Abstract
Purpose
To investigate the relationship between vitamin D and glaucoma.
Methods
This retrospective, cross-sectional study included subjects who underwent a health screening at the Health Screening Center of Kangbuk Samsung Hospital from August 2012 to July 2013. All fundus photographs were reviewed by ophthalmologists. The ophthalmologists determined if an eye was glaucomatous based on the criteria set forth by the International Society of Geographical and Epidemiological Ophthalmology and by the appearance of the retinal nerve fiber layer and optic disc. If the subjects previously underwent an ophthalmologic examination, they were enrolled based on the documented history. In addition to fundus photographs, each participant underwent a systemic examination including blood sampling and sociodemographic and behavioral questionnaires. The subjects were divided into five groups according to serum 25-hydroxyvitamin D (25(OH)D) level. Multivariate logistic regression models were constructed to assess possible associations between elevated glaucoma risk and systemic factors with a p < 0.2 on univariate analysis.
Results
Of the 169,208 subjects older than 20 years, 123,331 were eligible for the study. There was no difference in the prevalence of glaucoma according to quintile of serum 25(OH)D level based on sex (p = 0.412 for males, p = 0.169 for females). According to the multivariable-adjusted logistic analysis, the odds ratio of glaucoma for the fourth quintile was significantly lower than that of the first quintile in females (odds ratio, 0.713; 95% confidence interval, 0.520 to 0.979).
Conclusions
Lower 25(OH)D level was significantly associated with an elevated risk of glaucoma in females compared with higher 25(OH)D level. Further evaluation is needed to investigate the relationship between glaucoma and vitamin D.
Keywords: Glaucoma, Serum 25-hydroxyvitamin D, Vitamin D
Glaucoma is a chronic progressive optic neuropathy that causes irreversible damage. Therefore, early diagnosis and appropriate treatment to control various risk factors associated with glaucoma are important. Risk factors associated with the development of glaucoma have been reported by many researchers and include both ocular and non-ocular (systemic) factors such as myopia, central corneal thickness, disc hemorrhage, and genetic factors [1,2,3,4,5,6,7,8,9,10,11,12,13]. The majority of these risk factors cannot be adjusted; however, intraocular pressure (IOP) is one aspect of intraocular risk factors that can be modified. Many researchers have investigated the modifiable systemic factors associated with glaucoma, such as metabolic syndrome.
Recently, vitamin D has become a major area of interest in medical research. Vitamin D is an important secosteroid hormone that plays a role in the signaling pathways related to bone and mineral metabolism, cellular proliferation, immune modulation, and oxidative stress [14,15]. In general, 25-hydroxyvitamin D (25(OH)D) is considered the most reliable biomarker for assessing an individual's vitamin D status. Based on the results of serum 25(OH)D measurements in large, population-based studies, vitamin D deficiency is associated with neurodegenerative effects on the central nervous system [16,17]. Several biological experiments have indicated that vitamin D regulates neuroprotective functions in the central nervous system, including the optic nerve [18,19]. Moreover, vitamin D status can affect chronic metabolic diseases, including diabetes, hypertension, and dyslipidemia, which are considered important metabolic risk factors of elevated IOP and reduced ocular blood flow [20,21].
Studies focusing on vitamin D status and its relation to glaucoma are limited and inconsistent [22,23,24]. The purpose of this study was to investigate the association between vitamin D and the development of glaucoma.
Materials and Methods
This study followed the tenets of the Declaration of Helsinki and was approved by the institutional review board/ethics committee of Kangbuk Samsung Hospital. Subjects who underwent health screening at one of the Kangbuk Samsung Hospital screening centers in Seoul and Suwon from August 2012 to July 2013 were enrolled in this retrospective, cross-sectional study. The purpose of the screening program was to promote health through early detection of chronic diseases and their risk factors. Additionally, the Korean Industrial Safety and Health Law requires working individuals to participate in an annual or biennial health examination. Approximately 60% of the participants were employees (or their spouses) of companies or local governmental organizations, while the remaining participants registered individually for the program. During the screening examination, digital color fundus photographs were taken with a digital fundus camera (CR6-45NM; Canon, Tokyo, Japan). Of the 169,208 subjects who received health screenings and were older than 20 years, 168,044 (99.31%) underwent fundus photography. Of the 168,044 subjects who underwent fundus photography, 123,331 (72.89%) who received a serum 25(OH)D blood test were finally enrolled in this study. The IOP was measured using a noncontact tonometer (CT-80; Topcon, Tokyo, Japan), and the mean value of two IOP readings was recorded. In addition to fundus photographs, systemic examinations and sociodemographic and behavioral questionnaires were administered to all subjects; their medical histories were also reviewed to determine the presence of any associated systemic disease.
All fundus photographs were reviewed by two ophthalmologists, two glaucoma specialists, and one retinal specialist, all of whom were blinded to the subjects' demographic features and laboratory findings. Discrepancies among the observers' findings were resolved by consensus. Fundus photographs were divided into two groups based on disc and retinal nerve fiber layer (RNFL) appearance: non-glaucoma and glaucoma, defined as glaucomatous optic disc diagnosed according to International Society of Geographical and Epidemiological Ophthalmology (ISGEO) criteria or definite RNFL defect. A glaucomatous optic disc was defined based on disc appearance such as disc notching or cup-to-disc ratio (CDR) greater than 0.7 vertical CDR or greater than 0.2 vertical CDR asymmetry between the right and left eyes. A glaucomatous RNFL defect was defined as a localized, wedge-shaped RNFL defect within 60 degrees of the optic disc border. In cases with newly-detected glaucomatous features, glaucoma evaluations were recommended for confirmation, and some subjects were diagnosed with glaucoma after undergoing a glaucoma evaluation in the glaucoma clinic. The subjects diagnosed with glaucoma in the glaucoma clinic were enrolled as the glaucoma group. Non-glaucomatous RNFL defects, such as superior segmental optic hypoplasia, slit defects, and spindle-like defects, were excluded.
The following variables were analyzed to evaluate the risk factors of development of glaucoma: physical measurements (body mass index, waist circumference, and systolic and diastolic blood pressure), serum biochemical measurements (fasting blood glucose, hemoglobin A1c, and serum 25(OH)D), serum lipid profiles (total cholesterol and triglycerides), medical history (presence of diabetes, systemic hypertension, hyperlipidemia, hyperthyroidism, or hypothyroidism), and a questionnaire that addressed sociodemographic characteristics (age and sex) and health-related behaviors (smoking, alcohol intake, and physical activity). Alcohol intake was classified based on a cut-off of 20 g/day. In addition, regular physical activity was based on moderate levels of physical activity, such as carrying light loads, bicycling, or doubles tennis.
All statistical analyses were performed using PASW ver. 18.0 (SPSS Inc., Chicago, IL, USA). Each variable was initially evaluated by the chi-square test or independent t-test. After adjusting for confounding variables such as sex, age, current smoking, diabetes, hypertension, and IOP, multivariate logistic regression analysis was used to analyze the association between developing glaucoma and vitamin D or developing glaucoma and quintile of vitamin D. Model 1 was not adjusted; model 2 was adjusted for age, sex, current smoking status, diabetes, and hypertension; model 3 was adjusted for age, sex, current smoking status, diabetes, hypertension, and IOP. Odds ratios (ORs) with 95% confidence intervals were generated using a logistic regression model. Additionally, the glaucoma group was divided into high IOP and without high IOP groups based on 21 mmHg IOP, and the difference in vitamin D levels between the two groups was analyzed using independent t-test. A p-value <0.05 was considered statistically significant.
Results
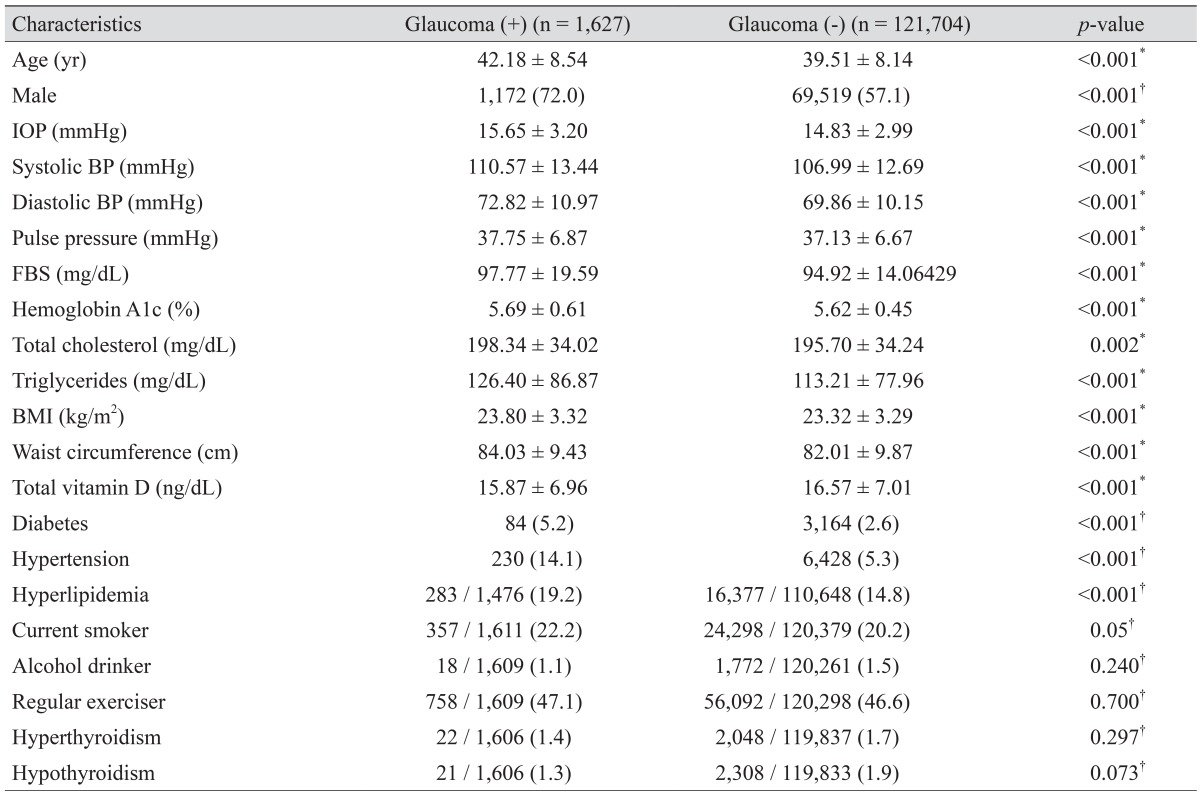
Subject characteristics according to the presence or absence of glaucoma are shown in Table 1. A total of 123,331 subjects were enrolled in the present study. Among the eligible subjects, 1,627 (1.31%) had glaucoma. The glaucoma group consisted of 1,029 subjects who were diagnosed in the glaucoma clinic based on a standard glaucoma evaluation and 598 who were diagnosed using ISGEO criteria and had RNFL defects. The mean of serum 25(OH)D level in the group diagnosed in the glaucoma clinic and the group diagnosed using ISGEO criteria and had RNFL defects was 15.85 ± 6.95 and 15.90 % 6.99 ng/dL, respectively. There was no significant difference between the two glaucoma groups (p = 0.388, independent t-test).
Table 1. Demographic and general health characteristics of the study subjects.
Values are presented as mean ± standard deviation or number (%).
IOP = intraocular pressure; BP = blood pressure; FBS = fasting blood glucose; BMI = body mass index.
*Student's t-test; †Chi-square test.
The following variables were all statistically different between the glaucoma and the healthy groups: age; percentage of males; IOP; systolic and diastolic blood pressure; pulse pressure; fasting blood glucose; hemoglobin A1c; total cholesterol; triglycerides; body mass index; waist circumference; serum 25(OH)D; presence of diabetes, hypertension, hyperlipidemia; and current smoking status. However, alcohol intake, regular exercise, and presence of hyperthyroidism and hypothyroidism were not statistically significantly different between the two groups.
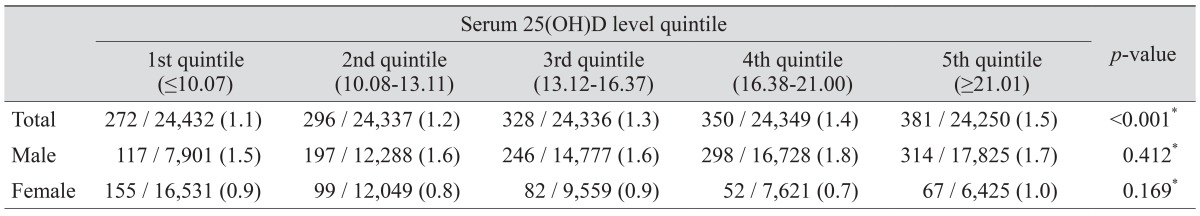
The prevalence of glaucoma according to quintile of serum 25(OH)D is shown in Table 2. The prevalence of glaucoma was statistically significantly different between various 25(OH)D quintiles (p < 0.001); however, after adjusting for sex, the prevalence of glaucoma was not statistically significantly different between the 25(OH)D quintiles.
Table 2. Prevalence of glaucoma according to quintile of serum 25(OH)D.
25(OH)D = 25-hydroxyvitamin D.
*Chi-square test.
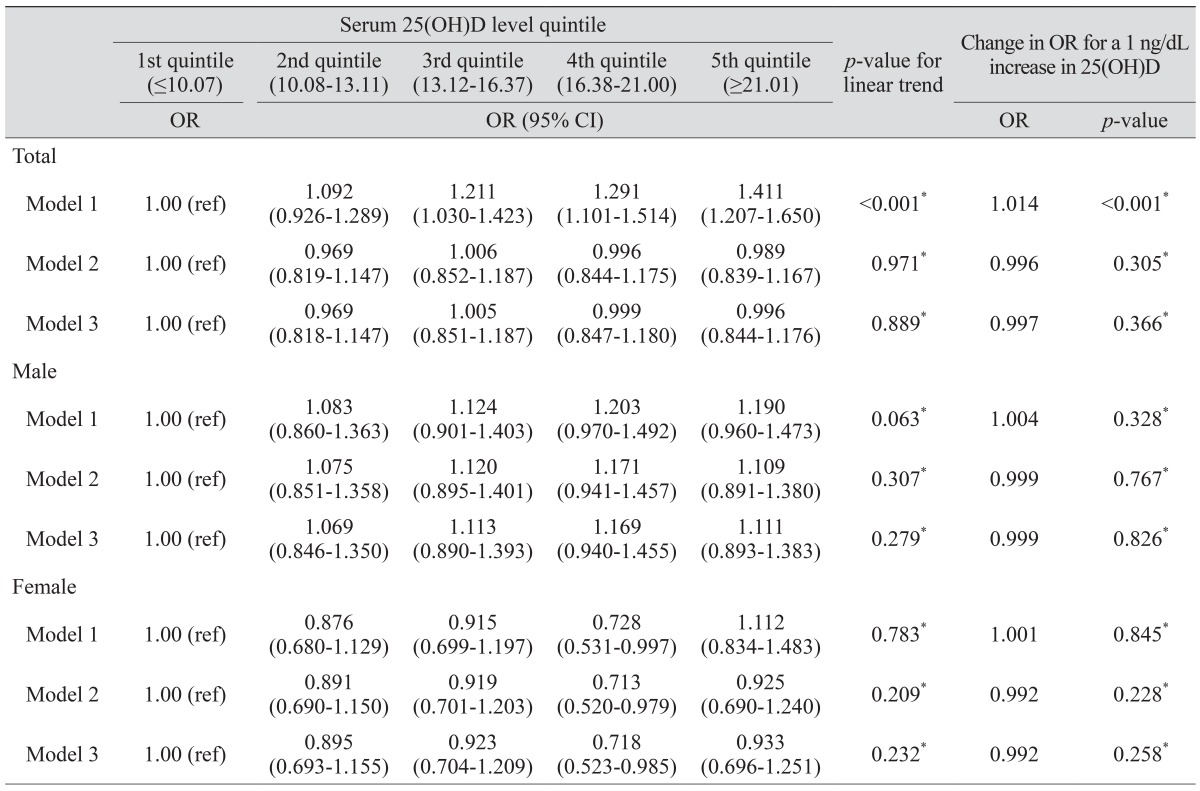
The ORs of glaucoma according to serum 25(OH)D and serum 25(OH)D quintiles are shown in Table 3. Univariate logistic regression analysis showed that the ORs for subjects in the third, fourth, and fifth quintiles of serum 25(OH)D were significantly higher than the ORs for first quintile subjects (ORs, 1.211, 1.291, and 1.411, respectively; p < 0.001). When the univariate logistic regression analyses were conducted using continuous serum 25(OH)D level, the OR of glaucoma for every 1 ng/dL increase in 25(OH)D was significantly changed (OR, 1.014). On multivariate logistic regression analysis according to sex, continuous value or quintile of serum 25(OH)D was not significantly associated with glaucoma in males. However, in females, the OR of subjects in the fourth quintile of serum 25(OH)D was significantly lower than that in the first quintile subjects (OR, 0.713).
Table 3. ORs of glaucoma according to quintile of serum 25(OH)D in all subjects.
Model 1 was not adjusted; Model 2 was adjusted for age, sex, current smoking status, diabetes, and hypertension; Model 3 was adjusted for age, sex, current smoking status, diabetes, hypertension, and intraocular pressure.
OR = odds ratio; 25(OH)D = 25-hydroxyvitamin D; CI = confidence interval.
*Logistic regression analysis.
Glaucoma subjects were subdivided into high IOP and without high IOP groups (1,565, 96.2% and 62, 3.8%, respectively). The mean of serum 25(OH)D level in the high IOP and without high IOP groups was 16.59 ± 6.89 and 15.86 ± 6.96 ng/dL, respectively; the difference between the two groups was not significant (p = 0.201, independent t-test).
Discussion
Glaucoma is considered a complex and multifactorial disorder that is influenced not only by IOP, but also various systemic conditions. To identify controllable risk factors other than IOP, many researchers have investigated the systemic factors that affect the development of glaucoma. Several mechanisms underlying this association remain unclear. In our study, the association between either 25(OH)D or quintile of 25(OH)D and the development of glaucoma was not statistically significant in males. However, in females, these associations showed statistically significant results. Females with lower 25(OH)D level were at a significantly greater risk of glaucoma compared with those with higher 25(OH)D level.
Potential mechanisms for the development of glaucoma might be influenced by several protective roles of vitamin D, either directly by activation of the vitamin D receptor or indirectly by regulation of calcium homeostasis [25,26]. First, vitamin D might affect immunomodulation in the pathogenesis of glaucoma [27]. Recent studies have shown that an imbalance of the immune system is a major contributor to neurodegenerative injuries of the optic nerve axons and ganglion cell bodies. Since vitamin D significantly affects the regulation of immune cell functions, this effect might play a key role in protecting the optic nerve. Second, vitamin D regulates both neurotrophic factors in the central nervous system and plasticity in neural networks [16,17]. Studies using animal models have found that vitamin D has a neurotrophic property associated with the synthesis of neurotrophic growth factors and neurotransmitter metabolism [16,17]. It is possible that this effect aids in the regeneration of the optic nerve after injury. Third, vitamin D regulates oxidative stress in neurons by activating calcium channels, an important factor in glaucomatous optic nerve damage [26,27,28]. Vitamin D is closely associated with a number of neurodegenerative and psychiatric diseases such as Alzheimer disease, Parkinson disease, depression, and schizophrenia [26]. Several studies have shown that these diseases can affect the development of glaucoma [27].
Another possible explanation for the association between lower vitamin D level and glaucoma might lie in the mechanism of impaired ocular blood flow. Yang et al. [25] reported that vitamin D regulates the renin-angiotensin system and improves endothelial cell-dependent vasodilation, which affects peripheral and microvessel circulation. In an animal-based study, the suppression of the renin-angiotensin system was shown to decrease the risk of glaucoma by improving ocular blood flow [29]. The anti-inflammatory effects of vitamin D also protect endothelial cells from metabolic damage and oxidative stress [25]. Furthermore, vitamin D status might reflect chronic non-specific illnesses that could affect systemic circulation [30]. These studies showed that improving vitamin D status might have a beneficial effect on the vascular dysregulation that leads to local vasospasm [30,31].
In glaucoma field studies, Krefting et al. [22] reported no association between vitamin D and IOP. Wiggs [24] reported that the risk of open-angle glaucoma due to exfoliation syndrome was associated with vitamin D. Yoo et al. [23] reported that serum vitamin D was associated with IOP in male subjects, and that low vitamin D level in glaucoma subjects might result from low external activity due to the disease. This result is in contrast with our findings. In our study, there was no association between vitamin D status and the development of glaucoma in all subjects or in males. The reason for this difference is unknown. It may be that there were more male than female subjects in our study, while the previous study population had a different composition. Furthermore, our subjects were enrolled from health-screening centers; thus, our study was not population-based. Another possibility is that the subjects in our study were in a higher socioeconomic class and had a more invested interest in their health. Whatever the reason, the association between vitamin D status and glaucoma remains unclear, and further studies are necessary.
Our study showed different results after adjusting for sex. One reason for this difference might be due to the effects of female sex hormones. Pasquale et al. [32] have reported that female sex hormones were associated with glaucoma. An altered sexual hormone status has been shown to lead to nutrition deficiency and various chronic diseases [33,34]. According to several studies, vitamin D status influences female reproductive and pregnancy outcomes, and low vitamin D status is associated with impaired fertility, endometriosis, and polycystic ovary syndrome [35,36]. Based on various studies, vitamin D levels have an influence on the health of females.
Our study has several limitations. First, the chosen individuals enrolled retrospectively in a health screening program; thus, the study population was biased to individuals with access to health care. All subjects were self-selected for health screening, and most of the study population consisted of workers and their spouses. The educational and economic demographics of the study subjects might have resulted in some bias. However, our study population was very large, which is expected to minimize selection bias. A future longitudinal study is needed. Second, some subjects were diagnosed with glaucoma based only on fundus photographs. However, most subjects showed RNFL defects in their fundus photographs. To exclude the possibility of misdiagnosis, each examiner diagnosed the fundus photograph blindly, and any discrepancies among the observers were resolved by consensus. Third, we could not exactly distinguish the type of glaucoma because we did not perform slit-lamp examination in some subjects who were diagnosed using ISGEO criteria and had RNFL defect. Instead of analysis according to the type of glaucoma, we only analyzed the difference in vitamin D level in the subtypes of glaucoma based on IOP. After dividing the glaucoma group based on IOP, serum 25(OH)D levels were not significantly different between the high IOP and without high IOP groups. One of the reasons might be the significant difference in the number of subjects in the two groups. Most of the subjects were in the without high IOP group. Fourth, ocular parameters such as axial length, corneal thickness and refractive error were not measured. Fifth, this was a cross-sectional, case-control study; therefore, no causal relationship between development of glaucoma and serum 25(OH)D level could be determined.
In conclusion, lower serum 25(OH)D level was significantly associated with an elevated risk of glaucoma in women compared to those with higher serum 25(OH)D. Our results suggest that vitamin D status independently affects the pathophysiology of glaucoma in women. We cannot explain the exact mechanism; however, considering both the results of previous reports and the present study, vitamin D influences the pathophysiology of glaucoma as a secondary aggravating factor rather than a primary cause. With the presence of a primary factor, a low vitamin D level might render the optic nerve or its environment more vulnerable to glaucomatous insult. Our study helps to elucidate the risk factors of glaucoma and to disclose the mechanisms underlying the influence of vitamin D on the development of glaucoma.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Salowe R, Salinas J, Farbman NH, et al. Primary open-angle glaucoma in individuals of African descent: a review of risk factors. J Clin Exp Ophthalmol. 2015;6 doi: 10.4172/2155-9570.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields MB. Normal-tension glaucoma: is it different from primary open-angle glaucoma? Curr Opin Ophthalmol. 2008;19:85–88. doi: 10.1097/ICU.0b013e3282f3919b. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki Y, Iwase A, Araie M, et al. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi Study. Ophthalmology. 2006;113:1613–1617. doi: 10.1016/j.ophtha.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 4.Kuzin AA, Varma R, Reddy HS, et al. Ocular biometry and open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2010;117:1713–1719. doi: 10.1016/j.ophtha.2010.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buus DR, Anderson DR. Peripapillary crescents and halos in normal-tension glaucoma and ocular hypertension. Ophthalmology. 1989;96:16–19. doi: 10.1016/s0161-6420(89)32930-7. [DOI] [PubMed] [Google Scholar]
- 6.Jonas JB, Xu L. Parapapillary chorioretinal atrophy in normal-pressure glaucoma. Am J Ophthalmol. 1993;115:501–505. doi: 10.1016/s0002-9394(14)74453-8. [DOI] [PubMed] [Google Scholar]
- 7.Park KH, Tomita G, Liou SY, Kitazawa Y. Correlation between peripapillary atrophy and optic nerve damage in normal-tension glaucoma. Ophthalmology. 1996;103:1899–1906. doi: 10.1016/s0161-6420(96)30409-0. [DOI] [PubMed] [Google Scholar]
- 8.Drance S, Anderson DR, Schulzer M Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 9.Lin PW, Friedman M, Lin HC, et al. Normal tension glaucoma in patients with obstructive sleep apnea/hypopnea syndrome. J Glaucoma. 2011;20:553–558. doi: 10.1097/IJG.0b013e3181f3eb81. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Kim TW, Park KH, Kim JM. Risk factors for primary open-angle glaucoma in South Korea: the Namil study. Jpn J Ophthalmol. 2012;56:324–329. doi: 10.1007/s10384-012-0153-4. [DOI] [PubMed] [Google Scholar]
- 11.Leung DY, Tham CC, Li FC, et al. Silent cerebral infarct and visual field progression in newly diagnosed normal-tension glaucoma: a cohort study. Ophthalmology. 2009;116:1250–1256. doi: 10.1016/j.ophtha.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Chon B, Qiu M, Lin SC. Myopia and glaucoma in the South Korean population. Invest Ophthalmol Vis Sci. 2013;54:6570–6577. doi: 10.1167/iovs.13-12173. [DOI] [PubMed] [Google Scholar]
- 13.Kim MJ, Kim MJ, Kim HS, et al. Risk factors for open-angle glaucoma with normal baseline intraocular pressure in a young population: the Korea National Health and Nutrition Examination Survey. Clin Exp Ophthalmol. 2014;42:825–832. doi: 10.1111/ceo.12347. [DOI] [PubMed] [Google Scholar]
- 14.Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88:720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 16.Balion C, Griffith LE, Strifler L, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79:1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Schaft J, Koek HL, Dijkstra E, et al. The association between vitamin D and cognition: a systematic review. Ageing Res Rev. 2013;12:1013–1023. doi: 10.1016/j.arr.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Schreiner DS, Jande SS, Lawson DE. Target cells of vitamin D in the vertebrate retina. Acta Anat (Basel) 1985;121:153–162. doi: 10.1159/000145958. [DOI] [PubMed] [Google Scholar]
- 19.Lucas RM, Ponsonby AL, Dear K, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology. 2011;76:540–548. doi: 10.1212/WNL.0b013e31820af93d. [DOI] [PubMed] [Google Scholar]
- 20.Yanagi M, Kawasaki R, Wang JJ, et al. Vascular risk factors in glaucoma: a review. Clin Exp Ophthalmol. 2011;39:252–258. doi: 10.1111/j.1442-9071.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 21.Oh SW, Lee S, Park C, Kim DJ. Elevated intraocular pressure is associated with insulin resistance and metabolic syndrome. Diabetes Metab Res Rev. 2005;21:434–440. doi: 10.1002/dmrr.529. [DOI] [PubMed] [Google Scholar]
- 22.Krefting EA, Jorde R, Christoffersen T, Grimnes G. Vitamin D and intraocular pressure: results from a case-control and an intervention study. Acta Ophthalmol. 2014;92:345–349. doi: 10.1111/aos.12125. [DOI] [PubMed] [Google Scholar]
- 23.Yoo TK, Oh E, Hong S. Is vitamin D status associated with open-angle glaucoma? A cross-sectional study from South Korea. Public Health Nutr. 2014;17:833–843. doi: 10.1017/S1368980013003492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiggs JL. The cell and molecular biology of complex forms of glaucoma: updates on genetic, environmental, and epigenetic risk factors. Invest Ophthalmol Vis Sci. 2012;53:2467–2469. doi: 10.1167/iovs.12-9483e. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Ma J, Zhang X, et al. Protective role of the vitamin D receptor. Cell Immunol. 2012;279:160–166. doi: 10.1016/j.cellimm.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 26.DeLuca GC, Kimball SM, Kolasinski J, et al. Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol. 2013;39:458–484. doi: 10.1111/nan.12020. [DOI] [PubMed] [Google Scholar]
- 27.McKinnon SJ. The cell and molecular biology of glaucoma: common neurodegenerative pathways and relevance to glaucoma. Invest Ophthalmol Vis Sci. 2012;53:2485–2487. doi: 10.1167/iovs.12-9483j. [DOI] [PubMed] [Google Scholar]
- 28.Kim JM, Kim YJ, Kim DM. Increased expression of oxyproteins in the optic nerve head of an in vivo model of optic nerve ischemia. BMC Ophthalmol. 2012;12:63. doi: 10.1186/1471-2415-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah GB, Sharma S, Mehta AA, Goyal RK. Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma. J Cardiovasc Pharmacol. 2000;36:169–175. doi: 10.1097/00005344-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flammer J, Orgul S. Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res. 1998;17:267–289. doi: 10.1016/s1350-9462(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 32.Pasquale LR, Loomis SJ, Weinreb RN, et al. Estrogen pathway polymorphisms in relation to primary open angle glaucoma: an analysis accounting for gender from the United States. Mol Vis. 2013;19:1471–1481. [PMC free article] [PubMed] [Google Scholar]
- 33.Lim SK, Kung AW, Sompongse S, et al. Vitamin D inadequacy in postmenopausal women in Eastern Asia. Curr Med Res Opin. 2008;24:99–106. doi: 10.1185/030079908x253429. [DOI] [PubMed] [Google Scholar]
- 34.Battaglia C, Mancini F, Regnani G, et al. Hormone therapy and ophthalmic artery blood flow changes in women with primary open-angle glaucoma. Menopause. 2004;11:69–77. doi: 10.1097/01.GME.0000079741.18541.92. [DOI] [PubMed] [Google Scholar]
- 35.Grundmann M, von Versen-Hoynck F. Vitamin D: roles in women's reproductive health? Reprod Biol Endocrinol. 2011;9:146. doi: 10.1186/1477-7827-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wulaningsih W, Van Hemelrijck M, Michaelsson K, et al. Association of serum inorganic phosphate with sex steroid hormones and vitamin D in a nationally representative sample of men. Andrology. 2014;2:967–976. doi: 10.1111/andr.285. [DOI] [PMC free article] [PubMed] [Google Scholar]