Abstract
Background
The aim of this study was to calculate 95% reference intervals and double-sided limits of serum alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) according to the CLSI EP28-A3 guideline.
Material/Methods
Serum AFP and CEA values were measured in samples from 26 000 healthy subjects in the Shuyang area receiving general health checkups. The 95% reference intervals and upper limits were calculated by using MedCalc.
Results
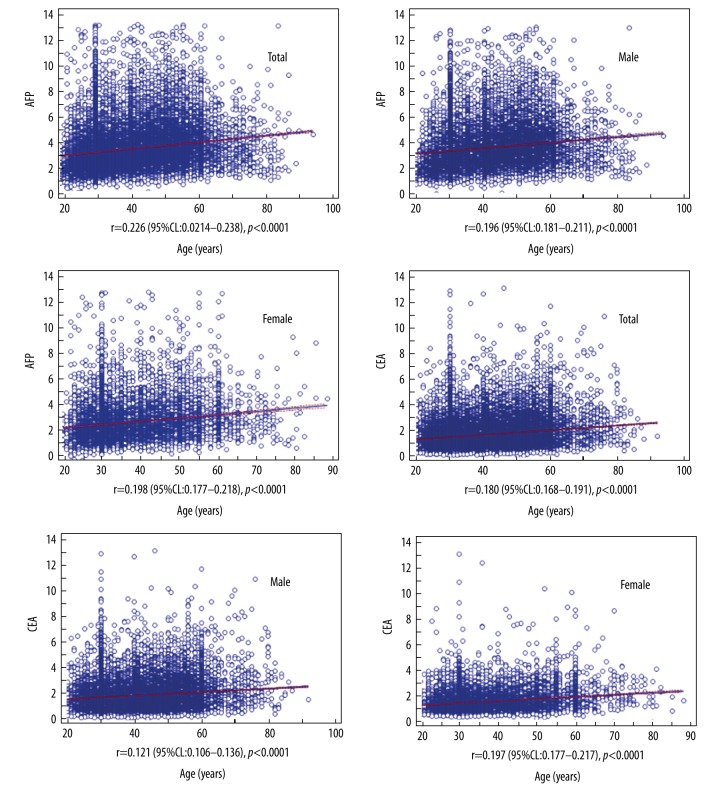
We provided continuous reference intervals from 20 years old to 90 years old for AFP and CEA. The reference intervals were: AFP, 1.31–7.89 ng/ml (males) and 1.01–7.10 ng/ml (females); CEA, 0.51–4.86 ng/ml (males) and 0.35–3.45ng/ml (females). AFP and CEA were significantly positively correlated with age in both males (r=0.196 and r=0.198) and females (r=0.121 and r=0.197).
Conclusions
Different races or populations and different detection systems may result in different reference intervals for AFP and CEA. Continuous reference intervals of age changes are more accurate than age groups.
MeSH Keywords: alpha-Fetoproteins, Carcinoembryonic Antigen, Reference Values
Background
Serum alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) are the most widely used tumor markers in tumor treatment and post-treatment monitoring [1]. Reference intervals of CEA and AFP vary by ethnicity, living standard, geographical location, detection system, and many other factors. However, at present, most laboratories use the AFP and CEA reference intervals provided by the reagent manual. According to Chinese laws and regulations and industry standards, clinical laboratories must set reference intervals suitable for the region to meet the needs of clinical diagnosis and treatment. In this paper, according to CLSI EP28-A3, we established particular reference intervals based on a large sample of local healthy people.
Material and Methods
From April 2013 to May 2016, 26 000 healthy subjects were chosen from the Shuyang area health examination findings, focusing on the exclusion of liver and gastrointestinal diseases (such as positive HBV surface-antigen and data from questionnaires). Specimens were fasting venous blood. AFP and CEA were measured by electrochemiluminescence immunoassay on Beckman DXI system 800 immunoassay analyzers (Beckman Co., Ltd., USA). The detection limits of the assays are 0.1–1000 ng/mL(CEA) and 0.5–3000 ng/mL(AFP). Regular internal quality control (IQC) procedures and external quality assessment scheme (EQAS) were performed to validate the quality of AFP and CEA results. The Ethics Committee of Shuyang People’s Hospital approved this study. Reference samples were selected according to a previous report [2].
Statistical analysis
AFP and CEA data were pooled and showed by the non-parametric method using 95% (double-sided) reference intervals according to CLSI EP28-A3 guidelines [3]. Gaussian distribution of all data was tested using the D’Agostino-Pearson test. For outlier exclusion, Tukey’s outlier method was used. The ratio D/R proposed by Dixon was used to detect outliers, but we decided not to exclude any data unless clear analytical or biological reasons could be demonstrated, and all data analyses were performed using SPSS 17.0 and MedCalc Version 16.4.3 (95% double-sided, Tucker, Box-Cox transformation, Lambda AND shift parameter get from data, D’Agostino-Pearson test). The Mann-Whitney U test was used to determine different sex groups. p<0.05 was considered statistically significant.
Results
The results of the D’Agostino-Pearson test show the AFP and CEA in our study were not in normal distribution. The reference intervals of AFP and CEA according to CLSI EP28-A3 guideline were determined using the non-parametric method (95% double-sided), and these values of AFP and CEA were significant for males and females (p<0.0001 and p <0.0001, respectively).
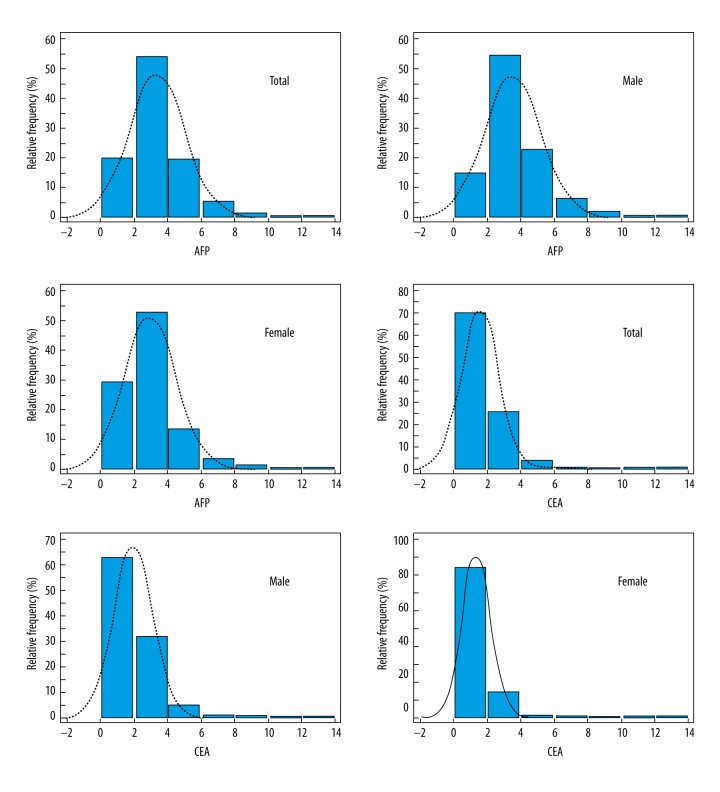
Figure 1 is a histograms of AFP and CEA distributions among subjects. We established the reference intervals based on the non-parametric method (95%, double-sided). The reference intervals for AFP and CEA are listed in Table 1.
Figure 1.
Histograms of alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) in subjects.
Table 1.
Reference Intervals of AFP and CEA for apparently healthy population.
| Gender group | AFP (ng/ml) | CEA (ng/ml) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Med | Lower limit (90% Cl) | Upper limit (90% Cl) | N | Med | Lower limit (90% Cl) | Upper limit (90% Cl) | |
| Male | 16057 | 3.21 | 1.31 (1.29–1.33) | 7.89 (7.79–8.00) | 16775 | 1.64 | 0.51 (0.50–0.52) | 4.86 (4.79–4.94) |
| Female | 8717 | 2.58 | 1.01 (0.99–1.03) | 7.10 (6.90–7.27) | 9095 | 1.10 | 0.35 (0.34–0.36) | 3.45 (3.38–3.54) |
| Total | 24774 | 2.98 | 1.15 (1.13–1.17) | 7.68 (7.60–7.79) | 25870 | 1.43 | 0.42 (0.42–0.43) | 4.53 (4.47–4.61) |
95% Reference interval, Double-sided. Non-parametric percentile method(CLSI EP28-A3).
As shown in Figure 2, AFP and CEA were significantly positively correlated with age (P<0.01).
Figure 2.
Scatter plots for alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA).
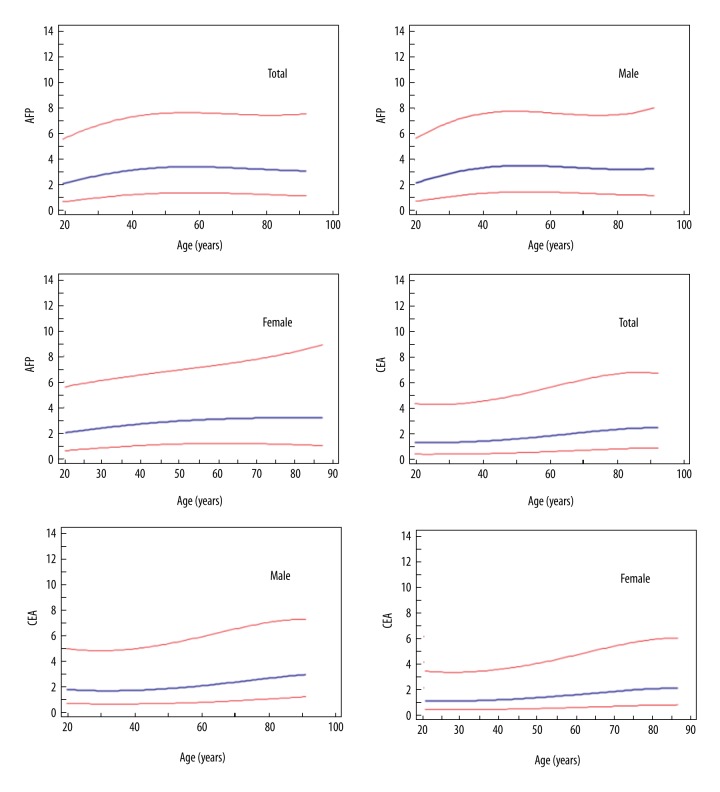
Figure 3 shows Continuous reference intervals for alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) concentration. The establishment of reference intervals of AFP and CEA should be divided into the age groups of 20–50 years old and over 50 years old (Table 2).
Figure 3.
Continuous reference intervals for alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) concentration. The blue line represents the 50th percentile ad the lower and upper red lines represent the 2.5th and 97.5th percentiles. The unit of AFP is ng/ml; the unit of CEA is ng/ml.
Table 2.
Reference Intervals of AFP and CEA for the different group.
| Group | AFP (ng/ml) | CEA (ng/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median | Lower limit (90% Cl) | Upper limit (90% Cl) | N | Med | Lower limit (90% Cl) | Upper limit (90% Cl) | ||
| Male | 20≤ age <50 | 11690 | 3.09 | 1.25 (1.22–1.28) | 7.74 (7.64–7.88) | 12151 | 1.56 | 0.48 (0.47–0.49) | 4.61 (4.51–4.71) |
| 50≤ age | 4317 | 3.53 | 1.57 (1.50–1.61) | 8.35 (8.11–8.60) | 4622 | 1.88 | 0.60 (0.58–0.61) | 5.43 (5.23–5.69) | |
| Female | 20≤ age <50 | 7345 | 2.5 | 0.99 (0.97–1.01) | 6.82 (6.64–7.05) | 7610 | 1.05 | 0.34 (0.33–0.35) | 3.29 (3.18–3.40) |
| 50≤ age | 1422 | 3.02 | 1.30 (1.21–1.35) | 8.13 (7.53–8.57) | 1487 | 1.44 | 0.43 (0.42–0.48) | 4.26 (3.90–4.48) | |
95% Reference interval, Double-sided. Non-parametric percentile method (CLSI EP28-A3).
Discussion
In this study, we analyzed serum AFP and CEA in a large cohort of apparently healthy individuals and established their reference intervals in Shuyang, Jiangsu Province, China. We found that AFP and CEA were positively correlated with age. Therefore, reference intervals of different ages and sexes should be established. A study of AFP and CEA in the Fang Chenggang area [5] concluded that the reference interval of the instrument should be established. In the modern era of individualized medical care doctors assess the patient’s current test results, and the patient’s baseline value or the past test report are particularly important. If lacking basic values or previous data, inspection reports should be marked with the reference interval and detection system, especially in developing countries in regions such as Latin America, Asia, and Africa, where laboratory information systems are not common.
In this study, we found that AFP and CEA were positively correlated with age (Figure 2), showing that AFP and CEA tend to increase with age [6–8]. As shown in Figure 3, it is more reasonable to establish a different reference ranges for people over and under 50 years of age. In our study, the upper limits of the reference intervals are different from those of a previous report [4], possibly because the detection systems are different.
There are several limitations to this study. First, this was a single-center study using a single analysis system. Second, the subjects in this study were apparently healthy individuals. Third, smoking was not considered.
Conclusions
Different ethnicities and different detection systems may result in different reference intervals for AFP and CEA. In addition to providing the baseline, it is important to note the variations between the different detection systems and to establish the continuous reference intervals by age.
Footnotes
Declaration of interest
The authors declare no conflict of interest.
Source of support: Departmental sources
References
- 1.Duffy MJ. Tumor markers in clinical practice: A review focusing on common solid cancers. Med Princ Pract. 2013;22:4–11. doi: 10.1159/000338393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang GM, Xia YJ, Guo XX, et al. Reference intervals for total bilirubin, ALT, AST and creatinine in healthy Chinese elderly. Med Sci Monit. 2014;20:1778–82. doi: 10.12659/MSM.892148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CLSI. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline. 3rd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2010. [Google Scholar]
- 4.Bevilacqua V, Chan MK, Chen Y, et al. Pediatric population reference value distributions for cancer biomarkers and covariate-stratifiedreference intervals in the CALIPER cohort. Clin Chem. 2014;60(12):1532–42. doi: 10.1373/clinchem.2014.229799. [DOI] [PubMed] [Google Scholar]
- 5.Qin X, Lin L, Mo Z, et al. Reference intervals for serum alpha-fetoprotein and carcinoembryonic antigen in Chinese Han ethnic males from the Fangchenggang Area Male Health and Examination Survey. Int J Biol Markers. 2011;26(1):65–71. doi: 10.5301/jbm.2011.6364. [DOI] [PubMed] [Google Scholar]
- 6.Anisimov VN, Sikora E, Pawelec G. Relationshipsbetweencancer and aging: A multilevel approach. Biogerontology. 2009;10:323–38. doi: 10.1007/s10522-008-9209-8. [DOI] [PubMed] [Google Scholar]
- 7.Sizaret P, Martel N, Tuyns A, Reynaud S. Mean alphafetoproteinvalues of 1,333 males over 15 years by age groups. Digestion. 1977;15:97–103. doi: 10.1159/000197990. [DOI] [PubMed] [Google Scholar]
- 8.Bjerner J, Høgetveit A, WoldAkselberg K, et al. Reference intervals for carcinoembryonic antigen (CEA), CA125, MUC1, Alfa-foeto-protein (AFP), neuron-specific enolase (NSE) and CA19.9 from the NORIP study. Scand J Clin Lab Invest. 2008;68(8):703–13. doi: 10.1080/00365510802126836. [DOI] [PubMed] [Google Scholar]