Abstract
More than half of all new lung cancer diagnoses are made in patients with locally advanced or metastatic disease, at which point therapeutic options are scarce. It is anticipated, however, that the widespread use of Low-Dose Computed Tomography (LDCT) screening, will lead to a greater proportion of lung cancers being diagnosed at an early, operable, stage. Still, the overall rate of recurrence for surgically treated Stage I lung cancer patients is up to 30% within 5 years of diagnosis. Thus, the identification and clinical application of biomarkers of early stage lung cancer is a pressing medical need. The integrative analysis of “omic,” clinical and epidemiological data for single patients is a core principle of precision medicine. Through rigorous bioinformatics and statistical analyses we have identified biomarkers of early-stage lung cancer based on DNA methylation, expression of mRNA and miRNA, inflammatory cytokines, and urinary metabolites. Beyond a more comprehensive understanding of the molecular taxonomy of lung cancer, these biomarkers can have very practical implications in the context of unmet clinical needs of early stage lung cancer patients: First, current guidelines for LDCT screening broadly include individuals based on age and history of heavy smoking. Tumor-derived circulating biomarkers in the blood and urine associated with lung cancer risk could narrow and prioritize individuals for LDCT screening. Second, a high number of nodules are identified by LDCT, of which fewer than 5% are finally diagnosed as lung cancer. Biomarkers may help discriminate malignant nodules from benign or indolent lesions. Third, the expected rise in the numbers of lung cancer patients diagnosed at an early stage will necessitate new treatment options. Circulating, urinary and tissue-based biomarkers that molecularly categorize Stage I patients after tumor resection can help identify high-risk patients who may benefit from adjuvant chemotherapy or innovative immunotherapy regimens.
Keywords: Low-Dose Computed Tomography, cytokine, metabolomics, methylation, gene expression, microRNA
Recent advances in Lung Cancer detection reveal unmet clinical needs
Lung cancer remains the leading cause of cancer-associated deaths worldwide, despite a slow but continuous decline in incidence and mortality in the US and other Western countries over the past two decades [1, 2]. Global variations in lung cancer incidence largely follow historical patterns of smoking, and incidence and mortality rates are still on the rise in Asia and some countries in Latin America and Africa, where the smoking epidemic began later [2]. Most lung cancer patients are diagnosed with locally advanced or metastatic disease, with few therapeutic options and a dismal survival rate. The introduction of innovative therapies targeted to specific molecular alterations continues to improve outcomes for a subset of patients with advanced stage lung cancer [3]. In addition, recent promising results of T cell-based immunotherapy associated high mutational burden in lung cancer patients suggest that exome-guided neoantigen identification may improve treatment responses [4].
When diagnosed at an early, operable stage, the 5-year survival rate from lung cancer climbs above 50% [1]. Cigarette smoking is the major risk factor for lung cancer and other smoking-related diseases [5]. Even as this risk gradually decreases after smoking cessation, former smokers account for most new lung cancer diagnoses. Thus, lung cancer screening efforts have focused on older individuals with a history of heavy smoking. The results of the landmark National Lung Screening Trial (NLST) published in 2011 [6], demonstrated a statistically significant mortality benefit of low-dose computed tomography (LDCT) over chest radiography (CXR) screening in high-risk individuals (defined by age and history of heavy smoking). This evidence and a systematic review of LDCT screening studies [7], prompted the American Cancer Society and other health care organizations to issue guidelines for clinicians to discuss lung cancer screening by LDCT with older patients with a history of heavy smoking, along with smoking-cessation counseling [8, 9].
In early 2015, the Centers for Medicare and Medicaid Services (CMS) made public a decision to cover the cost of lung cancer screening with LDCT for patients at high-risk according to guidelines similar to NSLT eligibility criteria [10]. As a result, the use of LDCT screening is expanding, creating a need for prioritization of the over 9 million individuals who would be eligible for screening under the current guidelines [11]. Non-invasive circulating or urinary biomarkers associated with lung cancer risk, i.e., tumor-derived metabolites, could help prioritize individuals for LDCT screening among those at large high-risk to increase the efficacy of screening and to reduce the cost and morbidity associated with it (Risk biomarkers, Figure 1). The magnitude of the task is compounded by the fact that LDCT scanning identifies a high number of nodules that prompt further, invasive testing but do not result in a lung cancer diagnosis. In the NLST, 96.4% of initial positive screenings were deemed non-cancer on further testing [6]. A recent retrospective study of the clinical management of patients with Intermediate Pulmonary Nodules (IPNs, 8-20 mm) in community practice, found wide variation in nodule management that led to a high number of unnecessary invasive procedures [12]. Thus, there is a need for non-invasive biomarkers that can help discriminate malignant nodules from benign or indolent lesions (Diagnostic biomarkers, Figure 1). Up to 60% of lung cancers detected by LDCT in the NLST were Stage I, primarily adenocarcinoma histology [6]. With the projected rise in LDCT screening [13], a shift in the stage at diagnosis, towards early, operable disease is expected. The recommended treatment for patients with Stage I Non-small-cell lung cancer (NSCLC) is surgery, which may be followed by chemotherapy in patients with pathologically high-risk, margin-negative Stage IB tumors [14]. Still, up to 30% surgically-treated Stage I patients will die from recurrent disease [15]. Non-invasive or tissue-based biomarkers that molecularly categorize Stage I patients after tumor resection and identify those at high-risk for recurrence could lead to improved clinical management (Prognostic biomarkers, Figure 1). High-risk patients may benefit from adjuvant chemotherapy or innovative checkpoint immunotherapy, while low-risk patients might safely be spared further treatment and instead be followed by surveillance LDCT. In summary, we will discuss three critical needs in early stage lung cancer: 1. To prioritize high-risk individuals for screening by LDCT (screening); 2. To assess the malignant potential of IPNs to reduce overdiagnosis and unnecessary surgery (diagnosis); 3. To identify Stage I patients at high risk of recurrence (prognosis).
Figure 1.
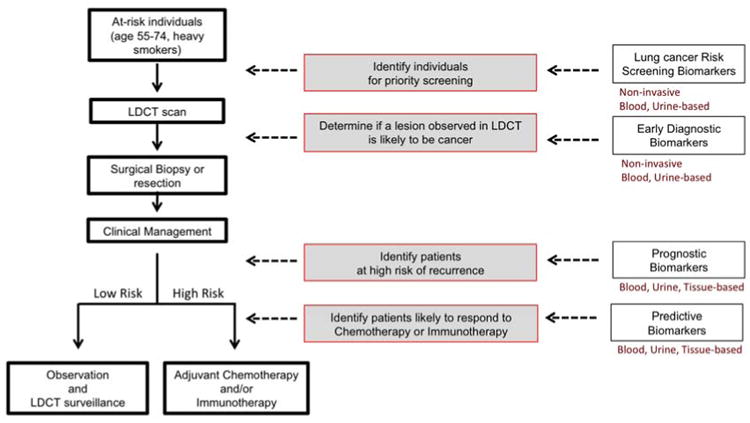
Early stage lung cancer unmet needs. Screening, diagnostic, prognostic and predictive biomarkers can inform clinical decisions.
Biomarkers in Precision Medicine
Biomarker discovery and validation are main components of the precision medicine strategy (Figure 2). The precision medicine approach, first outlined in the 2011 report from the National Research Council [16], includes four basic premises. First, a disease Information Commons is populated with comprehensive measurements of various types of molecules from individual patients (collectively referred to as “-omic” data). This multi-layer reservoir of molecular data may include global analysis of the exposome, genome, epigenome, transcriptome, metabolome, proteome and microbiome, as well as clinical and epidemiological information. Second, these data are integrated into a Knowledge Network that examines the interconnectivity across data layers from the Information Commons. Third, this Knowledge Network is used to develop new molecular classifications of disease. The ultimate goal of these new Taxonomic Classifiers is to refine risk assessment, more precisely diagnose patients, and make informed decisions on therapeutic strategies. Finally, this knowledge is used to inform biomedical research, preventive care, and clinical medicine and to fuel relevant mechanistic and observational studies. Progress is based on iterative process of acquiring information in individuals or cohorts of patients, making improvements in taxonomy and utilizing that knowledge to care for patients and design new studies that further feed the Information Commons. This approach has come to the forefront with the recent announcement of the oncology “precision medicine” research initiative by the National Institutes of Health [17]. The just-launched NCI-MATCH (Molecular Analysis for Therapy Choice) trial is an example of a precision oncology clinical trial that aims to evaluate the extent to which treating cancers according to their molecular abnormalities will be able to improve patient outcome [18]. The Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial (ALCHEMIST) [19] applies this concept to the treatment of patients with early-stage non-squamous NSCLC, while feeding back into the Information Commons through comprehensive genomic analysis.
Figure 2.
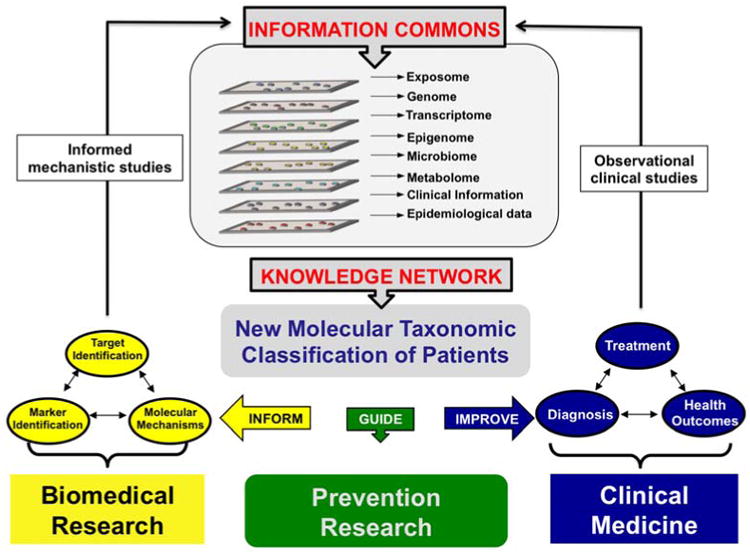
A Precision Medicine research strategy. Modified from Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease [16]. Precision Medicine starts with the creation of an Information Commons that interactively houses multiple “-omics” data types along with historical exposure and lifestyle information from individual patients. Bioinformatic integration of these data will lead to the development of a Knowledge Network that will be used to improve disease taxonomy, the application of clinical medicine and the study of molecular mechanisms of disease. An iterative process of acquiring information on individuals or cohorts of patients, making improvements in taxonomy and utilizing that knowledge to care for patients and design new studies that further feed the Information Commons will refine the molecular taxonomic classifiers and improve clinical medicine.
Although the field of biomarker research has for years operated within a similar framework as outlined by the precision medicine approach, the more recent focus on high-dimensional “-omic” data as a source for disease markers has brought with it a new set of analytical and clinical validation challenges [20]. As the number of features measured greatly exceeds the number of samples, analytical strategies have had to evolve to avoid over-fitting of the models and ensure that biomarkers are widely applicable beyond the sample cohort used to generate them. A set of recommendations has been put forward by the Institute of Medicine to guide the translation of omics-based biomarkers into clinical tests [21]. At the Discovery Phase, biomarkers should be confirmed in a set of samples that is independent from that in which the original discovery was made. The primary data and computational procedures should be fully disclosed and the derived algorithms should be defined precisely. At the Validation Phase, it is recommended that the candidate omics-based test and its intended clinical use be discussed with the US Food and Drug Administration (FDA) and that validations are conducted under Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory standards. Evaluation for clinical utility requires close consultation with FDA and a fully defined, validated and locked-down test [21]. To date, no molecular biomarkers that identify individuals for priority screening or facilitate the assessment of IPNs or prospectively identify lung cancer patients at a high risk of recurrence after surgery have been successfully translated into clinical use, although several are at advanced stages of development [22-33].
A strategy to identify OMICS-based biomarkers with potential clinical utility
A strategy for biomarker discovery and validation is outlined in Figure 3. Briefly, a cohort (nested case-control or case series) of sufficient size and with well-curated epidemiological and clinical data is the starting point for comprehensive profiling studies. Measures of association with presence of disease or disease outcome are utilized to select candidate biomarkers. This rigorous assessment requires evidence for statistically significant risk separation as well as improved predictive value over known risk factors, including age and smoking. A candidate biomarker that passes this threshold is then validated internally in the same patient cohort using a secondary, targeted assay, such as quantitative RT-PCR (qRT-PCR) or pyrosequencing, to avoid platform-specific biases. This step also allows the development of an assay that may be more easily adaptable to a large number of samples, compared to the initial comprehensive profiling platform. When possible, we recommend focusing on assays that can be developed within CLIA standards, with a vision of future clinical deployment. To demonstrate robustness, the biomarker is further evaluated in at least one completely independent cohort. Selection of this cohort also takes into account important factors associated with lung cancer that may affect the broad applicability of the biomarker, such as patient ethnicity and history of smoking. A fully specified assay and associated computational procedures emerges from this validation and is ready for further evaluation in other cohorts, such as publicly-available microarray cohorts, in the case of gene expression-based signatures, or prospectively collected samples, for biomarkers of risk. Ultimately, the clinical utility should be tested in the context of a prospective clinical trial.
Figure 3.
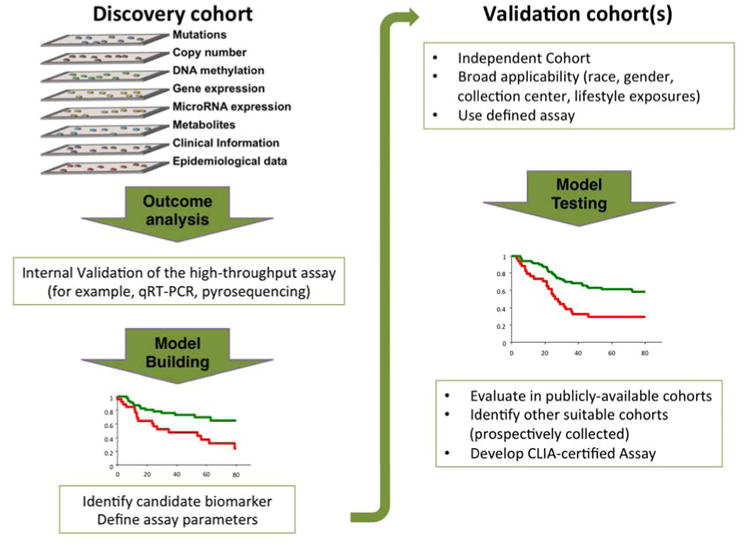
A strategy to identify biomarkers with clinical utility.
Based on the unmet needs for management of early stage lung cancer patients, we have investigated non-invasive, blood- and urine-based biomarkers as well as tissue-based biomarkers (Table 1). These encompass various types of molecular data for single patients. When combined, they may aid patient stratification into risk categories and for cancer diagnosis and treatment, and generate hypothesis that illuminate biology.
Table 1. Biomarkers of early stage lung cancer risk, diagnosis and prognosis.
| Specimen | Training set | Validation set | References | |
|---|---|---|---|---|
| Prognostic (Case Studies) | ||||
| mRNA-4gene | RNA (frozen tissue) | Japan (n=149) | NCI-MD/Norway (n=67) | [43, 44] |
| miRNA | RNA (frozen tissue) | Japan (n=149) | NCI-MD/Norway (n=67) | [42, 43] |
| Methylation | DNA (frozen tissue) | NCI-MD/Norway (n=99) | Japan (n=113) | [45] |
| Metabolomic | Urine | NCI-MD (n=469) | Study ongoing | [38] |
| Cytokine | Serum | NCI-MD (n=67) | PLCOa (n=548) | [34, 36] |
| Diagnostic (Case-Control Studies) | ||||
| Metabolomic | Urine | NCI-MD (n=1,005) | SCCSb (n=534) | [38, 39] |
| Cytokine | Serum | NCI-MD (n=566) | PLCOc (n=1127) | [35, 37] |
| Risk (Prospective Studies) | ||||
| Metabolomic | Urine | SCCS (n=534) | [39] | |
| Cytokine | Serum | PLCOc (n=1127) | [35, 37] | |
Case series nested within prospective Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
Case-control series nested within prospective Southern Community Cohort Study (SCCS).
Case-control series nested within PLCO Cancer Screening Trial.
Cytokines
Together with our collaborators, we have investigated whether circulating markers of inflammation, such as pro-inflammatory cytokines and C-reactive protein, are predictors of lung cancer diagnosis and prognosis [34-37]. Discovered in the NCI-MD case-control study and validated in the NCI's Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial, increased levels of IL-6, CRP and IL-8 were associated with lung cancer diagnosis. Significantly, IL-8 levels were elevated up to 5 years before lung cancer diagnosis, suggesting their potential use in screening [35]. Moreover, a combined IL-6 and IL-8 prognostic classifier was associated with poor outcome in stage I lung cancer patients. Using the PLCO prospective sample cohort, we investigated the relationship between serum cytokines and risk of mortality. Combining IL-8 and IL-6 was a superior prognostic classifier than IL-8 or IL-6 alone. In addition, this classifier was associated with survival in stage I lung cancer patients, and in those with ≥ 30 pack-years of smoking (the relevant patient demographic targeted by LDCT screening) [36]. Specific inflammatory profiles were also associated with risk of lung cancer in African Americans and in European Americans [37]. This latest study underscores the fact that risk prediction models may need to account for racial and other differences in biomarker profile.
Metabolomics
Global and targeted metabolomics studies using mass spectrometry in > 1,000 urine samples from the NCI-MD case-control study, uncovered a set of urine metabolites associated with lung cancer diagnosis and prognosis [38]. Novel and previously un-annotated creatine riboside (CR), and N-acetylneuraminic acid (NANA), were each significantly elevated in the urine of lung cancer patients and associated with worse prognosis. Results were validated in an independent sample set comprising more recently diagnosed cases and further confirmed by quantitation. Both metabolites were enriched in tumor tissue compared with adjacent nontumor tissue, and positively correlated with levels in urine, thus revealing their direct association with tumor metabolism [38]. Recent evaluation of this panel of urinary metabolite lung cancer biomarkers in the well-characterized prospective Southern Community Cohort Study (SCCS) has confirmed the association of CR and NANA levels with lung cancer risk prior to clinically detectable disease [39].
Tissue-based biomarkers
The identification of tissue-based biomarkers has recently been focused on the prognostic value of biomarkers derived from resected lung adenocarcinoma tissues. Many prognostic signatures based on gene expression have been developed but most of them have failed to validate in larger patient cohorts [40]. We have addressed this limitation by validating our classifiers in independently-collected cohorts from populations of diverse ethnicity and smoking habits. We also clearly defined the aim of the prognostic signature within the clinical context of our study as identifying Stage IA patients at high risk for recurrence who might benefit from adjuvant chemotherapy and/or immunotherapy, and Stage IB patients at low risk for recurrence who could be spared [41]. With this goal, we have conducted comprehensive profiling of the transcriptome, microRNAome and DNA methylome of tissues collected in three independent NCI-MD, Norway, and Japan cohorts of patients, and identified and validated prognostic biomarkers based on expression of genes and microRNA, as well as DNA promoter methylation [42-46]. Increased miR-21 expression is associated with disease progression and survival in patients with stage I adenocarcinoma [43]. An amplification peak encompassing MIR21 was also recently described by The Cancer Genome Atlas in lung adenocarcinomas [47]. Focusing on genes with a mechanistic role in lung cancer, we discovered, defined and validated a prognostic classifier based on the expression of 4 genes: BRCA1, HIF1A, DLC1, and XPO1 that could stratify stage I, stage IA and stage IB lung adenocarcinoma patients into high and low-risk groups [44]. This 4-gene classifier has been further validated using gene expression values derived from over 1,000 Stage I lung adenocarcinomas from a variety of publically available microarray datasets [44, 45]. Genome-wide screening of DNA methylation in early stage lung adenocarcinoma samples from the NCI-MD case cohort led to the discovery of the prognostic value of HOXA9 promoter methylation, which was further technically validated by pyrosequencing analysis [46].
Large scale characterization of the lung adenocarcinoma genome, transcriptome and methylome has revealed disease subtypes characterized by idiosyncratic combinations of molecular alterations which underscore the heterogeneity of this disease [48]. This implies that any one molecular biomarker may correctly classify tumors as high-risk based on a particular underlying biology, but misclassify others driven by a different set of genomic or epigenomic changes. Thus, in our recent study, we hypothesized that statistical independence would be required for multiple biomarkers to provide incremental improvement in patient stratification [46]. Upon statistical verification that the 4-gene classifier, miR-21 expression, and HOXA9 promoter methylation were each independently associated with outcome (HR, 2.8; p = 0.002; HR, 2.3; p = 0.01; and HR, 2.4; p = 0.005, respectively), we further demonstrated in that study that these biomarkers could be combined into a simple score that identified high-risk, therapy näive, stage I patients (HR, 10.2; p = 3 × 10) [46]. Such integrative analysis of “omic,” clinical and epidemiological data is a core principle of precision medicine. A practical consideration, however, is whether the benefit of combining several biomarkers supports the logistical burden of running multiple tests. Most recurrences in resected stage I lung cancer patients occur within 3 years of surgery [49]. In our study [46], the combined miR-21/4-gene transcriptomic signature correctly identified as high-risk 68% of the patients who recurred or died within 3 years. The addition of HOXA9 promoter methylation into a combined score resulted in correct identification of 78% of high-risk patients. Thus, the extra effort required to add DNA-based biomarker to an existing RNA-based signature may result in better management of resected patients.
OMICs-based diagnostic and prognostic lung cancer biomarkers in advanced clinical development
Targeted and comprehensive profiling technologies have been employed in numerous studies to identify and measure early detection and prognostic lung cancer biomarkers in a variety of biospecimens (tumor tissue, blood, urine, sputum, nasal swabs, bronchial brushings). The vast majority of prognostic biomarkers derive from comprehensive molecular analysis of mRNA, miRNA and DNA methylation in resected tissues, whereas, the identification of early detection or diagnostic biomarkers relies on the evaluation of surrogate tissues, such as nasal swabs or bronchial brushings, or, most commonly, biofluids, such as blood or urine.
The following omics-based tests have recently become commercially-available and are being clinically evaluated:
Pervenio Lung RS (14-gene signature)
This 14-gene signature was developed on formalin-fixed and paraffin-embedded (FFPE) non-squamous NSCLC tissue samples, using genes identified as prognostic for early stage lung cancer in prior profiling studies [50]. A subsequent study assessed the analytical validity of the assay by evaluating its precision and reproducibility in 2 large international cohorts [51]. A clinical trial to evaluate benefit of adjuvant therapy in high risk stage I non-squamous NSCLC identified by the signature has been initiated (NCT01817192 at www.clinicaltrials.gov) [24]. The test is marketed by Pinpoint Genomics/Life Technologies Corporation (West Sacramento, CA, USA).
myPlan Lung Cancer (CCP score)
This gene signature summarizes the expression of cell cycle proliferation (CCP) genes, originally identified in microarray profiling cohorts, into a score that utilizes FFPE lung adenocarcinoma tissues to predict lung cancer death in resected patients [52]. Subsequent studies tested the analytical validity of the assay [53], and the clinical utility of a prognostic score that takes into account pathological stage [30]. Two registries intended to measure the effect of using this test on treatment decisions by oncologists and surgeons and assess disease-free patient survival have been opened (ONC006 and ONC003, for Oncologists and Surgeons, respectively, at www.clinicaltrials.gov). The test is marketed by Myriad Genetics, Inc. (Salt Lake City, UT, USA).
Other omics-based tests with evidence of clinical utility include:
Percepta (bronchial genomic classifier)
A classifier based on the combination of expression of 17 cancer genes in airway epithelial cells of current and former smokers undergoing bronchoscopy for suspect lung cancer, as a diagnostic aid to reduce invasive procedures [33, 54].
MSC (microRNA Signature Classifier, plasma microRNA signature)
A classifier based on expression in plasma of a panel of microRNAs that stratifies smokers according to the risk to develop lung cancer (high-intermediate-low risk) and improves the efficacy of lung cancer screening by reducing the number of LDCT false positives [27, 55].
miR-Test (serum microRNA signature)
A diagnostic test for lung cancer based on expression of a panel of microRNAs in serum which can identify patients with cancer among those at high-risk who are candidates for LDCT screening [31, 56].
Proteomic plasma test
A diagnostic test based on a multiprotein plasma classifier that can identify likely benign nodules discovered by lung cancer screening [32, 57].
New directions
Biomarkers of response to Immunotherapy
A recent report indicated that tumor-infiltrating lymphocytes (TILs) have prognostic value for stage I in lung cancer patients [58]. We have discovered that the endogenous p53 isoforms Δ133p53 and p53β are physiological regulators of proliferation and senescence in human T lymphocytes in vivo [59]. In that study, CD8+ T lymphocytes associated with human lung tumors harbored senescent cells characterized by a distinct profile of p53 isoforms and surface markers. In light of the efficacy shown in advance stage lung cancer patients of novel therapeutic strategies that modulate the immune system [60, 61], it is imperative to understand how the immunogenic milieu affects lung cancer prognosis and response to therapy. For example, molecular pathways that affect senescence of TILs could be modulated to oppose tumor-induced T cell exhaustion and increase the effectiveness of checkpoint immunotherapy in lung cancer patients.
Prognostic biomarkers of early-stage lung squamous cell carcinoma
Despite an increase in the detection of early stage tumors, there was no benefit of LDCT screening for patients with lung squamous cell carcinoma (SCC) [62], the second most common histological subtype of NSCLC. Further, data are still lacking on molecular signatures that stratify risk of recurrence in these patients. The relative risk for SCC is increasingly higher with cumulative exposure to cigarette smoke [63]. The disease is heterogeneous, and patient outcome is complicated by comorbities such as Chronic Obstructive Pulmonary Disease and Cardiovascular Disease [64]. At the recent Fourth AACR-IASLC International Joint Conference on Lung Cancer Translational Science from the Bench to the Clinic, which was held in January 4-7, 2016 we unveiled a prognostic classifier based on gene expression that is broadly applicable to diverse patient cohorts as a clinical tool for guiding postoperative management and therapeutic decisions in patients with early-stage lung SCC [65]. Efforts towards analytical validation of this signature are ongoing.
Microbiomic biomarkers of lung cancer
There is strong epidemiological evidence that pulmonary infections are associated with increased risk of lung cancer lung cancer [66, 67]. The Human Microbiome Project revealed that a microbiota of astonishing diversity and abundance resides in healthy human tissues [68]. This has enabled and propelled the characterization of the contributions of microbial species harbored in humans, collectively referred to as Microbiome, to a variety of diseases, including cancer. Studies of microbial species altered in the respiratory track in individuals with lung cancer are now starting to emerge [69, 70]. Thus, the microbiome may be a suitable source for clinical biomarkers of lung cancer risk, diagnosis and prognosis.
Proteomic biomarkers of lung cancer
Recent advances in mass spectrometry-based proteomics technologies are facilitating the quantitative analysis of lung tumor-derived proteins and peptides in tissues, pleural fluids, and blood [71, 72]. These represent candidate diagnostic, prognostic, and predictive markers for lung cancer. Mass spectrometry analysis of serum collected from patients with advanced NSCLC before and after treatment with tyrosine kinase inhibitors led to a test that predicts treatment response [73], and evidence for its clinical utility is accumulating [74].
Radiomics-based biomarkers of lung cancer risk, diagnosis and prognosis
Images obtained non-invasively in the course of diagnosis and treatment of cancer patients can provide a wealth of data that can be systematically captured, mined and interpreted as another layer of the precision medicine [75, 76]. Recently, advanced computational methodologies were applied to the analysis and interpretation of features extracted from CT scans of lung cancer patients to derive a prognostic signature [77, 78]. Imaging-based biomarkers are now being evaluated for performance in risk stratification of lung cancer patients diagnosed by LDCT [79] as well as ability to predict the malignancy of IPNs [80]. Improvements in the identification of diagnostic and predictive quantitative imaging features extracted from data and images from the NLST, were also described at the Fourth AACR-IASLC International Joint Conference on Lung Cancer Translational Science [81].
Highlights.
Biomarkers are needed to prioritize high-risk individuals for lung cancer screening.
Biomarkers derive from integrative analysis of molecular data for single patients.
Validated biomarker combinations may aid patient diagnosis and risk stratification.
Acknowledgments
The authors are supported by the Intramural Research Program of the National Cancer Institute, NIH.
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Farhat FS, Houhou W. Targeted therapies in non-small cell lung carcinoma: what have we achieved so far? Ther Adv Med Oncol. 2013;5:249–270. doi: 10.1177/1758834013492001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta (GA): 2014. [Google Scholar]
- 6.National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, Sabichi AL, Smith-Bindman R, Wood DE, Qaseem A, Detterbeck FC. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wender R, Fontham ET, Barrera E, Jr, Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Gazelle GS, Kelsey DK, LaMonte SJ, Michaelson JS, Oeffinger KC, Shih YC, Sullivan DC, Travis W, Walter L, Wolf AM, Brawley OW, Smith RA. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:107–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deffebach ME, Humphrey L. Lung Cancer Screening. Surg Clin North Am. 2015;95:967–978. doi: 10.1016/j.suc.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N) Centers for Medicare and Medicaid Services; [Google Scholar]
- 11.Doria-Rose VP, White MC, Klabunde CN, Nadel MR, Richards TB, McNeel TS, Rodriguez JL, Marcus PM. Use of lung cancer screening tests in the United States: results from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21:1049–1059. doi: 10.1158/1055-9965.EPI-12-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanner NT, Aggarwal J, Gould MK, Kearney P, Diette G, Vachani A, Fang KC, Silvestri GA. Management of Pulmonary Nodules by Community Pulmonologists: A Multicenter Observational Study. Chest. 2015;148:1405–1414. doi: 10.1378/chest.15-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt C. Lung cancer screening poised to expand. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv114. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. Non–Small-Cell Lung Cancer (Version 4.2014) [Google Scholar]
- 15.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P C. International Staging, I. Participating. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 16.Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. The National Academies Press; Washington (DC): 2011. [PubMed] [Google Scholar]
- 17.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil C. NCI-MATCH launch highlights new trial design in precision-medicine era. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv193. [DOI] [PubMed] [Google Scholar]
- 19.Govindan R, Mandrekar SJ, Gerber DE, Oxnard GR, Dahlberg SE, Chaft J, Malik S, Mooney M, Abrams JS, Janne PA, Gandara DR, Ramalingam SS, Vokes EE. ALCHEMIST Trials: A Golden Opportunity to Transform Outcomes in Early-Stage Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21:5439–5444. doi: 10.1158/1078-0432.CCR-15-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McShane LM, Polley MY. Development of omics-based clinical tests for prognosis and therapy selection: the challenge of achieving statistical robustness and clinical utility. Clin Trials. 2013;10:653–665. doi: 10.1177/1740774513499458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institute of Medicine. Evolution of Translational Omics: Lessons Learned and the Path Forward. The National Academies Press; Washington (DC): 2012. [PubMed] [Google Scholar]
- 22.Hassanein M, Callison JC, Callaway-Lane C, Aldrich MC, Grogan EL, Massion PP. The state of molecular biomarkers for the early detection of lung cancer. Cancer Prev Res (Phila) 2012;5:992–1006. doi: 10.1158/1940-6207.CAPR-11-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich D, Kneip C, Raji O, Liloglou T, Seegebarth A, Schlegel T, Flemming N, Rausch S, Distler J, Fleischhacker M, Schmidt B, Giles T, Walshaw M, Warburton C, Liebenberg V, Field JK. Performance evaluation of the DNA methylation biomarker SHOX2 for the aid in diagnosis of lung cancer based on the analysis of bronchial aspirates. Int J Oncol. 2012;40:825–832. doi: 10.3892/ijo.2011.1264. [DOI] [PubMed] [Google Scholar]
- 24.Kratz JR, Mann MJ, Jablons DM. International trial of adjuvant therapy in high risk stage I non-squamous cell carcinoma identified by a 14-gene prognostic signature. Transl Lung Cancer Res. 2013;2:222–225. doi: 10.3978/j.issn.2218-6751.2013.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirales Casillas CE, Flores Fernandez JM, Padilla Camberos E, Herrera Lopez EJ, Leal Pacheco G, Martinez Velazquez M. Current status of circulating protein biomarkers to aid the early detection of lung cancer. Future Oncol. 2014;10:1501–1513. doi: 10.2217/fon.14.21. [DOI] [PubMed] [Google Scholar]
- 26.Massion PP. Biomarkers to the rescue in a lung nodule epidemic. J Clin Oncol. 2014;32:725–726. doi: 10.1200/JCO.2013.54.0047. [DOI] [PubMed] [Google Scholar]
- 27.Sozzi G, Boeri M, Rossi M, Verri C, Suatoni P, Bravi F, Roz L, Conte D, Grassi M, Sverzellati N, Marchiano A, Negri E, La Vecchia C, Pastorino U. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol. 2014;32:768–773. doi: 10.1200/JCO.2013.50.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahar AL, Compton C, McShane LM, Halabi S, Asamura H, Rami-Porta R, Groome PA C. Molecular Modellers Working Group of American Joint Committee on. Refining Prognosis in Lung Cancer: A Report on the Quality and Relevance of Clinical Prognostic Tools. J Thorac Oncol. 2015;10:1576–1589. doi: 10.1097/JTO.0000000000000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y, Bueno R. Commercially available prognostic molecular models in early-stage lung cancer: a review of the Pervenio Lung RS and Myriad myPlan Lung Cancer tests. Expert Rev Mol Diagn. 2015;15:589–596. doi: 10.1586/14737159.2015.1028371. [DOI] [PubMed] [Google Scholar]
- 30.Bueno R, Hughes E, Wagner S, Gutin AS, Lanchbury JS, Zheng Y, Archer MA, Gustafson C, Jones JT, Rushton K, Saam J, Kim E, Barberis M, Wistuba I, Wenstrup RJ, Wallace WA, Hartman AR, Harrison DJ. Validation of a molecular and pathological model for five-year mortality risk in patients with early stage lung adenocarcinoma. J Thorac Oncol. 2015;10:67–73. doi: 10.1097/JTO.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montani F, Marzi MJ, Dezi F, Dama E, Carletti RM, Bonizzi G, Bertolotti R, Bellomi M, Rampinelli C, Maisonneuve P, Spaggiari L, Veronesi G, Nicassio F, Di Fiore PP, Bianchi F. miR-Test: a blood test for lung cancer early detection. J Natl Cancer Inst. 2015;107:djv063. doi: 10.1093/jnci/djv063. [DOI] [PubMed] [Google Scholar]
- 32.Vachani A, Pass HI, Rom WN, Midthun DE, Edell ES, Laviolette M, Li XJ, Fong PY, Hunsucker SW, Hayward C, Mazzone PJ, Madtes DK, Miller YE, Walker MG, Shi J, Kearney P, Fang KC, Massion PP. Validation of a multiprotein plasma classifier to identify benign lung nodules. J Thorac Oncol. 2015;10:629–637. doi: 10.1097/JTO.0000000000000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vachani A, Whitney DH, Parsons EC, Lenburg M, Ferguson JS, Silvestri GA, Spira A. Clinical Utility of a Bronchial Genomic Classifier in Patients with Suspected Lung Cancer. Chest. 2016 doi: 10.1016/j.chest.2016.02.636. [DOI] [PubMed] [Google Scholar]
- 34.Enewold L, Mechanic LE, Bowman ED, Zheng YL, Yu Z, Trivers G, Alberg AJ, Harris CC. Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2009;18:215–222. doi: 10.1158/1055-9965.EPI-08-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso NE, Harris CC. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103:1112–1122. doi: 10.1093/jnci/djr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan BM, Pine SR, Chaturvedi AK, Caporaso N, Harris CC. A combined prognostic serum interleukin-8 and interleukin-6 classifier for stage 1 lung cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. J Thorac Oncol. 2014;9:1494–1503. doi: 10.1097/JTO.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pine SR, Mechanic LE, Enewold L, Bowman ED, Ryan BM, Cote ML, Wenzlaff AS, Loffredo CA, Olivo-Marston S, Chaturvedi A, Caporaso NE, Schwartz AG, Harris CC. Differential Serum Cytokine Levels and Risk of Lung Cancer Between African and European Americans. Cancer Epidemiol Biomarkers Prev. 2016;25:488–497. doi: 10.1158/1055-9965.EPI-15-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathe EA, Patterson AD, Haznadar M, Manna SK, Krausz KW, Bowman ED, Shields PG, Idle JR, Smith PB, Anami K, Kazandjian DG, Hatzakis E, Gonzalez FJ, Harris CC. Noninvasive urinary metabolomic profiling identifies diagnostic and prognostic markers in lung cancer. Cancer Res. 2014;74:3259–3270. doi: 10.1158/0008-5472.CAN-14-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haznadar M, Cai Q, Krausz KW, Bowman ED, Margono E, Noro R, Thompson MD, Mathe EA, Munro HM, Steinwandel MD, Gonzalez FJ, Blot WJ, Harris CC. Urinary Metabolite Risk Biomarkers of Lung Cancer: A Prospective Cohort Study. Cancer Epidemiol Biomarkers Prev. 2016;25:978–986. doi: 10.1158/1055-9965.EPI-15-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanian J, Simon R. Gene expression-based prognostic signatures in lung cancer: ready for clinical use? J Natl Cancer Inst. 2010;102:464–474. doi: 10.1093/jnci/djq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramanian J, Simon R. What should physicians look for in evaluating prognostic gene-expression signatures? Nat Rev Clin Oncol. 2010;7:327–334. doi: 10.1038/nrclinonc.2010.60. [DOI] [PubMed] [Google Scholar]
- 42.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 43.Saito M, Schetter AJ, Mollerup S, Kohno T, Skaug V, Bowman ED, Mathe EA, Takenoshita S, Yokota J, Haugen A, Harris CC. The association of microRNA expression with prognosis and progression in early-stage, non-small cell lung adenocarcinoma: a retrospective analysis of three cohorts. Clin Cancer Res. 2011;17:1875–1882. doi: 10.1158/1078-0432.CCR-10-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akagi I, Okayama H, Schetter AJ, Robles AI, Kohno T, Bowman ED, Kazandjian D, Welsh JA, Oue N, Saito M, Miyashita M, Uchida E, Takizawa T, Takenoshita S, Skaug V, Mollerup S, Haugen A, Yokota J, Harris CC. Combination of protein coding and noncoding gene expression as a robust prognostic classifier in stage I lung adenocarcinoma. Cancer Res. 2013;73:3821–3832. doi: 10.1158/0008-5472.CAN-13-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okayama H, Schetter AJ, Ishigame T, Robles AI, Kohno T, Yokota J, Takenoshita S, Harris CC. The expression of four genes as a prognostic classifier for stage I lung adenocarcinoma in 12 independent cohorts. Cancer Epidemiol Biomarkers Prev. 2014;23:2884–2894. doi: 10.1158/1055-9965.EPI-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robles AI, Arai E, Mathe EA, Okayama H, Schetter AJ, Brown D, Petersen D, Bowman ED, Noro R, Welsh JA, Edelman DC, Stevenson HS, Wang Y, Tsuchiya N, Kohno T, Skaug V, Mollerup S, Haugen A, Meltzer PS, Yokota J, Kanai Y, Harris CC. An Integrated Prognostic Classifier for Stage I Lung Adenocarcinoma Based on mRNA, microRNA, and DNA Methylation Biomarkers. J Thorac Oncol. 2015;10:1037–1048. doi: 10.1097/JTO.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, Shukla SA, Guo G, Brooks AN, Murray BA, Imielinski M, Hu X, Ling S, Akbani R, Rosenberg M, Cibulskis C, Ramachandran A, Collisson EA, Kwiatkowski DJ, Lawrence MS, Weinstein JN, Verhaak RG, Wu CJ, Hammerman PS, Cherniack AD, Getz G, Artyomov MN, Schreiber R, Govindan R, Meyerson M N. Cancer Genome Atlas Research. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48:607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martini N, Bains MS, Burt ME, Zakowski MF, McCormack P, Rusch VW, Ginsberg RJ. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–129. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 50.Kratz JR, He J, Van Den Eeden SK, Zhu ZH, Gao W, Pham PT, Mulvihill MS, Ziaei F, Zhang H, Su B, Zhi X, Quesenberry CP, Habel LA, Deng Q, Wang Z, Zhou J, Li H, Huang MC, Yeh CC, Segal MR, Ray MR, Jones KD, Raz DJ, Xu Z, Jahan TM, Berryman D, He B, Mann MJ, Jablons DM. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet. 2012;379:823–832. doi: 10.1016/S0140-6736(11)61941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kratz JR, Tham PT, Mulvihill MS, Ziaei F, Ray MR, Hurst JW, Segal MR, Berryman DM, Chu W, He B, Jablons DM, Mann MJ. Analytical validation of a practical molecular assay prognostic of survival in nonsquamous non-small cell lung cancer. Diagn Mol Pathol. 2013;22:65–69. doi: 10.1097/PDM.0b013e318273fb61. [DOI] [PubMed] [Google Scholar]
- 52.Wistuba II, Behrens C, Lombardi F, Wagner S, Fujimoto J, Raso MG, Spaggiari L, Galetta D, Riley R, Hughes E, Reid J, Sangale Z, Swisher SG, Kalhor N, Moran CA, Gutin A, Lanchbury JS, Barberis M, Kim ES. Validation of a proliferation-based expression signature as prognostic marker in early stage lung adenocarcinoma. Clin Cancer Res. 2013;19:6261–6271. doi: 10.1158/1078-0432.CCR-13-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warf MB, Fosso PG, Hughes E, Perry M, Brown KL, Reid JE, Kolquist KA, Wagner S, Gutin A, Roa B. Analytical validation of a proliferation-based molecular signature used as a prognostic marker in early stage lung adenocarcinoma. Biomark Med. 2015;9:901–910. doi: 10.2217/bmm.15.46. [DOI] [PubMed] [Google Scholar]
- 54.Silvestri GA, Vachani A, Whitney D, Elashoff M, Porta Smith K, Ferguson JS, Parsons E, Mitra N, Brody J, Lenburg ME, Spira A, Team AS. A Bronchial Genomic Classifier for the Diagnostic Evaluation of Lung Cancer. N Engl J Med. 2015;373:243–251. doi: 10.1056/NEJMoa1504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boeri M, Verri C, Conte D, Roz L, Modena P, Facchinetti F, Calabro E, Croce CM, Pastorino U, Sozzi G. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc Natl Acad Sci U S A. 2011;108:3713–3718. doi: 10.1073/pnas.1100048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bianchi F, Nicassio F, Marzi M, Belloni E, Dall'olio V, Bernard L, Pelosi G, Maisonneuve P, Veronesi G, Di Fiore PP. A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer. EMBO Mol Med. 2011;3:495–503. doi: 10.1002/emmm.201100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li XJ, Hayward C, Fong PY, Dominguez M, Hunsucker SW, Lee LW, McLean M, Law S, Butler H, Schirm M, Gingras O, Lamontagne J, Allard R, Chelsky D, Price ND, Lam S, Massion PP, Pass H, Rom WN, Vachani A, Fang KC, Hood L, Kearney P. A blood-based proteomic classifier for the molecular characterization of pulmonary nodules. Sci Transl Med. 2013;5:207ra142. doi: 10.1126/scitranslmed.3007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpreville V, Validire P, Besse B, Mami-Chouaib F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 59.Mondal AM, Horikawa I, Pine SR, Fujita K, Morgan KM, Vera E, Mazur SJ, Appella E, Vojtesek B, Blasco MA, Lane DP, Harris CC. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J Clin Invest. 2013;123:5247–5257. doi: 10.1172/JCI70355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L K.-. Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 61.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pinsky PF, Church TR, Izmirlian G, Kramer BS. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer. 2013;119:3976–3983. doi: 10.1002/cncr.28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pesch B, Kendzia B, Gustavsson P, Jockel KH, Johnen G, Pohlabeln H, Olsson A, Ahrens W, Gross IM, Bruske I, Wichmann HE, Merletti F, Richiardi L, Simonato L, Fortes C, Siemiatycki J, Parent ME, Consonni D, Landi MT, Caporaso N, Zaridze D, Cassidy A, Szeszenia-Dabrowska N, Rudnai P, Lissowska J, Stucker I, Fabianova E, Dumitru RS, Bencko V, Foretova L, Janout V, Rudin CM, Brennan P, Boffetta P, Straif K, Bruning T. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. 2012;131:1210–1219. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003;103:792–802. doi: 10.1002/ijc.10882. [DOI] [PubMed] [Google Scholar]
- 65.Noro R, Walsh N, Ishigame T, Shiraishi K, Robles AI, Ryan BM, Bowman E, Welsh JA, Schetter AJ, Skaug V, Mollerup S, Haugen A, Yokota J, Kohno T, Harris CC. Gene expression classifier for prognosis of early-stage squamous cell carcinoma of the lung. Journal of Thoracic Oncology. 2016;11:S38–S39. doi: 10.1016/j.jtho.2016.08.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One. 2011;6:e17479. doi: 10.1371/journal.pone.0017479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogtmann E, Goedert JJ. Epidemiologic studies of the human microbiome and cancer. Br J Cancer. 2016;114:237–242. doi: 10.1038/bjc.2015.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hosgood HD, 3rd, Sapkota AR, Rothman N, Rohan T, Hu W, Xu J, Vermeulen R, He X, White JR, Wu G, Wei F, Mongodin EF, Lan Q. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environ Mol Mutagen. 2014;55:643–651. doi: 10.1002/em.21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan X, Yang M, Liu J, Gao R, Hu J, Li J, Zhang L, Shi Y, Guo H, Cheng J, Razi M, Pang S, Yu X, Hu S. Discovery and validation of potential bacterial biomarkers for lung cancer. Am J Cancer Res. 2015;5:3111–3122. [PMC free article] [PubMed] [Google Scholar]
- 71.Kikuchi T, Hassanein M, Amann JM, Liu Q, Slebos RJ, Rahman SM, Kaufman JM, Zhang X, Hoeksema MD, Harris BK, Li M, Shyr Y, Gonzalez AL, Zimmerman LJ, Liebler DC, Massion PP, Carbone DP. In-depth proteomic analysis of nonsmall cell lung cancer to discover molecular targets and candidate biomarkers. Mol Cell Proteomics. 2012;11:916–932. doi: 10.1074/mcp.M111.015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Indovina P, Marcelli E, Pentimalli F, Tanganelli P, Tarro G, Giordano A. Mass spectrometry-based proteomics: the road to lung cancer biomarker discovery. Mass Spectrom Rev. 2013;32:129–142. doi: 10.1002/mas.21355. [DOI] [PubMed] [Google Scholar]
- 73.Taguchi F, Solomon B, Gregorc V, Roder H, Gray R, Kasahara K, Nishio M, Brahmer J, Spreafico A, Ludovini V, Massion PP, Dziadziuszko R, Schiller J, Grigorieva J, Tsypin M, Hunsucker SW, Caprioli R, Duncan MW, Hirsch FR, Bunn PA, Jr, Carbone DP. Mass spectrometry to classify non-small-cell lung cancer patients for clinical outcome after treatment with epidermal growth factor receptor tyrosine kinase inhibitors: a multicohort cross-institutional study. J Natl Cancer Inst. 2007;99:838–846. doi: 10.1093/jnci/djk195. [DOI] [PubMed] [Google Scholar]
- 74.Gregorc V, Novello S, Lazzari C, Barni S, Aieta M, Mencoboni M, Grossi F, De Pas T, de Marinis F, Bearz A, Floriani I, Torri V, Bulotta A, Cattaneo A, Grigorieva J, Tsypin M, Roder J, Doglioni C, Levra MG, Petrelli F, Foti S, Vigano M, Bachi A, Roder H. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. Lancet Oncol. 2014;15:713–721. doi: 10.1016/S1470-2045(14)70162-7. [DOI] [PubMed] [Google Scholar]
- 75.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441–446. doi: 10.1016/j.ejca.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans CR, Dekker A, Quackenbush J, Gillies RJ, Lambin P. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Schabath MB, Liu Y, Berglund AE, Bloom GC, Kim J, Stringfield O, Eikman EA, Klippenstein DL, Heine JJ, Eschrich SA, Ye Z, Gillies RJ. Semiquantitative Computed Tomography Characteristics for Lung Adenocarcinoma and Their Association With Lung Cancer Survival. Clin Lung Cancer. 2015;16:e141–163. doi: 10.1016/j.cllc.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maldonado F, Duan F, Raghunath SM, Rajagopalan S, Karwoski RA, Garg K, Greco E, Nath H, Robb RA, Bartholmai BJ, Peikert T. Noninvasive Computed Tomography-based Risk Stratification of Lung Adenocarcinomas in the National Lung Screening Trial. Am J Respir Crit Care Med. 2015;192:737–744. doi: 10.1164/rccm.201503-0443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas A, Yognand B, Sanja A, Heidi C, Matthew S, Yuhua G, Hua W, Ronald W, Robert G, Pierre PM. Lung Cancer Screening: Lessons Learned and Yet to be Learned. American Thoracic Society; 2015. Radiomic Analysis for Improved Lung Cancer Prediction of Indeterminate Pulmonary Nodules, D100; pp. A6119–A6119. [Google Scholar]
- 81.Schabath MB, Liu Y, Wang H, Stringfield O, Balagurunathan Y, Garcia A, Hall L, Goldgof D, Gillies RJ. Diagnostic and predictive quantitative-imaging features in lung cancer screening. Journal of Thoracic Oncology. 2016;11:S41–S42. [Google Scholar]


