Abstract
Ultrafiltration (UF) of skimmed milk altered the composition of UF retentate and decreased the heat stability. Heat stability further reduced upon its subsequent homogenization or diafiltration. Poor heat stability of UF retentate restricts its processing at elevated temperatures. Therefore, this study was aimed to investigate the effect of protein concentration, homogenization and addition of stabilizing salts on the heat stability and rheological properties of UF retentates. Changes in the heat stability of fivefold homogenized UF retentate (5× HUFR) was studied in the pH range of 6.1–7.0. Disodium phosphate and trisodium citrate significantly increased the heat coagulation time (HCT) from 1.45 min (pH 6.41) to 120 min (at pH 6.5, 6.6, 7.0) and 80 min (pH 6.6), respectively. Significant reduction in ζ-potential of UF retentates was observed with an increase in calcium and reduction in pH during UF process. Rheological behaviour of retentates above threefold concentration exhibited Herschel–Bulkley behavior with linear increase in flow behavior index (n). Changes in the viscosity of the homogenized retentates were measured at the respective pH of maximum heat stability as a function of temperature (20–80 °C). Promising approaches that might improve the heat stability, solubility and other functional properties of protein rich powders have been discussed in this article.
Keywords: Diafiltration, Stabilizing salts, Heat stability, ζ-Potential, Rheological properties
Introduction
Ultrafiltration (UF) of milk results in the concentration of milk proteins, fat and insoluble salts while reduces lactose, water soluble minerals and vitamins in retentates by allowing their selective passage into permeate (Meena et al. 2015). UF retentates obtained from pasteurized cow skimmed milk (PCSM) had different composition than concentrates either obtained by evaporation or reverse osmosis (RO). Heat stability or heat coagulation time (HCT) is an important property of liquid and concentrated milk that is defined as the resistance shown by these samples in minutes towards their heat induced coagulation at 140 and 120 °C, respectively (Singh 2004). It can be measured by various objective and subjective assays (O’Connell and Fox 2011). Higher HCT shows suitability of these milks for high heat treatment owing to appropriate proteins stability and vice versa. Slight modification in the delicate mineral balance of the milks by either any process or additives, influences its heat stability (Solanki and Gupta 2009). Owing to changes in salts and protein content, UF retentates showed altered heat stability and HCT-pH profile due to shift in delicate ionic equilibrium between serum and miceller proteins during concentration (Holt et al. 1981). Partial removal of calcium was reported to increase the HCT of cow milk and its concentrate. Concentrated milk had maximum HCT between 6.4 and 6.6 pH, but was less heat stable compared to unconcentrated milks on whole pH range (Singh and Creamer 1992). An intrinsic and extrinsic factor significantly affects the heat stability of concentrated milks (Singh 1995). Salt composition has significant impact on the heat stability of concentrated milks (Hardy et al. 1984; Sweetsur and Muir 1980), but β-Lg was inversely correlated with their heat stability (Muir and Sweetsur 1978). Heat stability of UF retentate was better than the concentrate produced through classical evaporation at 40 °C, in 9.3–18.4% TS range. Pre-heating (90 °C/10 min) doubled the heat stability of the concentrate but the same had little effect on heat stability of UF retentates (Sweetsur and Muir 1980). Ozimek et al. (1988) observed non-significant difference between the HCT of fresh and a week old samples of UF retentate.
Montella (2008) investigated the impact of batch pasteurization (before and after UF treatment) and ultrafiltration temperature (either 10 or 50 °C) on compositional and rheological properties (i.e. viscosity, at 10, 40 and 70 °C; 500 s−1 shear rate) of 3× UF retentates obtained from whole bovine milk. Filtration temperature and pasteurization treatment have significant effect on proximate composition, while former had marked effect on viscosity (shear thinning behavior) of UF retentate. Khatkar et al. (2014) reported that HCT of buffalo skim milk UF retentate (4.05–4.18 folds; 10% fat & 23.63% TS), homogenized at 175.76/35.15 kg/cm2 was successfully enhanced from 23 min to maximum 66 min by the addition of 0.4% mixture of monosodium and disodium phosphate (2:1 w/w). Similar increase in HCT of full fat liquid dairy whitener formulation was earlier also reported by Khatkar and Gupta (2012).
UF retentate is used as a protein ingredient in various food applications either in liquid or in dried forms so; it should have appropriate heat stability to withstand applied high heat treatment. Heat stability improvement of concentrated milk during the manufacture of evaporated milks by the addition of suitable stabilizing salts is now well established (Solanki and Gupta 2009), but the mechanism involved in the stabilization of UF retentate having different chemical makeup than evaporated milk needs to be studied.
Caseins are major milk proteins that contribute about 80% of total milk protein. Caseins are present as micelles with an average diameter of about 200 nm in colloidal suspension in milk. Casein micelles are spherical in shape, fully covered by hairy layer of κ- casein (Ferrer et al. 2011) that delivers steric and charge stabilization as well as zeta (ζ) potential of −20 mV to it (De Kruif and Holt 2003). Slight change in salt equilibrium of milk, induces significant change in structure and functional properties of casein micelles. Thus, its techno-functional properties can easily be altered by pH, temperature, high-pressure, high-shear treatment and spraying drying or their combination. ζ-potential indicates the charges present on casein micelles and its magnitude indicates the potential stability of a particular colloidal system.
Rheological properties plays a key role in the design and development of equipment and process line and significantly affects the general unit operations used in processing of milk and milk products. UF retentates exhibit altered rheological properties compared to PCSM due to change in its constituents. Hence, change in flow behaviour and viscosity of homogenized, stabilizing salts added UF retentate needs to be studied.
Therefore, current investigation was undertaken with two Objectives. (i) To investigate the effect of concentration in UF, homogenization, and diafiltration with 1:1 ratio (75 mM: 75 mM) of NaCl and KCl on heat stability, ζ-potential and rheological properties of PCSM ultrafiltered-diafiltered (UF-DF) retentate. (ii) To improve the heat stability of homogenized fivefold UF retentate by the addition of stabilizing salts such as monosodium phosphate (NaH2PO4), disodium phosphate (Na2HPO4) and tri-sodium citrate (Na3C6H5O7).
Materials and methods
Ultrafiltration and diafiltration
Pasteurized cow skim milk (73 ± 1 °C/15 s) was obtained from the Experiential Dairy of ICAR-National Dairy Research Institute, Karnal (India) and indirectly heated up to 50 ± 1 °C in a batch system. The PCSM (250 kg) was ultrafiltered in pilot UF plant (Tech-Sep., France, tubular module equipped with 50 kDa, ZrO2 membrane having 1.68 m2 membrane surface area) at 50 ± 1 °C temperature and 3.1 kg/cm2 constant Trans Membrane Pressure (TMP) as reported by Meena et al. (2015). The quantity of permeate coming out was measured by taking its weight on each concentration factor (CF). Permeate flux was directly monitored from the flow meter equipped with the UF plant and expressed as l/h/m2 (LMH). Mean flux was calculated using the following formula reported by St-Gelais et al. (1991).
Total 20 kg solution (150 mM strength) containing 75 mM solution of each NaCl and KCl (1:1 ratio) was prepared in RO water by proper dissolving of these salts and used during the diafiltration (DF) of 20 kg of fivefold UF retentate (5× UFR). All experiments were conducted three times (n = 3).
Concentration factor (CF)
The folds or degree of concentration for milk was calculated by following formula (Smith 2013).
CF were reported as 1×, 2×, 3×, 4× and 5×, where 1× represents PCSM while 2×, 3×, 4× and 5× represents the retentate samples obtained after 2, 3, 4 and 5 times or folds concentration, respectively.
Sample collection
Representative samples of all concentration factors were collected in 500 mL pre-sterilized glass bottles. Total 40 kg retentate was recovered after 5× concentration from 250 kg feed that was further divided into four parts. Retentate obtained after 5× UF concentration was named 5× UFR and treated as control. A part of this was homogenized at 2000 psi and 500 psi pressure in double stage homogenizer (make- APV Crepaco, Inc. Chicago, ILL. U.S.A., capacity 50 L/h) and named 5× HUFR. The pH of a part of 5× UFR was adjusted to 6.5 using 10% DSP solution and termed as 5× DSP sample. Representative sample of 5× UF–DF retentate was obtained after the removal of 20 kg NaCl–KCl added solution as permeate, called 5× Na–K. All samples were stored at 4 ± 1 °C and preserved by the addition of 0.03% (w/w) sodium azide to check the microbial growth till further analysis.
Compositional analysis
Total solids (TS) and ash contents of all the samples were determined by Gravimetric method as described by Indian standards (IS: 12333 1997) and Indian standards (IS: 1479 part III 1961), respectively. Crude protein content was determined using Macro Kjeldahl Method (IDF 1993). Crude protein contents of the samples were multiplied with factor 6.38 to obtain their respective total protein contents. Fat content of skim milk and ultrafiltered milks were determined by Gerber method (IS: 1224 part I 1977). Lactose contents of the samples were determined by difference by subtracting protein, fat and ash from their respective TS contents as reported by Khatkar and Gupta (2012) for dairy whitener.
Determination of calcium content
Calcium content of all samples were analyzed in a Shimadzu AA-7000, atomic absorption spectrophotometer (AAS) using the method of AOAC (2005) adopting the procedure earlier reported by Kaushik et al. (2014).
Measurement of ζ-potential
ζ-potential of all samples were measured in a particle size analyzer at 25 °C which works on Laser Doppler Micro-electrophoresis (Nano ZS90 Zetasizer, Malvern Instruments Ltd, Worcestershire, UK). ζ-potential of PCSM was initially measured by its 40 times dilution in distilled water and then all other retentate samples were diluted in such a manner that their TS level were equal to the TS (0.208%) of diluted PCSM.
pH measurement and its adjustment by addition of stabilizing salts
pH of all samples were measured using Eutech pH meter (model- cyberscan 1100) of Thermo Scientific at 20 ± 1 °C. All the analytical grade salts were used in this study such as monosodium phosphate (MSP), disodium phosphate (DSP), tri-sodium citrate (TSC), sodium chloride (NaCl) and potassium chloride (KCl) were procured from Sigma-Aldrich, USA. Initially, 10% solution of these salts were prepared in distilled water and then pH of the samples was adjusted to different values. The pH of the samples was rechecked and corrected after 1 h to nullify the effect of buffering action of milk proteins.
Determination of heat coagulation time (HCT)
It was obtained through measuring the time taken by the samples to form visible clots. HCT of PCSM and other retentate samples was measured at 120 °C using the standard method as reported by Khatkar et al. (2014).
Measurement of rheological properties
Flow behavior of the samples was determined at 20 °C using a Rheometer (MCR 52, Anton Paar, Germany) attached with cone plate CP75-1° (SS) probe at variable shear rate ranging from 1 to 1000 s−1. The rheological data obtained in the range of 1 to 100 s−1 were fitted to rheological models. The equations for these models commonly reported in the literature are:
where, σ is the shear stress (Pa), σo is the yield stress, is the shear rate (s−1), K is the consistency index (Pa-sn) and n is the flow behaviour index. Moreover, η o and η ∞ are viscosities at zero and infinite shear rate with no yield stress. Critical shear rate was denoted by λ −1 at which viscosity begins to decrease. The temperature sweep test was also performed to study the flow behaviour of samples under the influence of operational temperature. The samples were heated from 20 to 90 °C with 5 °C per min increase in temperature at constant shear rate (100 s−1).
Statistical analysis
Results of this study (mean value, n = 3) were subjected to one-way analysis of variance (ANOVA) using SAS Enterprise guide (5.1, 2012) developed by SAS Institute Inc., North Carolina, USA and represented by using Duncan’s multiple range test values as earlier reported by Meena et al. (2015).
Results and discussion
Chemical composition
Chemical composition and pH of PCSM, its retentates (2×, 3×, 4×), 5× UFR, 5× HUFR and 5× Na–K samples is reported in Table 1. It was observed that during continuous UF and DF, TS, protein, fat and ash contents increased in all samples up to 5× CF, but incessant decrease was observed in lactose content as it passed through the membrane into permeate. Similar findings have been previously reported by Mistry (2002) during skim milk concentration in UF during the manufacture of high protein milk powder and also during the manufacture of dairy whitener from PCSM by other researchers. Moreover, highly significant (P < 0.01) difference was observed in percent TS, protein, fat, ash contents and pH values of all samples, but non- significant difference (P > 0.05) was observed among 5×, 5× HUFR and 5× DSP samples. This is because after achieving fivefold concentration in UF process, a part of retentate was subjected to homogenization (5× HUFR) and the pH of another part was intentionally adjusted to 6.5 by adding 10% DSP solution (5× DSP), therefore these samples basically had similar chemical composition (Table 1). Three times DF of 6× UF retentates resulted in greater reduction in ash content (Mistry 2002). Slight increase in ash content of 5× Na–K samples obtained after one time DF of the 5× UFR sample was observed, which may be attributed to the external addition of salts as well as lower times of DF treatment that was not as effective as six times DF in removal of minerals into permeate.
Table 1.
Chemical composition, pH and ζ-potential of PCSM and retentate samples
| Samples | TS (%) | Protein (%) | Ash (%) | Fat (%) | Lactose (%) | pH | Ca (%) | HCT (min) | Flux (LMH) | ζ-Potential (mV) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1× | 8.33f ± 0.01 | 3.16f ± 0.01 | 0.66f ± 0.01 | 0.30e ± 0.00 | 4.27a ± 0.01 | 6.60a ± 0.00 | 0.11a ± 0.03 | 113a ± 0.00 | 52a ± 0.08 | −21.237g ± 0.04 |
| 2× | 12.14e ± 0.00 | 6.36e ± 0.00 | 0.99e ± 0.02 | 0.60d ± 0.00 | 4.19a ± 0.02 | 6.55b ± 0.00 | 0.26b ± 0.07 | 113a ± 0.00 | 34b ± 0.02 | −6.032d ± 0.05 |
| 3× | 15.27d ± 0.01 | 9.12d ± 0.00 | 1.24d ± 0.01 | 0.90c ± 0.00 | 4.01b ± 0.09 | 6.54c ± 0.00 | 0.34c ± 0.02 | 79.92b ± 0.60 | 30c ± 0.05 | −5.293e ± 0.09 |
| 4× | 19.15c ± 0.04 | 12.54c ± 0.01 | 1.56c ± 0.04 | 1.20b ± 0.00 | 3.88c ± 0.05 | 6.51d ± 0.00 | 0.43d ± 0.06 | 52.35c ± 0.21 | 26d ± 0.02 | −4.885c ± 0.08 |
| 5× UFR | 22.33b ± 0.02 | 15.64b ± 0.02 | 1.67b ± 0.03 | 1.50a ± 0.00 | 3.49d ± 0.05 | 6.41f ± 0.00 | 0.47e ± 0.02 | 5.45d ± 0.14 | 18f ± 0.04 | −0.598a ± 0.02 |
| 5× HUFR | 22.32b ± 0.00 | 15.67b ± 0.04 | 1.68b ± 0.05 | 1.50a ± 0.00 | 3.46d ± 0.06 | 6.41f ± 0.00 | 0.47e ± 0.02 | 1.45e ± 0.01 | 20e ± 0.02 | −1.007b ± 0.04 |
| 5× Na–K | 22.64a ± 0.00 | 15.87a ± 0.02 | 1.99a ± 0.02 | 1.50a ± 0.00 | 3.27e ± 0.08 | 6.34g ± 0.00 | 0.49f ± 0.04 | 1.05e ± 0.02 | NA | −18.500f ± 0.05 |
| 5× DSP | 22.27b ± 0.00 | 15.56b ± 0.05 | 1.70b ± 0.01 | 1.50a ± 0.00 | 3.52d ± 0.06 | 6.50e ± 0.00 | 0.46g ± 0.02 | 81b ± 0.00 | NA | −0.568a ± 0.09 |
Mean value of 3 trials ±SE; values for a particular column followed by different letters differ significantly (P < 0.01); NA Not applicable
In UF process, highly significant (P < 0.01) reduction in the pH of retentates was observed from 1× (pH 6.60) to 5× (pH 6.41) concentration (Table 1). The observed decrease in pH was in good agreement with the previously reported results (Huppertz et al. 2004; Karlsson et al. 2005; Ferrer et al. 2011). The concentration of milk salts (Ca2+, Mg+ and K+) increases during UF process which changes the cationic profile of milk based on their association with proteins, but total calcium to protein ratio decreases with increase in CF due to passage of soluble calcium in permeate (Ferrer et al. 2011; Sikand et al. 2013). Moreover, marked decreased (P < 0.01) in the pH of 5× Na–K was also observed which was attributed to the DF of 5× UFR sample with NaCl–KCl added RO water (Table 1).
Change in permeate flux during UF and DF processes has been shown in Table 1. It is well established that during UF process, membrane flux decreases with increase in TS of feed. Continuous decrease in permeate flux was observed with a rise in the protein concentration in UF and DF (Table 1) owing to increase in TS and viscosity of feed, that increases concentration polarization and fouling of the UF membrane. Mean flux, calculated using the values of initial and final flux was 47.06 ± 0.12 LMH, which was observed to be better than earlier published reports (St-Gelais et al. 1991).
Preliminary studies on UF-DF concentration of PCSM
Fresh PCSM was ultrafiltered up to 5× CF, containing 22.33% TS and 15.64% protein content. In liquid form, such retentates were utilized in many applications like manufacture of high protein milk, protein standardization in cheese milk to produce hard and soft varieties of cheese, quality improvement of fermented dairy products like dahi (curd) and yoghurt as well as in the production of sports drinks and beverages, production of reduced lactose milks, powders and specialty food products for lactose intolerant people. UF retentates was also converted into dry form for the production of different MPCs such as MPC42, MPC55, MPC70, MPC80, MPC85 (Mistry 2002; Sikand et al. 2013); milk protein isolates (MPIs); medium fat as well as full fat liquid and dried dairy whitener used for the preparation of better quality tea and coffee (Khatkar et al. 2014), while low phenylalanine yoghurt was developed using ultrafiltered milk permeate and non-dairy creamer powder by Goldar et al. (2016).
Preservation of high protein milk, particularly by ultra-high temperature (UHT) treatment can cater the lager consumer group as such milk does not require refrigerated storage, but this process demands for high quality milk and retentates, particularly in terms of their heat stability. Hence, the 5× UFR samples were subjected to different treatments to study the effect of such treatments on the heat stability 5× UFR samples as shown in Table 1. Statistically, PCSM and 2× samples had non-significant difference in their HCT (113 min, both samples were not coagulated up to reported period) but they differed significantly (P < 0.01) with all other samples. For other retentate (2×–5×) samples, HCT decreased significantly (P < 0.01) with increase in CF. Ozimek et al. (1988) also observed the reduction in HCT of UF retentates with increase in CF or volume concentration ratio. The reduction was due to alteration in mineral balance of milk during UF process. Homogenization, a common unit operation in dairy industry also showed detrimental effect on heat stability of 5× sample and had significantly (P < 0.01) lesser heat stability even than that of 5× UFR sample (Table 1). Reduction in heat stability of milk, concentrated milk (Sweetsur and Muir 1980) and evaporated milk after homogenization, was also observed earlier. Homogenization of unsweetened condensed milk resulted in the enhancement of viscosity (Kieczewska et al. 1999), but reduction in HCT that further decreased with an increase in the homogenization pressure (Kieczewska et al. 2003).
HCT improvement of 5× HUFR sample
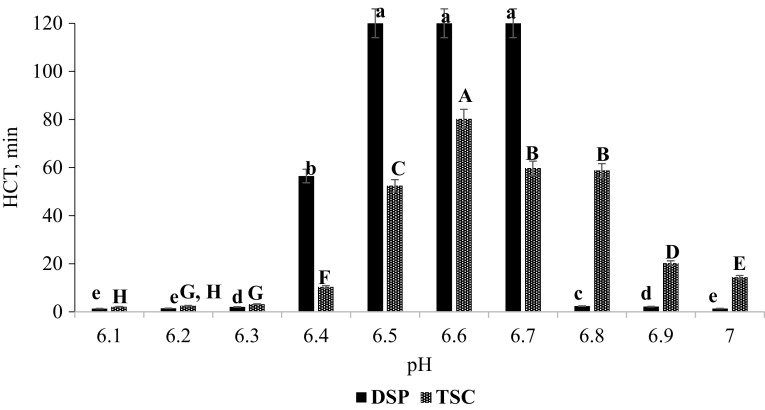
The adjustment of pH in 5× HUFR sample with 10% solution of MSP and DSP in particularly 6.5–6.7 pH range resulted in statistically significant (P < 0.01) improvement in its heat stability. In this pH range, HCT of 5× HUFR sample was very high (120 min), still samples were not coagulated (Fig. 1), as compared to the HCT of 5× HUFR sample containing no added stabilizing salt (pH-6.41, HCT-1.45 min) as shown in Table 1. Moreover, the increase in HCT was similar at pH 6.5, 6.6 and 6.7, but it differed significantly (P < 0.01) than other HCT values observed at 6.1, 6.2, 6.3, 6.4, 6.8, 6.9 and 7 adjusted pH values (Fig. 1). This sample also exhibited statistically similar resistance time towards its heat coagulation in two pH groups (6.1, 6.2, 7 and 6.3, 6.9) within the group. At pH 6.4, samples showed significantly (P < 0.01) lower HCT value compared to HCT at pH 6.5, 6.6 and 6.7 while it resulted in significantly (P < 0.01) higher HCT than other studied pH (6.1, 6.2, 6.3, 6.8, 6.9 and 7) values (Fig. 1). From processing point of view, the desired increase in HCT of 5× HUFR sample was observed within pH 6.5–6.7 range only otherwise DSP failed to enhance the HCT of the sample on both extreme sides of studied pH. Thus, variation in HCT values indicates the significance of salt balance and pH over the heat stability control of retentates. Therefore, optimum level of stabilizer addition helps in the maintaining proper pH and salt balance in the product as earlier also observed by Khatkar et al. (2014) during the production of dairy whiteners from UF retentates.
Fig. 1.
Effect of pH adjustment by 10% solution of DSP and TSC on HCT (s) of 5× HUFR sample (n = 3). abcdefg, ABCDEFGMean values of HCT within a graph with different letters differ significantly (P < 0.01) for DSP and TSC
Similarly, adjustment of pH by 10% solution of MSP and TSC also increased the HCT of 5× HUFR sample, but the maximum enhancement was lower than that obtained with the combination of MSP and DSP. With TSC, maximum heat stability was observed at pH 6.6 (80.21 min), which was significantly (P < 0.01) higher than the other observed HCT values on remaining studied pH range. Sample had non-significant difference in HCT at pH 6.7 and 6.8, but resulted in second highest (58 min) improvement in HCT of 5× HUFR sample. Moreover, significant difference (P < 0.1) among the HCT values of the sample were present at their respective pH i.e. at pH 6.1, 6.3, 6.4, 6.5, 6.8, 6.9 and 7. TSC performed better in improving the HCT of 5× HUFR on the extreme values of studied pH range as compared to DSP. Heat stability of 5× DSP sample (non-homogenized) was also investigated at pH 6.5 and it exhibited significant (P < 0.01) improvement in HCT (80 min) of the sample as compared to HCT (5.35 min) at its natural pH (Table 1). Moreover, this increase in HCT of 5× sample was higher than the obtained HCT value with TSC, but lower than exhibited by DSP at same pH (Table 2 and Fig. 1).
Table 2.
Rheological parameters modelling of UF retentates obtained at various stages of processing with addition of stabilizing salts
| Sample | Bingham | Herschel–Bulkley | Power Law | Carreau–Yasudaa | Apparent viscosity, mPa.s at 50 s−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| σ0 (Pa) | η (mPa.s) | R2 | σ0 (Pa) | k | n | R2 | k | n | R2 | η0 | η∞ | R2 | ||
| 1× | 0.631 | 3.56 | 0.848 | 0.598 | 0.013 | 0.738 | 0.878 | 0.572 | 0.099 | 0.749 | – | – | – | 17.3 |
| 2× | 0.753 | 3.93 | 0.891 | 0.701 | 0.020 | 0.662 | 0.928 | 0.673 | 0.098 | 0.816 | – | – | – | 19.1 |
| 3× | 0.739 | 6.17 | 0.943 | 0.737 | 0.007 | 0.988 | 0.944 | 0.677 | 0.123 | 0.722 | – | – | – | 21.7 |
| 4× | 0.662 | 15.92 | 0.986 | 0.610 | 0.032 | 0.841 | 0.991 | 0.523 | 0.279 | 0.874 | – | – | – | 29.4 |
| 5× UFR | 0.721 | 54.19 | 0.961 | 0.494 | 0.193 | 0.709 | 0.999 | 0.591 | 0.466 | 0.968 | – | – | – | 69.7 |
| 5× HUFR | 0.703 | 50.37 | 0.961 | 0.484 | 0.182 | 0.706 | 0.999 | 0.572 | 0.458 | 0.967 | – | – | – | 66.2 |
| 5× Na–K | 0.841 | 61.96 | 0.957 | 0.515 | 0.217 | 0.713 | 0.999 | 0.692 | 0.461 | 0.970 | – | – | – | 81.7 |
| 5× DSP | 3.221 | 332.20 | 0.841 | 0.541 | 2.975 | 0.500 | 0.999 | 3.513 | 0.465 | 0.998 | ∞ | 39.74 | 0.999 | 437.0 |
aCritical shear rate: 0.021 s−1
Due to lack of scientific reports dealing with the heat stability of homogenized UF concentrated milks, our findings have been compared with the earlier studies conducted on UF retentates but, not subjected to homogenization treatment. Sweetsur and Muir (1980) added TSC, mixture of MSP and DSP in 2:1 ratio at the rate of 200 mg/100 ml in control UF retentate as well as in forewarmed (90 °C, 10 min) concentrated milk (20.9% TS) to check the improvement in heat stability, measured at 130 °C. They observed that addition of single or combination of stabilizing salts did not markedly improved the HCT of UF retentates. Improvement in HCT of homogenized, medium (Khatkar et al. 2014) fat as well as full fat (Khatkar and Gupta 2012) liquid dairy whiteners were earlier observed with the addition of 0.4% mixture of monosodium and disodium phosphate (2:1 w/w) stabilizing salts.
Our findings were in good agreement with the work earlier conducted in author’s lab by Khatkar et al. (2014); Khatkar and Gupta (2012). These results will be of great importance during processing of homogenized UF retentates at high-temperature treatments such as conventional sterilization and UHT processing as such or as a part of other formulations. Hence, it is concluded that both the stabilizing salts are capable in improving the HCT of 5× HUFR sample markedly and their selection may be based on the desired viscosity in the end product.
Effect of concentration, homogenization and salts addition on ζ-potential of the samples
The ζ-potential of casein micelles was varied from −21.23 to −0.568 mV in the studied pH range and the same was in the broad agreement with the earlier reported values by Bouzid et al. (2008); Huppertz and Fox (2006). ζ- potential of all the samples were measured at their native pH and the same differed significantly (P < 0.01) except 5× UFR and 5× DSP samples (Table 1). Moreover, with increase in calcium content and reduction in pH, ζ-potential was decreased in milk (Dalgleish 1984; Anema and Klostermeyer 1996) and 2× concentrated UF retentate (Huppertz and Fox 2006), similar trend of reduction was observed in this study also (Table 1). As compared to 5× UFR sample (−0.598 mV) the ζ-potential of 5× Na–K sample (−18.5 mV) was further reduced. This might be attributed to the reduction in pH and increase calcium content during DF process. Concentration of ions either attached to caseins or present in colloidal state (i.e. Ca2+, sulphate and magnesium) increased during UF concentration, while the concentration of ions (such as sodium, potassium, chloride, sulphate and citrates) present in soluble state decreased due to their passage into permeate. This might have disturbed delicate salt balance (salt ratio) and decreased ζ-potential (Table 1). Due to shearing and cavitation action in homogenization, surface modification of proteins took place which might be responsible for reduction in ζ-potential (Table 1).
Rheological properties of different samples
The rheological data obtained during flow behavior measurement of the samples were further analyzed for the best fit of rheological model. The model parameters thus obtained are presented in the Table 2. The apparent viscosity values increased with increase in CF due to increase in TS. UF retentates up to 3× CF exhibited Herschel–Bulkley behavior with liner increase in flow behavior index (n) with increase in CF. However, the same was reduced upon subsequent concentration to 5×. Marked decrease in n was observed in the DSP added 5× UF retentate sample. Randhahn (1976) determined flow properties of skim milk retentate obtained through UF at 18–22 °C containing maximum 27% TS between 5 and 60 °C temperature range at 5–1000 s−1 shear rate. Based on temperature and TS, the retentate showed time-independent pseudoplastic behavior that was properly explained by power law. Moreover, in the present investigation 25–27% TS concentration was obtained during UF at 20 °C after that the viscosity of the retentate increased sharply with strong pseudoplastic behavior. Slight decrease in n and apparent viscosity (at 50 s−1) was observed in homogenized sample but salts (NaCl–KCl) addition altered the rheological behavior of 5× UF retentate. The DF treatment with NaCl–KCl solution increased the apparent viscosity (at 50 s−1) from 69.7 to 81.7 mPa-s whereas added DSP sharply increased it to 430.0 mPa.s. The DSP added UF retentates also exhibited Carreau–Yasuda behavior (i.e. pseudoplastic at lower shear rate while Newtonian at high shear rate, R 2 = 0.999). It had infinite zero-shear viscosity value which explains its consistency. The infinite shear viscosity was 39.74 mPa.s at a critical shear rate of 0.021 s−1. However, the Bingham model for this sample showed 3.22 Pa yield stress to commence the flow of gel as compared to <0.8 Pa for other samples (Table 3).
Table 3.
Rheological parameters modelling of 5× HUFR samples at the pH of maximum heat stability
| Samplea | Bingham | Herschel–Bulkley | Power Law | T (°C)b | Viscosity (mPa.s) at 50 s−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| σ0 (Pa) | η (mPa.s) | R2 | σ0, Pa | k | η (mPa.s) | R2 | k | n | R2 | |||
| 5× TSC (6.6) | 1.183 | 75.59 | 0.973 | 0.941 | 0.203 | 0.774 | 0.999 | 0.965 | 0.434 | 0.949 | 53.1 | 46.4 |
| 5× DSP (6.5) | 0.962 | 54.86 | 0.978 | 0.782 | 0.143 | 0.781 | 0.999 | 0.766 | 0.419 | 0.941 | 55.5 | 35.2 |
| 5× DSP (6.6) | 0.991 | 51.18 | 0.977 | 0.838 | 0.119 | 0.809 | 0.998 | 0.785 | 0.402 | 0.937 | 50.6 | 46.1 |
| 5× DSP (6.7) | 0.802 | 64.20 | 0.966 | 0.580 | 0.202 | 0.735 | 0.999 | 0.679 | 0.470 | 0.962 | 53.1 | 42.6 |
aValues in parentheses shows the pH at which samples exhibited maximum heat stability
bT—Temperature corresponding to minimum viscosity
The 5× HUFR sample was again adjusted for its pH value using TSC and DSP salts. The samples having maximum heat stability at 120 °C (more than 120 min.) were selected for their rheological behavior. All the samples exhibited the Herschel–Bulkley behavior. However, TSC contributed to the increase in the viscosity as well as yield stress to the highest level among all four samples. The viscosity of all the samples were reduced initially upon heating, which increased continuously as temperature increased. The temperature corresponding to minimum viscosity was least for 5× DSP sample at pH 6.6 while highest for the same at pH 6.5. This will play a vital role during heating of UF retentates prior to spray drying as the viscosity tend to rise with increase in temperature. It will also hamper the flow in pipelines and atomization of such samples. Moreover, the impact of different processes on viscosity of UF retentates as function of temperature. It was also observed that without compromising with the heat stability of 5× HUFR, selection of stabilizing salt can be made based on desired high or low viscosity at a particular temperature. The applied homogenization treatment reduced the heat stability of 5× UFR (Table 1), which was significantly improved by TSC and DSP (Table 1). Moreover, homogenization of UF retentates improved solubility of MPC (Sikand et al. 2012), but our findings might be of great significance in solubility enhancement of such high protein powders without decreasing its heat stability.
Conclusion
Concentration of milk proteins in UF process markedly increased the calcium content, but decreased pH, thereby reduced ζ-potential and heat stability of retentate. Homogenization and diafiltration of 5× UFR with NaCl–KCl also exhibited detrimental effect on the heat stability. However, addition of stabilizing salts (MSP, DSP and TSC) improved the heat stability of 5× HUFR, even higher than that of skim milk. Rheological modelling revealed that UF retentates followed Herschel–Bulkley behavior. UF and DF processes increased, while homogenization decreased the viscosity of retentate significantly without changing the flow behavior. The study revealed that even homogenized 5× UFR containing stabilizing salts can be employed for the manufacture of heat stable formulations such as dairy whiteners and milk protein concentrates with improved functional properties.
Acknowledgements
The authors are very grateful to the Director, ICAR-National Dairy Research Institute, Karnal for providing the required facilities to carrying out this work.
References
- Anema SG, Klostermeyer H. Zeta-potentials of casein micelles from reconstituted skim milk heated at 120 °C. Int Dairy J. 1996;6:673–687. doi: 10.1016/0958-6946(95)00070-4. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 18. Gaithersburg: The association of official analytical chemists; 2005. [Google Scholar]
- Bouzid H, Rabiller-Baudry M, Paugama L, Rousseau F, Derriche Z, Bettahar NE. Impact of zeta potential and size of caseins as precursors of fouling deposit on limiting and critical fluxes in spiral ultrafiltration of modified skim milks. J Membr Sci. 2008;314:67–75. doi: 10.1016/j.memsci.2008.01.028. [DOI] [Google Scholar]
- Dalgleish DG. Measurement of electrophoretic mobilities and zeta-potentials of particles from milk using laser Doppler electrophoresis. J Dairy Res. 1984;51:425–438. doi: 10.1017/S0022029900023724. [DOI] [Google Scholar]
- De Kruif CG, Holt C. Casein micelle structure, functions and interactions. In: Fox PF, McSweeney PLH, editors. Advanced dairy chemistry, vol. 1: proteins, part A. 3. New York: Kluwer Academic/Plenum Publishers; 2003. pp. 233–276. [Google Scholar]
- Ferrer MA, Alexander M, Corredig N. Does ultrafiltration have a lasting effect on the physico-chemical properties of the casein micelles? Dairy Sci Technol. 2011;91:151–170. doi: 10.1007/s13594-011-0002-0. [DOI] [Google Scholar]
- Goldar P, Givianrad MH, Shams A. Effect of ultrafiltered milk permeate and non-dairy creamer powder concentration on low phenylalanine yoghurt’s physicochemical properties during storage. J Food Sci Technol. 2016;53(7):3053–3059. doi: 10.1007/s13197-016-2278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy EE, Muir DD, Sweetsur AWM, West LG. Changes of calcium phosphate partition and heat stability during manufacture of sterilized concentrated milk. J Dairy Sci. 1984;67:1666–1673. doi: 10.3168/jds.S0022-0302(84)81490-3. [DOI] [Google Scholar]
- Holt C, Dalgleish DG, Jenness R. Inorganics constituents of milk. 2. Calculation of the ion equilibrium in milk diffusate and comparison with experiment. Anal Biochem. 1981;113:154–163. doi: 10.1016/0003-2697(81)90059-2. [DOI] [PubMed] [Google Scholar]
- Huppertz T, Grosman S, Fox PF, Kelly AL. Heat and ethanol stabilities of high-pressure-treated bovine milk. Int Dairy J. 2006;14(2):125–133. doi: 10.1016/S0958-6946(03)00170-5. [DOI] [Google Scholar]
- Huppertz T, Fox PF. Effect of NaCl on some physico-chemical properties of concentrated bovine milk. Int Dairy J. 2006;16:1142–1148. doi: 10.1016/j.idairyj.2005.09.011. [DOI] [Google Scholar]
- IDF . Lait: De´termination de la teneur en azote; IDF Standard 20 B. Brussels: International Dairy Federation; 1993. [Google Scholar]
- Indian Standard (1961) Methods of test for dairy industry-part II: chemical analysis of milk. Bureau of Indian Standards, IS-1479, Manak Bhavan, BIS, New Delhi
- Indian Standard (1977) Determination of fat by the Gerber method-part I: milk. Bureau of Indian Standards, IS-1224, Manak Bhavan, BIS, New Delhi
- Indian Standard (1997) Milk, cream and evaporated milk-determination of total solid content (Reference method). Bureau of Indian Standards, IS-12333, Manak Bhavan, BIS, New Delhi
- Karlsson AO, Ipsen R, Schrader K, Ardo Y. Relationship between physical properties of casein micelles and rheology of skim milk concentrate. J Dairy Sci. 2005;88:3784–3797. doi: 10.3168/jds.S0022-0302(05)73064-2. [DOI] [PubMed] [Google Scholar]
- Kaushik R, Sachdeva B, Arora S, Kapila S, Wadhwa BK. Bioavailability of vitamin D2 and calcium from fortified milk. Food Chem. 2014;147:307–311. doi: 10.1016/j.foodchem.2013.09.150. [DOI] [PubMed] [Google Scholar]
- Khatkar SK, Gupta VK. Physicochemical and functional quality attributes of dairy whitener prepared from ultrafiltration process. J Food Process Preserv. 2012;38:1145–1154. doi: 10.1111/jfpp.12074. [DOI] [Google Scholar]
- Khatkar SK, Gupta VK, Khatker AB. Studies on preparation of medium fat liquid dairy whitener from buffalo milk employing ultrafiltration process. J Food Sci Technol. 2014;51(9):1956–1964. doi: 10.1007/s13197-014-1259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieczewska K, Kruk A, Czerniewicz M. Effect of homogenization on physico-chemical properties of condensed milk. Nat Sci. 1999;3:71–80. [Google Scholar]
- Kieczewska K, Kruk A, Czerniewicz M, Warminska M, Haponiuk E. The effect of high-pressure homogenization on changes in milk colloidal and emulsifying systems. Pol J Food Nutr Sci. 2003;12(Suppl. 1):43–44. [Google Scholar]
- Meena PK, Gupta VK, Meena GS, Raju PN, Parmar PT. Application of ultrafiltration technique for the quality improvement of dahi. J Food Sci Technol. 2015;52:7974–7983. doi: 10.1007/s13197-015-1951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry VV. Manufacture and application of high milk protein powder. Lait. 2002;82:515–522. doi: 10.1051/lait:2002028. [DOI] [Google Scholar]
- Montella JW.2008. Effects of filtration temperature and heat treatment on composition and rheological properties of whole milk ultrafiltration retentates. M. Sc. Thesis, California Polytechnic State University, San Luis Obispo
- Muir DD, Sweetsur AWM. The effect of concentration on the heat stability of skim milk. J Dairy Res. 1978;45:37–45. doi: 10.1017/S0022029900016174. [DOI] [Google Scholar]
- O’Connell JE, Fox PF. Heat stability of milk. In: Fuquay JW, Fox PF, McSweeney PLH, editors. Encyclopaedia of dairy science. 2. San Diego: Academic Press; 2011. pp. 744–749. [Google Scholar]
- Ozimek L, Sirlaorkul S, Dziuba J, Pikus W. Heat stability of skim milk concentrated by ultrafiltration. J Dairy Sci. 1988;71(suppl. 1):109. [Google Scholar]
- Randhahn H. The flow properties of skim milk concentrates obtained by ultrafiltration. J Texture Stud. 1976;7(2):205–217. doi: 10.1111/j.1745-4603.1976.tb01262.x. [DOI] [Google Scholar]
- Sikand V, Tong P, Vink S, Walker J. Effect of powder source and processing conditions on the solubility of milk protein concentrates 80. Milchwissenschaft. 2012;67:300–303. [Google Scholar]
- Sikand V, Tong PS, Roy S, Rodriguez-Saona LE, Murray BA. Effect of adding salt during the diafiltration step of milk protein concentrate powder manufacture on mineral and soluble protein composition. Dairy Sci Technol. 2013;93:401–413. doi: 10.1007/s13594-013-0110-0. [DOI] [Google Scholar]
- Singh H (1995) Heat-induced changes in caseins including interactions with whey proteins, in heat-induced changes in milk. In: Fox PF (ed) Special Issue 9501, International Dairy Federation, Brussels, pp 86–99
- Singh H. Heat stability of milk. Int J Dairy Technol. 2004;57:111–119. doi: 10.1111/j.1471-0307.2004.00143.x. [DOI] [Google Scholar]
- Singh H, Creamer LK. Heat stability of milk. In: Fox PF, editor. Advanced dairy chemistry-I proteins. London: Elsevier; 1992. pp. 621–656. [Google Scholar]
- Smith K. Commercial membrane technology. In: Tamime AY, editor. Membrane processing: dairy and beverage applications. 1. Chichester: Wiley; 2013. pp. 52–71. [Google Scholar]
- Solanki P, Gupta VK. Effects of stabilizing salts on heat stability of buffalo skim milk ultrafiltered-diafiltered retentate. J Food Sci Technol. 2009;46(5):466–469. [Google Scholar]
- St-Gelais D, Hache S, Gros-Louis M. Combined effects of temperature, acidification, and diafiltration on composition of skim milk retentate and permeate. J Dairy Sci. 1991;75:1167–1172. doi: 10.3168/jds.S0022-0302(92)77863-1. [DOI] [Google Scholar]
- Sweetsur AWM, Muir DD. Effect of concentration by ultrafiltration on the heat stability of skim milk. J Dairy Res. 1980;47:327–335. doi: 10.1017/S002202990002121X. [DOI] [Google Scholar]