Abstract
PURPOSE
Recent studies demonstrate that addition of neoadjuvant (NA) carboplatin (Cb) to anthracycline/taxane chemotherapy improves pathological complete response (pCR) in triple negative breast cancer (TNBC). Effectiveness of anthracycline-free, platinum combinations in TNBC is not well known. Here we report efficacy of NA carboplatin + docetaxel (CbD) in TNBC.
PATIENTS AND METHODS
The study population includes 190 patients with stage I-III TNBC treated uniformly on two independent prospective cohorts. All patients were prescribed NA chemotherapy regimen of Cb (AUC 6) + D (75mg/m2) given every 21 days × 6 cycles. Pathological complete response (pCR: no evidence of invasive tumor in the breast and axilla) and Residual Cancer Burden (RCB) were evaluated.
RESULTS
Among 190 patients, median tumor size was 35mm, 52% Lymph Node positive and 16% had germline BRCA1/2 mutation. The overall pCR and RCB 0+1 rates were 55% and 68%, respectively. pCR in patients with BRCA associated and wild-type TNBC were 59% and 56%, respectively (p=0.83). On multivariable analysis stage III disease was the only factor associated with a lower likelihood of achieving a pCR. 21% and 7% of patients, respectively, experienced at least one grade 3 or 4 adverse event.
CONCLUSION
The CbD regimen was well tolerated and yielded high pCR rates in both BRCA associated and wildtype TNBC. These results are comparable to pCR achieved with addition of Cb to anthracycline-taxane chemotherapy. Our study adds to the existing data on the efficacy of platinum agents in TNBC and supports further exploration of the CbD regimen in randomized studies.
Introduction
Triple negative breast cancer (TNBC) accounts for 15% of all breast cancers, and is associated with poor long-term outcomes compared to other breast cancer subtypes(1–5). TNBC is a chemosensitive disease and therefore adjuvant chemotherapy is generally recommended for TNBC patients with stage I (T>1cm)-III disease(6, 7). However, despite receiving standard anthracycline-taxane based chemotherapy, a significant proportion (30–40%) of patients with early stage TNBC develop metastatic disease and succumb to the cancer(4, 5, 8). Thus, improved therapeutic approaches are desired for TNBC. Since no molecular actionable targets have been identified in TNBC so far, modifications of the traditional breast cancer chemotherapy regimens (i.e., the addition of carboplatin) are a potential way of improving patient outcomes.
Sporadic and germline BRCA mutation associated TNBC share several pathological and molecular similarities(9–13). These similarities have led to the exploration of DNA damaging agents like platinum compounds in the general population of patients with TNBC(14–17). While only 15–20% of TNBC patients harbor germline BRCA mutations, homologous recombination DNA repair deficiency is likely present in a much larger fraction of TNBC patients(15, 17–21). Currently it is not very clear if the efficacy of platinum salts will predominantly be restricted to BRCA mutation-associated TNBC or will these agents provide meaningful benefit in a larger fraction of sporadic TNBC(15, 22–24). Recent studies demonstrate that addition of carboplatin to anthracycline and taxane based neoadjuvant chemotherapy (NAC) improves pathological complete response (pCR) in unselected TNBC patients (14, 16, 25, 26). However, this improvement in pCR rate comes at the cost of a significant increase in toxicity (14, 16). Furthermore, anthracyclines and cyclophosphamide although very active for treatment of breast cancer, have established small but serious long term risks (secondary leukemia/myelodysplastic syndrome, cardiomyopathy)(27–29).
Therefore, the exploration of the activity of anthracycline-free chemotherapy regimens in TNBC is desired. Taxanes exhibit significant activity in TNBC and also demonstrate preclinical synergy with platinum agents thus, providing a rationale for evaluation of platinum-taxane combination in TNBC (30–33). Here-in we report a combined analysis of two separate TNBC cohorts (University of Kansas and Spanish MMJ-CAR-2014-01) treated with a uniform NAC regimen of carboplatin and docetaxel.
Patients and Methods
Patient Population
University of Kansas (KU) cohort
Patients with Stage I (T≥1 cm), II and III TNBC presenting at an academic center and five community practices within the Kansas City metropolitan area were approached for participation in an IRB approved prospective registry protocol (P.R.O.G.E.C.T. NCT02302742). Eligible female patients had biopsy proven invasive breast cancer not previously treated with taxanes, anthracyclines or carboplatin for any malignancy. Triple negativity was defined as estrogen receptor (ER) and progesterone receptor (PgR) immunohistochemical (IHC) nuclear staining of less than 10% and HER2 IHC staining 0 to 1+ or fluorescence in situ hybridization (FISH) ratio<2.0 if IHC 2+ or IHC not performed (ASCO/CAP Guideline recommendations for HER2 Testing in breast cancer) (34). From 2011 to 2015, 69 enrolled patients with stage I (T>1cm) II and III TNBC were treated with NAC regimen of carboplatin + docetaxel.
MMJ-CAR-2014-01cohort
GOMHGUGM022011 is a prospective, multicenter, non-randomized trial exploring the anti-tumor activity of neoadjuvant CbD (AEMPS code MMJ-CAR-2014-01, NCT01560663). Eligible patients included females with pathologically confirmed diagnosis of primary invasive breast cancer, stage II-III (T≥ 2cm) aged >18 years, and not previously treated with taxanes, anthracyclines or carboplatin for any malignancy. The patients were diagnosed at any of the participant academic institutions (see supplemental material for list of participating institutions) and were able to sign the IRB approved informed consent at each of the centers. Triple negativity was defined as ER and PgR nuclear staining of less than 1% by IHC, and HER2 IHC staining 0 to 1+ or FISH ratio < 2.0 if IHC 2+ or IHC not performed (ASCO/CAP Guideline recommendations for HER2 Testing in breast cancer (34). Between 2010 and 2015, 123 patients with stage II and III TNBC were enrolled, and treated with NAC regimen of carboplatin + docetaxel.
Study Procedures
Patients in both cohorts were prescribed NAC regimen of carboplatin (AUC 6) + docetaxel (75mg/m2) given every 21 days for 6 cycles. All patients received myeloid growth factor support (KU cohort: 6 mg peg-filgrastim on D2, Spanish cohort: filgastrim 300 micrgram/day × 5–7 days after chemotherapy according to the guidelines of each institution). In patients with clinically suspicious axillary lymph node/s, histological confirmation by biopsy or fine-needle aspiration was encouraged. Patients with clinically negative axillary lymph nodes could undergo pretreatment sentinel lymph node (SLN) sampling. Following NAC, all patients underwent breast surgery. Axillary sampling was required except in patients with pretreatment negative SLNs, but the extent of axillary surgery and subsequent irradiation were determined by the treating physician as was postoperative adjuvant therapy.
Pathologic Evaluation
Pathologic response was determined locally, without central pathologic review. All surgical pathology reports were centrally reviewed by the PIs of the respective cohorts. pCR was defined as the absence of residual invasive disease with or without ductal carcinoma in situ in the breast and axilla (ypT0/isN0). Patients with pCR in the breast and negative pretreatment SLNs were considered to have achieved pCR. Residual cancer burden (RCB) was recorded by local pathologists for all patients using the classification by Symmans et al. (35). Patients achieving pCR (RCB 0) or near pCR (RCB 1) are assessed within the group RCB 0+1.
Germline BRCA1/2 Testing
KU cohort
Germline testing for BRCA1/2 was done utilizing commercially available tests and laboratories. The majority of the patients (97%) had testing through Myriad Genetic Laboratories (comprehensive BRACAnalysis™ or myRisk™ Hereditary Cancer test), 3% had testing through other laboratories (BreastNext™, Ambry genetics), (OncoGeneDx™, GeneDx).
Spanish cohort
Germline testing for BRCA1/2 was done at Sistemas Genómicos facilities using Targeted Next Generation by sequencing of 7 genes (BRCA1, BRCA2, PALB2, BARD1, RAD50, RAD51C, RAD51D) and MLPA by quantification of probes corresponding to BRCA1, BRCA2 genes using the MLPA kit according to the manufacturer’s recommendations (MRC-Holland), fragment analysis by ABI 3730xl genetic analyzer and data normalization and interpretation of results using Coffalyser.net software as recommended by MRC-Holland. Only BRCA1 and BRCA2 testing are available and reported for this analysis.
According to BRCA1 and 2 results, patients were counseled in both cohorts as per institutional guidelines.
Data Collection and Statistical Methods
Relevant demographic, treatment and outcome variables of the Spanish and KU cohorts were combined for analysis. An independent statistical analysis of the combined data set was performed by both groups and any discrepancies between the two independent analyses were resolved by review of source documents.
Statistical section
Primary objective was to determine the rate of pathological complete response (pCR) and near pCR as defined above. All analyses were conducted using SPSS statistics version 22 (IBM corporation). All reported p values were two-sided without corrections for multiple comparisons. p values less than 0.05 were considered to indicate statistically significant results. Overall frequencies and percentages were summarized for race, ethnicity, menopausal status, T-stage, lymph node status, TNM stage, hormone receptor status, germline BRCA mutation status, surgery type, and family history of breast/ovarian cancer (36). Patient outcomes were summarized in terms of pCR, and RCB. Confidence intervals for the proportion of patients, who achieved pCR, and RCB 0+1, were calculated according to the exact two-sided binomial test. Categorical variables were compared between the two cohorts by Fisher’s exact test. Continuous variables were compared by non-parametric Mann-Whitney test. Logistic regression analysis was used to examine the effect of multiple variables on attainment of pathological response (pCR; RCB 0 + 1).
Results
Study Cohort
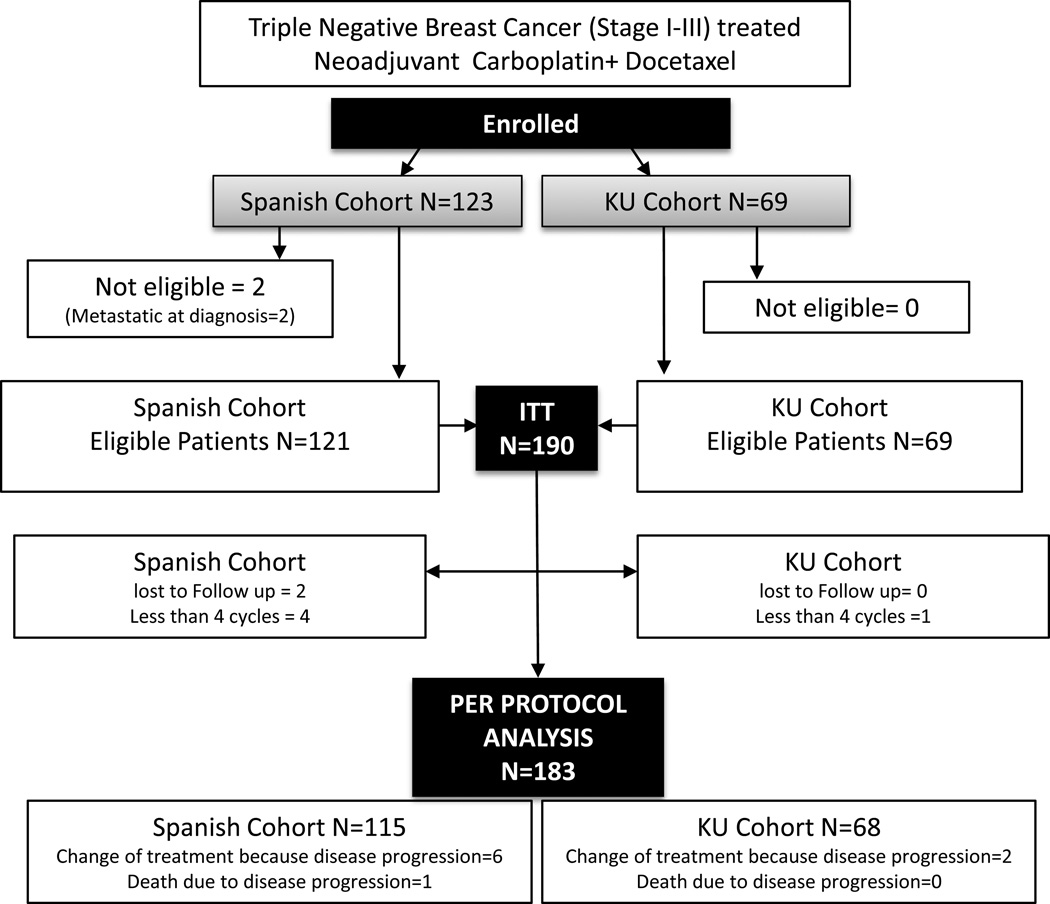
Consort diagram depicting cohort identification has been provided (figure 1). Two patients in the Spanish cohort were found to have metastatic disease after enrollment and were excluded from intention to treat (ITT) population. Seven patients in the ITT population were not included in the per protocol analysis (received less than four cycles of NAC without progression=5, lost to follow up =2). Eight patients switched to a different chemotherapy regimen due to clinically progressive disease and are designated as “no pCR” in per protocol analysis. In addition one subject died due to disease progression during NAC chemotherapy and is also included in per protocol analysis (designated as “no pCR”).
Figure 1.
CONSORT diagram
*In addition to the various centers in Spain, the Spanish cohort includes a center from Lima, Peru
Patient Characteristics
The study population included 190 subjects treated in KU and Spanish cohorts between 2010 and 2015. Table 1 describes the demographic and baseline clinical characteristics of the study population. For the overall study population median age was 51 years (range 29–81 years), 14% Hispanic, and 7% were black. Fifty-six percent had clinical stage II disease, and 52% were clinically node positive. Germline BRCA testing results were available for 87% of the study population and 16% of the study population carried a deleterious BRCA1/2 mutation. A small percentage (5%, all from KU cohort) of patients had ER/PgR expression between 1–10%. Compared to the KU cohort, patients in the Spanish cohort had larger tumors, and higher percentage of node positive and stage III disease. Family history was assessed in the two cohorts based on the local guidelines (specific guidelines are provided in supplement section). Twenty-eight percent of patients reported a positive family history of breast or epithelial ovarian cancer (KU: 39%, Spanish cohort: 21%). To provide consistent evaluation of family history, when the Spanish family history criteria was applied to the entire study population 24% (45/190) of patients demonstrated positive family history.
Table 1.
Patient Demographics
| All Patients | KU | Spanish | P* | |
|---|---|---|---|---|
| (N=190) | (N=69) | (N=121) | ||
| Age at Diagnosis (years) (Median , Range) |
51 (29–81) |
52 (30–80) |
50 (29–81) |
0.86 |
| Race | ||||
| Caucasian | 174 (92%) | 57 (83%) | 117 (96%) | <0.001 |
| Black | 14 (7%) | 12 (17%) | 2 (2%) | |
| Asian | 2 (1%) | 0 (0%) | 2 (2%) | |
| Ethnicity | ||||
| Hispanic | 26 (14%) | 2 (3%) | 24 (20%) | 0.001 |
| Non-Hispanic | 163 (86%) | 67 (97%) | 97 (80%) | |
| Menopausal status | ||||
| Pre/Peri | 86 (45%) | 30 (44%) | 56 (46%) | 0.59 |
| Post | 98 (52%) | 38 (55%) | 60 (50%) | |
| Unknown | 6 (3%) | 1 (1%) | 5 (4%) | |
| T Stage Number | ||||
| 1 | 23 (12%) | 19 (27%) | 4 (3%) | <0.001 |
| 2 | 104 (55%) | 37 (54%) | 67 (55%) | |
| 3 | 34 (18%) | 11 (16%) | 23 (19%) | |
| 4 | 29 (15%) | 2 (3%) | 27 (23%) | |
| Median Tumor Size (mm) (range) |
35 (9–180) | 30.5 (10–111) | 41(9–180) | <0.001 |
| Lymph Node Status | ||||
| Negative | 90 (47%) | 47 (68%) | 43 (35%) | <0.001 |
| Positive | 98 (52%) | 22 (32%) | 76 (63%) | |
| Unknown | 2 (1%) | 0 (0%) | 2 (2%) | |
| TNM Stage | ||||
| I | 20 (11%) | 18 (26%) | 2 (2%) | <0.001 |
| II | 107 (56%) | 41 (59%) | 66 (54%) | |
| III | 63 (33%) | 10 (15%) | 53 (44%) | |
| ER/PR | ||||
| 0% | 184 (97%) | 63 (91%) | 121(100%) | 0.001 |
| 1–10% | 6 (3%) | 6 (9%) | 0 (0%) | |
| Germline BRCA Mutation | ||||
| Absent | 136 (71%) | 49 (71%) | 87 (72%) | 0.084 |
| Present | 30 (16%) | 15 (22%) | 15 (12%) | |
| Unknown | 24 (13%) | 5 (7%) | 19 (16%) | |
| Surgery type# | ||||
| Lumpectomy | 84 (45%) | 23 (33%) | 61 (53%) | 0.045 |
| Mastectomy | 101 (55%) | 46 (67%) | 55 (47%) | |
| Axillary surgery | ||||
| Pre NAC SLNB | 24 (13%) | 0 (0%) | 24 (20%) | <0.001 |
| Post NAC | ||||
| SLNB without ALND | 50 (27%) | 46 (66%) | 4 (3%) | <0.001 |
| SLNB plus ALND | 15 (8%) | 6 (9%) | 9 (8%) | |
| ALND | 94 (50%) | 15 (22%) | 79 (66%) | |
| No axillary procedure | 5 (2%) | 2 (3%) | 3 (3%) | |
| Positive family History⍰ | ||||
| Yes | 52 (28%) | 27 (39%) | 25 (21%) | 0.11 |
| No | 136 (72%) | 42 (61%) | 94 (79%) | |
Mann-Whitney non-parametric test for continuous data; Pearson Chi-Square/Fisher Exact test for categorical data.
Surgical information was not available for 5 patients in the IIT population.
Family history information was not available for 2 patients from Spanish-cohort. Family history is reported utilizing cohort specific definitions of positive family history as outlined in patient characteristics section. Upon application of Spanish family history definition to the entire study population, 24% of patients had positive family history
Treatment Response
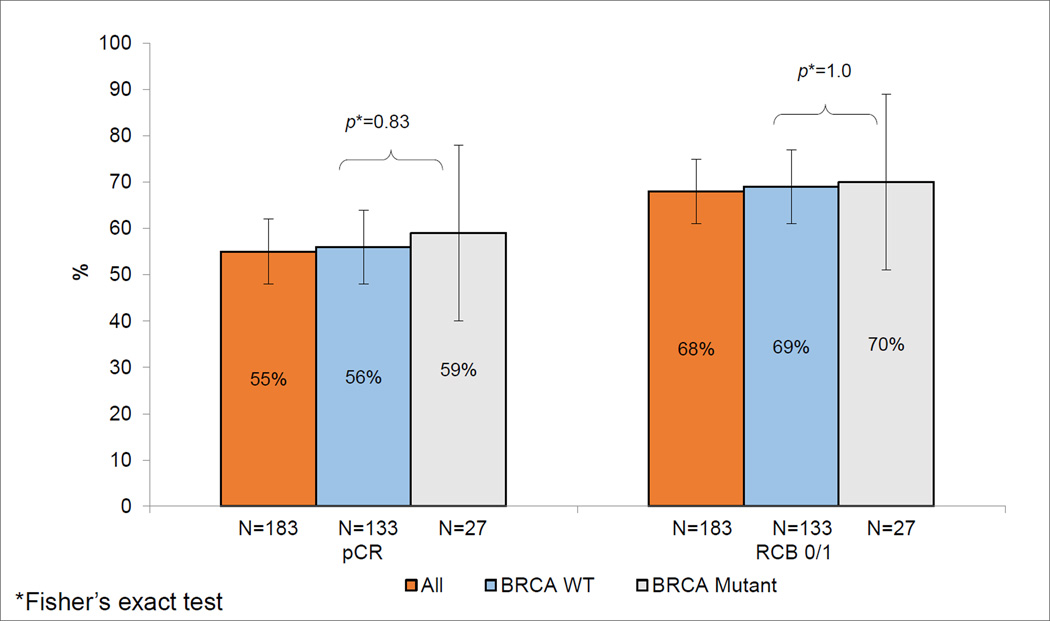
Pathological complete response and RCB 0+1 for the per protocol study population was 55% (95% CI: 48–62%) and 68% (95% CI: 61%, 75%), respectively. Both pCR and RCB 0+1 rates were higher in the KU cohort compared to the Spanish trial (Table 2). pCR and RCB 0+1 rates were lower in stage III disease compared to stage I-II disease (pCR: 37% vs. 63% p=.002; RCB 0+1: 49% vs. 77%, p=<.001). When assessed by individual TNM stage, the pCR rates were similar in the KU and Spanish cohorts (Table 2). When assessed by germline BRCA status, pCR rate was 56% in BRCA wild type and 59% in BRCA mutation-associated TNBC (p=0.83) (Figure 2). Family history of breast and ovarian cancer did not impact pCR or RCB 0+1 rates using individual cohort positive family history criteria (Supplemental Table 1) or using Spanish trial positive family history criteria (data not shown).
Table 2.
Pathological response by germline BRCA mutation status and Stage
| All Patients | KU | Spanish | P* | |
|---|---|---|---|---|
| All patients | N=183 | N=68 | N=115 | |
| pCR | N (%) (95% CI) | N (%) (95% CI) | N (%) (95% CI) | |
| Yes | 100 (55%) (48%, 62%) | 44 (65%) (54%, 76%) | 56 (49%) (40%, 58%) | 0.046 |
| No | 83 (45%) (38%, 52%) | 24 (35%) (24%, 46%) | 59 (51%) (42%, 60%) | |
| RCB 0 +1 | ||||
| Yes | 125 (68%) (61%, 75%) | 55 (81%) (72%, 90%) | 70 (61%) (52%, 70%) | 0.005 |
| No | 58 (32%) (25%, 39%) | 13 (19%) (10%, 28%) | 45 (39%) (30%, 48%) | |
| BRCA wild type | N=133 | N=48 | N=85 | |
| pCR | N (%) (95% CI) | N (%) (95% CI) | N (%) (95% CI) | |
| Yes | 75 (56%) (48%, 64%) | 31 (65%) (52%, 78%) | 44 (52%) (41%, 63%) | 0.20 |
| No | 58 (44%) (36%, 52%) | 17 (35%) (22%, 48%) | 41 (48%) (37%, 59%) | |
| RCB 0 +1 | ||||
| Yes | 92 (69%) (61%, 77%) | 39 (81%) (70%, 92%) | 53 (62%) (52%, 72%) | 0.031 |
| No | 41 (31%) (23%, 39%) | 9 (19%) (8%, 30%) | 32 (38%) (28%, 48%) | |
| BRCA mutation positive | N=27 | N=15 | N=12 | |
| pCR | N (%) (95% CI) | N (%) (95% CI) | N (%) (95% CI) | |
| Yes | 16 (59%) (40%, 78%) | 11 (73%) (51%, 95%) | 5 (42%) (14%, 70%) | 0.13 |
| No | 11 (41%) (22%, 60%) | 4 (27%) (5%, 49%) | 7 (58%) (30%, 86%) | |
| RCB 0+1 | ||||
| Yes | 19 (70%) (53%, 87%) | 12 (80%) (60%, 100%) | 7 (58%) (30%, 86%) | 0.40 |
| No | 8 (30%) (13%, 47%) | 3 (20%) (0%, 40%) | 5 (42%) (14%, 70%) | |
| BRCA status unknown | N=23 | N=5 | N=18 | |
| pCR | N (%) (95% CI) | N (%) (95% CI) | N (%) ( 95% CI) | 1 |
| Yes | 9 (39%) (19%, 59%) | 2 (40%) (3%, 83%) | 7 (39%) (16%, 62%) | |
| No | 14 (61%) (41%, 81%) | 3 (60%) (17%, 103%) | 11 (61%) (38%, 84%) | |
| RCB 0 +1 | ||||
| Yes | 14 (61%) (41%, 81%) | 4 (80%) (45%, 115%) | 10 (56%) (33%, 79%) | 0.61 |
| No | 9 (39%) (19%, 56%) | 1 (20%) (15%, 55%) | 8 (44%) (21%, 67%) | |
| TNM stage I and II | N=124 | N=58 | N=66 | |
| pCR | N (%) (95% CI) | N (%) (95% CI) | N (%) ( 95% CI) | |
| Yes | 78 (63%) (54%, 71%) | 40 (69%) (57%, 81%) | 38 (58%) (46, 70%) | 0.20 |
| No | 48 (37%) (29%, 46%) | 18 (31%) (19%, 43%) | 28(42%) (30%, 54%) | |
| RCB 0 +1 | ||||
| Yes | 95 (77%) (70%, 84%) | 50 (86%) (77%, 95%) | 45 (68%) (57%, 79%) | 0.02 |
| No | 29 (23%) (16%, 30%) | 8 (14%) (5%, 23%) | 21 (32%) (21%, 43%) | |
| TNM stage III | N=59 | N=10 | N=49 | |
| pCR | N (%) (95% CI) | N (%) (95% CI) | N (%) (95% CI) | |
| Yes | 22 (37%) (25%, 49%) | 4 (40%) (10%, 70%) | 18 (37%) (23%, 51%) | 1.0 |
| No | 37 (63%) (51%, 75%) | 6 (60%) (30%, 90%) | 31(63%) (49%, 77%) | |
| RCB 0 + 1 | ||||
| Yes | 29 (49%) (36%, 62%) | 5 (50%) (19%, 81%) | 24 (49%) (35%, 63%) | 1.0 |
| No | 30 (51% (38%, 64%) | 5 (50%) (19%, 81%) | 25 (51%) (37%, 65%) | |
Fisher Exact test.
Figure 2.
Carboplatin plus Docetaxel pathological response by gBRCA status
Due to the inclusion of a small number of patients with 1–10% ER/PgR expression (KU cohort), pCR analysis was also performed according to ER/PgR expression status. Fifty and 55% percent of patients with ER/PgR1–10% (n=6) and ER/PgR <1% (n=177) achieved pCR, respectively (Supplemental Table 2, p=1.0).
On univariate analysis presence of lower T stage (T1/2 vs. T3/4), lower TNM stage (I/II vs. III) and being treated in the KU cohort were associated with higher likelihood of achieving a pCR (Table 3). For RCB 0+1 presence of lower T stage (T1/2 vs. T3/4), lower TNM stage (I/II vs. III), lymph node negative status and being treated in the KU cohort were associated with higher likelihood of achieving a RCB 0+1 (Supplemental Table 3). Age, germline BRCA mutation and family history of breast/ovarian cancer did not influence the likelihood of achieving pCR or RCB 0+1. Compared to the KU cohort the Spanish cohort had a higher proportion of patients with stage III disease and on multivariable logistic regression analysis the presence of TNM stage III disease was the only factor associated with a lower likelihood of achieving a pCR (HR 0.35, 95% CI: 0.19, 0.67) (factors included in multivariable analysis: age, lymph node status, T stage, TNM stage, germline BRCA mutation status, family history and cohort type).
Table 3.
Predictors of pathological complete response on univariate analysis#
| OR | 95% CI | P* | |
|---|---|---|---|
| Age | |||
| ≤50 | 1 | ||
| >50 | 0.74 | 0.4, 1.33 | 0.37 |
| LN status | |||
| Negative | 1 | ||
| Positive | 0.71 | 0.39, 1.27 | 0.30 |
| T stage | |||
| T1/2 | 1 | ||
| T3/4 | 0.39 | 0.21,0.73 | 0.003 |
| TNM stage | |||
| I/ II | 1 | ||
| III | 0.35 | 0.19, 0.67 | 0.001 |
| gBRCA mutation | |||
| Negative/unknown | 1 | ||
| Positive | 1.25 | 0.54, 2.86 | 0.68 |
| Family History | |||
| No | 1 | ||
| Yes | 0.96 | 0.5, 1.85 | 1.0 |
| Cohort | |||
| Spanish | 1 | ||
| KU | 1.93 | 1.04, 3.50 | 0.046 |
Odds ratios (ORs) and 2-sided P values from Fisher’s exact test.
On multivariable logistic regression analysis (factors included: age, lymph node status, T stage, TNM stage, germline BRCA mutation status, family history and cohort type) presence of TNM stage III disease was the only factor associated with a lower likelihood of achieving a pCR (HR 0.35, 95% CI: 0.19, 0.67)
Adverse Events
Twenty-eight percent (54/190) of patients experienced one or more grade 3/4 adverse events (Grade 3=21%, Grade 4=7%) (Table 4). Eighty-three percent of patients completed all 6 cycles of treatment. Twelve percent (22/183) of patients discontinued treatment prematurely: 4.4% (8/183) because of progressive disease, 6.0% (11/183) because of toxicity, 1.6% (3/183) because of other reasons. No treatment related deaths were reported.
Table 4.
Grade 3 or 4 Treatment-Related Toxicities
| N = 190 | G 3/4 n (%) | G3 n (%) | G4 n (%) |
|---|---|---|---|
| Leukopenia | 2 (1%) | 2 (1%) | 0 (0%) |
| Neutropenia | 22 (12%) | 13 (7%) | 9 (5%) |
| Thrombocytopenia | 11 (6%) | 9 (5%) | 2 (1%) |
| Anemia | 7 (4%) | 6 (3%) | 1 (1%) |
| Febrile Neutropenia | 8 (4%) | 6 (3%) | 2 (1%) |
| Nausea | 5 (3%) | 5 (3%) | 0 (0%) |
| Vomiting | 5 (3%) | 4 (2%) | 1 (1%) |
| Mucositis | 2 (1%) | 2 (1%) | 0 (0%) |
| Diarrhea | 5 (3%) | 5 (3%) | 0 (0%) |
| Peripheral Neuropathy | 3 (2%) | 3 (2%) | 0 (0%) |
| Fatigue | 2 (1%) | 2 (1%) | 0 (0%) |
| Other* | 9 (5%) | 9 (5%) | 0 (0%) |
Other: Hepatic/transaminase elevation (n=3), rash (n=2), hyponatremia (n=1), thrombosis (n=1), allergic reaction (n=1), dehydration (n=1)
Discussion
Triple negative breast cancer, which is defined by the lack of ER and PgR expression and absence of HER2 over expression and/or gene amplification, is currently the most lethal subtype of breast cancer. Due to the lack of molecular targets, chemotherapy is the only available treatment for TNBC. Despite achieving higher rates of pCR with conventional neoadjuvant chemotherapy, TNBC phenotype is associated with higher relapse rates than luminal tumors among patients with residual disease (1, 4, 5, 37). Although current guidelines recommend anthracycline and taxane-based chemotherapy for (neo)adjuvant treatment of TNBC, these schedules have limitations (6, 7). First of all, the studies that support this recommendation included patients irrespective of HER 2 and/or ER status. Second, anthracyclines are associated with established irreversible and long-term adverse events and finally 30–40% of patients with early TNBC experience relapse despite receiving anthracycline-taxane based (neo)adjuvant treatment (8, 27–29, 38, 39).Thus, there is a need to design clinical trials in this population of patients incorporating new drugs and/or optimizing the administration of standard chemotherapy to improve the efficacy and reduce the toxicity.
Recent studies demonstrate that addition of platinum salts, namely carboplatin, to anthracycline and taxane based neoadjuvant chemotherapy improves pathological complete response in TNBC(14, 16, 25, 26). However, this improvement in pCR rate comes at the cost of an increase in toxicity and decrease in tolerability of chemotherapy (14, 16). The efficacy of anthracycline-devoid neoadjuvant combinations in sporadic and BRCA associated TNBC has not been properly explored so far. Over a decade ago a few neoadjuvant studies evaluated platinum-taxane combination chemotherapy in unselected locally advanced breast cancer patients. These studies demonstrated overall modest pCR rates of 14–22% with higher pCR rates noted in the ER negative subgroup (40, 41). Over the last few years improved understanding of biology of breast cancer subtypes coupled with the preclinical data supporting activity of platinum agents in TNBC has led to re-emergence of interest in exploration of platinum agents for treatment of TNBC (14, 16, 25, 26).Taxanes are an integral part of adjuvant/neoadjuvant chemotherapy regimens for breast cancer treatment and appear to contribute particularly among patients with TNBC(30). On the other hand, there is abundant preclinical and clinical data demonstrating synergy between platinum compounds and taxanes in several solid tumor types (31–33) . An interesting study, the randomized TNT trial, compared single agent carboplatin to single agent docetaxel in metastatic TNBC patients. The trial showed both significant single agent activity of docetaxel and carboplatin in unselected TNBC and non-cross resistance between these agents (22). Furthermore, in a randomized neoadjuvant study comparing single agent docetaxel versus single agent doxorubicin, the taxane demonstrated a significant superior activity with respect to single agent doxorubicin in cDNA microarray-defined basal-like TNBC, but not in the remaining subtypes (42). The above clinical and preclinical data provided the rationale for the two different studies (the KU and the Spanish trials) that were conducted in parallel and whose merged results are presented in this article. Both evaluated the antitumor activity of an identical neoadjuvant combination chemotherapy regimen of carboplatin plus docetaxel in TNBC.
The merged results presented here show that the pCR rates achieved with this CbD chemotherapy regimen in TNBC seem to be numerically higher than as those reported in several trials using classical neoadjuvant anthracycline-taxane combinations, in which 28–39% of TNBC patients achieved a pCR (14, 43, 44). This pCR rate of 55% was achieved even though more than half of the patients had node positive disease and a third had stage III disease. Previous studies have demonstrated that pathological complete response is a very robust surrogate for improved long term outcomes, specifically for aggressive tumor subtypes like TNBC (1, 45). Thus, pCR is considered as a good end point for early evaluation of new treatment approaches for TNBC.
Addition of carboplatin to anthracycline/taxane based chemotherapy in TNBC increases pathological complete responses from around 40% to 50–54% (14, 16, 25),. However, the impact of this approach on long term outcomes is not yet clear. In GeparSixto a 44% improvement in 3-year Event free survival (EFS) was noted with the addition of concurrent carboplatin to anthracycline plus taxane plus bevacizumab chemotherapy backbone. On the other hand, in CALGB 40603 addition of carboplatin to taxane followed by sequential doxorubicin/cyclophosphamide did improve pCR rate but, improvement in 3 year EFS or overall survival (OS) was not noted (46, 47). It is important to point out that, neither of these two trials were powered sufficiently for EFS and OS end points and thus cannot be considered definitive studies to answer the question of clinical utility of platinum agents for early stage TNBC. The optimal dose, sequence and chemotherapy backbone for efficacious incorporation of platinum in treatment of early stage TNBC are also not yet known. Several ongoing randomized phase III trials are evaluating various schedules and combinations of platinum salts in early stage TNBC (NCT02488967, NCT02455141, NCT02441933, NCT02445391, NCT02125344, NCT02032277).
Pathological complete response in our study with the CbD regimen was 55% which is similar to pCR attained with addition of carboplatin to anthracycline/taxane chemotherapy. However, the addition of carboplatin to anthracycline-taxane regimens is associated with a significant increase in acute toxicity with only 50–76% of patients completing all study treatment (14, 16, 25). In contrast, chemotherapy regimen of CbD has a much favorable toxicity profile with 83% of patients completing all 6 cycles of therapy.
There were some differences between the KU and Spanish cohorts. Compared to the KU cohort, patients in the Spanish cohort had higher disease burden (larger tumors, more node positive and stage III disease). Patients in the Spanish cohort were also more likely to undergo sentinel lymph node biopsy (SLNB) prior to NAC (35% vs 0% p<0.0001) and to undergo breast conservation surgery (vs. mastectomy). These surgical differences are a reflection the local surgical practice patterns and patient preference regarding breast conservation in the respective countries. The percentage of patients with deleterious BRCA mutation in the KU series is consistent with other reported studies from the United States (15, 19). The Spanish series reported a slightly lower percent of patients with deleterious BRCA mutations, but this prevalence rate is also consistent with the reported rates in Spanish women with TNBC non-selected for family history(48, 49). We noted a pCR rate of 59% in BRCA mutation carriers, which is consistent with previously reported pCR rates with single agent platinum and platinum-anthracyline-taxane combination chemotherapy in BRCA mutation carriers(24, 50).
In our study, deleterious BRCA mutation and family history were not associated with pCR and RCB 0+1 with equally good pathological response rates noted in BRCA wild type and mutant patients. This might be explained by the idea that docetaxel and carboplatin combination offers antitumor coverage for both sporadic and BRCA-associated TNBC. Docetaxel seems to be better in the first group, while carboplatin is more active in BRCA-mutated patients. Furthermore, it is now becoming increasingly evident that 40–50% of BRCA wild type TNBC harbor homologous recombination deficiency and benefit of platinum agents may very well extend much beyond BRCA mutation associated breast cancers (15, 17, 21). The tolerance to CbD was good. Only 6% of patients discontinued therapy because of toxicity. A similar chemotherapy regimen, the TCH regimen (that combines carboplatin and docetaxel with trastuzumab), is widely used for the neo/adjuvant treatment of HER-2 positive breast cancer.
Although presenting very robust pathological response data our study does have several limitations. The decision to combine the two series with the purpose of reporting pathological response was made post hoc and was not preplanned. However, this report includes almost 200 TNBC patients treated with the same regimen in three different continents. In this combined analysis more than half of the patients had node positive disease, a third had stage III disease and 16% harbored deleterious BRCA mutation. These patient characteristics are very similar to contemporary randomized neoadjuvant clinical trials in TNBC. We also acknowledge the lack of a control arm in both series; a deficit which can only be addressed in setting of a randomized trial. In fact, an ongoing randomized phase II study is currently comparing neoadjuvant CbD regimen to the anthracycline-taxane-carboplatin regimen used in CALGB 40603 (NCT02413320).
In conclusion, the combination of carboplatin plus docetaxel with G-CSF support yielded a significant antitumor activity in stage I-III TNBC with manageable toxicity. This anthracycline-free regimen should be compared to standard taxane-anthracycline chemotherapy as neoadjuvant therapy of TNBC in a prospective randomized trial.
Supplementary Material
Translational Relevance.
Pathological and molecular similarities between sporadic and germline BRCA mutation breast cancers associated and Triple negative breast cancer (TNBC) have led to the exploration of DNA damaging agents like platinum compounds in TNBC. In this manuscript we report robust pathological complete response rates in 190 patients treated with anthracycline-free chemotherapy regimen of carboplatin plus docetaxel. Germline BRCA1/2 mutations may be an important biomarker of response to platinum agents in TNBC. It has been speculated that improvement in pCR noted with addition of carboplatin to traditional chemotherapy in TNBC may be driven primarily by high responses in germline BRCA mutation carriers. However, this platinum-taxane chemotherapy regimen rendered equally high pathological complete response rates in BRCA-associated (pCR of 59%) and BRCA-wildtype (pCR of 56%) TNBC. The results of our study support further evaluation of platinum-chemotherapy in both BRCA-associated and wildtype TNBC.
Acknowledgments
Acknowledgement of research support: The KU PROGECT Registry was supported by the University of Kansas Research Career Award and the University of Kansas Cancer Center’s CCSG (P30 CA168524) Biospecimen Repository Core Facility.
Funding for M Martin was supported by research grant from Instituto de Salud Carlos III (ISCIII, PI 12/02684) and FEDER (RETICC-RD12/0036/0076). “Ayuda cofinanciada por el Fondo Europeo de Desarrollo Regional (FEDER). Una manera de hacer Europa”). CM Perou was supported by funds from the NCI Breast SPORE program P50-CA58223.
Footnotes
Disclaimer: Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014 and at the IX Congress of the SEOM (Spanish Society for Medical Oncology), Madrid, Spain, November 2013.
Authors’ Disclosures of Potential Conflicts of Interest
No potential conflicts of interest for all of the authors
REFERENCES
- 1.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 2.Osborne CR, Kannan L, Xie XJ, Ashfaq R, Bian A, Baylor TD. Neoadjuvant chemotherapy for basal-like breast cancer cohort: clinical and pathological outcomes. Breast Cancer Research and Treatment. 2006;100:S53. [Google Scholar]
- 3.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. Journal of the National Cancer Institute. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 6.NCCN. [cited 2013; 3.2014];NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer. 2014 [Available from: http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
- 7.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 9.Prat A, Cruz C, Hoadley KA, Diez O, Perou CM, Balmana J. Molecular features of the basal-like breast cancer subtype based on BRCA1 mutation status. Breast cancer research and treatment. 2014;147:185–191. doi: 10.1007/s10549-014-3056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. Journal of the National Cancer Institute. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 11.Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 12.Richardson AL, Wang ZC, De Nicolo A, Lu X, Brown M, Miron A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer cell. 2006;9:121–132. doi: 10.1016/j.ccr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Manie E, Vincent-Salomon A, Lehmann-Che J, Pierron G, Turpin E, Warcoin M, et al. High frequency of TP53 mutation in BRCA1 and sporadic basal-like carcinomas but not in BRCA1 luminal breast tumors. Cancer Res. 2009;69:663–671. doi: 10.1158/0008-5472.CAN-08-1560. [DOI] [PubMed] [Google Scholar]
- 14.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the Addition of Carboplatin and/or Bevacizumab to Neoadjuvant Once-per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance) J Clin Oncol. 2014 doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telli ML, Jensen KC, Vinayak S, Kurian AW, Lipson JA, Flaherty PJ, et al. Phase II Study of Gemcitabine, Carboplatin, and Iniparib As Neoadjuvant Therapy for Triple-Negative and BRCA1/2 Mutation-Associated Breast Cancer With Assessment of a Tumor-Based Measure of Genomic Instability: PrECOG 0105. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1895–1901. doi: 10.1200/JCO.2014.57.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. The Lancet Oncology. 2014;15:747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 17.Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, et al. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1902–1909. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:1082–1089. doi: 10.1158/1078-0432.CCR-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma P, Klemp JR, Kimler BF, Mahnken JD, Geier LJ, Khan QJ, et al. Germline BRCA mutation evaluation in a prospective triple-negative breast cancer registry: implications for hereditary breast and/or ovarian cancer syndrome testing. Breast cancer research and treatment. 2014;145:707–714. doi: 10.1007/s10549-014-2980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:304–311. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vollebergh MA, Lips EH, Nederlof PM, Wessels LF, Wesseling J, Vd Vijver MJ, et al. Genomic patterns resembling BRCA1- and BRCA2-mutated breast cancers predict benefit of intensified carboplatin-based chemotherapy. Breast cancer research : BCR. 2014;16:R47. doi: 10.1186/bcr3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tutt AEPKL, Gilett C, Pinder S, Abraham J, Barrett S, Barrett-Lee P, Chan S, Cheang M, Fox L, Grigoriadis A, Harper-Wynne C, Hatton M, Kernaghan S, Owen J, Parker P, Rahman N, Roylance R, Smith I, Thompson R, Tovey H, Wardley A, Wilson G, Harries M, Bliss J. The TNT trial: A randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer (CRUK/07/012) 2014 Abstract S3-01, 2014. [Google Scholar]
- 23.Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Minckwitz GHE, Fasching PA, Hauke J, Schneeweiss A, Salat C, Rezai M, Blohmer JU, Zahm DM, Jackisch C, Gerber B, Klare P, Kummel S, Eidtmann H, Paepke S, Nekljudova V, Loibl S, Untch M, Schmultzler RK. Pathological complete response (pCR) rates after carboplatin-containing neoadjuvant chemotherapy in patients with germline BRCA (gBRCA) mutation and triple-negative breast cancer (TNBC): Results from GeparSixto. Journal of Clinical Oncology. 2014;32 [Google Scholar]
- 25.Rugo HSOO, DeMichele A, et al. Veliparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: First efficacy results from the I-SPY2 trial. San Antonio Breast Cancer Symposium; 2013. [Google Scholar]
- 26.Tamura KHJ, Tsuda H, Yoshida M, Yamauchi H, Aogi K, Shimizu S, Iwata H, Masuda N, Yamamoto N, Inoue K, Ohno S, Kuroi K, Sukigara T, Fujiwara Y, Andoh M. Randomized phase II study of weekly paclitaxel with or without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA HER2-negative breast cancer. Journal of Clinical Oncology. 2014;32 [Google Scholar]
- 27.Tan TC, Neilan TG, Francis S, Plana JC, Scherrer-Crosbie M. Anthracycline-Induced Cardiomyopathy in Adults. Comprehensive Physiology. 2015;5:1517–1540. doi: 10.1002/cphy.c140059. [DOI] [PubMed] [Google Scholar]
- 28.Wolff AC, Blackford AL, Visvanathan K, Rugo HS, Moy B, Goldstein LJ, et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol. 2015;33:340–348. doi: 10.1200/JCO.2013.54.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khouri MG, Douglas PS, Mackey JR, Martin M, Scott JM, Scherrer-Crosbie M, et al. Cancer Therapy-Induced Cardiac Toxicity in Early Breast Cancer: Addressing the Unresolved Issues. Circulation. 2012;126:2749–2763. doi: 10.1161/CIRCULATIONAHA.112.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D, et al. HER2 and Response to Paclitaxel in Node-Positive Breast Cancer. New England Journal of Medicine. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 31.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. The New England journal of medicine. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 32.Engblom P, Rantanen V, Kulmala J, Helenius H, Grenman S. Additive and supra-additive cytotoxicity of cisplatin-taxane combinations in ovarian carcinoma cell lines. British journal of cancer. 1999;79:286–292. doi: 10.1038/sj.bjc.6690046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delbaldo C, Michiels S, Syz N, Soria JC, Le Chevalier T, Pignon JP. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non-small-cell lung cancer: a meta-analysis. Jama. 2004;292:470–484. doi: 10.1001/jama.292.4.470. [DOI] [PubMed] [Google Scholar]
- 34.Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. Journal of Clinical Oncology. 2013 doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 35.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 36.Sobin LH, Fleming ID. TNM classification of malignant tumors. cancer. (fifth) 1997;80:1803–1804. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and Clinical Characterization of the Basal-Like Subtype of Invasive Breast Carcinoma. Clinical Cancer Research. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 39.Tan D, Marchió C, Jones R, Savage K, Smith I, Dowsett M, et al. Triple negative breast cancer: molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Research and Treatment. 2008;111:27–44. doi: 10.1007/s10549-007-9756-8. [DOI] [PubMed] [Google Scholar]
- 40.Lee YJ, Doliny P, Gomez-Fernandez C, Powell J, Reis I, Hurley J. Docetaxel and cisplatin as primary chemotherapy for treatment of locally advanced breast cancers. Clinical breast cancer. 2004;5:371–376. doi: 10.3816/cbc.2004.n.044. [DOI] [PubMed] [Google Scholar]
- 41.Hurley J, Reis I, Silva O, Gomez C, DeZarraga F, Velez P, et al. Weekly docetaxel/carboplatin as primary systemic therapy for HER2-negative locally advanced breast cancer. Clinical breast cancer. 2005;5:447–454. doi: 10.3816/cbc.2005.n.003. [DOI] [PubMed] [Google Scholar]
- 42.Martin M, Romero A, Cheang MC, Lopez Garcia-Asenjo JA, Garcia-Saenz JA, Oliva B, et al. Genomic predictors of response to doxorubicin versus docetaxel in primary breast cancer. Breast Cancer Res Treat. 2011;128:127–136. doi: 10.1007/s10549-011-1461-y. [DOI] [PubMed] [Google Scholar]
- 43.Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3739–3746. doi: 10.1200/JCO.2011.35.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortazar P, Geyer CE., Jr Pathological complete response in neoadjuvant treatment of breast cancer. Annals of surgical Oncology. 2015;22:1441–1446. doi: 10.1245/s10434-015-4404-8. [DOI] [PubMed] [Google Scholar]
- 46.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC +/− carboplatin and/or bevacizumab in triple-negative breast cancer: Outcomes from CALGB 40603 (Alliance) 38 Annual SABCS. 2015 [Google Scholar]
- 47.Von Minckwitz GLS, Schneeweiss A, et al. Early survival analysis of the randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto) San Antonio, Texas: San Antonio Breast Cancer Symposium; 2015. [Google Scholar]
- 48.Andres R, Pajares I, Balmana J, Llort G, Ramon YCT, Chirivella I, et al. Association of BRCA1 germline mutations in young onset triple-negative breast cancer (TNBC) Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2014;16:280–284. doi: 10.1007/s12094-013-1070-9. [DOI] [PubMed] [Google Scholar]
- 49.Zugazagoitia J, Perez-Segura P, Manzano A, Blanco I, Vega A, Custodio A, et al. Limited family structure and triple-negative breast cancer (TNBC) subtype as predictors of BRCA mutations in a genetic counseling cohort of early-onset sporadic breast cancers. Breast cancer research and treatment. 2014;148:415–421. doi: 10.1007/s10549-014-3167-4. [DOI] [PubMed] [Google Scholar]
- 50.Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast cancer research and treatment. 2014;147:401–405. doi: 10.1007/s10549-014-3100-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.