Summary
The Escherichia coli aerotaxis receptor, Aer, monitors cellular oxygen and redox potential via FAD bound to a cytosolic PAS domain. Here, we show that Aer-PAS controls aerotaxis through direct, lateral interactions with a HAMP domain. This contrasts with most chemoreceptors where signals propagate along the protein backbone from an N-terminal sensor to HAMP. We mapped the interaction surfaces of the Aer PAS, HAMP and proximal signalling domains in the kinase-off state by probing the solvent accessibility of 129 cysteine substitutions. Inaccessible PAS-HAMP surfaces overlapped with a cluster of PAS kinase-on lesions and with cysteine substitutions that crosslinked the PAS β-scaffold to the HAMP AS-2 helix. A refined Aer PAS-HAMP interaction model is presented. Compared to the kinase-off state, the kinase-on state increased the accessibility of HAMP residues (apparently relaxing PAS-HAMP interactions), but decreased the accessibility of proximal signalling domain residues. These data are consistent with an alternating static-dynamic model in which oxidized Aer-PAS interacts directly with HAMP AS-2, enforcing a static HAMP domain that in turn promotes a dynamic proximal signalling domain, resulting in a kinase-off output. When PAS-FAD is reduced, PAS interaction with HAMP is relaxed and a dynamic HAMP and static proximal signalling domain convey a kinase-on output.
Introduction
Microbial sensory systems include numerous combinations of common modular domains, enabling microbes to respond to a remarkable variety of environmental stimuli (Zhulin, 2001; Wuichet et al., 2007). This has been likened to assembling sensory pathways from ‘Lego®’-like modules (Schultz and Natarajan, 2013) that can be arranged into endless possible constructions, each maintaining function and a fine-tuned response. One of the best-characterized sensory systems is E. coli chemotaxis, where stimuli are integrated to modulate flagella rotation via a common phosphorylation cascade (Hazelbauer and Lai, 2010; Krell et al., 2011; Parkinson et al., 2015). Chemoreceptors regulate the cascade by controlling the autophosphorylation of the histidine kinase, CheA. Phospho-CheA in turn phosphorylates the response regulator, CheY, and phospho-CheY binds to the flagellar motor, thus altering the direction of flagella rotation and changing the direction of bacterial swimming. This is a versatile strategy that enables bacteria to collectively respond to numerous and diverse stimuli using variations on common mechanisms of intraprotein and interprotein signalling.
Here, we investigate another variation on the chemotaxis paradigm in the modular aerotaxis receptor, Aer (Fig. 1A), and examine common signalling mechanisms that underlie the different signalling pathways in aerotaxis and chemotaxis. The sensor for the aerotaxis receptor is an N-terminal PAS [Per-Arnt-Sim (Nambu et al., 1991]) domain, which monitors cellular redox potential via a flavin adenine dinucleotide (FAD) cofactor ([Bibikov et al., 1997; Rebbapragada et al., 1997; Taylor and Zhulin, 1999; Taylor, 2007], Fig. 1B). PAS-FAD is reduced under hypoxic conditions, eliciting a conformational cascade that promotes the kinase-on state. We previously showed that the PAS sensor can interact directly (Campbell et al., 2010) with the Aer HAMP domain [HAMP is found in histidine kinases, adenylyl cyclases, methyl-accepting chemotaxis proteins, phosphatases, diguanylate cyclases and phosphodiesterases (Aravind and Ponting, 1999; Dunin-Horkawicz and Lupas, 2010)]. From that study and previous work we postulated that PAS modulates HAMP by direct PAS-HAMP interactions.
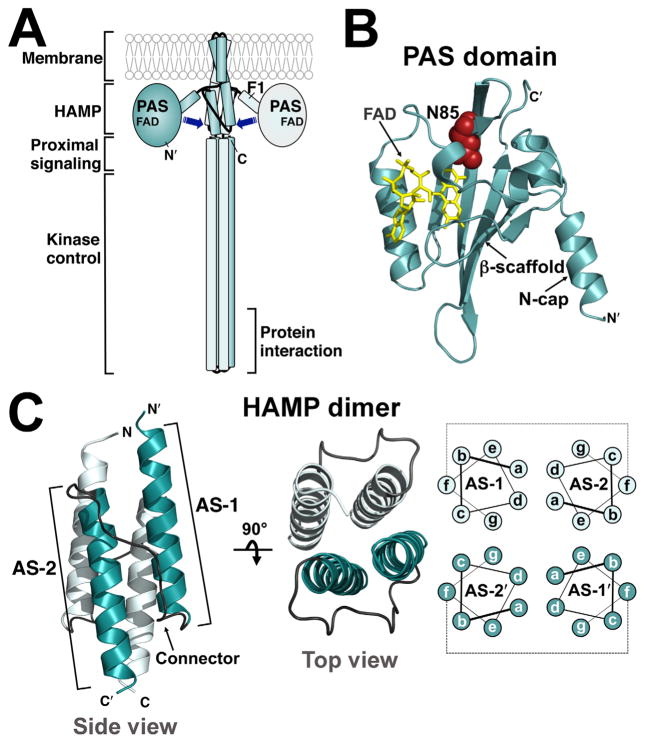
Fig. 1. Models of the aerotaxis receptor, Aer, and the Aer PAS and HAMP domains.
A. Cartoon of the domain organization of an Aer dimer. The PAS sensing domain is proposed to contact the downstream HAMP and proximal signalling domains (arrows).
B. Aer PAS homology model (res. 5–122) based on the co-ordinates of the Azotobacter vinelandi NifL PAS domain (Key et al., 2007) showing FAD (yellow) and the location of N85 (red spheres), which was substituted with serine to generate the kinase-on state of Aer.
C. Aer-HAMP dimer model (res. 204–258) based on the co-ordinates of the Archaeoglobus fulgidus Af1503 HAMP domain (Hulko et al., 2006). Each monomer is composed of two helices, AS-1 and AS-2, which are separated by a non-helical connector and arranged as a parallel four-helix bundle. Helical positions ‘a’ through ‘g’, and their proposed arrangement in the bundle are indicated. Abbreviations: FAD, flavin adenine dinucleotide; AS, amphipathic sequence.
In Aer, the PAS and HAMP domains are separated by the F1 linker (Bibikov et al., 2000; Campbell et al., 2011) and the hairpin membrane anchor [(Amin et al., 2006), Fig. 1A]. This intervening sequence is not directly involved in signalling, but the F1 linker supports maturation of the PAS and HAMP domains (Buron-Barral et al., 2006; Campbell et al., 2011), and the membrane anchor localizes Aer with other chemoreceptors (Amin et al., 2006). The proximal signalling domain [following HAMP; (Ma et al., 2005), Fig 1A] corresponds to the adaptation region in methyl-accepting chemoreceptors. Although the proximal signalling domain has no adaptation function in Aer (Bibikov et al., 2004), it does serve a critical role in Aer signalling (Bibikov et al., 2004; Ma et al., 2005; Buron-Barral et al., 2006). Lastly, the C-terminal kinase control module controls the rate of CheA phosphorylation. Here, each monomer forms antiparallel helices with a U-turn at the tip, and together the two monomers form a long, supercoiled, four-helix bundle that extends into the proximal signalling domain (Fig. 1A).
In E. coli chemoreceptors, the HAMP domain is positioned in the cytosol between the cytoplasmic membrane and the kinase control module. Here it acts as a central processing unit, receiving input from an N-terminal sensor domain and relaying this information to the C-terminal kinase control domain (Parkinson, 2010). Each HAMP monomer is made up of two amphipathic α-helices (AS-1 and AS-2) (Butler and Falke, 1998; Watts et al., 2008), and two HAMP monomers fold into a parallel four-helix coiled-coil [(Hulko et al., 2006; Swain and Falke, 2007; Airola et al., 2010; Watts et al., 2011; Wang et al., 2013; Mechaly et al., 2014), Fig. 1C]. Our studies on Aer have shown that signals received by HAMP domains can be of two types. In many chemoreceptors, the HAMP domain is controlled by a periplasmic sensor domain, which transmits signals through the membrane to the HAMP domain [reviewed by (Parkinson, 2010)]; but in the aerotaxis receptor, Aer, the HAMP domain is controlled via direct lateral interactions with the cytosolic PAS sensing domain [(Herrmann et al., 2004; Watts et al., 2004; Ma et al., 2005; Buron-Barral et al., 2006; Watts et al., 2006a, Campbell et al., 2010), Fig. 1A]. HAMP domains are therefore able to convert two disparate conformational inputs into similar output controls.
Models to explain HAMP signalling range from those with static kinase-on and kinase-off conformations, such as the gearbox rotation model (Hulko et al., 2006; Ferris et al., 2011; Mondejar et al., 2012), helix tilting models (Swain and Falke, 2007; Watts et al., 2011), and combined helix rotation with tilting models (Airola et al., 2010; Wang et al., 2012), to a biphasic static-dynamic signalling model in which the signalling state depends on the structural stability of the HAMP four-helix bundle (Zhou et al., 2009; 2011; Airola et al., 2013; Ames et al., 2014; Klose et al., 2014; Lai and Parkinson, 2014). In the static-dynamic signalling model, the kinase-off conformation of the receptor is associated with stable HAMP packing, in contrast to the kinase-on conformation, which is associated with a more dynamic HAMP bundle. A loosely packed HAMP domain (the kinase-on state) appears to be associated with a tightly packed adaptation region in methyl-accepting chemoreceptors (regionally equivalent to the Aer proximal signalling domain), causing a concomitant destabilization of the distal kinase control region (the protein-interaction region; Fig. 1A) and subsequent phosphorylation of CheA (Swain et al., 2009; Parkinson, 2010; Zhou et al., 2011; Falke and Piasta, 2014).
In this study, we investigate the signalling pathway from the PAS domain to the HAMP and proximal signalling domains of Aer. Several previous studies argued for direct signalling from the PAS to the HAMP domain: (i) HAMP AS-2 is required for PAS folding and for PAS FAD-binding (Bibikov et al., 2000; Herrmann et al., 2004; Ma et al., 2005; Buron-Barral et al., 2006), (ii) PAS-N34D is an allele-specific suppressor of HAMP-C253R, implying close proximity between PAS-N34 and AS-2-C253 (Watts et al., 2004) and (iii) specific cysteine substitutions in the PAS β-scaffold crosslink with a cysteine substitution in the HAMP domain, confirming close proximity of the PAS β-scaffold and HAMP domain (Campbell et al., 2010). Here, we extend previous studies by defining the interacting surfaces of the Aer PAS, HAMP and proximal signalling domains. We first map the in vivo solvent accessibility of residues in these regions to identify hidden (contact) surfaces, and use cysteine crosslinking to uncover the orientation between the PAS and HAMP domains. We compare accessibilities in the kinase-on and kinase-off states and find signal-induced changes in the HAMP and proximal signalling domains that support the alternating static-dynamic signalling model. Our results suggest that HAMP domains use a common signalling mechanism that can be modulated by either a lateral or linear sensory input.
Results
Mapping the in vivo accessibility of residues in Aer
We previously showed that under aerobic conditions the PAS and HAMP domains of Aer can physically interact (Campbell et al., 2010). Under these conditions PAS-FAD remains oxidized and the output is kinase-off. Here, we examined the pathway through which the oxidized PAS domain controls the HAMP domain and stabilizes the kinase-off state. If PAS-HAMP interactions are stable, the contact surfaces should be sequestered and less accessible to solvent than non-contact surfaces. To identify putative contact regions on the PAS, HAMP and proximal signalling domains, we made single cysteine replacements throughout these domains, and then probed each protein under aerobic conditions with methoxypolyethylene glycolmaleimide 5000 (PEG-mal). PEG-mal is a bulky sulfhydryl-reactive reagent that preferentially reacts with sulfhydryl residues that are accessible to solvent at the protein surface (Lu and Deutsch, 2001). The E. coli cells used for these experiments lacked both the Aer and Tsr aerotaxis receptors [BT3312 (aer tsr)]. BT3312 cells expressing each plasmid-encoded Aer-Cys protein were made permeable to PEG-mal by treatment with toluene and ethanol, and then incubated with 5 mM PEG-mal at 25°C for 15 min (see Experimental Procedures). To measure the PEGy-lation of native samples, reactions were stopped with excess β-mercaptoethanol before being boiled in sample buffer. To measure the maximum PEGylation of denatured samples, parallel reactions were continued by boiling in sample buffer without β-mercaptoethanol. The PEGylated samples were separated by SDS-PAGE, and a mobility shift of approximately 10 kDa on Western blots readily differentiated PEGylated from un-PEGylated Aer (Fig. 2, [Amin et al., 2006]). Under these conditions, the average chemical reactivity relative to denatured protein was below 50% and spanned a large dynamic range, ensuring that most reactions did not approach completion (compare Figs. 3A and 4B).
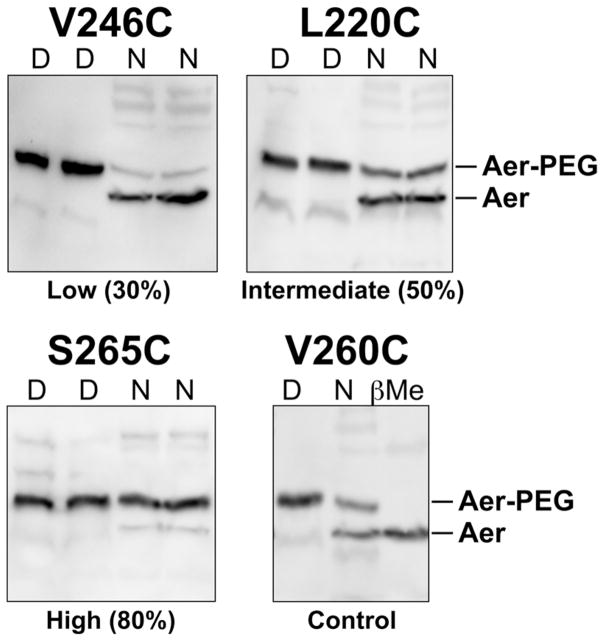
Fig. 2.
Western blots of Aer-Cys proteins showing examples of low (Aer-V246C), intermediate (Aer-L220C), and high (Aer-S265C) PEGylation under native (N) conditions. PEGylated Aer (Aer-PEG) has an apparent mass increase of ~10 kDa. Samples denatured (D) without β-mercaptoethanol quencher were PEGylated to apparent completion; samples pretreated with β-mercaptoethanol (before PEG-mal) were not PEGylated (see Aer-V260C, βMe lane). Faint bands migrating faster than an Aer monomer represent PEGylated Aer break-down product (Ma et al., 2004). Abbreviations: D, denatured; N, native; β-Me, β-mercaptoethanol.
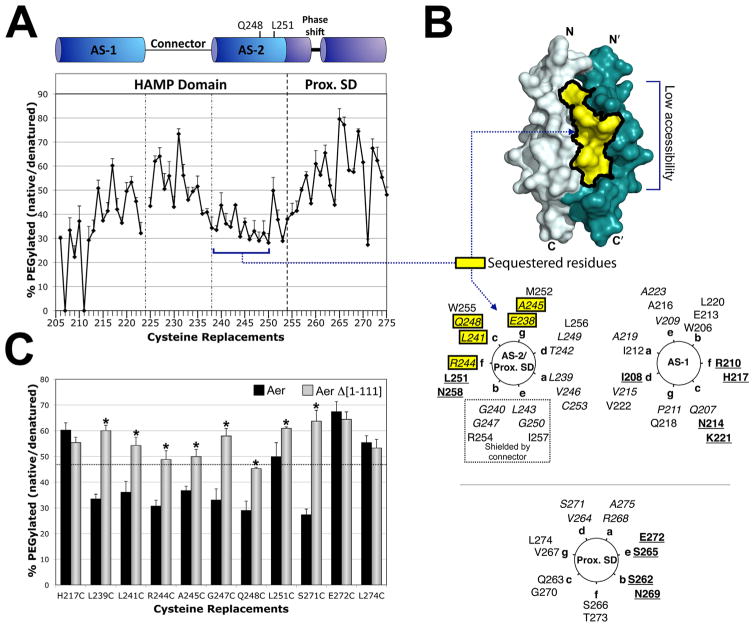
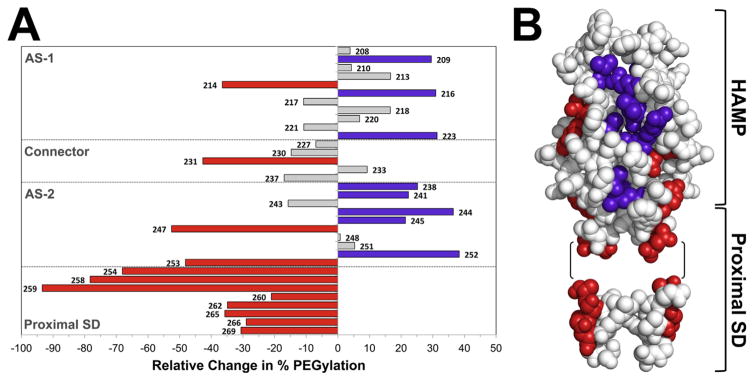
Fig. 3. Accessibility of residues in the HAMP and proximal signalling domains as inferred from reactivity with PEG-mal.
A. Extent of PEGylation for substituted cysteines at each residue of the Aer HAMP and proximal signalling domains. Error bars represent the standard deviation from multiple experiments.
B. Sequestered AS-2 surface residues mapped onto a HAMP dimer model and a helical wheel. The hidden region was inferred by low accessibility to PEG-mal (A), and included residues 238–250 (shaded yellow). Helical wheels use the standard heptad repeat nomenclature (see Fig. 1), and include HAMP AS-1 (right panel), AS-2 (left panel) and the proximal signalling domain (Prox. SD). The proximal signalling domain is divided between two helical wheels due to the presence of a helical phase shift after residue 259, and the resumption of a helical accessibility pattern (with maximum accessibility every third and forth residue), at residue 262 (Watts et al., 2008). AS-2 residues that are shielded by the connector in the HAMP model are indicated within a dotted box. Colour code: bold and underlined, the most accessible residues in each region; italicized, the least accessible residues in each region; yellow boxes, residues predicted to be accessible but found to have low accessibility.
C. Accessibility of HAMP and proximal signalling domain residues in the presence (black bars, full length Aer) and absence (grey bars, PAS-less Aer Δ[1-111]) of the Aer-PAS domain. Error bars represent standard deviations from multiple experiments. Asterisks indicate statistically significant differences in the absence of the PAS domain (P <0.05). The dotted line indicates the average accessibility of exposed AS-1 residues (the underlined residues in the AS-1 helical wheel shown in B).
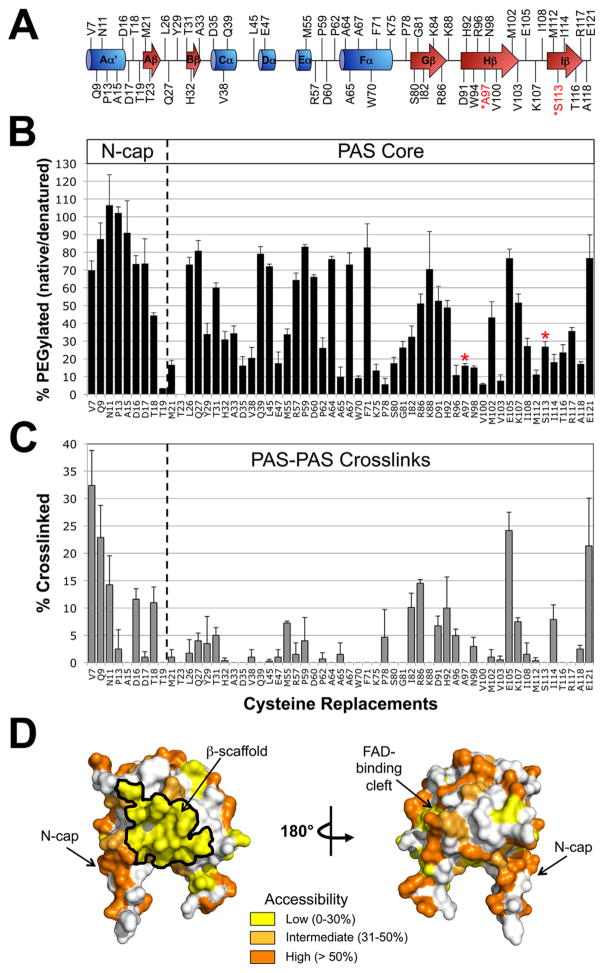
Fig. 4. Probing the PAS domain for solvent accessibility and PAS-PAS proximity using PEGylation and disulfide crosslinking.
A. Residues selected for cysteine replacement mapped onto the secondary structure of the Aer PAS domain. Residues in black font are predicted to be accessible on the surface of the Aer-PAS homology model; those in red font are predicted to face inwards towards the PAS interior, and were selected as surface-inaccessible controls.
B. Extent of PEGylation for substituted cysteines in the Aer PAS core and N-terminal cap (N-cap). Red asterisks identify the surface-inaccessible controls.
C. Extent of disulfide crosslinking between neighboring PAS domains. Error bars in B and C represent the standard deviation from multiple experiments.
D. Aer-PAS homology model showing the distribution of the tested residues based on whether they had low (yellow shading, 0–30% PEGylation), intermediate (light orange, 31–50% PEGylation) or high (orange, >50% PEGylation) accessibility.
Accessibility of HAMP and proximal signalling domain residues in Aer
A library with serial cysteine replacements at each of 70 HAMP and proximal signalling domain residues was previously constructed [res. 206–276], and all but three of these cysteine mutants retained function (Amin et al., 2006; Watts et al., 2008). In the current study, the extent to which each residue reacted with PEG-mal was used as a measure of solvent accessibility (Fig. 3). In the first HAMP region, AS-1 (res. 207–223), the PEGylation pattern was inversely related to previously determined disulfide crosslinking results (Watts et al., 2008). Thus, PEGylation of the substituted cysteines increased as the extent of crosslinking decreased, and vice versa, which is consistent with prior conclusions that AS-1 is an α-helix. The most accessible AS-1 residues were located at the ‘c’ and ‘f’ positions of a helical wheel, where each position of the heptad repeat was designated by the letters a to g (Fig. 3B). In the membrane-proximal end of AS-1 (res. 206–211), PEGylation ratios were lower than the remainder of AS-1 (res. 212–223, located within the four-helix bundle) (Fig. 3A). This may be due to a local membrane effect, as this region precedes the HAMP four-helix bundle and anchors HAMP to the membrane at or near residue 206 (Amin et al., 2006). The non-helical HAMP connector, which follows AS-1, generally showed greater PEGylation compared to AS-1 (Fig. 3A), but there was no discernible periodicity, nor did the data correlate well with the extent of disulfide formation previously determined in this region (Watts et al., 2008).
Following the HAMP connector, HAMP AS-2 (res. 238–253) forms an α-helix with a crosslinking periodicity of 3.5 residues per turn, and ‘a’ and ‘d’ positions on the interior of the four-helix bundle (Watts et al., 2008). In the current study, PEGylation was low for most of AS-2. This area is shaded yellow in Fig. 3B and has a calculated surface area of 1180 Å2. The low accessibility was not likely caused by a membrane effect because AS-2 begins approximately 10 Å from the membrane. Of note, the interior facing ‘a’ and ‘d’ positions of AS-2 were not always the least accessible residues. This was because the ‘b’, ‘c’, ‘e’ and ‘g’ positions also had low accessibility (Fig. 3). The HAMP connector is predicted to shield the ‘b’ and ‘e’ positions of AS-2, but the low accessibility of the ‘c’ and ‘g’ positions (and one ‘f’ position) suggested that this face was relatively hidden from solvent and may be shielded by another protein surface. In contrast, residues at the C-terminal end of AS-2 (res. 251–253) had PEGylation values that, like those in AS-1, inversely correlated with the extent of disulfide formation determined previously (Watts et al., 2008).
The region immediately following HAMP AS-2 is the proximal signalling domain (res. 254–271), which links Aer-HAMP to the kinase control module (Ma et al., 2005). The proximal end of this region showed a sequential increase in PEGylation values that did not inversely correlate with previously determined disulfide crosslinking results (residues 254–261, Fig. 3A). This was notable because we previously found a high extent of crosslinking in this region (Watts et al., 2008). Specifically, Cys replacements at residues 256 and 260 had the greatest extents of disulfide bond formation of any cytosolic cysteine replacement in Aer (Watts et al., 2008). Together these data raise the possibility that this segment is both accessible and flexible. Flexibility could result from the helical phase shift previously identified by disulfide crosslinking (Watts et al., 2008) and narrowed to residues 259–262 in the current study. This experimentally determined phase shift represents a discontinuity in the heptad repeat sequence and is five residues downstream of the HAMP-proximal signalling juncture. These junctures are notable because sequence analyses have revealed a phase stutter (a change in coiled-coil registry) at HAMP-output-helix connections (Parkinson, 2010). In this study, the Aer phase shift did not occur at the phase stutter (residues 254–256) but occurred between residues 259 and 262. Residue S262 is predicted to be in a ‘c’ position on the AS-2/proximal signalling domain helix (Fig. 3B, left helical wheel), but actually fits the accessibility pattern of an accessible ‘b’ position in the proximal signalling domain helix (Fig. 3B, bottom wheel). For the remainder of the proximal signalling domain and beyond (res. 262–275), PEGylation efficiency remained high, but the profile was inversely related to previously determined disulfide crosslinking values (Watts et al., 2008). Therefore, residues following the putative phase shift appeared to form a 3.5 residue-per-turn α-helix, with the least accessible residues in the ‘a’ and ‘d’ positions and the most accessible residues in the ‘b’ and ‘e’ positions (Fig. 3B).
Comparison of HAMP accessibility in the presence and absence of the PAS domain
The simplest explanation for the sequestered surface of AS-2 (Fig. 3B, yellow residues) is that this region is shielded by another part of Aer. To determine whether the PAS domain shields HAMP AS-2, accessibility measurements were repeated in the presence (full-length Aer) or absence (Aer Δ[1-111]) of the PAS domain. Aer Δ[1-111] lacks all but eight C-terminal PAS residues and forms a stable product (J.S. Parkinson, pers. comm.) that maintains near-normal steady-state levels under low induction (data not shown). Without the PAS domain, ‘inaccessible’ AS-2 residues became significantly more PEGylated, reaching levels routinely observed for surface-exposed AS-1 residues in full-length Aer (Fig. 3C). Control residues in AS-1 and the proximal signalling domain (with the exception of Aer-S271C at the interface of the proximal signalling domain) were not significantly more accessible in PAS-less Aer. Taken together, these data both provide evidence that the PAS domain shields HAMP AS-2, and potentially define a PAS-HAMP contact surface.
Mapping inaccessible surfaces of the PAS domain
To identify surfaces on the PAS domain that may form stable interactions with HAMP or other domains, we probed predicted PAS surface residues with PEG-mal. Using an Aer-PAS homology model (Fig. 1B), we selected 57 surface-exposed PAS residues and two interior-facing PAS residues as inaccessible controls (A97 and S113, Fig. 4A). Each residue was individually replaced with cysteine, expressed in BT3312 and screened for phenotype in succinate minimal soft agar. Of the 59 Aer-PAS Cys mutants constructed, only Aer-W94C did not support aerotaxis in semi-solid agar at each expression level (see Supporting Information Fig. S1 for details).
The extent of PEGylation for each PAS cysteine replacement is shown in Fig. 4B. To search for inaccessible PAS surfaces, we first sorted the PAS PEGylation values into one of three categories: low accessibility (0–30% PEGylation), intermediate accessibility (31–50% PEGylation) or high accessibility (> 50% PEGylation). Each category was then mapped onto the Aer-PAS model (Fig. 4D). As anticipated for residues shielded from PEG-mal, the two interior-facing controls, A97C and S113C, had low accessibility (16% and 27% PEGy-lation, respectively). Residues with high accessibility were scattered over the PAS surface. A notable region of high accessibility was the PAS N-terminal cap (N-cap, res. 1–19), where all residues tested (except T18C and T19C) had high accessibility (Figs. 4B and D). This suggests that the PAS N-cap is dynamic and does not stably interact with other domains in Aer, a conclusion that is compatible with our previous finding that the N-cap can collide with neighboring dimers (Watts et al., 2006b). In contrast, a large area of low accessibility measuring 1,370 Å2 was present on the PAS β-scaffold (Fig. 4D, yellow region outlined in black). This area was surrounded by residues with intermediate accessibility (Fig. 4D), perhaps delineating the boundary of a PAS contact surface.
PAS-PAS crosslinking
To determine if the area of low accessibility on the PAS β-scaffold was due to PAS-PAS interactions, each of the 59 PAS Cys substitutions that circumscribed the PAS domain was crosslinked in vivo by treating whole cells with 600 μM Cu(II)(1,10-phenanthroline)3 (CuPhe) for 20 min. Crosslinked products were identified by their migration on SDS-PAGE and detected with anti-Aer2-166 antisera. As anticipated, the interior-facing controls, A97C and S113C, did not crosslink (Fig. 4C). Notably, residues that clustered on the inaccessible PAS β-scaffold did not crosslink substantially (<8% dimers). This suggests that PAS-PAS interactions do not contribute to the inaccessible surface. The eight cysteine mutants that formed more than 10% dimers (Fig. 4C) also had high or intermediate accessibility to PEG-mal (Fig. 4B), indicating that some of these PAS-PAS interactions may have been transient, rather than stable. These residues included several in the flexible N-cap (V7C, Q9C, N11C, D16C and T18C), R86C in the β-scaffold, E105C in the PAS H-I loop, and E121C in the F1 loop (Fig. 4C).
In the PAS core, the greatest extent of PAS-PAS crosslinking was observed for E105C. Our previous studies predict that only flexible regions of PAS can form PAS-PAS crosslinks within an Aer dimer because the PAS domains are separated by the HAMP four-helix bundle. Non-flexible PAS regions are more likely to contact another dimer within the trimer-of-dimers hexameric structure of Aer (Campbell et al., 2011). To differentiate intradimeric from interdimeric contacts for E105C, the aspartate receptor, Tar, was over-expressed from a compatible plasmid to form mixed trimers-of-dimers with Aer (Gosink et al., 2006). In the presence of over-expressed Tar, Aer-E105C crosslinking decreased from 24% to 11%. This suggests that Aer-E105C crosslinking occurs between Aer dimers, a result that was also shown for E121C (Campbell et al., 2011).
PAS-HAMP interactions defined by disulfide crosslinking
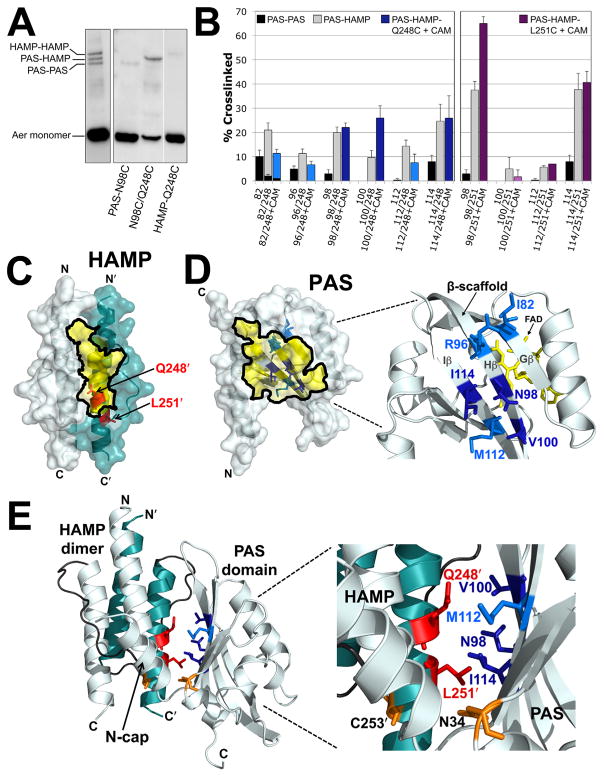
We previously demonstrated crosslinking between Cys replacements in the PAS and HAMP domains of Aer (Campbell et al., 2010). For those experiments, Q248C was selected as the HAMP Cys probe because Q248C (i) is located on the sequestered face of AS-2 (Figs. 3A and B), (ii) is significantly more accessible in the absence of the PAS domain (Fig. 3C) and (iii) does not significantly crosslink with itself within or between dimers [≤1% dimers, (Watts et al., 2008)]. Those initial studies demonstrated in vivo PAS-HAMP crosslinking between HAMP-Q248C and PAS-N98C or PAS-I114C (Campbell et al., 2010). The findings were possible because PAS-HAMP crosslinks can be differentiated from PAS-PAS and HAMP-HAMP crosslinks by their migration on SDS-PAGE. The different mobility is based on the N-terminal location of the PAS domain: Aer PAS-PAS crosslinked dimers migrate faster on SDS-PAGE than HAMP-HAMP crosslinked dimers, and PAS-HAMP crosslinked dimers have an intermediate mobility (Fig. 5A). Note that the latter point is only true if the PAS-HAMP crosslink forms between subunits, not within a subunit. An intrasubunit crosslink will migrate as a compact monomer (Bass et al., 2007). In the current study, we identify additional PAS-HAMP pairs that can cross-link and use these data to define the PAS-HAMP interaction surface.
Fig. 5. Disulfide crosslinking between the Aer PAS and HAMP domains.
A. Representative examples comparing SDS-PAGE mobilities for Aer dimers crosslinked between PAS-PAS, PAS-HAMP and HAMP-HAMP. The left panel illustrates the separation of the three crosslinked bands for a di-Cys mutant with similar band densities at each position (Aer-I82C/A245C). The right panel illustrates the separation of these bands for a di-Cys mutant with preferential PAS-HAMP crosslinking (Aer-N98C/Q248C). In all cases, uncrosslinked monomer bands are near the bottom of the gels.
B. Extent of disulfide formation for PAS residues that preferentially crosslinked with HAMP-Q248C (left panel) and -L251C (right panel). PAS-PAS crosslinking is indicated by black fill, PAS-HAMP crosslinking by grey fill, PAS-Q248C crosslinking after treatment with chloramphenicol in blue fill and PAS-L251C crosslinking after treatment with chloramphenicol in purple fill. Dark blue or dark purple fill indicates that the extent of PAS-HAMP crosslinking did not significantly decrease after chloramphenicol treatment, whereas lighter blue or purple fill indicates a decreased extent of PAS-HAMP crosslinking after chloramphenicol treatment. HAMP-Q248C and -L251C controls routinely produced ≤ 1% dimers and are not shown. Error bars represent the standard deviation from multiple experiments.
C. HAMP homology model showing the location of HAMP-Q248 and -L251, which crosslinked with PAS, in relation to the region that was sequestered from PEG-mal (Fig. 3B, yellow shading).
D. PAS homology model showing the β-scaffold positions of the PAS residues that preferentially crosslinked with HAMP-Q248C in relation to the region that was sequestered from PEG-mal (Fig. 4D, yellow shading). Residue colours match those used in the left panel of B.
E. PAS-HAMP dimer model showing the proposed orientation of the PAS and HAMP domains based on PEGylation and PAS-HAMP crosslinking data. For clarity, the model includes the PAS domain from just one subunit (res. 5–119) and a HAMP dimer (monomers from both subunits, res. 204–258). The PAS model was manually oriented relative to the HAMP AS-2 helix of the cognate monomer to account for the crosslinking (B) and sequestration (C and D) data. Residue colours match those used in B, C and D. The locations of PAS-N34D and HAMP-C253R, which were previously identified as site-specific suppressors (Watts et al., 2004), are shown in orange. Abbreviation: CAM, chloramphenicol.
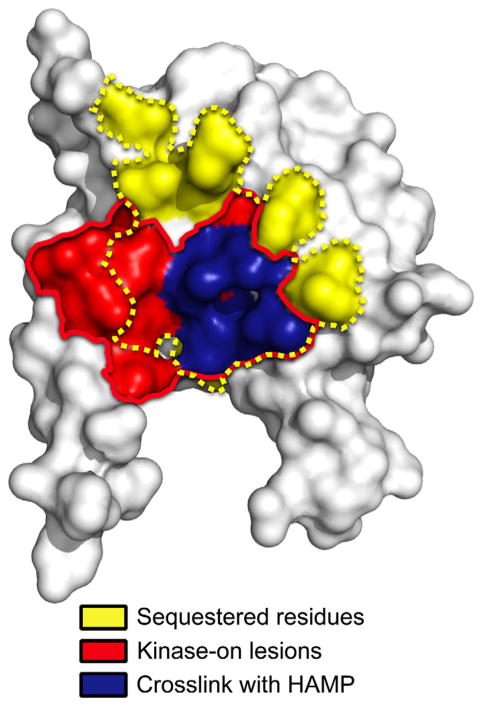
Q248C was paired with 25 different PAS Cys residues at sites circumscribing the PAS domain (see Experimental Procedures for details). The 25 di-Cys Aer constructs supported BT3312 aerotaxis in succinate minimal soft agar, with migration rates within the aerotactic range shown in Supporting Information Fig. S1. The Aer-Cys proteins were oxidized by treating whole cells with CuPhe as described above. Many of the substitutions produced small quantities of PAS-PAS and PAS-HAMP crosslinked dimers. However, several residues preferentially produced PAS-HAMP crosslinked dimers: I82C, R96C, N98C, V100C, M112C and I114C on the PAS β-scaffold (Fig. 5A, right panel and Fig. 5B, left panel, grey bars) and V38C, in the N-cap hinge region. V38C had the lowest extent of crosslinking with Q248C (5.5% dimers, not shown) and will not be discussed further. All of the PAS-HAMP crosslinks formed between, and not within, monomeric subunits. Several of the residues, e.g. I82C, exhibited some PAS-PAS crosslinking, but the extent consistently decreased in the presence of HAMP-Q248C (Fig. 5B). This indicated that the side chains of the PAS residues collided more often with a HAMP domain than with another PAS domain. The PAS residues that preferentially crosslinked with HAMP-Q248C resided almost exclusively within the inaccessible region of the PAS β-scaffold (Fig. 5D). This region is notable because it includes the cluster of kinase-on lesions that we previously identified as components of the signalling pathway [(Campbell et al., 2010), and the red area in Fig. 7].
Fig. 7.
PAS space-filled model overlaying a region of previously determined kinase-on lesions ([Campbell et al., 2010], within the red line) with residues shown in the current study to be sequestered (dotted yellow line), or that preferentially crosslinked with the HAMP domain (blue).
Aer undergoes a complex maturation process in which proper folding of the Aer PAS domain requires the presence of the HAMP domain (Herrmann et al., 2004). This process is easily destabilized by key mutations in either the PAS or HAMP domains (Buron-Barral et al., 2006; Campbell et al., 2010). In this study, we considered the possibility that some PAS-HAMP crosslinking occurred during the folding process before a mature Aer product was formed. To increase the fraction of mature Aer protein before crosslinking, new protein synthesis was inhibited with 500 μg ml−1 chloramphenicol for 15 min before adding CuPhe. After chloramphenicol treatment, three of the di-Cys mutants containing Q248C (N98C/Q248C, V100C/Q248C and I114C/Q248C) had an equivalent or increased proportion of PAS-HAMP cross-linked dimers (Fig. 5B, dark blue bars). This indicates that N98, V100 and I114, which are located on the PAS H and I β-strands (Fig. 5D), are each proximal to HAMP AS-2 in the folded protein. To test whether PAS-HAMP disulfide cross-links formed within or between dimers, Aer-I114C/Q248C was expressed with increasing concentrations of Tar. The amount of crosslinked I114C-Q248C product was unaffected by increased Tar expression (not shown), indicating that PAS-HAMP disulfide bonds most likely occurred between cognate monomers of the same dimer.
To identify HAMP residues other than Q248C that can crosslink with the PAS β-scaffold, we tested di-Cys combinations with several other HAMP residues (see Experimental Procedures for details). Of these, only HAMP-L251C (Fig. 5C) showed preferential PAS-HAMP crosslinking (Fig. 5B, right panel). Like Q248C, L251C has favorable properties in that it does not significantly crosslink with cognate L251C either within or between dimers [≤1% dimers, (Watts et al., 2008)]. In the presence of chloramphenicol, HAMP-L251C preferentially crosslinked with PAS-N98C, -M112C and -I114C, but not with -V100C (Fig. 5B, purple bars). The extent of crosslinking between HAMP-L251C and either PAS-N98C or PAS-I114C was higher than that between HAMP-Q248C and these PAS residues (Fig. 5B). This indicates that these PAS residues are closer to HAMP-L251C than they are to HAMP-Q248C. In contrast, crosslinking between HAMP-Q248C and either PAS-V100C or PAS-M112C was higher than that between HAMP-L251C and these PAS residues (Fig. 5B). This suggests that these PAS residues are closer to Q248C than they are to L251C. Using this information, homology models of the Aer PAS and HAMP domains were manually manipulated to obtain the best fit (Fig. 5E). To fit the data, the PAS domain was rotated ~180° around the PAS-HAMP interface such that the PAS-HAMP interaction surfaces are now flipped relative to our previous PAS-HAMP models [e.g. in (Watts et al., 2008; Campbell et al., 2011)]. The revised PAS-HAMP orientation resolves an unexplained anomaly that was present in previous models. Pseudoreversion analysis previously identified PAS-N34D as an allele-specific suppressor of HAMP-C253R (Watts et al., 2004). This implies close proximity between N34 and C253, but the previous PAS-HAMP models separated them. In the revised PAS-HAMP model, HAMP-C253 is in close proximity to PAS-N34 (Fig. 5E), resolving the anomaly.
Comparison of the kinase-on and kinase-off states
The solvent accessibility measurements in Figs. 3 and 4 were obtained under aerobic conditions and are expected to represent the kinase-off state of Aer (Repik et al., 2000). To gain insight into changes that might occur on HAMP surfaces as a result of signalling, we re-measured PEG-mal reactivities for 34 HAMP residues in the presence of the PAS kinase-on lesion N85S [(Campbell et al., 2010), Fig. 6A]. N85 is located on the PAS Gβ strand, and its side chain is predicted to face the interior of the PAS domain, contacting the isoalloxazine ring of FAD [(Campbell et al., 2010), Fig. 1B]. N85 is a possible link from the bound FAD to the β-scaffold, and the N85S substitution results in a kinase-on output.
Fig. 6. Influence of the PAS kinase-on lesion, N85S, on the accessibility of residues in the HAMP and proximal signalling domains to PEG-mal.
A. Histogram showing the average percent change in PEGylation for 34 Cys substitutions in Aer-N85S. Bars projecting to the right of the origin denote residues that became more accessible in the presence of N85S (significant increases are coloured purple; P <0.05). Bars projecting to the left denote residues that became less accessible (significant decreases are coloured red; P <0.05). Residues in grey had statistically insignificant changes.
B. Location of residues from A that showed significant changes in accessibility (in the kinase-on state) when mapped onto models of the Aer-HAMP domain and part of the proximal signalling domain. The models include residues 204–258 and 262–269. Residues 259–261 were omitted because of the phase shift in this region. Residues 262–269 are modeled onto a 3.5 residue per turn coiled coil α-helix; the precise orientation of this region relative to HAMP is unknown.
Residues that were significantly more accessible in the presence of N85S formed a patch on the HAMP surface that included both AS-1 and cognate AS-2′ residues (Fig. 6B, purple residues). This patch overlapped with AS-2 residues that were sequestered by the PAS domain in the kinase-off state (Fig. 3), suggesting that the N85S lesion disrupts the interaction of PAS with HAMP. In contrast to the AS-1 and AS-2′ residues with increased accessibility, residues with decreased accessibility in this region were located on a different face from the more accessible patch (Fig. 6B). The decrease in accessibility was greatest at the phase shift, where the PEGylation of D259C decreased from approximately 45% in the unstimulated state to approximately 3% in the presence of PAS-N85S. However, the face on which residues after N258 were located could not be determined because the three-dimensional structure of the phase shift is unknown.
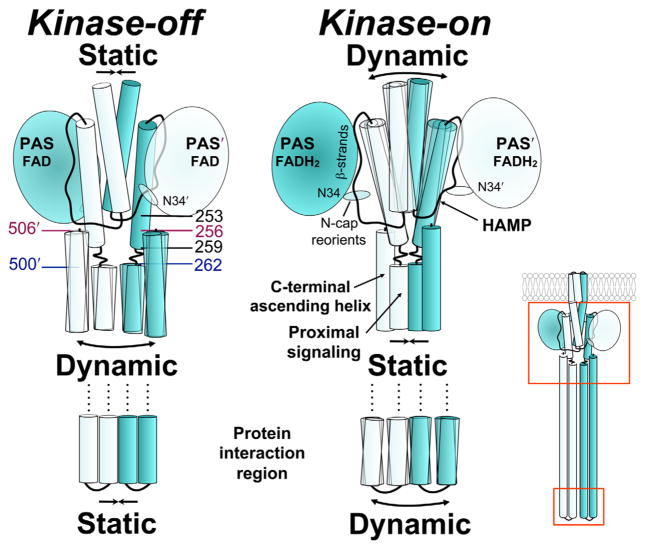
Notably, all residues that were significantly more accessible in the kinase-on state preceded the end of the HAMP domain (C253), while residues after the HAMP domain in the proximal signalling domain showed decreased accessibility. The proximal signalling and C-terminal kinase control domains are predicted to form an elongated antiparallel four-helix bundle in Aer (Fig. 1) that is analogous to the adaptation and protein interaction regions of other chemoreceptors (Falke and Piasta, 2014). Crosslinking studies of the proximal signalling domain indicate that this four-helix extends through the phase shift (res. 259–262). Our model pairs proximal signalling residue I257 with L505, and ends with H506 (aligned with L256) at the C-terminus of Aer [inferred from (Alexander and Zhulin, 2007), see Fig. 8]. Therefore, it is possible that residues following I257 that are less accessible in the kinase-on state form a more compact four-helix bundle. Conversely, increased accessibility in the HAMP domain in the kinase-on state may be associated with a more dynamic four-helix bundle.
Fig. 8.
Working model of Aer showing the relationship between PAS and HAMP in the kinase-off and kinase-on states, based on current and previous data. Colours match those of Fig. 5. The reduction of PAS-bound FAD elicits a conformational change that decreases interaction between the PAS domain and the HAMP domain. The relative orientation between the N-cap and PAS core also changes, perhaps altering the stability of PAS-HAMP interactions. Decreased PAS-HAMP interactions are accompanied by tilting of the HAMP helices and a more relaxed, dynamic HAMP structure. The HAMP, proximal signalling domain and protein interaction regions are in opposition across contiguous boundaries such that a relaxed HAMP domain results in a static proximal signalling domain and a dynamic protein interaction region. Relevant residues: N34D, C253R, allele-specific suppressors; 259–262, phase shift; pairs 262/500 and 256/506 are highlighted to show their relative latitudes on descending and ascending helices. See Results and Discussion for details.
Discussion
The Aer PAS-HAMP interaction surface
In this study, we investigated an unusual signalling mechanism in which a cytosolic PAS domain controls signalling by direct lateral interaction with a HAMP domain (Fig. 8). This differs from the prototypical membrane-bound chemoreceptor, in which HAMP is directly tethered through the membrane to a periplasmic sensor domain (Parkinson, 2010). In Aer, the measured solvent accessibilities of the PAS, HAMP and proximal signalling domains (Figs. 3 and 4) defined potential PAS-HAMP interaction surfaces. Data from PAS surface residues showed a hidden region on the PAS β-scaffold (1,370 Å2, Fig. 4D) that was consistent with a HAMP footprint rather than the footprint of a cognate PAS domain. Specifically, none of the sequestered residues crosslinked substantially with another PAS domain (Fig. 4C) and any such interactions may be transient rather than stable. Notably, the hidden region overlapped both with a cluster of PAS kinase-on lesions that we previously identified as part of the aerotaxis signalling pathway (Campbell et al., 2010), and with PAS residues in this study that crosslinked with the HAMP domain (Fig. 7). Together, the data strongly support the hypothesis that the inaccessible PAS region forms the PAS component of the PAS-HAMP interface.
Of the 70 HAMP and proximal signalling domain replacements tested, accessibilities inversely correlated with disulfide crosslinking in two regions: HAMP AS-1 and the proximal signalling domain region that followed the phase-shift. In contrast, most of HAMP AS-2 and the N-terminal proximal signalling domain did not inversely correlate with crosslinking. Notably, 10 of 11 AS-2 residues residing on the HAMP exterior had low accessibility to PEG-mal (Fig. 3). Four of these were likely shielded by the HAMP connector (Fig. 3B), but five of the remaining residues were sequestered by the PAS domain (Fig. 3B, boxed residues shaded yellow) and are proposed to form the HAMP component of the PAS-HAMP interface (Fig. 8). In support of this, HAMP AS-2 substitutions Q248C and L251C crosslinked with residues on the cognate PAS β-scaffold (Fig. 5). A best fit of the crosslinking data required that the PAS domain be rotated ~180° relative to a previous model (Watts et al., 2008; Campbell et al., 2011). The location of allele-specific suppressors PAS-N34D and HAMP-C253R are now shown in close proximity (Fig. 5E) and in harmony with genetic studies (Watts et al., 2004). PAS-HAMP crosslinking occurred between cognate subunits within dimers rather than between adjacent dimers because crosslinking was unaffected when collisions between Aer dimers were decreased by the presence of a second receptor (Tar). This is consistent with previous work indicating that HAMP dimers are centrally positioned, flanked by two PAS monomers (Campbell et al., 2011), and are required for PAS folding and FAD binding (Bibikov et al., 2000; Herrmann et al., 2004; Ma et al., 2005; Buron-Barral et al., 2006). Interestingly, an arrangement similar to the Aer PAS β-scaffold-HAMP AS-2 α-helix interface (Fig. 5E) has been described for several proteins including the periplasmic sensing domain of the Sinorhizobium meliloti C4-dicarboxylate sensor DctB (Zhou et al., 2008) and the Vibrio harveyi quorum sensor LuxQ (Neiditch et al., 2006), where in both cases the β-scaffold of two PAS domains abuts a long α-helical spine.
Changes in PAS N-cap orientation during signalling
The PAS N-cap and the loop connecting the N-cap to the PAS core were highly accessible (Fig. 4) yet appear to be part of the Aer signalling pathway. Kinase-on lesions that define the Aer signalling pathway cluster not only on the PAS β-scaffold and in the FAD cleft, but also in the N-cap (Watts et al., 2006b, Campbell et al., 2010). Truncating the first six N-cap residues bestows a kinase-on phenotype, while deleting the first 14 residues yields an inverted response phenotype (Watts et al., 2006b). Aer-L14 is significant in that it likely hydrogen bonds to N34, forming an unusual extended helix that is unbroken in structure, but discontinuous in sequence (Watts et al., 2006b; Key et al., 2007; Etzkorn et al., 2008; Campbell et al., 2010). In DcuS-PASc, an N248D substitution that is equivalent to Aer-N34D reorients the N-cap and activates the protein (Etzkorn et al., 2008). Notably, an N34D substitution in Aer is both kinase-on and an allele-specific suppressor of HAMP-C253R (Watts et al., 2004). The crosslinking data from the current study indicate that N34 and C253 are in close proximity (Fig. 5E), and by homology to DcuS-PASc, the N-cap may reorient when PAS is in the kinase-on state (Fig. 8). In this scenario, N-cap reorientation would help destabilize PAS-HAMP interactions (Fig. 8), giving the HAMP domain more degrees of freedom and dynamic movement.
Changes in HAMP conformation and PAS-HAMP interactions during signalling
We previously found that the kinase-on substitution, PAS-N85S, altered rates of crosslinking between HAMP AS-1 and AS-2′ helices (Watts et al., 2011). Rates decreased at the proximal end of HAMP, and increased at the distal end of HAMP. This was interpreted as either (i) tilting of AS-2 with respect to AS-1′ or (ii) a more relaxed HAMP structure [Fig. 8, (Watts et al., 2011)]. The data from the current study reveal changes in PAS-HAMP interactions that are correlated with these states: the kinase-on substitution (PAS-N85S) exposed a patch of HAMP residues that had been hidden in the kinase-off state by the PAS domain (Fig. 6). This is consistent with a decrease in the strength of the PAS-HAMP AS-2 interaction in the kinase-on state, and a less compact, more dynamic conformation of HAMP. The dynamic conformation is likely to be favored by Aer-HAMP because it lacks strong hydrophobic residues at several key packing positions in the HAMP bundle (Watts et al., 2008; Parkinson, 2010). Still, some HAMP residues apparently remained in contact with PAS in the kinase-on state, because residues in the exposed patch remained less solvent accessible than they were in the PAS-less mutant (compare res. 241, 244 and 245 in Figs. 3C and 6A). However, PAS is effectively tethered to HAMP in the Aer dimer, so some collisional contacts are also expected.
The impact of PAS-N85S on AS-2 accessibility was not uniform. In contrast to AS-2 residues that exhibited increased accessibility with N85S, a patch of residues at the distal end of AS-2 through the proximal signalling domain had decreased accessibility (Fig. 6). The proximal signalling domain links HAMP AS-2 to the kinase control domain and has a helical phase shift between residues 259 and 262; the shift is five residues downstream of the phase stutter at the HAMP-output junction (Brown et al., 1996; Parkinson, 2010). In Aer, the residues with decreased accessibility included not only residues throughout the proximal signalling domain toward the kinase control junction (N269), but also residues upstream, extending to the HAMP junction (C253) (Fig. 6). The proximal signalling domain is also functionally distinguishable from the HAMP domain: missense mutations in the proximal signalling domain do not influence PAS-FAD binding (Ma et al., 2005; Buron-Barral et al., 2006), nor are they suppressed by PAS suppressor lesions that rescue HAMP defects (Watts et al., 2004). This suggests that the proximal signalling domain, unlike HAMP, does not associate with the PAS domain.
The data from the current study are consistent with the dynamic bundle model (Zhou et al., 2009; Parkinson, 2010), although they are not definitive. The broad changes in accessibility associated with signalling (Fig. 6) are more easily explained by dynamics [regulated unfolding, (Schultz and Natarajan, 2013)] than by conformational changes in semi-rigid structures that mimic a specific pattern of changes propagated by the transmembrane helix of a chemoreceptor. In Aer, signalling caused opposite accessibility changes in the HAMP and proximal signalling domains (Figs. 6 and 8). This suggests an inverse structural relationship between these domains, such that when one is compact the other is dynamic. Inverted shifting between compact and dynamic structures has been proposed for the other E. coli chemoreceptors, whereby three contiguous segments have opposite structural transitions: the HAMP domain, the adaptation region and the protein interaction region of the kinase control module (Parkinson, 2010; Falke and Piasta, 2014). The first inversion is associated with a helical phase shift at the juncture between HAMP AS-2 and the adaptation region. This shift couples the helical bundles in opposition, so that increasing the packing stability of one bundle decreases the stability of the other. The second inversion may occur at a glycine hinge that is equivalent to Aer residues G330, G331 and G429. The behavioural output for Aer indicates that the structural state of the protein interaction region is similar to the state of HAMP, as it is in other chemoreceptors (Fig. 8B). Switching to a more static HAMP domain would reverse the states of the other two regions, so the HAMP and protein interaction regions continue to share the same conformational profile [(Falke and Piasta, 2014), Fig. 8].
Recent studies on the Tsr receptor suggest that the HAMP domain acts as a brake that inhibits the default kinase-on state of the receptor. Thus, the HAMP-independent output state of the Tsr kinase control module is kinase-on, and HAMP must actively override this state in response to attractant stimuli (Ames et al., 2014). Given the homology among the Tsr, Tar, and Aer kinase-control modules, the default output for Aer is also likely to be kinase-on, although similar definitive analyses of Aer-HAMP deletions would be confounded by the requirement of HAMP for PAS maturation [(Herrmann et al., 2004; Buron-Barral et al., 2006), J.S. Parkinson, pers. comm.].
Aer signalling model
From the present and previous studies, we propose a complete pathway for Aer-mediated aerotaxis. When E. coli swims into a region where the ambient oxygen concentration cannot maintain the electron transport system, FAD bound to the Aer-PAS domain is reduced and protonated. A hydrogen-bond network linked to FAD is reorganized, resulting in a conformational change in the PAS β-scaffold at the PAS-HAMP AS-2 interface ([Campbell et al., 2010]; see also [Key et al., 2007; Ukaegbu and Rosenzweig, 2009]). Altered PAS-HAMP interactions switch a patch of HAMP surface residues from low accessibility to high accessibility, consistent with decreased affinity between the PAS and HAMP domains. We propose that interactions between oxidized PAS and HAMP AS-2 promote a more ordered (static) HAMP structure and an active kinase-off output, whereas weaker PAS-HAMP interactions in the reduced state allow a more dynamic HAMP structure and kinase-on output (Fig. 8). Aer-HAMP controls the proximal signalling domain, and in turn, the kinase control domain. The tip of the kinase control domain is the protein interaction region, which shares the same relative state as HAMP (dynamic or static) [reviewed by (Falke and Piasta, 2014)]. After the bacteria approach the hypoxic region and FAD is reduced, the cells tumble briefly and swim in a different direction. This avoids anaerobiosis and ensures that cells move towards a higher oxygen concentration. It is the movement up the oxygen gradient that is dominant in determining net migration. In an aerobic environment, FAD becomes oxidized and PAS-HAMP interactions strengthen, resulting in a static HAMP domain and a kinase-off output. The static HAMP domain shifts the bias of the protein interaction region, which inhibits CheA kinase and suppresses changes in swimming direction.
The model for direct signalling between the PAS and HAMP domains of Aer presents a new paradigm for controlling HAMP states. The paradigm suggests that signal-sensitive, intradimeric contacts between PAS and HAMP AS-2 controls the static or dynamic nature of HAMP. This mechanism likely occurs in other proteins that have laterally interacting PAS and HAMP domains, and may be the mechanism by which other sensing domains can control HAMP activity in a wide variety of systems.
Experimental procedures
Bacterial strains and plasmids
Cysteine-less (C-less) Aer (Aer-C193S/C203A/C253A) was expressed from pMB1 (Ma et al., 2004; Watts et al., 2006b), a pTrc99A-derivative that expresses Aer under the control of an IPTG-inducible ptrc promoter. All Aer-Cys mutants in this study were derived from pMB1. The WT Tar expression plasmid, pLC113, was a gift from John S. Parkinson and is a pACYC184-based plasmid that confers chloramphenicol resistance and carries a sodium salicylate-inducible promoter (Ames and Parkinson, 2006). Plasmids were expressed in E. coli BT3312, a strain that lacks the two aerotaxis receptors, Aer and Tsr [Δaer-1 Δtsr-7021, (Repik et al., 2000] or in chemoreceptorless BT3388 [aer:: erm Δtsr-7021 Δtar-tap-5201 trg::Tn10) (Yu et al., 2002)].
Mutant construction
A library of Aer mutants with single cysteine substitutions between residues 206 and 275 was previously constructed in pMB1 (Amin et al., 2006; Watts et al., 2008). Aer-N85S and additional Aer-Cys mutants were constructed by site-directed mutagenesis of pMB1 or pMB1-derived plasmids according to the instructions of the QuikChange® II site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). The N85S codon was introduced into plasmids that contained a single Cys codon substitution, whereas di-Cys mutants were created from a pMB1-derivative that expressed either Aer-Q248C or Aer-L251C. Aer[112–506] mutants were created by amplifying the coding region for residues 112–506 from individual pMB1-derived plasmids using primers containing recognition sequences for AflIII and SalI, and ligating the products into pTrc99A with the NcoI-SalI DNA segment removed. Mutations were confirmed by sequencing the entire aer gene of each plasmid.
Expression and aerotaxis assays
Plasmids were introduced into BT3312 (aer tsr) and Aer production was confirmed by Western blotting with a 1 in 133,000 dilution of anti-Aer2-166 antisera (Repik et al., 2000). Aer[112–506] mutants express stable protein (K.K. Gosink and J.S. Parkinson, pers. comm.), but have fewer epitopes than full-length Aer, so were detected with a 1 in 50,000 dilution of anti-Aer2-166 antisera. For Aer/Tar co-expression assays, Aer and Tar expression plasmids were introduced into BT3388 (aer tsr tar trg tap) and expressed as described (Campbell et al., 2011). Tar expression was confirmed using a 1 in 10,000 dilution of anti-Tsr290-470 antisera [a gift from J. S. Parkinson, (Ames and Parkinson, 1994)]. Aerotaxis phenotypes were determined for each Aer mutant by inoculating cells into succinate minimal soft agar containing 50 μg ml−1 ampicillin, incubating the plates at 30°C for 15–20 h, and then observing colony morphologies (Taylor et al., 2007).
In vivo accessibility assays using PEG-mal
In vivo PEGylation assays were performed using an unpublished permeabilized cell protocol developed by Claudia A. Studdert at the Universidad Nacional de Mar del Plata, Argentina, but modified in this study to work optimally for Aer. BT3312 cells expressing each of the Aer-Cys mutants were grown at 30°C to mid-log phase in tryptone broth containing 100 μg ml−1 ampicillin, and induced for 3 h with 50 μM IPTG. Aer-P211C, Aer-R235C and Aer-G240C have lower steady-state accumulation levels (Watts et al., 2008), as did Aer-R57C, Aer-D60C and Aer-A97C (this study) and were induced with 100 μM IPTG. Two milliliters of each culture was centrifuged at 10,000 × g in each of two tubes (corresponding to denatured and native samples), then washed twice with 20 mM potassium phosphate [pH 7.0], 0.1 mM EDTA, and 0.1 mM MgCl2 buffer. The cells in each tube were resuspended in 1 ml of wash buffer and perme-abilized by adding 50 μl of 1:4 toluene:ethanol. After 15 min of vigorous mixing at room temperature on an Eppendorf mixer (Eppendorf model 5432, Hauppauge, NY), the cells were centrifuged, the supernatant and excess fluid were removed, and the pellets were resuspended in 50 μl of wash buffer. Five mM PEG-mal (Laysan Bio, Arab, AL) was thoroughly mixed with the cells, and the tubes were incubated at 25°C for 15 min. PEGylation reactions were stopped by adding 100 μl of sample buffer with excess β-mercaptoethanol (native samples, 1.43 M β-mercaptoethanol) or with no reducing agent (denatured samples). The samples were then boiled for four min and analyzed by SDS-PAGE. A quenching control, in which 1.43 M β-mercaptoethanol was added before PEG-mal, efficiently quenched the PEGylation reaction (see Fig. 2). Bands were visualized on Western blots and quantified on a Bio-Spectrum® digital imager (UVP, Upland, CA). For each residue, the proportion of PEGylated product was calculated by dividing the average density of the PEGylated band by the average densities of the non-PEGylated plus PEGylated fractions from duplicate lanes. Reactions were repeated on at least two, but usually three or more, occasions. To compare extents of PEGylation in the presence and absence of the PAS domain or PAS-N85S, statistical analyses were carried out using a two-tailed Student’s t-test. A P value of less than 0.05 was considered statistically significant.
In vivo disulfide crosslinking
BT3312 cells expressing each of the di-Cys and corresponding single-Cys mutants were grown to mid-log phase in H1 minimal salts medium supplemented with 30 mM succinate, 0.1% (w/v) casamino acids and 100 μg ml−1 ampicillin, before being induced for 3 h with 50 μM IPTG. Crosslinking was performed at 25°C by exposing whole cells to 600 μM Cu(II)(1,10-phenanthroline)3 (CuPhe) for 20 min, similar to that described previously (Hughson and Hazelbauer, 1996; Amin et al., 2006; Watts et al., 2008), but with modifications as described in (Lai and Hazelbauer, 2007; Taylor et al., 2007). The following 25 PAS-Cys mutants were tested for crosslinking with Q248C in di-Cys receptors: T19C, M21C, T23C, H32C, N34C, D35C, T36C, V38C, L45C, M55C, K75C, P78C, S80C, I82C, K88C, N89C, R96C, N98C, V100C, V103C, I108C, M112C, I114C, A118C and E121C. PAS-Cys subsets were also tested for their ability to crosslink with R244C, G250C, M252C, C253, R254C and D259C. Aer-I114C/Q248C and Aer-E105C were also analyzed in BT3388, with Aer as the sole receptor, or in the presence of WT Tar (as expressed from pLC113) with no induction or with 1.2 μM sodium salicylate induction. Crosslinked products were separated from monomers by SDS-PAGE and quantified on the UVP digital imager after Western blotting. Percent crosslinking was calculated by dividing the intensity of the crosslinked dimer band by the sum of the intensities of the monomer and dimer bands, multiplied by 100. Aer-V260C (Watts et al., 2008) and C-less Aer (Ma et al., 2004) were used as positive and negative crosslinking controls, respectively. Dimer bands were never evident with C-less Aer, whereas the extent of dimerization for Aer-V260C was routinely ~65% after 20 min. Reactions were repeated on two or more occasions.
In silico modeling
Aer PAS and HAMP domain models were previously created from the coordinates of NifL PAS (2GJ3) and Af1503 HAMP (2ASX), respectively (Watts et al., 2008; Campbell et al., 2010). PyMOL viewer (The PyMOL Molecular Graphics System, Version 1.0, Schrödinger, LLC) was used to manipulate models, map experimental data and determine surface areas. The experimental results were used as a guide to manually manipulate the positions of the PAS and HAMP domains in an Aer dimer to obtain the best fit (Figs. 5 and 8).
Supplementary Material
Acknowledgments
We would like to thank John S. Parkinson for helpful suggestions and for providing a Tar expression plasmid and anti-Tsr antisera, Claudia A. Studdert for helpful suggestions and for communicating an unpublished PEGylation protocol, Lauren A. Abraham for technical assistance, and Jennifer Ngo for constructing and testing several PAS-Cys mutants. This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers RO1 GM029481 (to B.L.T.) and 2 R25 GM060507 (for support of D.G.). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Airola MV, Sukomon N, Samanta D, Borbat PP, Freed JH, Watts KJ, Crane BR. HAMP domain conformers that propagate opposite signals in bacterial chemoreceptors. PLoS Biol. 2013;11:e1001479. doi: 10.1371/journal.pbio.1001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airola MV, Watts KJ, Bilwes AM, Crane BR. Structure of concatenated HAMP domains provides a mechanism for signal transduction. Structure. 2010;18:436–448. doi: 10.1016/j.str.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RP, Zhulin IB. Evolutionary genomics reveals conserved structural determinants of signaling and adaptation in microbial chemoreceptors. Proc Natl Acad Sci USA. 2007;104:2885–2890. doi: 10.1073/pnas.0609359104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames P, Parkinson JS. Constitutively signaling fragments of Tsr, the Escherichia coli serine chemoreceptor. J Bacteriol. 1994;176:6340–6348. doi: 10.1128/jb.176.20.6340-6348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames P, Parkinson JS. Conformational suppression of inter-receptor signaling defects. Proc Natl Acad Sci USA. 2006;103:9292–9297. doi: 10.1073/pnas.0602135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames P, Zhou Q, Parkinson JS. HAMP domain structural determinants for signalling and sensory adaptation in Tsr, the Escherichia coli serine chemoreceptor. Mol Microbiol. 2014;91:875–886. doi: 10.1111/mmi.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin DN, Taylor BL, Johnson MS. Topology and boundaries of the aerotaxis receptor Aer in the membrane of Escherichia coli. J Bacteriol. 2006;188:894–901. doi: 10.1128/JB.188.3.894-901.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravind L, Ponting CP. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol Lett. 1999;176:111–116. doi: 10.1111/j.1574-6968.1999.tb13650.x. [DOI] [PubMed] [Google Scholar]
- Bass RB, Butler SL, Chervitz SA, Gloor SL, Falke JJ. Use of site-directed cysteine and disulfide chemistry to probe protein structure and dynamics: applications to soluble and transmembrane receptors of bacterial chemotaxis. Methods Enzymol. 2007;423:25–51. doi: 10.1016/S0076-6879(07)23002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikov SI, Barnes LA, Gitin Y, Parkinson JS. Domain organization and flavin adenine dinucleotide-binding determinants in the aerotaxis signal transducer Aer of Escherichia coli. Proc Natl Acad Sci USA. 2000;97:5830–5835. doi: 10.1073/pnas.100118697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikov SI, Biran R, Rudd KE, Parkinson JS. A signal transducer for aerotaxis in Escherichia coli. J Bacteriol. 1997;179:4075–4079. doi: 10.1128/jb.179.12.4075-4079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikov SI, Miller AC, Gosink KK, Parkinson JS. Methylation-independent aerotaxis mediated by the Escherichia coli Aer protein. J Bacteriol. 2004;186:3730–3737. doi: 10.1128/JB.186.12.3730-3737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Cohen C, Parry DA. Heptad breaks in alpha-helical coiled coils: stutters and stammers. Proteins. 1996;26:134–145. doi: 10.1002/(SICI)1097-0134(199610)26:2<134::AID-PROT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Buron-Barral MDC, Gosink KK, Parkinson JS. Loss- and gain-of-function mutations in the F1-HAMP region of the Escherichia coli aerotaxis transducer Aer. J Bacteriol. 2006;188:3477–3486. doi: 10.1128/JB.188.10.3477-3486.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SL, Falke JJ. Cysteine and disulfide scanning reveals two amphiphilic helices in the linker region of the aspartate chemoreceptor. Biochemistry. 1998;37:10746–10756. doi: 10.1021/bi980607g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AJ, Watts KJ, Johnson MS, Taylor BL. Gain-of-function mutations cluster in distinct regions associated with the signalling pathway in the PAS domain of the aerotaxis receptor, Aer. Mol Microbiol. 2010;77:575–586. doi: 10.1111/j.1365-2958.2010.07231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AJ, Watts KJ, Johnson MS, Taylor BL. Role of the F1 region in the Escherichia coli aerotaxis receptor Aer. J Bacteriol. 2011;193:358–366. doi: 10.1128/JB.01028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunin-Horkawicz S, Lupas AN. Comprehensive analysis of HAMP domains: implications for transmembrane signal transduction. J Mol Biol. 2010;397:1156–1174. doi: 10.1016/j.jmb.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Etzkorn M, Kneuper H, Dunnwald P, Vijayan V, Kramer J, Griesinger C, et al. Plasticity of the PAS domain and a potential role for signal transduction in the histidine kinase DcuS. Nat Struct Mol Biol. 2008;15:1031–1039. doi: 10.1038/nsmb.1493. [DOI] [PubMed] [Google Scholar]
- Falke JJ, Piasta KN. Architecture and signal transduction mechanism of the bacterial chemosensory array: progress, controversies, and challenges. Curr Opin Struct Biol. 2014;29C:85–94. doi: 10.1016/j.sbi.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris HU, Dunin-Horkawicz S, Mondejar LG, Hulko M, Hantke K, Martin J, et al. The mechanisms of HAMP-mediated signaling in transmembrane receptors. Structure. 2011;19:378–385. doi: 10.1016/j.str.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Gosink KK, del Carmen Buron-Barral M, Parkinson JS. Signaling interactions between the aerotaxis transducer Aer and heterologous chemoreceptors in Escherichia coli. J Bacteriol. 2006;188:3487–3493. doi: 10.1128/JB.188.10.3487-3493.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer GL, Lai WC. Bacterial chemoreceptors: providing enhanced features to two-component signaling. Curr Opin Microbiol. 2010;13:124–132. doi: 10.1016/j.mib.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann S, Ma Q, Johnson MS, Repik AV, Taylor BL. PAS domain of the Aer redox sensor requires C-terminal residues for native-fold formation and flavin adenine dinucleotide binding. J Bacteriol. 2004;186:6782–6791. doi: 10.1128/JB.186.20.6782-6791.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson AG, Hazelbauer GL. Detecting the conformational change of transmembrane signaling in a bacterial chemoreceptor by measuring effects on disulfide cross-linking in vivo. Proc Natl Acad Sci USA. 1996;93:11546–11551. doi: 10.1073/pnas.93.21.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulko M, Berndt F, Gruber M, Linder JU, Truffault V, Schultz A, et al. The HAMP domain structure implies helix rotation in transmembrane signaling. Cell. 2006;126:929–940. doi: 10.1016/j.cell.2006.06.058. [DOI] [PubMed] [Google Scholar]
- Key J, Hefti M, Purcell EB, Moffat K. Structure of the redox sensor domain of Azotobacter vinelandii NifL at atomic resolution: signaling, dimerization, and mechanism. Biochemistry. 2007;46:3614–3623. doi: 10.1021/bi0620407. [DOI] [PubMed] [Google Scholar]
- Klose D, Voskoboynikova N, Orban-Glass I, Rickert C, Engelhard M, Klare JP, Steinhoff HJ. Light-induced switching of HAMP domain conformation and dynamics revealed by time-resolved EPR spectroscopy. FEBS Lett. 2014;588:3970–3976. doi: 10.1016/j.febslet.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Krell T, Lacal J, Munoz-Martinez F, Reyes-Darias JA, Cadirci BH, Garcia-Fontana C, Ramos JL. Diversity at its best: bacterial taxis. Environ Microbiol. 2011;13:1115–1124. doi: 10.1111/j.1462-2920.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- Lai RZ, Parkinson JS. Functional suppression of HAMP domain signaling defects in the E. coli serine chemoreceptor. J Mol Biol. 2014;426:3642–3655. doi: 10.1016/j.jmb.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WC, Hazelbauer GL. Analyzing transmembrane chemoreceptors using in vivo disulfide formation between introduced cysteines. Methods Enzymol. 2007;423:299–316. doi: 10.1016/S0076-6879(07)23013-7. [DOI] [PubMed] [Google Scholar]
- Lu J, Deutsch C. Pegylation: a method for assessing topological accessibilities in Kv1.3. Biochemistry. 2001;40:13288–13301. doi: 10.1021/bi0107647. [DOI] [PubMed] [Google Scholar]
- Ma Q, Johnson MS, Taylor BL. Genetic analysis of the HAMP domain of the Aer aerotaxis sensor localizes flavin adenine dinucleotide-binding determinants to the AS-2 helix. J Bacteriol. 2005;187:193–201. doi: 10.1128/JB.187.1.193-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Roy F, Herrmann S, Taylor BL, Johnson MS. The Aer protein of Escherichia coli forms a homodimer independent of the signaling domain and FAD binding. J Bacteriol. 2004;186:7456–7459. doi: 10.1128/JB.186.21.7456-7459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechaly AE, Sassoon N, Betton JM, Alzari PM. Segmental helical motions and dynamical asymmetry modulate histidine kinase autophosphorylation. PLoS Biol. 2014;12:e1001776. doi: 10.1371/journal.pbio.1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondejar LG, Lupas A, Schultz A, Schultz JE. HAMP domain-mediated signal transduction probed with a Mycobacterial adenylyl cyclase as a reporter. J Biol Chem. 2012;287:1022–1031. doi: 10.1074/jbc.M111.284067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu JR, Lewis JO, Wharton KA, Jr, Crews ST. The Drosophila single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline development. Cell. 1991;67:1157–1167. doi: 10.1016/0092-8674(91)90292-7. [DOI] [PubMed] [Google Scholar]
- Neiditch MB, Federle MJ, Pompeani AJ, Kelly RC, Swem DL, Jeffrey PD, et al. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JS. Signaling mechanisms of HAMP domains in chemoreceptors and sensor kinases. Annu Rev Microbiol. 2010;64:101–122. doi: 10.1146/annurev.micro.112408.134215. [DOI] [PubMed] [Google Scholar]
- Parkinson JS, Hazelbauer GL, Falke JJ. Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update. Trends Microbiol. 2015;23:257–266. doi: 10.1016/j.tim.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbapragada A, Johnson MS, Harding GP, Zuccarelli AJ, Fletcher HM, Zhulin IB, Taylor BL. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc Natl Acad Sci USA. 1997;94:10541–10546. doi: 10.1073/pnas.94.20.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repik A, Rebbapragada A, Johnson MS, Haznedar JO, Zhulin IB, Taylor BL. PAS domain residues involved in signal transduction by the Aer redox sensor of Escherichia coli. Mol Microbiol. 2000;36:806–816. doi: 10.1046/j.1365-2958.2000.01910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JE, Natarajan J. Regulated unfolding: a basic principle of intraprotein signaling in modular proteins. Trends Biochem Sci. 2013;38:538–545. doi: 10.1016/j.tibs.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Swain KE, Falke JJ. Structure of the conserved HAMP domain in an intact, membrane-bound chemoreceptor: a disulfide mapping study. Biochemistry. 2007;46:13684–13695. doi: 10.1021/bi701832b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain KE, Gonzalez MA, Falke JJ. Engineered socket study of signaling through a four-helix bundle: evidence for a yin-yang mechanism in the kinase control module of the aspartate receptor. Biochemistry. 2009;48:9266–9277. doi: 10.1021/bi901020d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BL. Aer on the inside looking out: paradigm for a PAS-HAMP role in sensing oxygen, redox and energy. Mol Microbiol. 2007;65:1415–1424. doi: 10.1111/j.1365-2958.2007.05889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BL, Watts KJ, Johnson MS. Oxygen and redox sensing by two-component systems that regulate behavioral responses: behavioral assays and structural studies of Aer using in vivo disulfide cross-linking. Methods Enzymol. 2007;422:190–232. doi: 10.1016/S0076-6879(06)22010-X. [DOI] [PubMed] [Google Scholar]
- Taylor BL, Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukaegbu UE, Rosenzweig AC. Structure of the redox sensor domain of Methylococcus capsulatus (Bath) MmoS. Biochemistry. 2009;48:2207–2215. doi: 10.1021/bi8019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Sang J, Wang J, Su M, Downey JS, Wu Q, et al. Mechanistic insights revealed by the crystal structure of a histidine kinase with signal transducer and sensor domains. PLoS Biol. 2013;11:e1001493. doi: 10.1371/journal.pbio.1001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sasaki J, Tsai AL, Spudich JL. HAMP domain signal relay mechanism in a sensory rhodopsin-transducer complex. J Biol Chem. 2012;287:21316–21325. doi: 10.1074/jbc.M112.344622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts KJ, Johnson MS, Taylor BL. Minimal requirements for oxygen sensing by the aerotaxis receptor Aer. Mol Microbiol. 2006a;59:1317–1326. doi: 10.1111/j.1365-2958.2005.05012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts KJ, Johnson MS, Taylor BL. Structure-function relationships in the HAMP and proximal signaling domains of the aerotaxis receptor Aer. J Bacteriol. 2008;190:2118–2127. doi: 10.1128/JB.01858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts KJ, Johnson MS, Taylor BL. Different conformations of the kinase-on and kinase-off signaling states in the Aer HAMP domain. J Bacteriol. 2011;193:4095–4103. doi: 10.1128/JB.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts KJ, Ma Q, Johnson MS, Taylor BL. Interactions between the PAS and HAMP domains of the Escherichia coli aerotaxis receptor Aer. J Bacteriol. 2004;186:7440–7449. doi: 10.1128/JB.186.21.7440-7449.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts KJ, Sommer K, Fry SL, Johnson MS, Taylor BL. Function of the N-terminal cap of the PAS domain in signaling by the aerotaxis receptor Aer. J Bacteriol. 2006b;188:2154–2162. doi: 10.1128/JB.188.6.2154-2162.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuichet K, Alexander RP, Zhulin IB. Comparative genomic and protein sequence analyses of a complex system controlling bacterial chemotaxis. Methods Enzymol. 2007;422:1–31. doi: 10.1016/S0076-6879(06)22001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Saw JH, Hou S, Larsen RW, Watts KJ, Johnson MS, Zimmer MA, Ordal GW, Taylor BL, Alam M. Aerotactic responses in bacteria to photore-leased oxygen. FEMS Microbiol Lett. 2002;217:237–242. doi: 10.1111/j.1574-6968.2002.tb11481.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Ames P, Parkinson JS. Mutational analyses of HAMP helices suggest a dynamic bundle model of input-output signalling in chemoreceptors. Mol Microbiol. 2009;73:801–814. doi: 10.1111/j.1365-2958.2009.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Ames P, Parkinson JS. Biphasic control logic of HAMP domain signalling in the Escherichia coli serine chemoreceptor. Mol Microbiol. 2011;80:596–611. doi: 10.1111/j.1365-2958.2011.07577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YF, Nan B, Nan J, Ma Q, Panjikar S, Liang YH, Wang Y, Su XD. C4-dicarboxylates sensing mechanism revealed by the crystal structures of DctB sensor domain. J Mol Biol. 2008;383:49–61. doi: 10.1016/j.jmb.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Zhulin IB. The superfamily of chemotaxis transducers: from physiology to genomics and back. Adv Microb Physiol. 2001;45:157–198. doi: 10.1016/s0065-2911(01)45004-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.