Abstract
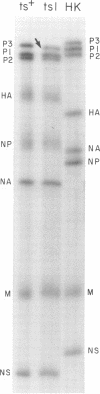
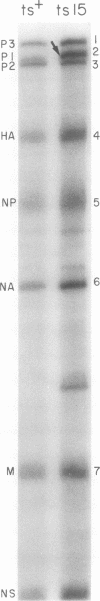
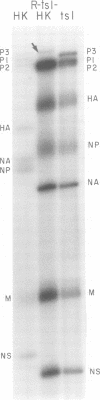
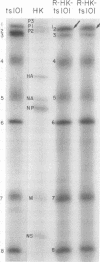
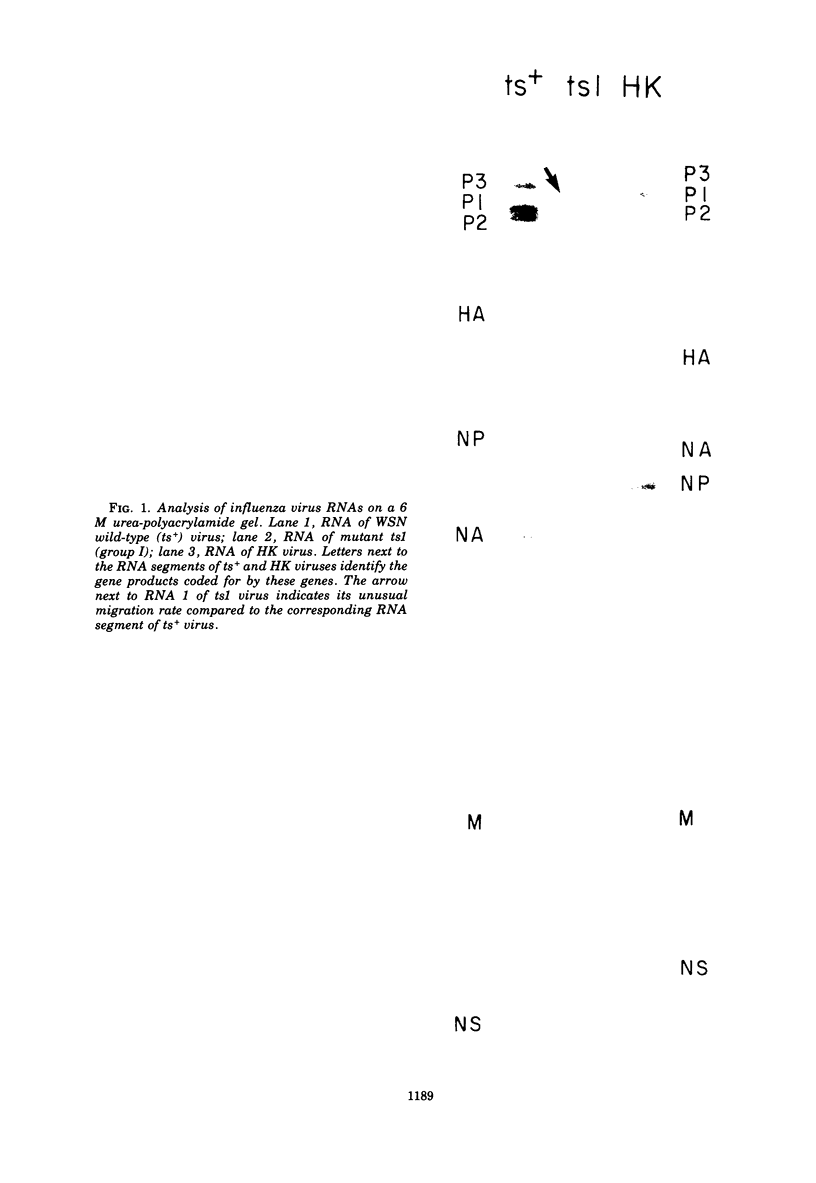
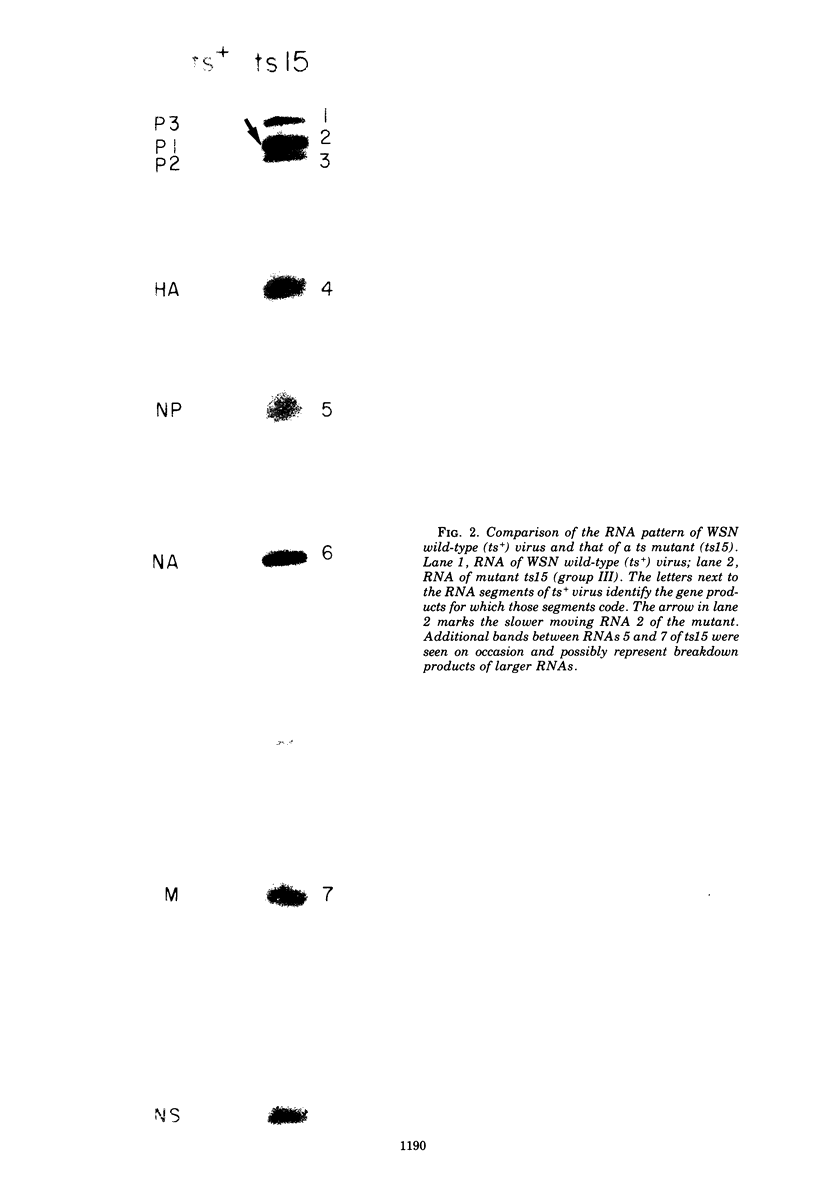
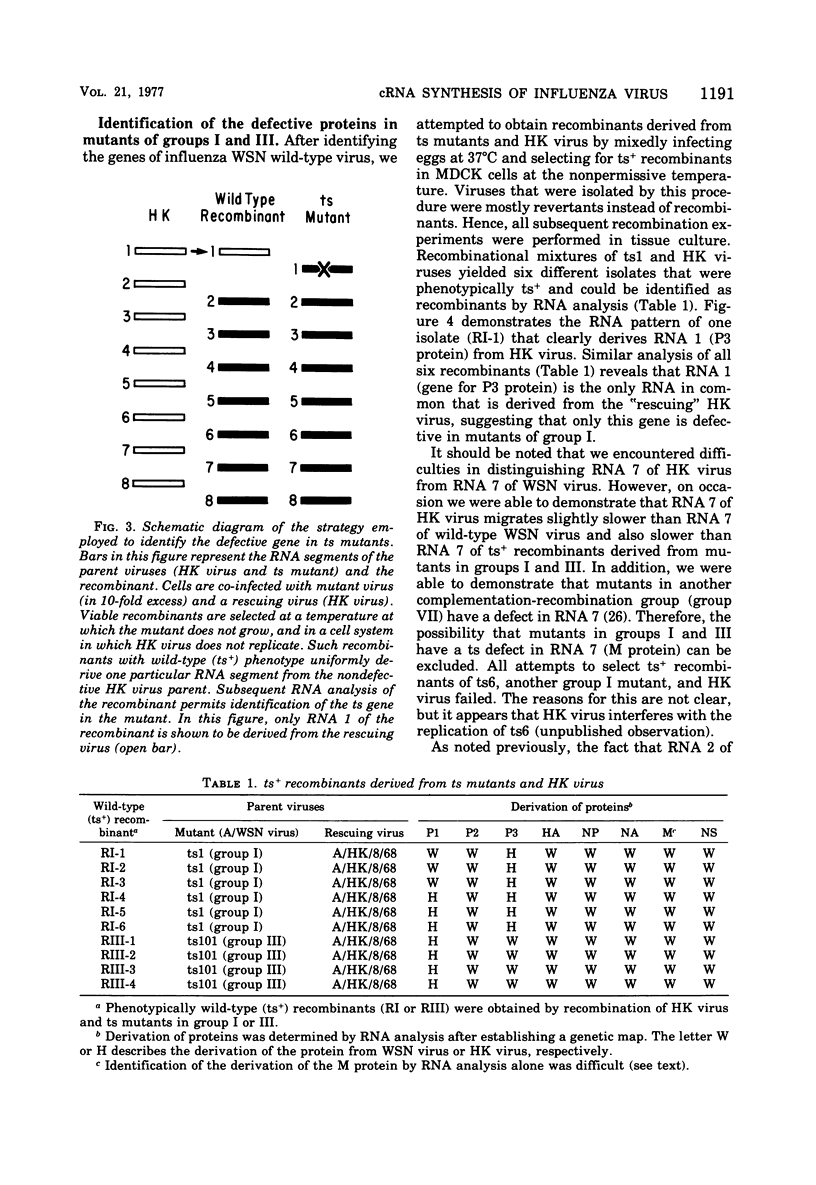
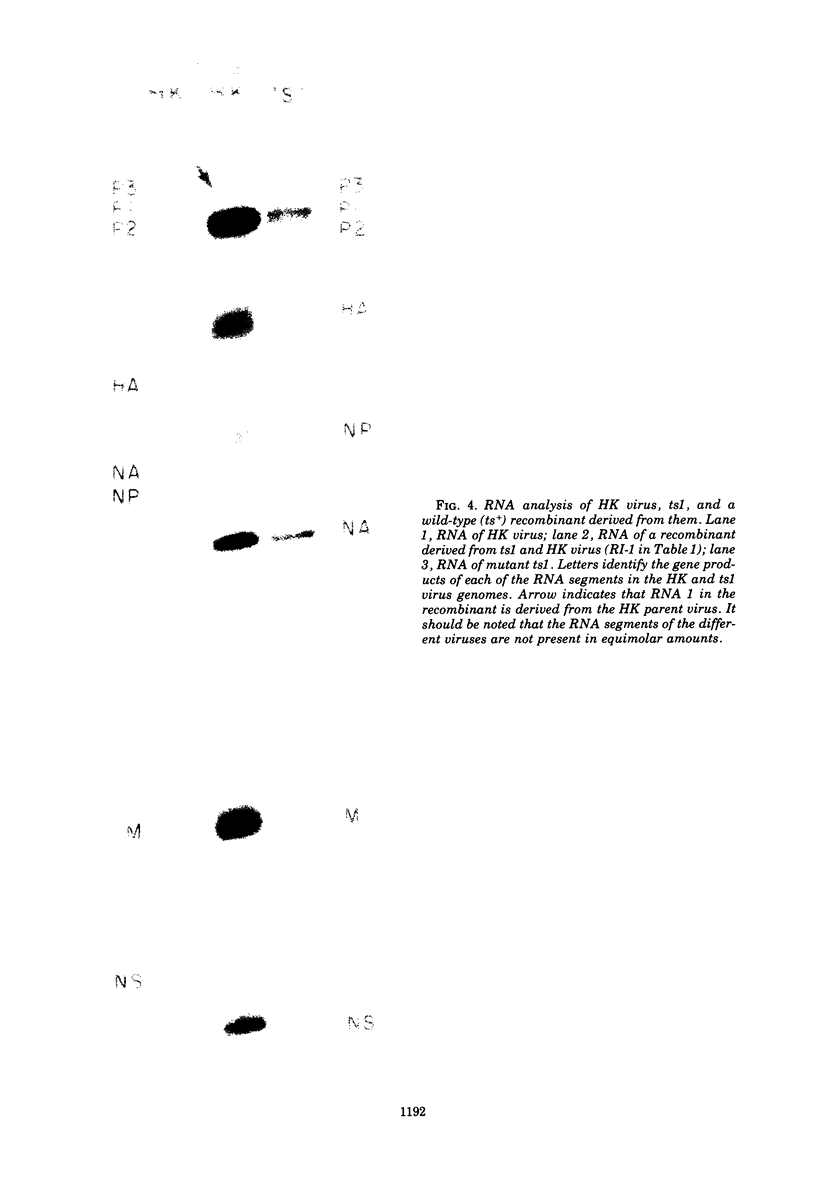
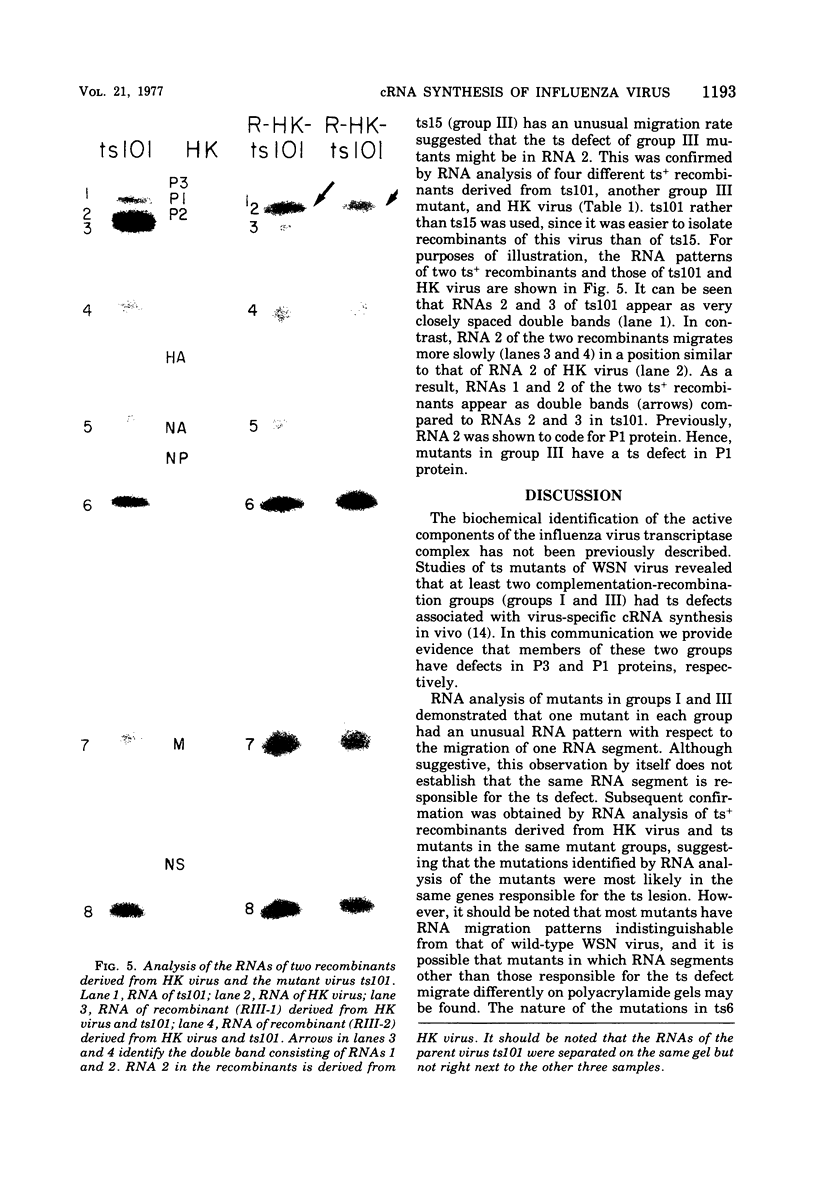
Members of two temperature-sensitive (ts) mutant groups of influenza A/WSN virus defective in complementary RNA synthesis were analyzed with respect to the identity of their defective genes. RNA analysis of recombinants having a ts+ phenotype derived from the mutants and HK virus permitted the identification of RNA 1 and RNA 2 as the single defective gene in mutant groups I and III, respectively. Based on knowledge obtained by mapping the WSN virus genome, it then was possible to determine that biologically functional P3 protein (coded for by RNA 1) and P1 protein (RNA 2) are required for complementary RNA synthesis of influenza virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P., Bean W. J., Jr, Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. 3. Completeness of the transcription process. J Virol. 1972 Oct;10(4):689–697. doi: 10.1128/jvi.10.4.689-697.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Compans R. W. Analysis of the in vitro product of an RNA-dependent RNA polymerase isolated from influenza virus-infected cells. J Virol. 1974 Aug;14(2):191–197. doi: 10.1128/jvi.14.2.191-197.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow N. L., Simpson R. W. RNA-dependent RNA polymerase activity associated with virions and subviral particles of myxoviruses. Proc Natl Acad Sci U S A. 1971 Apr;68(4):752–756. doi: 10.1073/pnas.68.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J. Cell-free translation of influenza virus mRNA. J Virol. 1976 May;18(2):604–618. doi: 10.1128/jvi.18.2.604-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Influenza viral messenger RNA. Virology. 1974 Nov;62(1):38–45. doi: 10.1016/0042-6822(74)90301-8. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Purification of influenza viral complementary RNA: its genetic content and activity in wheat germ cell-free extracts. J Virol. 1975 Dec;16(6):1464–1475. doi: 10.1128/jvi.16.6.1464-1475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R. W., Stone M. P., Joklik W. K. Separation of single-stranded ribonucleic acids by acrylamide-agarose-urea gel electrophoresis. Anal Biochem. 1974 Jun;59(2):599–609. doi: 10.1016/0003-2697(74)90313-3. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Wunner W. H., Skehel J. J. Virus replication in enucleate cells: vesicular stomatitis virus and influenza virus. J Virol. 1974 Feb;13(2):394–399. doi: 10.1128/jvi.13.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Avery R. J., Dimmock N. J. Failure of an influenza virus to initiate infection in enucleate BHK cells. J Virol. 1974 Jun;13(6):1155–1161. doi: 10.1128/jvi.13.6.1155-1161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W., Webster R. G. Cell-free translation of influenza virus messenger RNA. Virology. 1973 Dec;56(2):654–657. doi: 10.1016/0042-6822(73)90069-x. [DOI] [PubMed] [Google Scholar]
- Krug R. M. Influenza viral RNPs newly synthesized during the latent period of viral growth in MDCK cells. Virology. 1971 Apr;44(1):125–136. doi: 10.1016/0042-6822(71)90159-0. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Ueda M., Palese P. Temperature-sensitive mutants of influenza WSN virus defective in virus-specific RNA synthesis. J Virol. 1975 Oct;16(4):790–796. doi: 10.1128/jvi.16.4.790-796.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus proteins in infected cells: translation of viral polypeptides, including three P polypeptides, from RNA produced by primary transcription. Virology. 1976 Oct 15;74(2):504–519. doi: 10.1016/0042-6822(76)90356-1. [DOI] [PubMed] [Google Scholar]
- Mowshowitz S. L., Ueda M. Temperature-sensitive virion transcriptase activity in mutants of WSN influenza virus. Arch Virol. 1976;52(1-2):135–141. doi: 10.1007/BF01317872. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L., Kilbourne E. D. Genetic composition of a high-yielding influenza A virus recombinant: a vaccine strain against "Swine" influenza. Science. 1976 Oct 15;194(4262):334–335. doi: 10.1126/science.968486. [DOI] [PubMed] [Google Scholar]
- Palese P., Ritchey M. B., Schulman J. L. Mapping of the influenza virus genome. II. Identification of the P1, P2, and P3 genes. Virology. 1977 Jan;76(1):114–121. doi: 10.1016/0042-6822(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Mapping of the influenza virus genome: identification of the hemagglutinin and the neuraminidase genes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2142–2146. doi: 10.1073/pnas.73.6.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. RNA pattern of 'swine' influenza virus isolated from man is similar to those of other swine influenza viruses. Nature. 1976 Oct 7;263(5577):528–530. doi: 10.1038/263528a0. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. Isolation and characterization of influenza virus recombinants with high and low neuraminidase activity. Use of 2-(3'-methoxyphenyl)-n-acetylneuraminic acid to identify cloned populations. Virology. 1974 Jan;57(1):227–237. doi: 10.1016/0042-6822(74)90123-8. [DOI] [PubMed] [Google Scholar]
- Palese P. The genes of influenza virus. Cell. 1977 Jan;10(1):1–10. doi: 10.1016/0092-8674(77)90133-7. [DOI] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Richey M. B., Palese P. In vitro translation of influenza virus messenger RNAs. Virology. 1976 Jul 15;72(2):410–420. doi: 10.1016/0042-6822(76)90170-7. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P. Identification of the defective genes in three mutant groups of influenza virus. J Virol. 1977 Mar;21(3):1196–1204. doi: 10.1128/jvi.21.3.1196-1204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Kilbourne E. D. RNAs of influenza A, B, and C viruses. J Virol. 1976 May;18(2):738–744. doi: 10.1128/jvi.18.2.738-744.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Difference in protein patterns of influenza A viruses. Virology. 1977 Jan;76(1):122–128. doi: 10.1016/0042-6822(77)90289-6. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochovansky O. M., Hirst G. K. Infectivity and marker rescue activity of influenza virus ribonucleoprotein-polymerase complexes. Virology. 1976 Sep;73(2):339–349. doi: 10.1016/0042-6822(76)90395-0. [DOI] [PubMed] [Google Scholar]
- Schulman J. L., Palese P. Selection and identification of influenza virus recombinants of defined genetic composition. J Virol. 1976 Oct;20(1):248–254. doi: 10.1128/jvi.20.1.248-254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Tobita K., Kilbourne E. D. Isolation and preliminary characterization of temperature-sensitive mutants of influenza virus. J Virol. 1972 Oct;10(4):639–647. doi: 10.1128/jvi.10.4.639-647.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Ueda M., Tobita K., Enomoto C. Further isolation and characterization of temperature-sensitive mutants of influenza virus. Virology. 1975 Jun;65(2):363–373. doi: 10.1016/0042-6822(75)90042-2. [DOI] [PubMed] [Google Scholar]
- Tekamp P., Penhoet E. E. Message activity of influenza viral RNA. J Virol. 1976 May;18(2):812–816. doi: 10.1128/jvi.18.2.812-816.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Kilbourne E. D. Temperature-sensitive mutants of influenza virus: a mutation in the hemagglutinin gene. Virology. 1976 Apr;70(2):425–431. doi: 10.1016/0042-6822(76)90283-x. [DOI] [PubMed] [Google Scholar]