Abstract
This study aims to develop a puffer fish skin gelatin (PSG) film that contains Moringa oleifera Lam. leaf extract (ME) as a new biodegradable film. With the increase in ME concentration, the tensile strength and elongation at break of the PSG film increased, whereas the oxygen permeability and water vapor permeability decreased. In addition, the PSG film with ME exhibited antimicrobial activity against Listeria monocytogenes and antioxidant activity. To apply the ME-containing PSG film to food packaging, Gouda cheese was wrapped with the ME-containing PSG film. During storage, the cheese packaging with the ME-containing PSG film effectively inhibited the microbial growth and retarded the lipid oxidation of cheese compared with the control sample. Thus, the ME-containing PSG film can be used as an antimicrobial and antioxidative packaging material to improve the quality of food products.
Keywords: Antimicrobial activity, Moringa oleifera, Physical property, Protein film, Puffer fish
Introduction
Plastic packaging materials are used because of their low cost and good physical properties. However, the use of synthetic plastic packaging causes serious environmental problems because of its non-biodegradability (Tajik et al. 2013). To solve these problems, there has been a growing interest in biodegradable films, which can act as carriers of active ingredients such as antioxidant and antimicrobial agents (Bahram et al. 2014). These active components of the film can be released onto the food surfaces and extend the shelf life by reducing microbial growth and lipid oxidation (Kuorwel et al. 2011).
Among different types of puffer fish, Lagocephalus gloveri, which belongs to the family of Tetraodontidae, is commonly distributed in the East China Sea and popularly consumed as a special dish in Korea (Senaratne et al. 2006; Kim et al. 2010). Puffer fish is known to contain non-protein neurotoxins called tetrodotoxin in the skin, muscle, and liver. (Nagai et al. 2002). However, L. gloveri does not contain tetrodotoxin. In particular, L. gloveri is covered with hard skin, and its skin contains a considerable amount of collagen (Nagai et al. 2002). Gelatin, which can be obtained from the hydrolysis of collagen, can be used to prepare biodegradable films. In addition, most puffer fish skin is discarded during food processing (Kim et al. 2010). Therefore, the unused puffer fish skin can be a new film base material for biodegradable films.
Moringa oleifera Lam. is known as a high-value plant and is distributed in many countries (Moyo et al. 2012). The leaves of M. oleifera Lam can be consumed fresh, cooked, or as dried powder (Verma et al. 2009). The leaves contain large quantities of protein, vitamin C, β-carotene, and minerals. In addition, flavonoids such as kaempferol, quercetin, and many other phytochemicals are the main constituents and exhibit antioxidant and antimicrobial activities (Bukar et al. 2010). Therefore, this study aimed to develop a puffer fish skin gelatin film with antimicrobial and antioxidant activities by adding M. oleifera Lam. leaf extract and apply the film to the packaging of Gouda cheese.
Materials and methods
Materials
Puffer fish skin and M. oleifera Lam. leaf powder were purchased from a local market (Daejeon, Korea).
Extraction of puffer fish skin gelatin
The puffer fish gelatin was extracted according to the method described by Senaratne et al. (2006) with minor modifications. Puffer fish skin pieces (4 × 4 cm) were soaked in 0.1 M NaOH (1:5, w/v) at room temperature (RT) for 20 min to remove non-collagenous proteins. After 20 min, the puffer fish skin was rinsed with tap water and treated with 0.1 M acetic acid (1:5, w/v) at RT for 20 min to swell, and it was washed again with tap water. Then, the swollen puffer fish skin was stirred in distilled water (1:5, w/v) at 70 °C for 3 h and centrifuged at 10,000×g for 20 min. After the centrifugation, the supernatant was freeze-dried to obtain a puffer fish skin gelatin (PSG), whose yield was approximately 11%.
Preparation of M. oleifera Lam. leaf extracts (ME)
The M. oleifera Lam. leaf powder was prepared using a 30 mesh sieve and mixed with 70% ethanol (1:20, w/v) and stirred at RT for 24 h. After 24 h, the mixed solution was filtered using a Whatman No. 2 filter paper. The organic solvents in the filtrate were removed with a rotary evaporator, and the filtrate was freeze-dried. After lyophilization, the M. oleifera Lam. leaf extracts (ME) was ground with a mortar and filtered using a 30 mesh sieve.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
To analyze the molecular weight patterns of PSG and PSG films, SDS-PAGE was performed according to the method described by Lee et al. (2016). The PSG, PSG film, and PSG-1% ME film samples (200 mg) were completely dissolved in distilled water (10 mL). The sample solution was mixed with an equal volume of 2 × Laemmli sample buffer (65.8 mM Tris–HCl, pH 6.8, 2.1% SDS, 26.3% glycerol, 0.001% bromophenol blue) and heated at 95 °C for 5 min. The supernatant of the sample was collected after centrifugation at 2000×g for 5 min. The sample solution (4 μL) was then applied on the polyacrylamide gel (7.5%). After electrophoresis at a constant current of 20 mA, the gel was stained with Coomassie Brilliant Blue R-250 and then destained.
Preparation of the ME-containing PSG film
To determine a suitable plasticizer for the PSG film, various plasticizers (fructose, glycerol, sucrose, and sorbitol) were attempted to prepare the film. Among them, sorbitol was selected for the PSG film based on a preliminary study (data not shown). After the PSG films were prepared with various amounts of sorbitol, 40% sorbitol was selected because it provided the best physical properties (data not shown). The PSG film-forming solution was prepared by completely dissolving PSG (5 g) in distilled water (100 mL) at RT, homogenized, and sonicated for 8 min. After sonication, the mixture was degassed and heated at 60 °C for 30 min and cooled. Sorbitol (2 g) and various concentrations of ME (0.03, 0.05, 0.07, and 0.1 g) were added to the mixture and stirred for 30 min. The resulting solution (80 mL), which was obtained by filtration, was cast onto a Teflon-coated plate and dried at RT for 16 h. After drying, the PSG film was stored in a chamber at RT and 50% relative humidity (RH).
Measurement of physical and optical properties
The tensile strength (TS) and elongation at break (E) of the PSG films were determined using a Testometric machine (M250-2.5 CT, The Testometric Company Ltd., Lancashire, UK). The films (2.54 × 10 cm) were prepared, and each test was performed at least 5 times. The initial grip length was 5 cm, and the cross-head speed was 50 cm/min.
Hunter L, a, and b value was measured with a colorimeter (CR-400, Minolta, Tokyo, Japan) at random positions of the film, and 5 replications were performed for each film sample. The film opacity was evaluated by measuring the absorbance at 600 nm with a spectrophotometer (UV-2450, Shimadzu Corporation, Kyoto, Japan) and obtained using the following equation.
where Abs600 is the absorbance at 600 nm, and x is the thickness of the film (mm).
Water vapor permeability (WVP)
The water vapor permeability (WVP) of the PSG film was evaluated using a polymethylacrylate cup according to the ASTM Method E96-95. The PSG films (2 × 2 cm) were placed on top of the cup, which contained distilled water (18 mL), and kept in a chamber at RT and 50% RH. The weight of the cup was recorded every hour up to 8 h, and 3 replications were conducted for each film.
Scanning electron microscopy (SEM)
To examine the surface morphology of the PSG film, a scanning electron microscope (Tescan LYRA3 XMU, Brno, Czech Republic) under high vacuum condition was used. The PSG film samples were placed onto a metal holder and coated with platinum for 90 s. Then, the surface of the PSG film was observed under an accelerating voltage of 10 kV.
Antimicrobial activity of film
The antimicrobial activity of the ME-containing PSG film against Listeria monocytogenes was determined according to the method described by Bahram et al. (2014) with minor modifications. Listeria monocytogenes (ATCC 19111) was cultured in the Brain Heart Infusion (BHI) at 37 °C for 24 h, and the final bacterial concentration was approximately 106–107 CFU/mL. A bacterial suspension was uniformly spread on the Oxford medium base using a sterile cotton swab. The PSG film-forming solution (75 μL) was poured onto a sterile disc (8 mm in diameter). The disc was put on the inoculated plate and incubated at 37 °C for 48 h. After the incubation, the diameter of the inhibition zone around the disc was determined using a Digimatic caliper (Model 500-181-20, Mitutoyo Corp., Kawasaki, Japan).
Antioxidant activity of film
ABTS radical scavenging activity
To prepare the film extract solution for determination of antioxidant activity, each film (25 mg) was solubilized with 3 mL of distilled water and used for both ABTS radical scavenging activity and ferrous iron chelating activity (Ruiz-Navajas et al. 2013). The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical-scavenging activity of the ME-containing PSG film was evaluated according to the method described by Giménez et al. (2009) with minor modifications. The stock solution was prepared from a mixture of 7 mM ABTS solution and 2.45 mM potassium persulfate solution (2:1, v/v) and maintained in the dark at RT for 16 h. Then, the preparing stock solution was diluted with distilled water to make an ABTS working solution with an absorbance of 0.7 at 734 nm. The solubilized film sample (120 μL) was mixed with the ABTS working solution (5880 μL) and incubated at 37 °C for 10 min in the dark. After incubation, the mixed solution was centrifuged at 2000×g for 5 min to obtain the supernatant. The absorbance at 734 nm was measured with a spectrophotometer. The ABTS radical-scavenging activity was calculated using the following equation.
Ferrous-ion (Fe2+)-chelating activity
The film extract solution (1 mL) was mixed with 3.7 mL of methanol and 0.1 mL of 2 mM FeCl2. After the incubation at RT for 10 min, 0.2 mL of 5 mM ferrozine was added to the mixture and incubated at RT for 10 min. The mixture was then centrifuged at 2000×g for 5 min. After centrifugation, the absorbance of the supernatant was measured at 562 nm. The Fe2+-chelating activity was calculated using the following equation.
Application of the PSG film on Gouda cheese
Cube-type Gouda cheese was purchased from a local market (Daejeon, Korea). The cheese samples were prepared in a uniform size (3 × 2 cm, 10 g) and sterilized using ultraviolet light for 15 min. L. monocytogenes (ATCC 19111) was incubated in BHI at 37 °C for 24 h and centrifuged at 2000×g for 15 min. The obtained bacterial pellets were washed 3 times using 0.1% peptone water. The cheese samples were inoculated with the inoculum (1 mL, approximately 105–106 CFU/mL). After drying at 25 °C for 30 min, the cheese samples were wrapped with the PSG film or the PSG film with 1% ME. As the control, the cheese samples without film packaging were packed in polyethylene terephthalate. All cheese samples were stored at 4 ± 1 °C for 16 days and analyzed for microbial enumeration and lipid oxidation.
Analysis of Gouda cheese samples
Microbial analysis
The Gouda cheese samples (10 g) were put into sterile bags with 0.1% sterile peptone water (90 mL) and homogenized with a stomacher (MIX 2, AES Laboratoire, Combourg, France). For the microbial analysis, 0.1 mL of serial diluted sample with peptone water was spread on an Oxford medium base and incubated at 37 °C for 48 h. All experiments were replicated 3 times, and the microbial counts were expressed as log colony-forming units (CFU)/g.
POV value
To analyze the lipid oxidation of Gouda cheese during storage, the peroxide value (POV) was evaluated according to the AOAC method (1990). A cheese sample (2 g) was mixed with 30 mL of acetic acid/chloroform mixed solution (3:2, v/v) and stirred for 10 min. After the lipids dissolved, a saturated potassium iodide solution (1 mL) was added to the mixture and stored in the dark for 5 min, and distilled water (30 mL) was added. As an indicator, 1% starch solution was added to the mixture, and 0.01 N sodium thiosulfate was used to titrate the solution. The POV was represented as meq peroxides/kg sample.
TBARS value
The TBARS value was evaluated using a method described by Lee et al. (2015b). The Gouda cheese samples (2 g) were homogenized with 7.5% trichloroacetic acid (10 mL) for 1 min and passed through cheesecloth. First, 0.02 M thiobarbituric acid (5 mL) was added to the filtrate (5 mL), heated at 95 °C for 45 min, and cooled. After the centrifugation, the absorbance of the supernatant was measured at 539 nm. The TBARS value was represented as a malonaldehyde (MDA)/kg sample.
Statistical analysis
To statistically analyze the experimental results, SAS program version 9.4 (SAS Institute, Inc., Cary, NC, USA) was used. Analysis of variance and Duncan’s multiple range tests with a significance level of p < 0.05 were examined. All data are shown as the mean ± standard deviation.
Results and discussion
Electrophoretic pattern of PSG
The protein molecular weight pattern of PSG, PSG film, and PSG-1% ME film are shown in Fig. 1. PSG consists of γ chain (300 kDa), β chain (250 kDa), and two α chains (α1, 130–120 kDa; α2, 110 kDa) as major proteins. According to the report of Senaratne et al. (2006), the puffer fish (L. gloveri) skin collagen has two α chains and intra- and inter- molecular cross-linked components, which are γ chain (trimers) and β chain (dimmers). The PSG film and PSG-1% ME film also had the similar protein pattern of PSG. The protein pattern of tuna skin gelatin film was similar with that of tuna skin gelatin (Gómez-Guillén et al. 2007). These results indicated that fish skin gelatin films have the same protein molecular weight profile with the original fish skin gelatin, although ME was incorporated into the film in this study. The addition of ME did not affect the molecular weight profile of the PSG film, the molecular interactions between PSG and ME appeared to be non-covalent, resulting in destruction under electrophoretic condition.
Fig. 1.
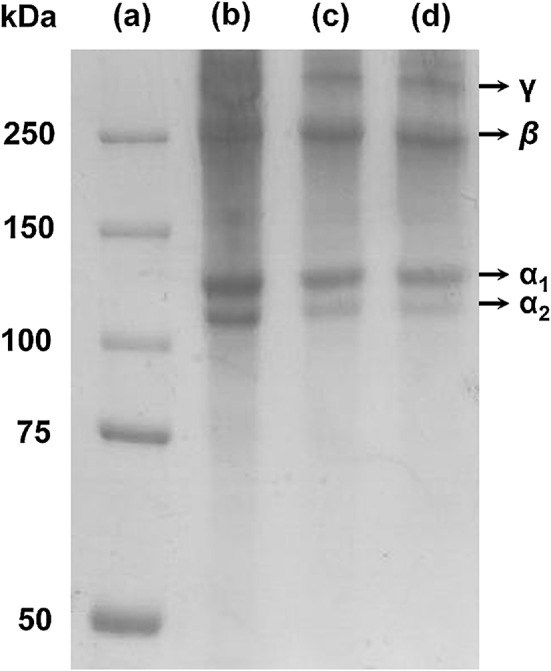
Protein patterns of PSG, PSG film, and PSG-1% ME film. The SDS-PAGE was performed using 7.5% separating gel and 5% stacking gel. a Molecular weight marker proteins; b PSG; c PSG film; d PSG-1% ME film
Physical and optical properties of ME-containing PSG films
Antimicrobial PSG films with various ME concentrations were prepared. The addition of ME significantly affected the physical properties of the PSG films. The increase in ME concentrations proportionally increased both TS and E values of the PSG films (Table 1). The TS of the PSG films increased from 50.60 to 80.49 MPa with the addition of 1% ME. This increase in TS can be attributed to the increase in interactions between gelatin molecules and polyphenolic compounds through hydrophobic and hydrogen bonds (Rattaya et al. 2009). In particular, cross-links were formed between PSG and the polyphenolic compounds of ME, which led to more rigid films. Similarly, the chitosan film that contained green tea extract showed an increase in TS because chitosan molecules interacted with the phenolic compounds of the green tea extract (Siripatrawan and Harte 2010). Rattaya et al. (2009) also reported that the TS of fish skin gelatin films increased with increase in seaweed extract concentration.
Table 1.
Physical and optical properties of the PSG films containing various concentrations of ME
| ME (%) | Tensile Strength (MPa) |
Elongation at break (%) | Water vapor permeability (×10−9 g m/m2 s Pa) |
L | a | b | Opacity |
|---|---|---|---|---|---|---|---|
| 0 | 50.60 ± 0.32e | 18.90 ± 1.42e | 1.70 ± 0.07a | 93.10 ± 0.23a | −1.26 ± 0.10a | 7.85 ± 0.29e | 0.77 ± 0.04e |
| 0.3 | 55.68 ± 0.82d | 29.85 ± 0.95d | 1.64 ± 0.07a | 84.25 ± 0.56b | −8.25 ± 0.17e | 32.79 ± 0.25d | 1.02 ± 0.04d |
| 0.5 | 63.28 ± 0.64c | 41.91 ± 1.59c | 1.49 ± 0.01b | 77.11 ± 0.22c | −7.34 ± 0.10d | 39.22 ± 0.15c | 1.24 ± 0.06c |
| 0.7 | 71.37 ± 0.85b | 46.13 ± 1.53b | 1.43 ± 0.02b | 73.43 ± 0.15d | −5.94 ± 0.08c | 40.36 ± 0.10b | 1.41 ± 0.08b |
| 1.0 | 84.49 ± 1.34a | 65.93 ± 1.08a | 1.38 ± 0.02b | 68.23 ± 0.29e | −4.60 ± 0.40b | 41.03 ± 0.11a | 1.59 ± 0.13a |
Mean ± SD
a–e Any means in the same column followed by different letters are significantly (p < 0.05) different by Duncan’s multiple range test
In addition, E increased from 18.90 to 65.93% when 1% ME was added to the PSG film. Lee et al. (2015a) reported the increase in TS and E values of jellyfish protein films with the increase in wasabi extract concentration. These changes in the mechanical properties might be due to non-covalent interactions between gelatin molecules and polyphenolic compounds. In particular, the PSG film had higher TS and E than the gelatin films produced from cuttle fish skins (Hoque et al. 2011), possibly because of different gelatin sources and the film preparation method.
The water vapor permeability (WVP) of the films is an important parameter associated with the moisture transfer into food products from the surrounding atmosphere, and low WVP values are preferable. The WVP of the PSG films decreased with the increase in ME concentration (Table 1). These results were similar to those obtained in silver carp skin gelatin films with green tea extract (Wu et al. 2013). The decrease in WVP of the ME-containing PSG films might be because of the compact structure formed due to cross-linking between gelatin molecules and polyphenolic compounds. The dense network can reduce the free volume of the film matrix, which reduced the diffusion rate of water molecules (Nie et al. 2015). Thus, the ME-containing PSG films with low WVP can be used to prevent microbial growth caused by moisture transfer in foods.
The effects of ME addition on the optical properties of PSG films are shown in Table 1. Hunter a value did not change significantly, whereas Hunter L and b value of the PSG film was affected by ME addition. With increasing ME concentration, Hunter L value decreased from 93.10 to 68.23 and b value increased from 7.85 to 41.03. The reason for the change was mainly due to light brown color of ME, which made the film more yellow. An increase in Hunter b value was also reported earlier for jellyfish protein films with wasabi extract (Lee et al. 2015a) and soy protein isolate films with raspberry extract (Wang et al. 2012). In addition, the opacity of the PSG film slightly increased when ME concentration increased. Siripatrawan and Harte (2010) also reported an increase in opacity of the chitosan film with increase in green tea extract concentration. The increase in opacity of the PSG film can affect the appearance of food products when applied for packaging.
Microstructure of ME-containing PSG film
The microstructure of the film represents the compatibility of the film with ME (data not shown). The control film without ME had a rough surface, whereas small agglomerates present on the film surface decreased with increase in ME concentration. ME-containing PSG film had a smoother and more homogeneous surface. ME-containing PSG film did not show any pore, crack and phase separation, which indicated better compatibility between PSG and ME. These results might be related to the physical properties of the ME-containing PSG films such as high TS and low WVP. Similarly, soy protein isolate films with raspberry extract had smoother and more homogeneous surface structure than the control film (Wang et al. 2012).
Antimicrobial activity of ME-containing PSG film
The antimicrobial activity of the ME-containing PSG film against L. monocytogenes was determined using the disc diffusion test. When the ME concentration increased from 0.3 to 1%, the inhibition zone increased from 9.63 to 20.00 mm. ME has antimicrobial activity possibly because there are several small proteins and peptides in ME (Jayawardana et al. 2015). ME contains pterygospermin, which is composed of two benzyl-isothiocyanate molecules. Benzyl-isothiocyanate is mainly responsible for the antimicrobial activity of ME (Jayawardana et al. 2015). It can inhibit microbial growth by disrupting the synthesis of cell membrane and essential enzymes in microorganisms (Suarez et al. 2003). The antimicrobial activities of ME against foodborne pathogens such as Escherichia coli, Staphylococcus aureus, and Salmonella typhimurium have been reported (Bukar et al. 2010). The results reflected that the ME-containing PSG film can reduce the growth of L. monocytogenes in cheese during storage.
Antioxidant activity of the PSG films containing ME
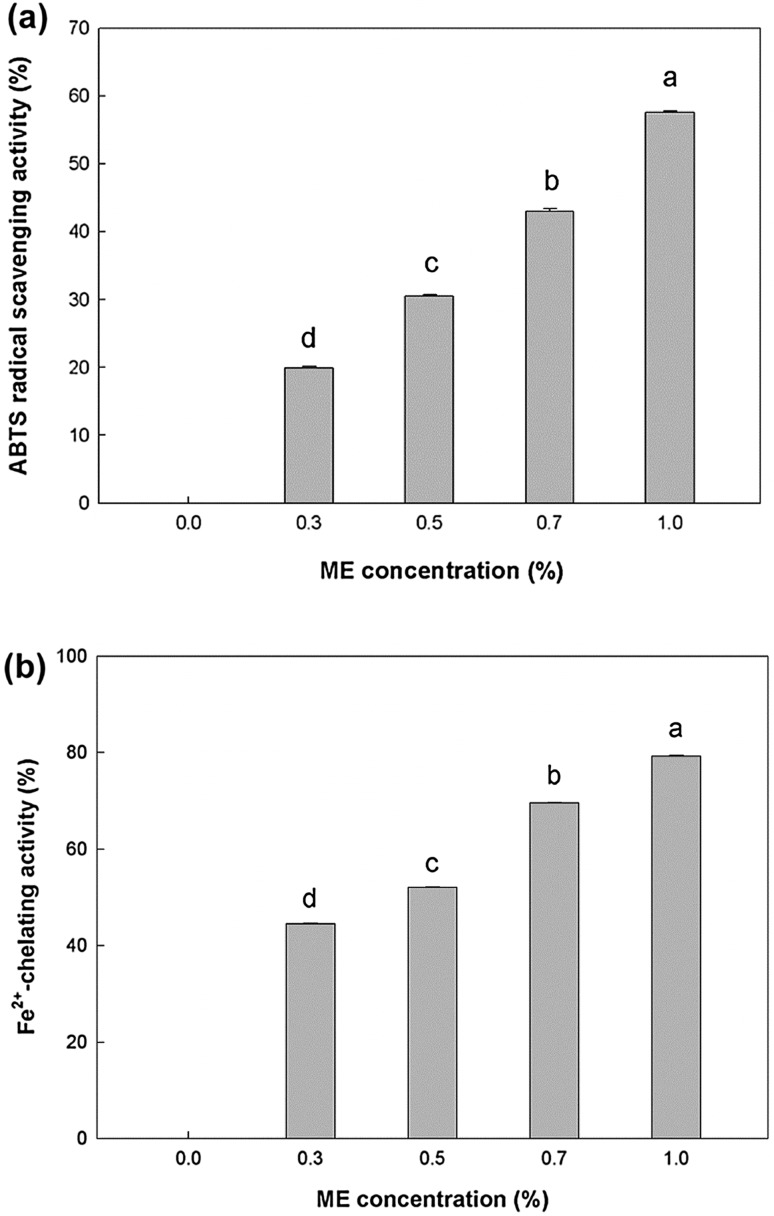
The ABTS radical-scavenging activity and ferrous-ion-chelating activity were measured to determine the antioxidant activity of the ME-containing PSG film. The control film without ME had no ABTS radical-scavenging activity, whereas the antioxidant activity of the film with 1% ME was 57.63% (Fig. 2a). There is a linear relationship between the antioxidant activity and the ME concentration. Moyo et al. (2012) also reported that the acetone extract of M. oleifera Lam. leaves has ABTS radical-scavenging activity.
Fig. 2.
Antioxidant activity of the PSG films containing various amounts of ME. a ABTS radical scavenging activity; b Fe2+-chelating activity, different letters denote significant (p < 0.05) difference
In general, Fe2+ causes the production of free radical and lipid peroxidation, minimizing the Fe2+ concentration protects food products against oxidative damage. The Fe2+-chelating activity of the PSG film with or without ME was measured (Fig. 2b). The results indicate that the ME-containing PSG film had Fe2+-chelating activity, and the antioxidant activity increased when the ME concentration increased. The antioxidant activity of ME is mainly attributed to flavonoids such as kaempferol, quercetin, and other polyphenolic compounds (Siddhuraju and Becker 2003). The addition of ME led to a significant increase in antioxidant activity of the PSG film. The antioxidant activity of the ME-containing PSG film can contribute to the inhibition of lipid oxidation of Gouda cheese during storage, which extends the cheese shelf life.
Microbiological analysis of Gouda cheese wrapped with PSG film
Cheese is easily contaminated by L. monocytogenes which can grow at refrigeration temperature (Meira et al. 2016). PSG film with 1% ME, was selected based on the physical, antioxidant, and antimicrobial properties and used to package Gouda cheese. The change in the populations of Gouda cheese was measured during storage (Table 2). The L. monocytogenes populations in the control sample and those wrapped with the PSG film continuously increased until day 8, slightly decreased on day 12 , and subsequently increased again. However, the L. monocytogenes populations in the cheese packed with the 1%-ME-containing PSG film did not show any change during storage. The growth pattern of bacteria during storage depends mainly on availability of nutrients. After 16 days of storage, the microbial count in the cheese packed with the PSG film with 1% ME decreased by 1.21 log CFU/g compared to the control sample. In addition, there was a reduction by 0.62 log CFU/g compared to the sample wrapped with the PSG film without ME. These results indicate that the addition of ME provided the antimicrobial property to the PSG film, thus inhibited the growth of L. monocytogenes on the cheese samples. Therefore, M. oleifera Lam. leaves can be used as a natural preservative to inhibit food-borne pathogens in foods (Bukar et al. 2010). In addition, these results suggest that the ME-containing PSG film can maintain the quality of Gouda cheese during storage as an active packaging material.
Table 2.
Changes in the populations of L. monocytogenes in Gouda cheese during storage
| (log CFU/g) | |||||
|---|---|---|---|---|---|
| Storage time (d) | |||||
| Sample | 0 | 4 | 8 | 12 | 16 |
| Control | 5.36 ± 0.32Ca | 6.03 ± 0.05Ba | 6.27 ± 0.13ABa | 6.10 ± 0.06Ba | 6.52 ± 0.05Aa |
| PSG film | 5.36 ± 0.32Ba | 5.72 ± 0.33ABab | 5.77 ± 0.16ABb | 5.45 ± 0.09Bb | 5.93 ± 0.08Ab |
| PSG-1% ME film | 5.36 ± 0.32Aa | 5.47 ± 0.02Ab | 5.25 ± 0.13Ac | 5.12 ± 0.14Ac | 5.31 ± 0.17Ac |
Mean ± SD
a–cAny means in the same column followed by different letters are significantly (p < 0.05) different by Duncan’s multiple range test
A–CAny means in the same row followed by different letters are significantly (p < 0.05) different by Duncan’s multiple range test
Lipid oxidation of Gouda cheese wrapped with the PSG film
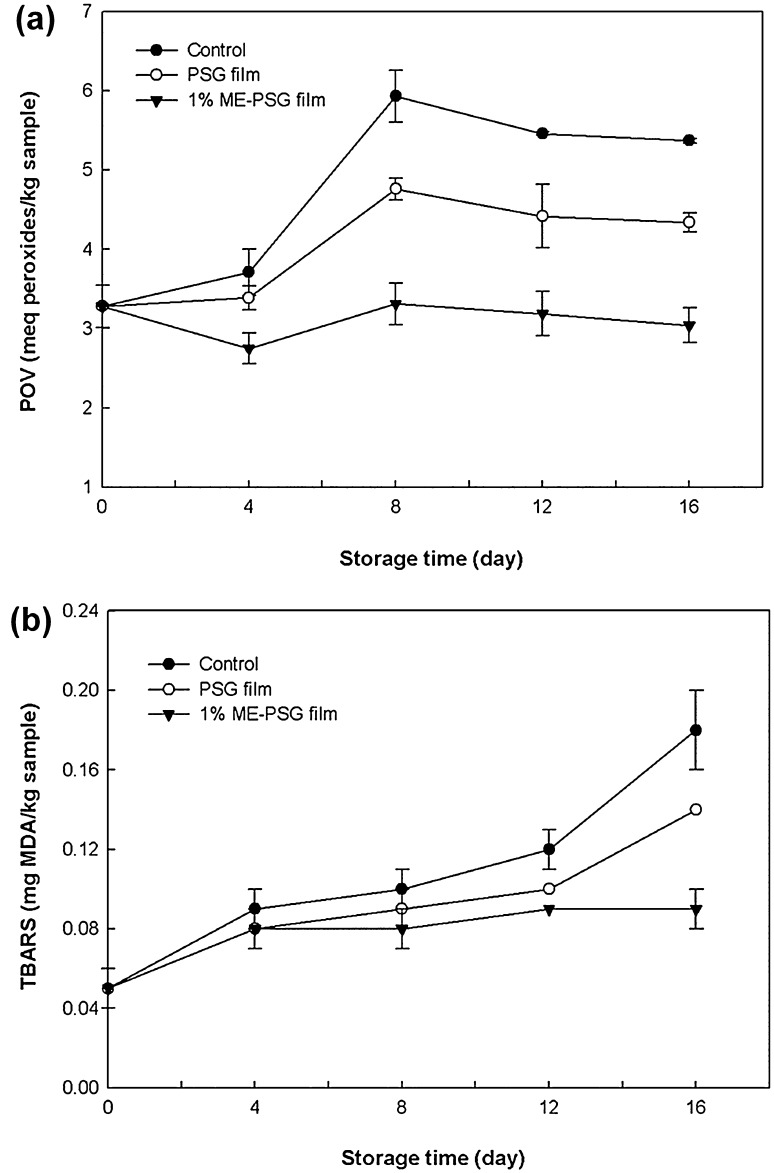
The PO and TBARS values were measured to determine the lipid oxidation of Gouda cheese during storage. The initial POV of the cheese sample was 3.28 meq peroxide/kg sample. The POV of the control sample and the cheese wrapped with the PSG film continuously increased until 8 days of storage and subsequently decreased. Cheese wrapped with the PSG film containing 1% ME, showed a decrease in POV on day 4, a slight increase on day 8, and a decrease thereafter (Fig. 3a). The decrease in POV after 12 days was observed may be due to unstable peroxides decomposed to secondary products such as ketones, hydrocarbons, and aldehydes (Nascimento et al. 2015). At the end of the storage period, the POVs of the control sample, the cheese wrapped with the PSG film, and the cheese wrapped with the PSG film with 1% ME were 5.37, 4.34, and 3.04 meq peroxide/kg sample, respectively. The cheese wrapped with the PSG film with 1% ME had lower POV than the other samples, which indicates that the PSG film with 1% ME effectively inhibited the production of primary lipid oxidation products in the cheese. These results are consistent with those of Nascimento et al. (2015), where the ME-containing fish oil had lower POV than those with other synthetic antioxidants during storage.
Fig. 3.
Lipid oxidation of Gouda cheese during storage. a Peroxide value; b TBARS value
There was a similar trend in the TBARS assay results. The initial TBARS value was 0.05 mg MDA/kg sample, and all samples showed an increase in TBARS value during storage (Fig. 3b). Among the samples, the cheese that was wrapped with the PSG film with 1% ME showed the lowest increase in TBARS value. The oxygen permeability (OP) of the film is an important parameter that may have affected the lipid oxidation of the cheese. The OP of the PSG film and 1% ME-containing PSG film was 35.12 and 22.21 cm3/m2 24 h atm, respectively. Therefore, cheese that was wrapped with the ME-containing PSG film had less contact with oxygen than the control samples and those wrapped with the PSG film. These results indicate that the oxygen barrier property of the film was enhanced by the addition of ME, which further inhibited the lipid oxidation of the cheese by decreasing the oxygen content in the packaging.
Conclusion
In this study, a biodegradable film was developed using gelatin derived from puffer fish skin as a new film base material. The PSG film had good physical and barrier properties compared to other fish skin gelatin films. The addition of ME effectively improved the TS, E, WVP, and OP of the film. Furthermore, the ME-containing PSG film had antimicrobial activity against L. monocytogenes and antioxidant activity such as ABTS radical-scavenging activity and Fe2+-chelating activity. The use of ME-containing PSG film for packaging of Gouda cheese, indicated inhibition of microbial growth and lipid oxidation during storage. Based on these results, the ME-containing PSG film can potentially be used as an alternative to synthetic plastic packaging materials in the food industry.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2015R1D1A1A01058736).
References
- AOAC (1990) Official methods of analysis, 15th edn. Method 965.33. Peroxide value of oils and fats. Association of Official Analytical Chemists, Washington DC
- Bahram S, Rezaei M, Soltani M, Kamali A, Ojagh SM, Abdollahi M. Whey protein concentrate edible film activated with cinnamon essential oil. J Food Process Preserv. 2014;38:1251–1258. doi: 10.1111/jfpp.12086. [DOI] [Google Scholar]
- Bukar A, Uba A, Oyeyi T. Antimicrobial profile of Moringa oleifera Lam. extracts against some food-borne microorganisms. Bayero J Pure Appl Sci. 2010;3:43–48. [Google Scholar]
- Giménez B, Gómez-Estaca J, Alemán A, Gómez-Guillén MC, Montero MP. Improvement of the antioxidant properties of squid skin gelatin films by the addition of hydrolysates from squid gelatin. Food Hydrocolloid. 2009;23:1322–1327. doi: 10.1016/j.foodhyd.2008.09.010. [DOI] [Google Scholar]
- Gómez-Guillén MC, Ihl M, Bifani V, Silva A, Montero P. Edible films made from tuna-fish gelatin with antioxidant extracts of two different murta ecotypes leaves (Ugni molinae Turcz) Food Hydrocolloids. 2007;21:1133–1143. doi: 10.1016/j.foodhyd.2006.08.006. [DOI] [Google Scholar]
- Hoque MS, Benjakul S, Prodpran T. Properties of film from cuttlefish (Sepia pharaonis) skin gelatin incorporated with cinnamon, clove and star anise extracts. Food Hydrocolloids. 2011;25:1085–1097. doi: 10.1016/j.foodhyd.2010.10.005. [DOI] [Google Scholar]
- Jayawardana BC, Liyanage R, Lalantha N, Iddamalgoda S, Weththasinghe P. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT-Food Sci Technol. 2015;64:1204–1208. doi: 10.1016/j.lwt.2015.07.028. [DOI] [Google Scholar]
- Kim RY, Sung NJ, Kim WT, Park JH, Kim YJ, Ju JC. Physicochemical characteristic of concentrate prepared by puffer muscle and skin. J Korean Soc Food Sci Nutr. 2010;39:267–273. doi: 10.3746/jkfn.2010.39.2.267. [DOI] [Google Scholar]
- Kuorwel KK, Cran MJ, Sonneveld K, Miltz J, Bigger SW. Antimicrobial activity of biodegradable polysaccharide and protein-based films containing active agents. J Food Sci. 2011;76:R90–R102. doi: 10.1111/j.1750-3841.2011.02102.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee JH, Yang HJ, Won M, Song KB. Characterisation of jellyfish protein films with added transglutaminase and wasabi extract. Int J Food Sci Techol. 2015;50:1683–1689. doi: 10.1111/ijfs.12826. [DOI] [Google Scholar]
- Lee JH, Lee J, Song KB. Development of a chicken feet protein film containing essential oils. Food Hydrocolloids. 2015;46:208–215. doi: 10.1016/j.foodhyd.2014.12.020. [DOI] [Google Scholar]
- Lee KY, Lee JH, Yang HJ, Song KB. Production and characterisation of skate skin gelatin films incorporated with thyme essential oil and their application in chicken tenderloin packaging. Int J Food Sci Techol. 2016;51:1465–1472. doi: 10.1111/ijfs.13119. [DOI] [Google Scholar]
- Meira SMM, Zehetmeyer G, Scheibel JM, Werner JO, Brandelli A. Starch-halloysite nanocomposites containing nisin: characterization and inhibition of Listeria monocytogenes in soft cheese. LWT-Food Sci Technol. 2016;68:226–234. doi: 10.1016/j.lwt.2015.12.006. [DOI] [Google Scholar]
- Moyo B, Oyedemi S, Masika PJ, Muchenje V. Polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Sci. 2012;91:441–447. doi: 10.1016/j.meatsci.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Nagai T, Araki Y, Suzuki N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes) Food Chem. 2002;78:173–177. doi: 10.1016/S0308-8146(01)00396-X. [DOI] [Google Scholar]
- Nascimento JA, Magnani M, Sousa J, Araújo KL, Epaminondas PS, Souza AS, Souze AL, Silva MCD, Souza AG. Assessment of the antioxidant effects of Moringa oleifera Lam. extracts in fish oil during storage. J Food Process Preserv. 2015;40:29–36. doi: 10.1111/jfpp.12580. [DOI] [Google Scholar]
- Nie X, Gong Y, Wang N, Meng X. Preparation and characterization of edible myofibrillar protein-based film incorporated with grape seed procyanidins and green tea polyphenol. LWT-Food Sci Technol. 2015;64:1042–1046. doi: 10.1016/j.lwt.2015.07.006. [DOI] [Google Scholar]
- Rattaya S, Benjakul S, Prodpran T. Properties of fish skin gelatin film incorporated with seaweed extract. J Food Eng. 2009;95:151–157. doi: 10.1016/j.jfoodeng.2009.04.022. [DOI] [Google Scholar]
- Ruiz-Navajas Y, Viuda-Martos M, Sendra E, Perez-Alvarez JA, Fernández-López J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control. 2013;30:386–392. doi: 10.1016/j.foodcont.2012.07.052. [DOI] [Google Scholar]
- Senaratne LS, Park PJ, Kim SK. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) skin. Bioresour Technol. 2006;97:191–197. doi: 10.1016/j.biortech.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Siripatrawan U, Harte BR. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocolloid. 2010;24:770–775. doi: 10.1016/j.foodhyd.2010.04.003. [DOI] [Google Scholar]
- Suarez M, Entenza JM, Doerries C, Meyer E, Bourquin L, Sutherland J, Marison I, Moreillon P, Mermod N. Expression of a plant-derived peptide harboring water-cleaning and antimicrobial activities. Biotechnol Bioeng. 2003;81:13–20. doi: 10.1002/bit.10550. [DOI] [PubMed] [Google Scholar]
- Tajik S, Maghsoudlou Y, Khodaiyan F, Jafari SM, Ghasemlou M, Aalami M. Soluble soybean polysaccharide: a new carbohydrate to make a biodegradable film for sustainable green packaging. Carbohyd Polym. 2013;97:817–824. doi: 10.1016/j.carbpol.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47:2196–2201. doi: 10.1016/j.fct.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Wang S, Marcone M, Barbut S, Lim LT. The impact of anthocyanin-rich red raspberry extract (ARRE) on the properties of edible soy protein isolate (SPI) films. J Food Sci. 2012;77:C497–C505. doi: 10.1111/j.1750-3841.2012.02655.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Chen S, Ge S, Miao J, Li J, Zhang Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocolloid. 2013;32:42–51. doi: 10.1016/j.foodhyd.2012.11.029. [DOI] [Google Scholar]