remarkable improvement in their functional skills, overall stamina, cognitive abilities, and muscle strength. Furthermore, the improvement in the patients’ conditions were assessed by magnetic resonance tractography and single photon emission computed tomography (SPECT).
Conclusions:
Therapy with hESCs might emerge as an effective and safe treatment for patients with both MS and LD. Well-designed clinical trials and follow-up studies are needed to prove the long-term efficacy and safety of hESC therapy in the treatment of patients with MS and LD.
MeSH Keywords: Embryonic Stem Cells, Lyme Disease, Multiple Sclerosis, Stem Cell Transplantation
Background
Multiple sclerosis (MS) is characterized as an inflammatory, neurodegenerative [1] and autoimmune disease, resulting in damage to the myelin sheath (composed of fats) and tissues of the central nervous system (CNS) [2]. Thymus derived T-cells (myelin reactive) migrate to the blood brain barrier and stimulate an inflammatory cascade in the CNS [3].
Globally, MS prevalence parallels the circulation of the Lyme disease (LD), [4] which is characterized by white matter lesions in the brain similar to those found in MS patients [5]. Borrelia burgdorferi (a tick borne spirochete) causes LD after a tick bite. It is estimated that the number of LD cases in the United States each year is close to 300,000 [6]. The disease can cause postponed neurological signs of serious illness, like those seen in MS such as visual disturbances, peripheral neuropathy, cognitive defects, and fatigue [7,8]. The clinical distinction between these two diseases (MS and LD), even with the use of magnetic resonance imaging (MRI) is very difficult [5]. Also, there is no effective cure for MS [9]. Although several antibiotic regimens are available for the treatment of LD, in some patients the symptoms are reported to be persistent even after antibiotic treatment. Thus, infected patients may require alternative antibiotics or other treatment options [10]. Long-term treatment with antibiotics has been found to result in adverse events (AEs) [11,12].
Conventional therapies that are used for the treatment of MS include beta interferons [13], immunosuppressants [14], monoclonal antibodies (natalizumab) [15] and corticosteroids [16]. However, utilizing these treatments long-term may be associated with an increased risk of depression, anxiety, heart damage, pneumonia, and serious and life-threatening diseases such as progressive multifocal leukoencephalopathy (PML) [14,17]. Past research has shown that cell-based therapies hold a potential for CNS repair and may be protective from inflammatory damage after injury [18–20]. Cell transplantation therapies play an important role in promoting remyelination and preventing demyelination of the axons [21,22]. Earlier studies have shown improvement in blurred vision, stamina, appetite, tremors, balance, and speech after receiving human embryonic stem cell (hESC) therapy [23–25]. An improvement in patients affected with either MS or LD has also been previously reported [23]. The present study presents two cases of patients affected with both MS and LD. The patients had an uneventful recovery after the treatment.
Methods
The hESCs transplanted in this study were chromosomally stable and had no xeno product(s). An in-house patented technology was followed for the culture and maintenance of the cells (United States Granted Patent No US 8592, 208, 52) in a good manufacturing practice (GMP), good laboratory practice (GLP), and good tissue practice (GTP) compliant laboratory at our facility (patent WO 2007/141657A PCT/1B 2007, published December 13, 2007). The use of hESCs at Nutech Mediworld has also been accepted and confirmed by the House of Lords, Regenerative Medicine, Science, and Technology Committee. The Independent Institutional Ethics Committee (IEC) approved this study. The procedure used for cell culture and differentiation has been elaborated in a previous study [23]. The treatment regime consisted of three phases (T1, T2, T3) followed by a gap phase of 4–6 months. As per the treatment protocol, 0.25 mL hESCs were injected intramuscularly two times a day, and 1 mL of hESCs (<16 million cells) were injected intravenously two times a day for 7 days. In addition, hESCs were also injected through supplemental routes (e.g., deep spinal, caudal, and intercostal through eye drops). The patients also received supportive treatment including antibiotic medications for LD as per Lyme disease protocol, and physiotherapy (Table 1). The safety and efficacy of these cells has been assessed previously [23]. All the patients provided written consent prior to start of their treatment. The patients’ condition was video recorded before, during, and after the treatment.
Table 1.
Lyme protocol of antibiotics for all patients.
| Name of the drug | Frequency |
|---|---|
| Monocef injection (1 gm) | bd |
| Tinidazole (50 mg) | i.v. bd |
| Minocycline (100 mg) | od |
| Pantop (40 mg) | od |
| Vizylac/econorm (Biocodex) | bd |
| Fludac (20 mg) | 1 cup od |
| MUI + N/S (10 mL) | i.v. infusion thrice a week |
Case Report
Case 1
A 42-year-old male who was presented to our hospital was diagnosed with MS in 1995. On his first visit to the hospital, the patient was having chief complaints of sub-normal walking using support (stick), weakness in all limbs, severe fatigue, sense of numbness in both feet, tingling sensations in all limbs, band around head, drooling and mixing up words while talking continuously, and subnormal bladder control. The patient was a chronic smoker and marijuana dependent. The first single photon emission computed tomography (SPECT) scan performed on February 17, 2015, showed sections of multiple small ovoid discrete and confluent T2/fast fluid-attenuated inversion recovery (FLAIR) hyper-intense lesions perpendicular to the long axis of lateral ventricles. A small plaque was seen in the posterior limb (posterior most portion) of internal capsule on both sides. Demyelination plaques were present in the right midbrain, bilateral (b/L) ventral pons, and brachium pontis (Bt >Lt). Few discrete lesions were noted in the white matter of b/L inferior cerebellar hemisphere region (Figure 1). Magnetic resonance (MR) tractography showed paucity of fiber tract, suggested in b/L centrum semi-ovals and subcortical regions of fronto parietal lobes, with no significant interval change.
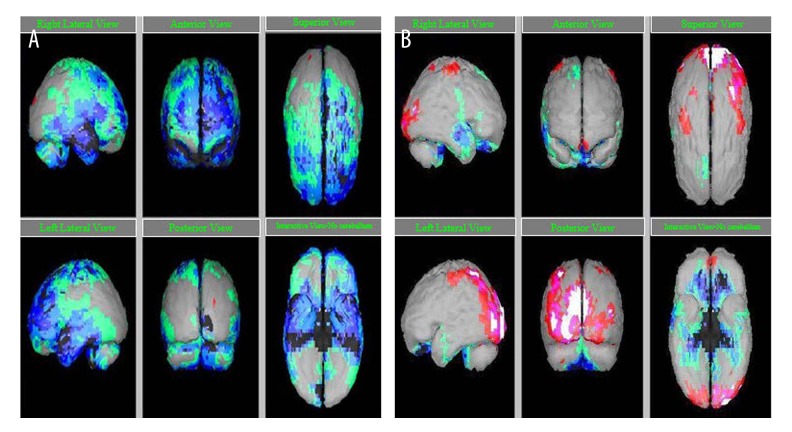
Figure 1.
SPECT scan before hESC therapy. (A) Before therapy, (B) after therapy. Grey: normal; red, pink, white: above normal (+2, +3, +4 of standard); Hypoperfused regions – green: −2 of standard; light blue: −3 of standard; dark blue: −4 of standard; black: −5 of standard.
Tractography of the spine showed a section of an ovoid elongated T2 hyper-intense plaque position in the cervical cord at C1–C2, C2 body, and at C4 level (posteriorly on the right side). A tiny plaque was noted anteriorly at the cervicomedullary junctions.
The patient received hESC therapy at our facility as a primary treatment. After receiving hESC therapy, the patient had no weakness or fatigue. His balance and cognitive functional skills were also improved. SPECT scan after his therapy showed moderate improvement in the degree of cerebral and cerebellar perfusion (Figure 2). The second MRI of the brain was performed after the therapy on April 17, 2015 showed fiber tracts to be better. A remarkable improvement was observed in b/L central semi-ovals and sub-cortical regions of front parietal fiber (Figures 1, 2). Spine tractography showed improvement in D9–D10 level with no aggression of demyelinating pitch. The spinal curvature was normal at all levels. Disc desiccation changes were found in the cervical spine with posterior disc bulge at C5–C6 level, not causing cord indentation. The vertebral bodies were normal in height, and no significant disc bulge or herniation was present in dorso-lumbar spine. 2 enlists the symptoms of the patient depicting improvement after the hESC therapy.
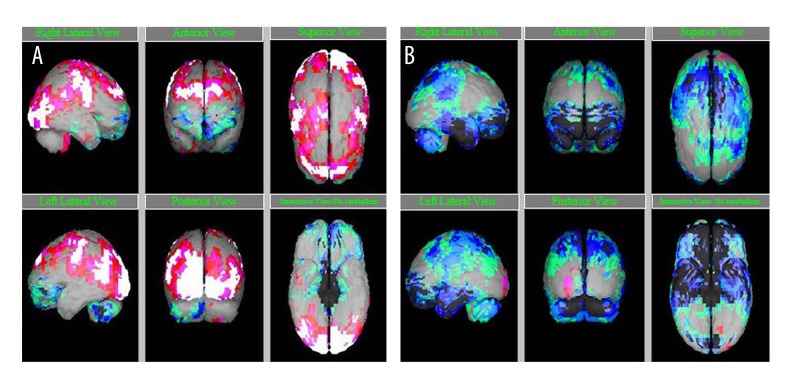
Figure 2.
SPECT scan after hESC therapy. (A) Before therapy, (B) after therapy. Grey: normal; red, pink, white: above normal (+2, +3, +4 of standard); Hypoperfused regions – green: −2 of standard; light blue: −3 of standard; dark blue: −4 of standard; black: −5 of standard.
Case 2
A 30-year-old female was presented to our facility with chief complaints of inability to walk, wheel chair dependence, unable to stand without support, spasticity of lower limbs with foot drop on the left foot, weakness of the left arm and spasticity of the left hand, severe fatigue with myalgia, and joint pains especially in the shoulder (left >right).
The patient had complaint of weakness in the left leg about five years back which caused her to limp and fall frequently. She was prescribed prednisolone and diagnosed as a possible case of MS. After further deterioration of her condition, she was tested and found positive for LD in 2012. She was prescribed an antibiotic regime without much beneficial results. The first SPECT scan performed on October 14, 2014 revealed confluent T2/fast FLAIR hyper-intensity (suggestive of demyelinating lesions) in right subcortical white matter and in the superior right putamen. The infratentorial compartment showed a small hyper-intense demyelinating lesion in left superior cerebellar peduncle and larger plaque in anterior medulla oblongata (midline up top onto medullary junction). MR tractography reports of October 14, 2014 showed abnormal confluent T2-hyperintensity along the ventral surface of medulla oblongata. C5 and C6, and the anterior medullary, represented demyelinating lesions. Tractography of the spine showed disc desiccation changes and moderate broad-based posterocentral-left paracentral disc protrusion at C5-C6, causing thecal sac indentation. Patient was a non-smoker and non-alcoholic and had no significant surgical history.
At our facility, the patient received hESC therapy as per the Lyme disease protocol along with antibiotics and physiotherapy. The treatment period for the patient ranged from October 3, 2014 to April 29, 2015. Following the first treatment phase, the patient reported remarkable improvement in her lower limb strength, decreased spasticity, and had no longer fatigue issues. Also, she was able to walk upright now with support. After her second visit, improvement in muscle strength, movement of left upper arm, spasticity of left lower, and left upper limb was observed. The patient was able to walk independently for up to 40–50 minutes around the room (Table 2). An improvement was observed in parameters like muscle weakness, walking distance, balance, fatigue, pain, blurring of eyes, and deformity. Short tau inversion recovery (STIR) signal SPECT scan showed significant improvement in the degree of cerebellar perfusions as illustrated in Figure 2. MRI of the spine showed normal caliber outline and parenchymal signal of the entire spinal cord. The nerve roots of cauda equina appeared normal with no intraspinal mass. The ventral bodies at all levels were normal in height and were in alignment with normal.
Table 2.
Symptoms of the patients before and after hESC therapy.
| Case 1 | |||||
|---|---|---|---|---|---|
| Clinical presentation | MRI brain | MRI spine | |||
| Before | After | Before | After | Before | After |
| Poor balance | Improved balance | Paucity of fibre tracts present in bilateral central semiovals | Visualization of fibre tracts is better and significantly improved in bilateral semiovals | Paucity of fibre tracts in the dorsal cord at D9–D10 | Visualization of fibre tract in the dorsal cord at D9–D10 level has improved |
| Inability to sit or stand at one go | Able to sit or stand at one go | Paucity of fibre tracts present in subcortical regions of frontoparietal lobes | Improvement in subcortical regions of frontoparietal lobes | Paucity at the site of demyelination pitch seen | No aggression of demyelination pitch seen |
| Unable to walk, uses stick to balance | Able to walk without stick | ||||
| Weakness and fatigue | Improved weakness and fatigue | ||||
| Impaired cognitive skills | Cognitive skills improved | ||||
| Impaired near visions | Near vision is better | ||||
| Drooling present | No drooling | ||||
| Poor bladder control | Bladder control is better | ||||
Discussion
Our study used in-house cultured hESCs in the treatment of patients affected with both MS and LD. Patients showed clinical signs of improvement in health after the therapy; such as improvement in overall stamina, muscle strength, functional skills, and cognitive abilities. SPECT scan was able to interpret the extent of improvement in perfusion after treatment with hESCs. MRI performed after therapy showed improvement in b/L and subcortical regions of the brain. Neither of the patients reported any AEs following the hESC therapy.
Demyelination refers to the destruction of the myelin sheath, i.e., a protective covering of neurons. Various studies have shown that stem cells possess an ability for propagation, transmission, and differentiation into mature myelinating oligodendrocytes [14,26]. Sharp and colleagues transplanted hESC derived oligodendrocyte progenitor cells (OPCs) into a cervical rat model. They observed that OPCs enhanced re-myelination and stimulated improvement of motor function (after 7 days of transplantation) [27]. In another study, Aharonowiz and colleagues transplanted hESCs obtained from neural progenitors into a mice model with experimental autoimmune encephalomyelitis (EAE). They noticed that the clinical symptoms of EAE diminished exceptionally after the transplantation. Histological assessment unveiled that transplanted neural progenitors travel to the mouse brain, particularly in the host white matter. The author concluded that the healing effect of neural progenitor transplantation was arbitrated by an immunosuppressive neuroprotective mechanism [28]. The immunoregulatory properties of mesenchymal stem cells (MSCs) efficiently intervene with the autoimmune attack in the course of EAE [29]. These stem cells stimulated functional restoration by rebuilding neural circuits, remyelinating axons, and increasing plasticity or axon regeneration [30].
There have been contrasting results regarding successful xenogeneic engraftment of hESC-derived OPCs. Some studies suggested that hESC derived OPCs were successfully engrafted and resulted in recovery in animal models [31,32], whereas other stated that allogenic OPCs were rejected and failed to remyelinate [33,34]. In addition, hESCs are known to possess low levels of major histocompatibility (MHC) antigens, thus even a single difference in MHC antigens between donor and recipient can result in graft rejection [35]. The hESC cell line used in our study has not shown any graft rejection to date, as the cell line was isolated from a single fertilized ovum after 24–48 hours of fertilization, at which time the cells had not acquired any antigenic properties [23].
Previous studies have provided evidence for the communication between stem cells and injured cells, and their homing to the affected/injured region [36]. These studies laid the foundation for therapeutic use of hESCs in a variety of neurological disorders. The author assumed that the hESCs used in our study also rely on a same mechanism of action. The hESCs transplanted in our study had a very small size (<1 μm) which facilitated their permeation across parenchyma and migration towards the damaged area thereby replacing the damaged cell. The cytokines, growth factors, and chemokines secreted from the affected/injured region have been shown to communicate with the stem cells injected in the body [37]. There were limitations to this study. The patients came to our facility after being previously diagnosed with MS and LD by other investigators. We could only perform MRI for these patients, which is not a definitive way to reach to a definitive diagnosis. Future studies that include a robust study design and definitive diagnosis may be helpful.
Conclusions
This study found that hESC therapy was effective and safe for the treatment of patients with both MS and LD. However, more well-designed clinical trials and follow-up studies are needed to prove the long-term efficacy and safety of hESCs in the treatment of patients with MS and LD.
Acknowledgments
The author acknowledges all the doctors, staff, and patients of the Nutech Mediworld. The author also acknowledges Knowledge Isotopes Pvt. Ltd. for writing assistance.
Footnotes
Conflict of interest
Author declares that there is no conflict of interest.
References:
- 1.Schmitt N. Role of T follicular helper cells in multiple sclerosis. J Nat Sci. 2015;1:139. [PMC free article] [PubMed] [Google Scholar]
- 2.Minagar A, Jy W, Jimenez JJ, Alexander JS. Multiple sclerosis as a vascular disease. Neurol Res. 2006;28:230–35. doi: 10.1179/016164106X98080. [DOI] [PubMed] [Google Scholar]
- 3.Fan HC, Ho LI, Chi CS, et al. Current proceedings of cerebral palsy. Cell Transplant. 2015;24:471–85. doi: 10.3727/096368915X686931. [DOI] [PubMed] [Google Scholar]
- 4.Fritzsche M. Chronic Lyme borreliosis at the root of multiple sclerosis – is a cure with antibiotics attainable? Med Hypotheses. 2005;64:438–48. doi: 10.1016/j.mehy.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Triulzi F, Scotti G. Differential diagnosis of multiple sclerosis: Contribution of magnetic resonance techniques. J Neurol Neurosurg Psychiatry. 1998;64:6–14. [PubMed] [Google Scholar]
- 6.Sharma B, Brown AV, Matluck NE, et al. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrob Agents Chemother. 2015;59:4616–24. doi: 10.1128/AAC.00864-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado MR, Hirtz D, Aisen M, et al. Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2010;74:336–43. doi: 10.1212/WNL.0b013e3181cbcd2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bae SH, Kong TH, Lee HS, et al. Long-lasting paracrine effects of human cord blood cells on damaged neocortex in an animal model of cerebral palsy. Cell Transplant. 2012;21:2497–515. doi: 10.3727/096368912X640457. [DOI] [PubMed] [Google Scholar]
- 9.Safavi M, Nikfar S, Abdollahi M. A Systematic review of drugs in late-stage development for the treatment of multiple sclerosis: a focus on oral synthetic drugs. Inflamm Allergy Drug Targets. 2015;13:351–66. doi: 10.2174/1871528114666150529102613. [DOI] [PubMed] [Google Scholar]
- 10.Lantos PM. Chronic lyme disease. Infect Dis Clin North Am. 2015;29:325–40. doi: 10.1016/j.idc.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stricker RB, Green CL, Savely VR, et al. Safety of intravenous antibiotic therapy in patients referred for treatment of neurologic Lyme disease. Minerva Med. 2010;101:1–7. [PubMed] [Google Scholar]
- 12.Fallon BA, Keilp JG, Corbera KM, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70:992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d. [DOI] [PubMed] [Google Scholar]
- 13.Clanet M, Radue EW, Kappos L, et al. A randomized, double-blind, dose-comparison study of weekly interferon beta-1a in relapsing MS. Neurology. 2002;59:1507–17. doi: 10.1212/01.wnl.0000032256.35561.d6. [DOI] [PubMed] [Google Scholar]
- 14.Pithadia A. Pathogenesis and treatment of multiple sclerosis (MS) Internet Journal of Neurology. 2009;10:10. [Google Scholar]
- 15.Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab – unforeseen consequences. N Engl J Med. 2005;353:414–16. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- 16.Sloka JS, Stefanelli M. The mechanism of action of methylprednisolone in the treatment of multiple sclerosis. Mult Scler. 2005;11:425–32. doi: 10.1191/1352458505ms1190oa. [DOI] [PubMed] [Google Scholar]
- 17.Adelman B, Sandrock A, Panzara MA. Natalizumab and progressive multi-focal leukoencephalopathy. N Engl J Med. 2005;353:432–33. doi: 10.1056/NEJMc055235. [DOI] [PubMed] [Google Scholar]
- 18.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 19.Pfeifer K, Vroemen M, Caioni M, et al. Autologous adult rodent neural progenitor cell transplantation represents a feasible strategy to promote structural repair in the chronically injured spinal cord. Regen Med. 2006;1:255–66. doi: 10.2217/17460751.1.2.255. [DOI] [PubMed] [Google Scholar]
- 20.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–43. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 21.Munzel EJ, Williams A. Promoting remyelination in multiple sclerosis-recent advances. Drugs. 2013;73:2017–29. doi: 10.1007/s40265-013-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui QL, Kuhlmann T, Miron VE, et al. Oligodendrocyte progenitor cell susceptibility to injury in multiple sclerosis. Am J Pathol. 2013;183:516–25. doi: 10.1016/j.ajpath.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Shroff G. Establishment and characterization of a neuronal cell line derived from a 2 cell stage human embryo: Clinically tested cell-based therapy for neurological disorders. International Journal of Recent Scientific Research. 2015;6:3730–38. [Google Scholar]
- 24.Shroff G, Gupta A, Barthakur JK. Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J Transl Med. 2014;12:318. doi: 10.1186/s12967-014-0318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shroff G, Lopamudra D. Human embryonic stem cell therapy in cerebral palsy children with cortical visual impairment: A case series of 40 patients. J Cell Sci Ther. 2014;5:189. [Google Scholar]
- 26.Liu S, Qu Y, Stewart TJ, et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci USA. 2000;97:6126–31. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharp J, Frame J, Siegenthaler M, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–63. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aharonowiz M, Einstein O, Fainstein N, et al. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS One. 2008;3:3145. doi: 10.1371/journal.pone.0003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–61. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 30.Harper JM, Krishnan C, Darman JS, et al. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc Natl Acad Sci USA. 2004;101:7123–28. doi: 10.1073/pnas.0401103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pluchino S, Gritti A, Blezer E, et al. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann Neurol. 2009;66:343–54. doi: 10.1002/ana.21745. [DOI] [PubMed] [Google Scholar]
- 32.Hori J, Ng TF, Shatos M, et al. Neural progenitor cells lack immunogenicity and resist destruction as allografts. 2003. Ocul Immunol Inflamm. 2007;15:261–73. doi: 10.1080/09273940701382242. [DOI] [PubMed] [Google Scholar]
- 33.Hatch MN, Schaumburg CS, Lane TE, Keirstead HS. Endogenous remyelination is induced by transplant rejection in a viral model of multiple sclerosis. J Neuroimmunol. 2009;212:74–81. doi: 10.1016/j.jneuroim.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Swijnenburg RJ, Schrepfer S, Govaert JA, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci USA. 2008;105:12991–96. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson NJ, Brook FA, Gardner RL, et al. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc Natl Acad Sci USA. 2007;104:20920–25. doi: 10.1073/pnas.0710265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borlongan CV, Glover LE, Tajiri N, et al. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95:213–28. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang SK, Shin IS, Ko MS, et al. Journey of mesenchymal stem cells for homing: Strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012;2012:342968. doi: 10.1155/2012/342968. [DOI] [PMC free article] [PubMed] [Google Scholar]