Abstract
Background
An animal (Sprague-Dawley rat) model of Pseudomonas aeruginosa biofilm associated with chronic pulmonary infection in vivo was established and the effects of the biofilm on P. aeruginosa and its relationship to cytokines were investigated.
Material/Methods
Biofilm of P. aeruginosa in alginate beads and planktonic PA0725 were purified by anion-exchange chromatograph. Sprague-Dawley (SD) rats were immunized with the biofilm and then inhaled the same strain of P. aeruginosa. Anti-biofilm antibody titer was detected using the enzyme linked immunosorbent assay (ELISA) method. The cell count and differential count in the bronchoalveolar lavage fluid (BALF) were measured. The levels of cytokines (IL-17, IL-1β, MIP-2, and G-CSF) and tumor necrosis factor (TNF)-α in sera were also measured using an ELISA kit.
Results
The sera anti-biofilm IgG antibody titer of immunized SD rats was increased significantly on the 5th and 8th days after inhalation. The IL-17 concentration was significantly higher on the 8th day after inhalation. The results indicated that when biofilm-pre-immunized rats were challenged with inhalation of PA0725 of P. aeruginosa, the biofilm acted as an antigen substance and mediated the antibody reaction of the antigen, which might cause serious airway inflammatory response and lung tissue injury. This effect may be related to IL-17.
Conclusions
P. aeruginosa biofilm protected the bacterium from antibiotics and might induce host immune damage in lung tissue and facilitate bacterium evading the host barrier.
MeSH Keywords: Bacterial Infections, Biofilms, Cytokines, Pseudomonas aeruginosa
Background
As an opportunistic pathogen, Pseudomonas aeruginosa is seldom pathogenic to people with normal immune systems. However, P. aeruginosa can cause serious and sometimes fatal infections to immunodeficient hosts, such as HIV-infected patients, organ transplant patients, and tumor patients [1–22].
The mucoid strains of P. aeruginosa can grow on the surface of the respiratory tract and form a biofilm, causing respiratory biofilm diseases such as diffuse pan-bronchiolitis and cystic fibrosis of the lung, which are very difficult to treat. Some scientists believe that the alginate produced by P. aeruginosa can act as a kind of antigen that is able to induce the body to generate antibodies to the alginate [5–16,18,19,22]. Therefore, after P. aeruginosa colonization in the respiratory tract causes infections, the alginate that is produced will result in an antigen-antibody reaction with alginate antibody in the local respiratory tract. This reaction is then mediated by inflammation, leading to infiltration of inflammatory cells around the respiratory tract, and further results in respiratory immune pathological injury [11,12,15,17,20]. Cytokines likely play a role in regulating these inflammatory reactions.
Currently, the formation of P. aeruginosa biofilm is considered to be one of the important causes of refractory pulmonary infection [16,18,20]. So far, there have been no standards for an in vivo model of biofilm, and a systematic description of pathogenic characteristics of biofilm in an in vivo model has rarely been reported. In this study, the lung infection animal model of chronic P. aeruginosa biofilm was established to observe the bacteriology of lung tissues in SD rats, and the pathological characteristics and TNF responses. In this study, we investigated the pathogenic effects of P. aeruginosa biofilm and discuss the potential for prevention and cure of biofilm-associated infections. Theoretically, this research also provides support to inform the clinical treatment of biofilm-associated infections.
Material and Methods
Purification of P. aeruginosa PA0725
Mucoid strain PA0725 of P. aeruginosa was inoculated in P. aeruginosa isolation agar and cultured for 24 hours at 27°C. Cultures were collected by scraping, and cells were suspended in phosphate buffer saline (PBS) with pH of 7.5, centrifuged for 40 minutes at 5°C at 13,500 r/minute. The supernatant was filtered through 0.15 μm membranes to remove bacteria and then heated for 20 minutes to denature the proteins. The resulting alginate was precipitated by ethanol (95%), and the product was dissolved in PBS containing 1 mM NaCl and 10 mM MgCl2. RNase A (200 μg/mL) and type VI DNase I (200 μg/mL) were added and the mixture was reacted for 2 hours at 27°C to remove RNA and DNA. The enzymes were inactivated by heating the samples for 20 minutes at 70°C, then the samples were centrifuged for 20 minutes at room temperature at 13,500 r/minute. The supernatant was again precipitated using ethanol (95%). The sediment was collected and dissolved in ammonium carbonate solution (0.05 M) and added to a column chromatography (AutoColumn), then eluted by ammonium carbonate solution (0.05–10 M). The eluate (2 mL in each tube) was treated with carbachol boric acid to denature the alginate content. A solution with alginate content larger than or equal to 80 μg/mL was collected and dialyzed three times using PBS (12 hours for each dialysis). The dialyzed alginate product was mixed with AFFI-Prep polymyxin (Biorad) for 24 hours to remove the lipopolysaccharide.
Immunization of SD rats
Sixty SPF-grade male SD rats (age: 56 weeks, weight: 170–200 g) were purchased from Experimental Animal Research Center at Guangxi Medical University. The rats were randomly divided into an immune treatment group and a control treatment group, with 30 rats in each group. For the immune treatment we used alginate (40 μg/HP) and complete Freund’s adjuvant; for the control treatment we used saline and complete Freund’s adjuvant. Both treatments were administered by intraperitoneal injection one time per week for five weeks. On the sixth week, the immune group was injected with alginate (20 μg/HP) in the caudal vein, and the control group was injected with saline. On the seventh week anti-alginate IgG antibody titers were collected from the caudal vein of the rats; the rats in the immune group that had antibody titers greater than 1:800 were selected for experiments. When the P. aeruginosa antibody titers decreased to less than 1:8, the inhalation experiments were started.
Determination of antibody titer of the serum anti-alginate IgG
Anti-alginate IgG antibody in the blood of rats was determined by the ELISA method. Seaweed alginate was dissolved in carbonate buffer (Na2CO3 1.25 g/L, Na2HCO3 1.85 g/L, and NaN3 0.15 g/L). The solution was added into plate wells and incubated overnight, rinsed with PBS, and then sealed for four hours with 2% bovine serum albumin (BSA), and then rinsed three times. The serum from the rats (200 μL) was double diluted to decrease the concentration from 1:5 to 1:20, and then the diluted serum was added to the plate to react for one hour at 27°C, followed by rinsing three times. Then IgG antibody combined catalase (200 μL) was added for one hour at 27°C, followed by rinsing five times. H2SO4 (50 μL) was used to terminate the reaction. The OD value was recorded in an enzyme standard instrument (Biorad450) at the wavelength of 450 nm. An OD value more than twice the negative control was defined as positive.
Determination of concentrations
The concentrations of anti-alginate antibody and cytokines after inhalation of Pseudomonas aeruginosa were determined by anti-alginate antibody in the serum of the study rats. The concentrations of IL-17, IL-1β, MIP-2, G-CSF, and tumor necrosis factor (TNF)-α were determined by ELISA method according to the kit instructions.
Cell count in BALF and histological examination of the lung
The total numbers of cells in BALF on the 2nd, 5th, and 8th day were measured after the completion of the inhalation experiment. Cell sorting and counting were done by Wright-Giemsa staining. Lung tissue sections were stained using standard H&E method.
Determination of T lymphocyte subsets CD4+ and CD8+
On the 2nd, 5th, and 8th day after inhalation of Pseudomonas aeruginosa, heparin anticoagulant blood was taken from the veins of the SD rats. Heparin anticoagulant (25 μL) was taken and FITC-labeled anti CD4+ and CD8+ McAb (10 uL, diluted with pH 7.2 PBS) was added. After mixing well, the blood was incubated for 30 minutes at room temperature, then distilled water (750 μL) was added into the anti-coagulation mixture for hemolysis. After mixing well, blood (750 μL) was diluted with PBS (pH 7.2). The percentages of T lymphocytes CD4+ and CD8+ in the peripheral blood of SD rats were detected using flow cytometry; 3,000 cells were detected for CD4+ and CD8+, respectively.
Statistical analysis
The software SPSS 15.0 was used for statistical analysis and data processing. The experimental results were expressed by mean ±SD. Single-factor Analysis of Variance (ANOVA) was used to compare the difference between the control group and the experimental group; p<0.05 indicates that there are no significant differences.
Results
The serum antibody titer of anti-alginate
The anti-alginate IgG antibody titers of the immune group were significantly higher than the control group on the 5th and 8th day after inhalation of Pseudomonas aeruginosa; the results are shown in Figure 1.
Figure 1.
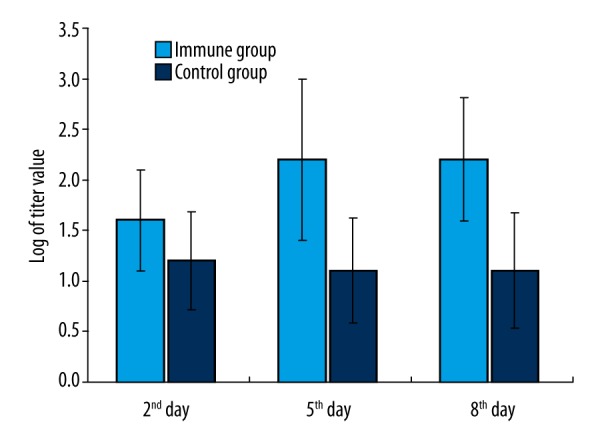
The anti-alginate antibody titer value in serum after inhalation of Pseudomonas aeruginosa (error bars were added).
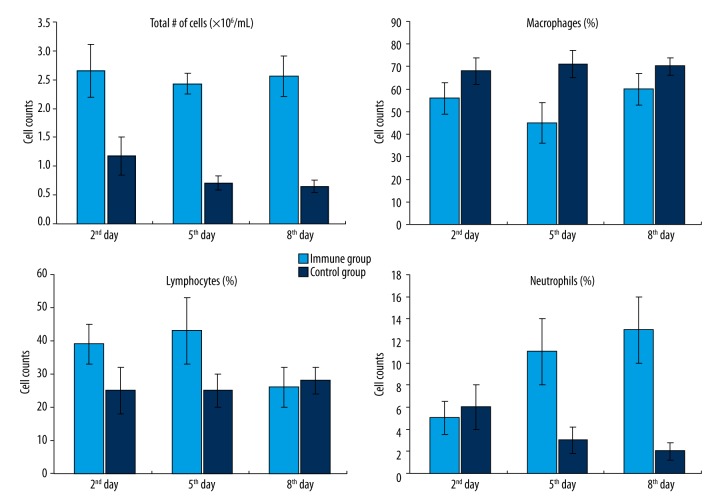
Cell counts and classification in BALF
The total number of cells in BALF of the immune group were significantly higher than in the control group on the 2nd, 5th, and 8th day after inhalation of Pseudomonas aeruginosa. Among the cells, the lymphocytes of the immune group were significantly higher than the control group on the 2nd and 5th day, but no significant difference was observed on the 8th day. It should be noted that the number of neutrophils in the immune group increased dramatically compared to the control group (Figure 2).
Figure 2.
Cell counts and classification in BALF after inhalation of Pseudomonas aeruginosa (error bars were added).
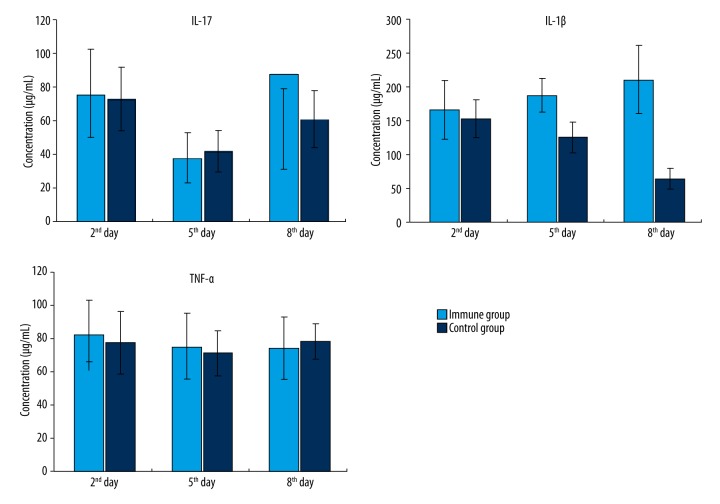
Concentration of cytokines
After inhalation of Pseudomonas aeruginosa, the concentrations of serum cytokines IL-17, IL-1β, and TNF-α of the immune group and the control group were all lower than the limits of the ELISA kit. There was no significant difference between the concentrations of serum cytokines IL-17 and TNF-α in the immune and the control groups. However, the IL-1β concentration in the serum of the immune group was significantly higher than in the control group on the 8th day (Figure 3).
Figure 3.
Cytokine concentrations in serum after inhalation of Pseudomonas aeruginosa (error bars were added).
Changes of T lymphocyte subsets in peripheral blood of SD rats
The results of the lymphocyte subset analysis showed that the CD4+ cells in the peripheral blood of the SD rats infected with Pseudomonas aeruginosa were significantly fewer than in the control group, and the CD8+ cells were significantly increased. The ratio of CD4+/CD8+ cells in rats infected with Pseudomonas aeruginosa decreased significantly compared to the control group (Table 1).
Table 1.
Cell percentages of CD4+ and CD8+ and the ratio of CD4+/CD8+ for the immune group and the control group.
| Days | CD4+ % | CD8+ % | CD4+/CD8+ | |||
|---|---|---|---|---|---|---|
| Immune group | Control group | Immune group | Control group | Immune group | Control group | |
| Day 2 | 45.28±2.24 | 48±1.38 | 24.03±1.86 | 17±1.23 | 1.87±0.18 | 2.82±0.16 |
| Day 5 | 43.61±2.03 | 49±1.27 | 22.38±1.75 | 17±1.14 | 1.95±0.13 | 2.87±0.21 |
| Day 8 | 41.42±2.33 | 47±1.19 | 21.55±1.77 | 16±1.19 | 1.94±0.15 | 2.92±0.24 |
Discussion
P. aeruginosa can generate alginate, which can adhere to the surface of the respiratory tract and form a biofilm. Alginate has antigenicity; previous research has shown that the antibody titers of anti-alginate were higher in the serum of patients with diffuse pan-bronchiolitis complicated with P. aeruginosa infection, and the antibody titer levels are positively correlated with the severity of the disease. Meers et al. [24] studied the effects of inhaled liposomal amikacin on chronic P. aeruginosa lung infections using fluorescently labelled liposomes to penetrate into P. aeruginosa biofilms. The penetration of labelled liposomes and lipid composition as liposomal amikacin into sputum and biofilm showed the potential for drugs to reach sites of infection in the lungs of cystic fibrosis patients. Moskowitz et al. [25,26], in a study of 41 cystic fibrosis patients, explored the feasibility of using a biofilm susceptibility assay in a clinical microbiology laboratory setting, and found that biofilm testing might be more effective than currently used methods for selection of antibiotic combinations. Drenkard et al. [27] found that antibiotic-resistant phenotypic variants of P. aeruginosa that have enhanced ability to form biofilms can arise at high frequency in vitro and in the lungs of cystic fibrosis patients. In our study, when the experimental SD rats were immune treated with alginate followed by inhalation of P. aeruginosa, the anti-alginate antibody titer in the serum was significantly higher than in the control group. At the same time, the lymphocytes and neutrophils in BALF of the immune group also increased significantly compared to the control group. Obvious inflammatory cell exudation around small respiratory tracts in the immune group was observed, while there were no clear changes in the control group. The local inflammation of the respiratory tracts in the immune group was stronger than that of the control group, which may be due to a rapid increase in anti-alginate antibody titers in the serum of the immune-treated rats after inhalation of P. aeruginosa. The virulence of P. aeruginosa as well as other pathogenic factors that may contribute to the inflammatory reaction caused by the alginate will need to be further investigated. However, our study did confirm that when the alginate antibodies exist in the blood of rats, alginate can lead to lung inflammation. These results are similar to the results found by Ohatami [23].
In our study, IL-17 in the serum of immunized rats significantly increased after inhalation of P. aeruginosa, while IL-1β, MIP-2, G-CSF, and TNF-α did not increase dramatically. IL-17 is a heterologous pro-inflammatory cytokine formed by covalent combination of two subunits p65 (65 kDa) and P75 (75 kDa). Bacteria and bacterial metabolites can strongly stimulate the secretion of IL-17, and IL-17 can induce cell cytotoxicity of natural killer cells and lymphokine-activated killer cells. It can also induce the transformation of TH1 cells from CDT cells, which plays an important function in the pathogenesis of infectious diseases. In infectious diseases, excessive production of IL-17 can produce septic shock syndrome, or induce immune pathological reactions. In our study, when SD rats were immunized with alginate inhaled P. aeruginosa, IL-17 probably increased due to the antigen-antibody reaction of alginate and anti-alginate antibody on the surface of respiratory tract; the mechanism of this reaction needs to be further investigated. As a pro-inflammatory factor, IL-17 can mediate inflammatory reactions, resulting in exudation of lymphocytes and neutrophils from blood vessels to lung tissues; the neutrophils can release super oxidase, lytic enzymes, and other factors which may lead to immune-related damage to lung tissue.
Conclusions
The results of our current research indicated that alginate beads have pathogenic effects in P. aeruginosa lung infections; this effect may be mediated by cytokine IL-17.
Footnotes
Source of support: This research was funded by National Natural Science Foundation of China (81260663)
References
- 1.Gellatly SL, Hancock RE. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog Dis. 2013;67:159–73. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 2.Whiteley M, Bangera MG, Bumgarner RE, et al. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001;413:860–64. doi: 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- 3.de Bentzmann S, Roger P, Puchelle E. Pseudomonas aeruginosa adherence to remodelling respiratory epithelium. Eur Respir J. 1996;9:2145–50. doi: 10.1183/09031936.96.09102145. [DOI] [PubMed] [Google Scholar]
- 4.Pang Z, Sun G, Junkins RD, Lin TJ. Aim2 inflammasome is dispensable for the host defense against Pseudomonas aeruginosa infection. Cell Mol Biol (Noisy-le-grand) 2015;61(3):63–70. [PubMed] [Google Scholar]
- 5.Lee A, Chow D, Haus B, et al. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am J Physiol. 1999;277:L204–17. doi: 10.1152/ajplung.1999.277.1.L204. [DOI] [PubMed] [Google Scholar]
- 6.Heiniger RW, Winther-Larsen HC, Pickles RJ, et al. Infection of human mucosal tissue by Pseudomonas aeruginosa requires sequential and mutually dependent virulence factors and a novel pilus-associated adhesin. Cell Microbiol. 2010;12:1158–73. doi: 10.1111/j.1462-5822.2010.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soong G, Parker D, Magargee M, Prince AS. The type III toxins of Pseudomonas aeruginosa disrupt epithelial barrier function. J Bacteriol. 2008;190:2814–21. doi: 10.1128/JB.01567-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange KH, Hougen HP, Høiby N, et al. Experimental chronic Pseudomonas aeruginosa lung infection in rats. Non-specific stimulation with LPS reduces lethality as efficiently as specific immunization. APMIS. 1995;103:367–74. [PubMed] [Google Scholar]
- 9.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat Rev. 2009;7:654–65. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bragonzi A, Farulla I, Paroni M, et al. Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response. PLoS One. 2012;7:e52330. doi: 10.1371/journal.pone.0052330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauser AR, Cobb E, Bodi M, et al. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–28. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Woods DE, Bryan LE. Studies on the ability of alginate to act as a protective immunogen against infection with Pseudomonas aeruginosa in animals. J Infect Dis. 1985;151:581–88. doi: 10.1093/infdis/151.4.581. [DOI] [PubMed] [Google Scholar]
- 13.Naraian R, Ram S, Kaistha SD, Srivastava J. Occurrence of plasmid linked multiple drug resistance in bacterial isolates of tannery effluent. Cell Mol Biol. 2012;58:134–41. [PubMed] [Google Scholar]
- 14.Rangel SM, Diaz MH, Knoten CA, et al. The role of ExoS in dissemination of Pseudomonas aeruginosa during pneumonia. PLoS Pathog. 2015;11:e1004945. doi: 10.1371/journal.ppat.1004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray TS, Ledizet M, Kazmierczak BI. Swarming motility, secretion of type 3 effectors and biofilm formation phenotypes exhibited within a large cohort of Pseudomonas aeruginosa clinical isolates. J Med Microbiol. 2010;59:511–20. doi: 10.1099/jmm.0.017715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman M, Bryan R, Rajan S, et al. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998;66:43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tartor YH, Elnaenaeey EY. RT-PCR detection of exotoxin genes expression in multidrug resistant Pseudomonas aeruginosa. Cell Mol Biol. 2016;62:56–62. [PubMed] [Google Scholar]
- 18.Hayashi N, Nishizawa H, Kitao S, et al. Pseudomonas aeruginosa injects type III effector ExoS into epithelial cells through the function of type IV pili. FEBS Lett. 2015;589:890–96. doi: 10.1016/j.febslet.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Bucior I, Pielage JF, Engel JN. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 2012;8:e1002616. doi: 10.1371/journal.ppat.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen HK, Høiby N, Pedersen SS. Experimental immunization with Pseudomonas aeruginosa alginate induces IgA and IgG antibody responses. APMIS. 1991;99:1061–68. [PubMed] [Google Scholar]
- 21.Johansen HK, Hougen HP, Cryz SJ, Jr, et al. Vaccination promotes TH1-like inflammation and survival in chronic Pseudomonas aeruginosa pneumonia in rats. Am J Respir Crit Care Med. 1995;152:1337–46. doi: 10.1164/ajrccm.152.4.7551392. [DOI] [PubMed] [Google Scholar]
- 22.Apodaca G, Bomsel M, Lindstedt R, et al. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: Glycosylation-defective host cells are resistant to bacterial killing. Infect Immun. 1995;63:1541–51. doi: 10.1128/iai.63.4.1541-1551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohatami H. [Study on the pathogenetic role of alginate produced by mucoid Pseudomonas aerugiosa in diffuse panbronchiolitis]. Kansenshogaku Zasshi. 1995;69(5):553–67. doi: 10.11150/kansenshogakuzasshi1970.69.553. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 24.Meers P, Neville M, Malinin V, et al. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother. 2008;61:859–68. doi: 10.1093/jac/dkn059. [DOI] [PubMed] [Google Scholar]
- 25.Moskowitz SM, Foster JM, Emerson J, Burns JL. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J Clin Microbiol. 2004;42:1915–22. doi: 10.1128/JCM.42.5.1915-1922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskowitz SM, Foster JM, Emerson JC, et al. Use of Pseudomonas biofilm susceptibilities to assign simulated antibiotic regimens for cystic fibrosis airway infection. J Antimicrob Chemother. 2005;56:879–86. doi: 10.1093/jac/dki338. [DOI] [PubMed] [Google Scholar]
- 27.Drenkard E, Ausubel FM. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;416:740–43. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]